Abstract
The Notch1 signaling pathway is regarded as one of the main regulators of neural stem cell behavior during development, but its role in the adult brain is less well understood. We found that Notch1 was mainly expressed in doublecortin (DCX)-positive cells corresponding to newborn neurons, whereas the Notch1 ligand, Jagged1, was predominantly expressed in glial fibrillary acidic protein (GFAP)-positive astrocytic cells in the subventricular zone (SVZ) of the normal adