Abstract
Prior research has shown that perceived social isolation (loneliness) motivates people to attend to and connect with others but to do so in a self-protective and paradoxically self-defeating fashion. Although recent research has shed light on the neural correlates of social perception, cooperation, empathy, rejection and love, little is known about how individual differences in loneliness relate to neural responses to social and emotional stimuli. Using functional MRI we show that there are at least two neural mechanisms differentiating social perception in lonely and nonlonely young adults. For pleasant depictions, lonely individuals appear to be less rewarded by social stimuli, as evidenced by weaker activation of the ventral striatum to pictures of people than of objects, whereas nonlonely individuals showed stronger activation of the ventral striatum to pictures of people than of objects. For unpleasant depictions, lonely individuals were characterized by greater activation of the visual cortex to pictures of people than of objects, suggesting their attention is drawn more to the distress of others; whereas nonlonely individuals showed greater activation of the right and left temporoparietal junction to pictures of people than of objects, consistent with the notion that they are more likely to reflect spontaneously on the perspective of distressed others.
As a social species, humans create emergent organizations beyond the individual– structures that range from dyads, families, and groups to cities, civilizations, and cultures. These emergent structures evolved hand in hand with neural and hormonal mechanisms to support them because the consequent social behaviors helped these organisms survive, reproduce, and care for offspring sufficiently long that they too reproduced (Cacioppo & Patrick, in press; Dunbar & Shultz, 2007). The multimodal neurophysiological processes involved in the execution of an action, for instance, give rise to parallel neurophysiological sensorimotor processes in the observer of these actions (Rizzolatti & Craighero, 2004). This mirror neuron system appears to play a role in a variety of social processes including mimicry, synchrony, contagion, coordination, and co-regulation (e.g., Rizzolatti & Fabbri-Destro, in press; Semin & Cacioppo, in press).
Empathy for another person’s pain is also associated with many of the same neural mechanisms associated with one’s personal experience, including activation of the dorsal anterior cingulate (dACC), thalamus, and anterior insula (Decety & Lamm, in press-a; Jackson, Rainville, & Decety, 2006). In an illustrative study, Jackson, Meltzoff, and Decety (2005) found that the level of activity in the dACC was strongly correlated with ratings of the intensity of pain experienced by the observed person, a result reminiscent of Eisenberger, Lieberman, and Williams’ (2003) finding that the social pain participants felt during an episode of social exclusion was strongly correlated with activity in the dACC. In the case of empathy and of social pain, evolutionarily older neural mechanisms appear to have been co-opted to serve important social functions. This exaptation of mammalian neural mechanisms to serve social, in addition to emotional, functions does not appear to be limited to the dACC (Norris & Cacioppo, 2007).
Behavioral and neuroimaging studies suggest that establishing a sense of social connection is fundamentally rewarding. For instance, a daily activity reconstruction method study to assess how 909 employed women spend their time and experienced their life revealed that respondents reported the most enjoyment from spending time with friends, relatives, and spouses, and among the least enjoyment when alone (Kahneman et al., 2004). Neuroimaging studies indicate that social cooperation (Rilling et al., 2002) and romantic love (Aron et al., 2005) are associated with activation of the ventral striatum, a region involved in reward (Smith & Berridge, 2005), expected reward (Knutson & Bossaerts, 2007), and motivational evaluation more generally (Yeates et al., 2007).
Not everyone feels socially connected, however. The loss or absence of meaningful relationships creates strong negative affect and hostility (Cacioppo et al., 2006b; Rotenberg, 1994), impairs self regulation (e.g., Baumeister, DeWall, Ciarocco, & Twenge, 2005; Cacioppo et al., 2000), promotes a search for social information (Gardner, Pickett, Jeffries, & Knowles, 2005) and human connection (Baumeister & Leary, 1995; Epley et al., 2007), and predicts adverse health outcomes (Adam et al., 2006; Hawkley et al., 2006; Seeman, 2000). This debilitating psychological condition, termed loneliness (Weiss, 1973), has a significant heritable component (Bartels, Cacioppo, Hudziak, & Boomsma, in press; Boomsma, Willemsen, Dolan, Hawkley, & Cacioppo, 2005); is stochastically and functionally distinguishable from other states (e.g., mood, perceived stress) and dispositions (e.g., extraversion, neuroticism, depressiveness, hostility, social support; Berscheid & Reis, 1998; Cacioppo et al., 2006a, 2006b; Marangoni & Ickes, 1989); and describes a chronic experience for more than 20% of the U.S. population (Davis & Smith, 1998).
Loneliness also influences how people perceive and think about the world. For instance, in addition to feeling unhappy, lonely, compared to nonlonely, individuals feel unsafe. They are more likely to construe others as threatening, appraise stressors as threats rather than challenges, and cope with stressors in a passive, isolative fashion rather than an active fashion that includes actively seeking the help and support of others (Berscheid & Reis, 1998; Cacioppo & Hawkley, 2005). In a study of college students, lonely individuals did not differ from their nonlonely counterparts in the number of major life stressors they experienced or the number of uplifts they encountered during the course of a day, but lonely individuals reported that these uplifts were less intense than nonlonely individuals (Cacioppo et al., 2000). An experience sampling study demonstrated that these lonely and nonlonely individuals did not differ in the frequency of social contacts or in the profile of activities in which they were engaged (Hawkley et al., 2003), suggesting that lonely compared to nonlonely individuals may derive less pleasure from social observations and encounters. To investigate this possibility, we used functional magnetic resonance imaging (fMRI) to investigate differences in the neural responses to social stimuli with emotional content, relative to matched nonsocial stimuli, in individuals differing in loneliness. We hypothesized that the lower the loneliness (greater social connection), the greater the activation in reward areas of the brain that would be observed in the pleasant social minus nonsocial picture contrast. This pattern of brain activation was not expected to occur in the unpleasant social minus nonsocial picture contrast.
METHODS
Participants
Twenty-three female University of Chicago undergraduates participated in the study. All were right-handed, had normal or corrected-to-normal vision, and were not diagnosed with a chronic disease (including any psychopathology). Participants were required to either have completed a previous fMRI study or to undergo an fMRI simulation session prior to their participation to minimize any individual differences in anxiety attributable to the scanner. Participants gave informed written consent before the experiment in accordance with the University of Chicago Health Sciences Institutional Review Board and were compensated for their time at the rate of $20/hour. Participants completed four tasks in the scanner; the picture viewing task was included to test the current hypotheses.
Stimulus Materials, Tasks, and Study Design
In the scanner, participants viewed a series of pictures that varied in their emotional (i.e., negative/unpleasant, positive/pleasant) and social (i.e., nonsocial, social) content. Pictures were chosen from the International Affective Picture System (Lang, Bradley, & Cuthbert, 1999). Sample stimuli include: a roach (IAPS #: 1270) and explosion (9630) as unpleasant nonsocial pictures; a soldier (9160) and man slapping a woman (6360) as unpleasant social pictures; money (8502) and a rocket liftoff (5450) as pleasant nonsocial pictures; and a roller coaster (8490) and a man and dog running (8460) as pleasant social pictures. Note that social pictures were not chosen to present social relationships or interactions; rather, we were interested in investigating basic social perception as a function of loneliness and pictures were chosen accordingly. The target pictures were embedded in a larger set of filler pictures. Each picture was presented for 6 s. Intertrial intervals, consisting of a white crosshairs on a black background, were jittered to allow for deconvolution of the hemodynamic response and ranged from 1.5 – 29 s in duration. Participants were asked simply to view each picture for the entire duration that it was presented and to make a categorical judgment regarding the valence of each picture by using their right hand to press one of three buttons on a response box to indicate whether it was negative (index finger), neutral (middle finger), or positive (ring finger). Pictures were presented in one of two pre-determined random orders that were counterbalanced across subjects.
Following the scanner protocol, participants viewed the same series of pictures and rated how negative and positive they felt about each using a 5 (negativity: 0 = not at all negative, 4 = extremely negative) × 5 (positivity: 0 = not at all positive, 4 = extremely positive) grid, and how arousing they found each using a 9-point arousal scale (1 = not at all arousing, 9 = extremely arousing. A valence rating was calculated by subtracting the negativity rating from the positivity rating (Larsen, Norris, McGraw, Hawkley, & Cacioppo, in press).
Individual Differences in Loneliness
At the end of the experimental session participants completed a set of questionnaires including the UCLA Loneliness Scale (Russell, 1996). The UCLA Loneliness Scale consists of 20 items measuring general loneliness and degree of satisfaction with one’s social relationships. An example statement is, “How often do you feel that there is no one you can turn to?” Participants are instructed that the statements describe how people sometimes feel, and that for each statement they should indicate how often they feel the way described by the statement (1 = never, 2 = rarely, 3 = sometimes, 4 = always). After reverse scoring appropriate items, the UCLA loneliness score is calculated by summing the scores of all items (α = 0.88).
fMRI Image Acquisition
A PC was used to present stimuli and to record participants’ responses. Visual stimuli were presented using binocular goggles mounted on the head coil approximately two inches above the participant’s eyes. Button-press responses were made on an fMRI-compatible response box.
Imaging was performed on a 3T GE Signa scanner (GE Medical Systems, Milwaukee, WI) with a standard quadrature GE head coil. High-resolution volumetric T1-weighted spoiled gradient-recalled (SPGR) images were obtained for each subject in 124 1.5-mm sagittal slices with 10° flip angle and 24 cm field of view (FOV) for use as anatomical images. Functional images were acquired using a gradient-echo spiral-in/out pulse sequence (Glover & Law, 2001) with 33 contiguous 5-mm coronal slices in an interleaved order spanning the whole brain (TR = 2.5 s, TE = 26 ms, flip angle = 77°, FOV = 22 cm; 64 × 64 matrix size, fat suppressed).
fMRI Image Preprocessing and Analyses
Spiral-in and spiral-out images were reconstructed first separately and then combined using a weighted-average algorithm that maximizes signal-to-noise ratio while reducing signal loss (Glover & Law, 2001; Preston et al., 2004). Further image processing was performed using AFNI software. For each subject, motion detection and correction were undertaken using a six-parameter, rigid-body transformation. Functional images were temporally smoothed using a low-pass filter consisting of a 3-point Hamming window; and were spatially smoothed using a 5 mm full-width at half-maximum (FWHM) Gaussian filter.
Individual-subject analyses were conducted using a deconvolution analysis to generate impulse response functions (IRFs) of the BOLD signal on a voxel-wise basis (Ward, 2001). This approach produces an estimate of the hemodynamic response for each condition relative to a baseline state without a priori assumptions about the IRF. The deconvolution analysis uses a separate regressor for each time point of each condition, and fits these regressors using a linear least squares model to each time point of the hemodynamic response. Each of the four conditions (i.e., unpleasant nonsocial, unpleasant social, pleasant nonsocial, pleasant social) had seven regressors, one for each TR. Output from the deconvolution analysis conducted for each participant was converted to Talairach stereotaxic coordinate space (Talairach & Tournoux, 1988) and interpolated to volumes with 3 mm3 voxels. Estimated signal intensity for the four TRs under the peak of the hemodynamic response (i.e., a measure of area under the curve [AUC]; TRs 2–5) was averaged for each voxel in each condition for use in group analyses.
Whole brain voxel-wise regressions
For each subject, two contrasts were conducted for use in group-level analyses. The first contrast was calculated as the difference in average percent signal change when viewing pleasant social minus pleasant nonsocial pictures; the second contrast was between unpleasant social minus unpleasant nonsocial pictures. The ventricles, cerebellum, brainstem, and white matter were masked.
To examine the relationship between loneliness and patterns of neural activation to pictures that varied in emotional and social content, we conducted two whole-brain voxel-wise regression analyses predicting neural activation for each contrast from subjects’ scores on the UCLA Loneliness Scale. The β1 term from each regression at each voxel represents the relationship between loneliness and neural activation. Results from each whole brain regression were subjected to a cluster analysis, using an individual voxelwise threshold of p < 0.025, a minimum cluster connection radius of 5.2 and a cluster volume of 459 μL (corresponding to 48 active, contiguous voxels). Minimum cluster volume was determined using a Monte Carlo simulation with 10,000 iterations, and assuming some interdependence between voxels (5 mm FWHM), resulting in a corrected whole-brain p-value of .05. Outliers were identified on a cluster-by-cluster basis, and the analyses were repeated without the outliers.
RESULTS
Behavioral Data
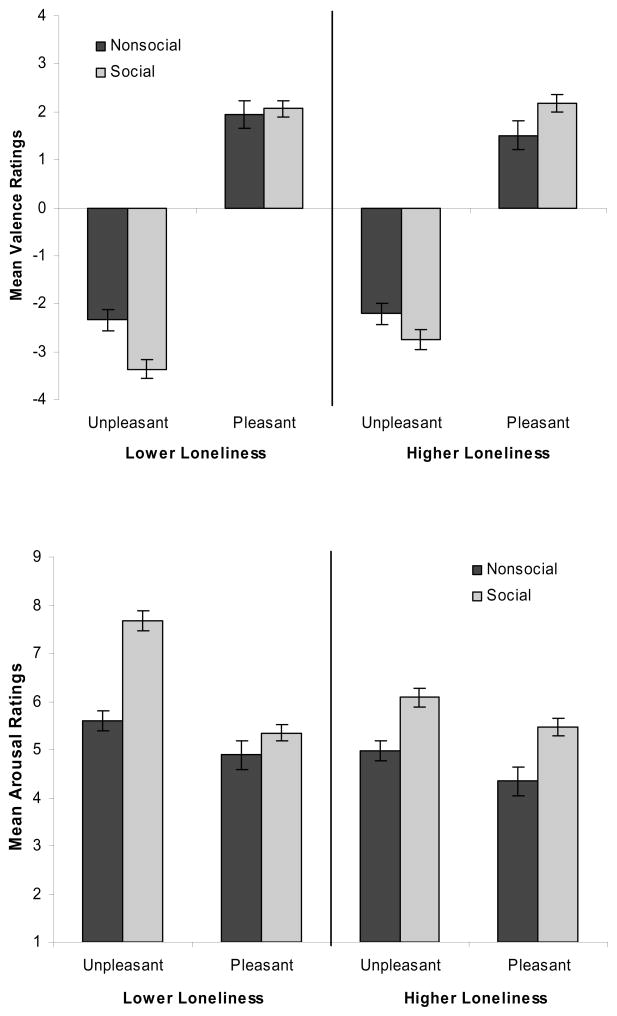
Valence ratings were subjected to a Valence (pleasant, unpleasant) × Content (social, nonsocial) × Loneliness (continuous) general linear regression model (GLM) analysis. Any effect involving the Loneliness factor was interpreted using estimates at one standard deviation above and below the mean in our sample. The Valence main effect, F(1, 21) = 45.86, p < .001, confirmed that unpleasant stimuli (M = −2.67, SE = 0.13) were rated more negatively than pleasant stimuli (M = 1.92, SE = 0.14). In addition, the Social main effect, F(1, 21) = 6.60, p < .05, showed that social stimuli (M = −0.48, SE = 0.10) were rated more negatively than nonsocial stimuli (M = −0.27, SE = 0.12). This main effect, however, was qualified by a Social × Loneliness interaction, F(1, 21) = 4.96, p < .05, which indicated that nonlonely individuals rated social stimuli more negatively than nonsocial stimuli whereas lonely individuals rated social and nonsocial stimuli equally (see Figure 1, top panel). Importantly, no differences were found in the ratings of the pleasant stimuli.
Figure 1.
Results from 2(Valence: unpleasant, pleasant) × 2(Content: nonsocial, social) × UCLA (continuous) GLMs conducted on participants’ valence ratings (top panel) and arousal ratings (bottom panel). Estimates at 1 SD above and below the mean UCLA score in our sample are presented; error bars represent one SE.
Analyses of the arousal ratings produced a main effect for Valence, F(1, 21) = 9.69, p < .01, a marginal Valence × Loneliness interaction, F(1, 21) = 4.23, p = .052, a Valence × Social interaction, F(1, 21) = 6.87, p < .05, and a Valence × Social × Loneliness interaction, F(1, 21) = 4.80, p < .05. Social stimuli generally were rated as more arousing than nonsocial stimuli, and this difference was particularly pronounced for nonlonely participants in response to unpleasant social stimuli (Figure 1, bottom panel).
fMRI Data
Loneliness and Pleasant Social – Pleasant Nonsocial Picture Processing
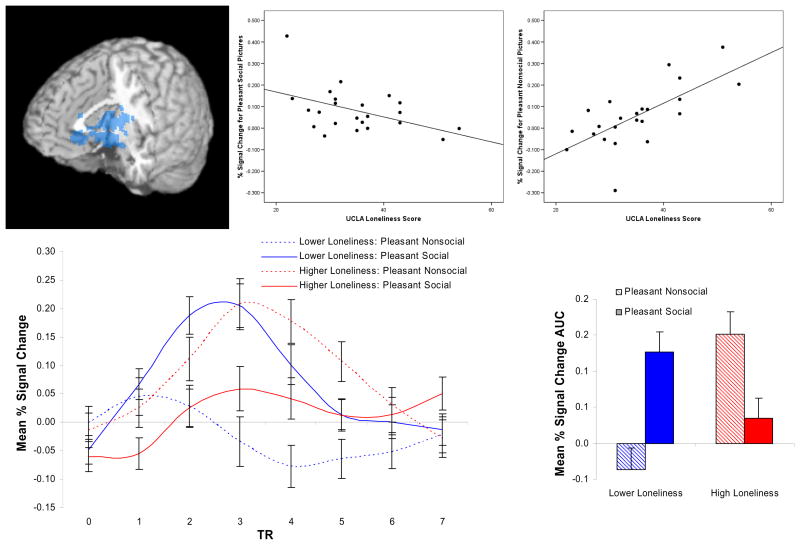
The whole brain regression analyses of loneliness scores against the BOLD signal detected in the pleasant social minus pleasant nonsocial contrast revealed five significant regions of covariation (see Table 1). The largest region was centered in the ventral striatum, part of the neural reward network, and showed that the lower the loneliness, the greater the BOLD signal in this contrast, r(21) = −.75, p < .001 (Figure 2). To better understand this relationship, we examined correlations between loneliness scores and neural activation to pleasant social pictures and to pleasant nonsocial pictures separately. These correlations indicated that loneliness was negatively related to neural activation in the region centered in the ventral striatum when viewing pleasant social pictures, r(21) = −.46, p < .05, and positively related to neural activation in this region when viewing pleasant nonsocial pictures, r(21) = .69, p < .001. That is, the less the participant felt socially isolated the greater the activation of the ventral striatum when viewing pleasant social pictures, whereas the more the participant felt socially isolated the greater the activation of the ventral striatum to pleasant nonsocial pictures.
Table 1.
Regions exhibiting a significant relationship between loneliness and neural activation.
| Region | BA | Volume | x | y | z |
|---|---|---|---|---|---|
| Pleasant Social – Pleasant Nonsocial Contrast | |||||
| Ventral striatum | 24678 | 1 | −5 | 2 | |
| Left dorsomedial PFC | 810 | −22 | 26 | 22 | |
| Right medial frontal gyrus | 6 | 702 | 10 | −21 | 57 |
| Left fusiform gyrus | 675 | −31 | −39 | −12 | |
| Left anterior insula | 13 | 513 | −36 | 13 | 7 |
| Unpleasant Social – Unpleasant Nonsocial Contrast | |||||
| Left primary visual cortex | 17 | 891 | −21 | −80 | 16 |
| Right caudate & caudate body | 810 | 15 | 2 | 16 | |
| Right inferior frontal gyrus | 46 | 729 | 43 | 39 | 7 |
| Left superior temporal gyrus | 22, 39 | 513 | −49 | −54 | 22 |
| Right superior temporal gyrus | 22, 39 | 513 | 54 | −57 | 17 |
| Right secondary visual cortex | 19 | 459 | 27 | −83 | 19 |
Figure 2.
A cluster of voxels centered in the ventral striatum, but extending to the amygdala and portions of the anterior thalamus, showed an inverse relationship between loneliness and activation in the Pleasant Social – Pleasant Nonsocial contrast. The scatterplots demonstrate the association between loneliness and activity in this cluster in response to pleasant social pictures, r(21) = −.46, p < .05, and in response to pleasant nonsocial pictures, r(21) = .69, p < .001. Estimated impulse response functions and mean percent signal change AUC for participants lower and higher in loneliness (estimates at 1 SD above and below the mean UCLA score in our sample are presented) shows a crossover interaction for the relationship between loneliness and brain responses to pleasant social and pleasant nonsocial stimuli, such that nonlonely participants exhibit greater activation to pleasant pictures that contain social content and lonely participants exhibit greater activation to pleasant nonsocial pictures.
In addition to this large cluster in ventral striatum, other regions of covariation between loneliness and pleasant social – pleasant nonsocial picture processing were the dorsal mPFC, r(21) = −.79, p < .001; right medial frontal gyrus, r(21) = .67, p < .001; left fusiform gyrus, r(21) = −.68, p < .001; and left anterior insula, r(21) = −.68, p < .001. Follow-up correlations for the dorsal mPFC cluster indicated that loneliness was negatively related to neural activation when viewing pleasant social pictures, r(21) = −.60, p < .01, and positively related to neural activation when viewing pleasant nonsocial pictures, r(21) = .68, p < .01. Follow-up tests for the right medial frontal gyrus, left fusiform gyrus, and left anterior insula revealed the same pattern of effects, such that loneliness was unrelated to the BOLD signal change when viewing pleasant social pictures, r(21) = −.13, −.03, & −.19, respectively, all ns; whereas loneliness was significantly related to the BOLD signal change when viewing pleasant nonsocial pictures, r(21) = −.58, .55, & .43, ps < .05.
Loneliness and Unpleasant Social – Unpleasant Nonsocial Picture Processing
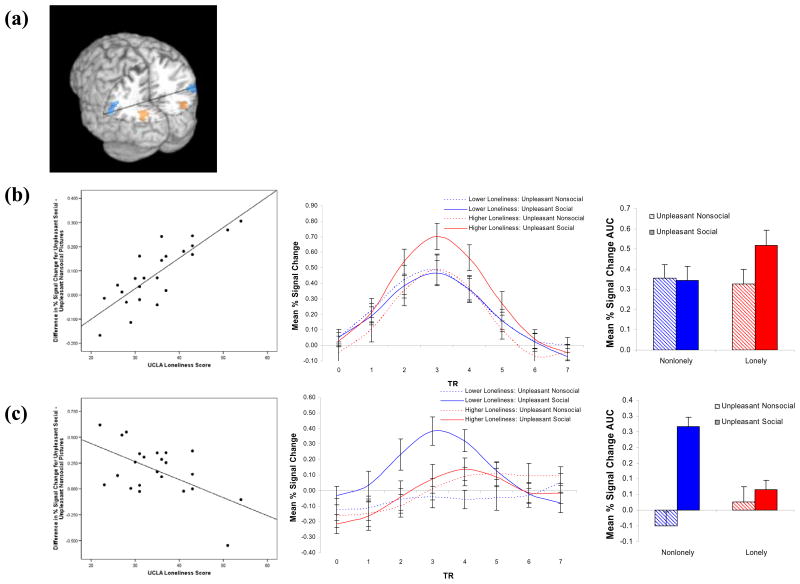
The whole brain regression analyses of loneliness scores against neural activation in the unpleasant social minus unpleasant nonsocial contrast revealed six significant regions of covariation (see Table 1). Four of these clusters included regions of bilateral visual cortex (left visual cortex, r(21) = .83, p < .001; right visual cortex, r(20) = .71, p < .001) and bilateral temporoparietal junction (left TPJ, r(20) = −.43, p < .05; and right TPJ, r(21) = −.57, p < .01). Follow-up tests revealed that lonely individuals tended to show greater activation of the bilateral visual cortex when viewing unpleasant social pictures than did nonlonely individuals, rs(21) = .35 & .39, for the left and right visual cortex, ps < .10; whereas loneliness was unrelated to neural activation when viewing unpleasant nonsocial pictures, rs(21) = −.06 & −.10, ns. In contrast, follow-up tests for the bilateral TPJ clusters revealed that nonlonely individuals tended to show greater activation of the TPJ to unpleasant social pictures than did lonely individuals, r(20) = −.31, ns, for the left TPJ and r(21) = −.46, p < .05, for the right TPJ. Loneliness scores were unrelated to the BOLD signal change in the TPJ when viewing unpleasant nonsocial pictures, r(20) = .17 and r(21) = .25, ns.
The remaining two clusters that survived whole brain correction for multiple comparisons were the right caudate and the right inferior frontal gyrus. Loneliness was inversely related to neural activation in the unpleasant social – unpleasant nonsocial contrast for the right caudate, r(21) = −.67, p < .001, and the right inferior frontal gyrus, r(20) = −.47, p < .05. Follow-up tests showed weak patterns for both of these clusters, such that loneliness tended to be negatively related to neural activation when viewing unpleasant social pictures, r(21) = −.41, p = .053, and r(20) = −.28, ns, respectively; and loneliness was unrelated to neural activation when viewing unpleasant nonsocial pictures, r(21) = .40 & r(20) = .28, respectively, both ns.
DISCUSSION
The present study provides further evidence that neural mechanisms that serve emotional functions can be co-opted to serve important social functions, as well. The ventral striatum, a key component of the mesolimbic dopamine system, is rich in dopaminergic neurons and is critical in reward processing and learning (e.g., Delgado, Miller, Inati, & Phelps, 2005; O’Doherty, 2004). The ventral striatum is activated by primary rewards such as stimulant drugs (Leyton, 2007) and food (O’Doherty, Deichmann, Critchley, & Dolan, 2002), abstinence-induced cravings for primary rewards (Wang et al., 2007), and secondary rewards such as money (Seymour, Daw, Dayan, Singer, & Dolan, 2007). Evidence that social rewards also activate the ventral striatum has begun to accumulate in studies of romantic love (Aron et al., 2005), social cooperation (Rilling et al., 2002), social comparison (Fliessbach et al., 2007), and punitive altruism (DeQuervain et al., 2004). In the present study, the lonelier the participant the less the activation elicited by pleasant pictures of people than of objects in a brain region centered in the ventral striatum and extending to the right amygdala, subgenual region of the ACC, caudate, thalamus, insula, lentiform, and putamen. Follow-up analyses indicated that participants who were low, compared to those who were high, in loneliness tended to show stronger activation of the ventral stratum in response to pleasant pictures of people, whereas the opposite pattern was observed when they were exposed to pleasant pictures of objects. Prior research has found that social interactions are more rewarding for individuals low than high in loneliness (Hawkley, Preacher, & Cacioppo, 2007), but the current study adds to this literature by showing that the simple exposure to pleasant depictions of people elicits stronger reward-related brain activity in the ventral striatum in nonlonely than lonely individuals. Differences in the rewarding qualities of others would explain prior findings such as daily uplifts – most of which involve other people –having less impact on lonely than nonlonely individuals (Cacioppo et al., 2000).
The association between individual differences in loneliness and activation of the ventral striatum in response to nonsocial stimuli indicates the reach of loneliness may not be limited to social stimuli. Given their feelings of social isolation, lonely individuals may be left to find relative comfort in nonsocial rewards. Recent work on anthropomorphism shows, for instance, that loneliness influences how people respond to a variety of nonsocial stimuli (Epley et al., 2007), and the present results show loneliness predicted greater reward-related brain activity in response to pleasant pictures of objects than of people. But the causal direction may also point in the other direction. Given the heritability of loneliness, the present study raises the intriguing possibility that loneliness may result from reduced reward-related brain activity in the ventral striatum in response to social (relative to nonsocial) rewards. If pleasant social stimuli do not serve as particularly powerful reinforcers, then subjugating self-interests to the interests of the pair bond or social group in exchange for the possibility of long term benefits or a greater good may be less compelling.
Prior functional neuroimaging work on thinking about the characteristics of people has reliably shown the dorsal mPFC to be involved (e.g., Mitchell, in press). The finding that individuals low in loneliness showed greater activity in the dorsal mPFC to pleasant social stimuli, whereas individuals high in loneliness showed the greatest activity in this region to pleasant nonsocial stimuli is consistent with individuals high in loneliness maintaining a psychological distance from others. Interestingly, the activity in regions that reflect more mandatory aspects of social perception (e.g., fusiform gyrus/face processing) was comparable for individuals high and low in loneliness when viewing social stimuli. It was when participants viewed nonsocial stimuli that individuals low compared to high in loneliness showed less activity in these regions.
The results of the present study also suggest that individual differences in loneliness do not map onto the activation of qualitatively different brain regions during the viewing of emotionally evocative social and nonsocial pictures. Instead, loneliness appears to modulate the extent to which a network of brain regions is activated and the circumstances in which a network is activated. The latter is evidenced by the different set of brain regions whose activation was found to vary as a function of loneliness in response to unpleasant social minus nonsocial pictures. Loneliness in this contrast was associated with regions involved in attention and first-person perspective taking. Specifically, the differences in neural activation in the visual cortices during the presentation of unpleasant social pictures suggest increased visual processing by individuals high, in contrast to low, in loneliness. These results are generally consistent with Gardner, Pickett, Jeffries, and Knowles’ (2005; Pickett & Gardner, 2005) social monitoring theory.
Our results may appear inconsistent with one aspect of Gardner et al.’s (2005) research. In their study, participants were instructed to form an impression of a person based on excerpts they read from a (hypothetical) person’s daily diary. They found lonely individuals showed heightened incidental social memory regardless of the valence of the behavioral description they read in the diary. In the present study, we found loneliness was related to the activation of the visual cortices in response to unpleasant pictures of people, relative to objects. Using a modified emotional Stroop task, Shintel, Cacioppo, and Nusbaum (2006) found that lonely, compared to nonlonely, individuals showed a greater interference effect in response to negative social words but comparable (and smaller) interference effects to positive social words. The diaries used as stimulus materials in Gardner et al. (2005) have greater personal relevance and behavioral implications than the IAPS pictures used in the current study or the words used in the Stroop task by Shintel et al. (2006). Together, the results suggest that negative social associations may be more accessible in memory in lonely than nonlonely individuals, as reflected by attentional indices, whereas tasks and stimulus materials that permit more extensive self-relevant processing may produce better recall for social information more generally.
We also found that individuals high in loneliness show less activity in the TPJ than individuals low in loneliness when they view unpleasant pictures. Activation of the TPJ has been associated with tasks involving theory of mind (Saxe & Kanwisher, 2003), inferences of social intentions (Ciaramidaro et al., 2007; Decety & Grezes, 2006), attentional reorienting (Decety & Lamm, 2007), and the sense of agency (Decety & Lamm, 2007). The results for the TPJ may imply that the lower an individual’s loneliness the more likely they may be to reorient their attention to consider the perspective of the people pictured in an unpleasant circumstance.
Finally, the regression analyses for the unpleasant pictures revealed nonlonely participants showed greater activation in the right caudate and right inferior frontal gyrus. Follow-up analyses indicated the neural activation in response to unpleasant social pictures tended to be greater in the caudate and right inferior frontal gyrus for nonlonely than lonely individuals, whereas the neural activation in both of these areas in response to unpleasant nonsocial pictures tended to be greater for lonely than nonlonely individuals. Loneliness was unrelated to activation in the right inferior frontal gyrus in response to unpleasant nonsocial pictures. However, the right caudate was the only region to be predicted by loneliness in response to pleasant and unpleasant social stimuli after controlling for the neural responses to equally emotional nonsocial stimuli. The neural activation of the caudate has been shown to be involved in reward-based learning (Galvan et al., 2005) and in incentive-based learning more generally (Tricomi et al., 2006). The reduction in neural response in the caudate observed for individuals high, relative to low, in loneliness may reflect the down-regulation of this system based on prior experience regarding the relatively less rewarding outcomes of social interactions or it may reflect less ability to learn from their social encounters.
In sum, social interactions are replete with opportunities for trust, understanding, hope, support, and cooperation just as they are full of opportunities for treachery, betrayal, conflict, and disappointment. Loneliness operates in part by shaping what people expect and think about other people. Lonely individuals seek to fulfill unmet needs but generally are less forgiving of minor hassles and transgressions than nonlonely individuals. The present results raise new questions about the role of the ventral striatum, TPJ, and caudate in differences in social cognition between lonely and nonlonely individuals, and about the brain mechanisms that enable skillful social interactions.
Figure 3.
(a) Clusters of voxels in the left and right visual cortices exhibited a positive relationship between loneliness and activation in the Unpleasant Social – Unpleasant Nonsocial contrast; whereas clusters of voxels in the left and right TPJ exhibited a negative relationship between loneliness and activation in the Unpleasant Social – Unpleasant Nonsocial contrast. (b) The scatterplot depicts the relationship between loneliness and activation of the left visual cortex in response to Unpleasant Social – Unpleasant Nonsocial pictures, r(21) = .83, p < .001. Estimated IRFs (and AUC values) show that individuals with higher loneliness showed greater activation to unpleasant social pictures. Results were comparable for the right visual cortex. (c) The scatterplot shows the inverse relationship between loneliness and activation of the right temporoparietal junction (TPJ) in response to Unpleasant Social – Unpleasant Nonsocial pictures, r(21) = −.58, p < .01. Estimated IRFs show that individuals lower in loneliness showed greater activation of the right TPJ to unpleasant social pictures in particular. Results were comparable for the left TPJ.
Acknowledgments
The authors would like to thank Robert Lyons, Carden Safran, John Scott Railton, J.S. Irick and Jia Hong Gao for their assistance. Support for this research was provided by NIMH Grant No. P50 MH72850, NIA Grant No. PO1 AG18911, and a grant from the John Templeton Foundation.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li HY, Brown LL. Reward motivation and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Bartels M, Cacioppo JT, Hudziak JJ, Boomsma DI. Genetic and environmental contributions to stability in loneliness throughout childhood. American Journal of Medical Genetic Part B: Neuropsychiatric Genetics. doi: 10.1002/ajmg.b.30608. in press. [DOI] [PubMed] [Google Scholar]
- Baumesiter RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88:589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The need to belong: Desire for interpersonal attachment as a fundamental human motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Berscheid E, Reis HT. Attraction and close relationships. In: Gilbert DT, Fiske ST, editors. The handbook of social psychology. 4. Vol. 2. New York, NY, US: McGraw-Hill; 1998. pp. 193–281. [Google Scholar]
- Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions to loneliness in adults: The Netherlands Twin Register Study. Behavior Genetics. 2005;35:745–752. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, Kowalewski RB, Paulsen A, Hobson JA, Hugdahl K, Speigel D, Berntson GG. Lonely traits and concomitant physiological processes: The MacArthur Social Neuroscience Studies. International Journal of Psychophysiology. 2000;35:143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. People thinking about people: The vicious cycle of being a social outcast in one’s own mind. In: Williams Kipling D, Forgas Joseph P, von Hippel William., editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York: Psychology Press; 2005. pp. 91–108. [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, Spiegel D. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006a;40:1054–1085. [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: Cross sectional and longitudinal analyses. Psychology and Aging. 2006b;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Patrick W. Loneliness. New York: Norton Press; in press. [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, Erk S, Pia L, Bara BG, Walter H. The intentional network: How the brain reads varieties of intentions. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.05.011. in press. [DOI] [PubMed] [Google Scholar]
- Davis JA, Smith TW. General social surveys, 1972–1998: Cumulative codebook. Chicago: National Opinion Research Center; 1998. [Google Scholar]
- De Quervain D, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J. The power of simulation: Imagining one’s own and other’s behavior. Brain Research. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C. The biological bases of empathy. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. New York: John Wiley & Sons; in press-a. [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition. The Neuroscientist. 2007;13:580–595. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24:862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Evolution of the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003 October 10;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Epley N, Waytz A, Cacioppo JT. On seeing human: A three-factor theory of anthropomorphism. Psychological Review. 2007;114:864–886. doi: 10.1037/0033-295X.114.4.864. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A. Social comparison affects reward-related brain activity in the ventral striatum. Science. 2007;318:1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of the ventral frontostriatal circuitry in reward-based learning in humans. Journal of Neuroscience. 2005;25:8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WL, Pickett CL, Jeffries V, Knowles M. On the outside looking in: Loneliness and social monitoring. Personality and Social Psychology Bulletin. 2005;31:1549–1560. doi: 10.1177/0146167205277208. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. Journal of Personality and Social Psychology. 2003;85:105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Multilevel modeling of social interactions and mood in lonely and socially connected individuals: The MacArthur social neuroscience studies. In: Ong AD, van Dulmen M, editors. Oxford Handbook of Methods in Positive Psychology. New York: Oxford University Press; 2007. pp. 559–575. [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: a window into the neural processes involved in empathy. NeuroImage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA. A survey method for characterizing daily life experience: The day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- Knutson B, Bossaerts P. Neural antecedents of financial decisions. Journal of Neuroscience. 2007;27:8174–8177. doi: 10.1523/JNEUROSCI.1564-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Digitized photographs, instruction manual and affective ratings. University of Florida; Gainesville, FL: 1999. Technical Report A-6. [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: A single-item measure of positive and negative evaluative reactions. Cognition & Emotion in press. [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Marangoni C, Ickes W. Loneliness: A theoretical review with implications for measurement. Journal of Social and Personal Relationships. 1989;6:93–128. [Google Scholar]
- Mitchell JP. Contributions of functional neuroimaging to social cognition. Current Directions in Psychological Science in press. [Google Scholar]
- Norris CJ, Cacioppo JT. I know how you feel: Social and emotional information processing in the brain. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: Integrating biological and psychological explanations of social behavior. New York: Guilford Press; 2007. pp. 84–105. [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Pickett CL, Gardner WL. The social monitoring system: Enhanced sensitivity to social cues as an adaptive response to social exclusion. In: Williams K, Forgas J, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York: Psychology Press; 2005. [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. NeuroImage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. The mirror neuron system. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. New York: John Wiley & Sons; in press. [Google Scholar]
- Rotenberg K. Loneliness and interpersonal trust. Journal of Social and Clinical Psychology. 1994;13:152–173. [Google Scholar]
- Russell D. UCLA Loneliness Scale (Version 3): Reliability, validity, and factor structure. Journal of Personality Assessment. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people: The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Semin GR, Cacioppo JT. From embodied representation to co-regulation. In: Pineda JA, editor. Role of mirroring processes in social cognition. Totowa, NJ: The Humana Press; in press. [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintel H, Nusbaum HC, Cacioppo JT. Accentuate the negative, eliminated the positive? Individual differences in attentional bias to positive and negative information. Presented at the 47th Annual Meeting of the Psychonomic Society; Houston, Texas. Nov. under review. [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. Journal of Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3D Proportional System: An Approach to Cerebral Imaging. New York, New York: Georg Thieme Verlag; 1988. [Google Scholar]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience. 2006;18:1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto E, Detre J, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. Journal of Neuroscience. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Deconvolution Analysis of FMRI Time Series Data (Technical Report) Milwaukee, Wisconsin: Biophysics Research Institute, Medical College of Wisconsin; 2001. [Google Scholar]
- Weiss RS. Loneliness: the experience of emotional and social isolation. Cambridge: The MIT Press; 1973. [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]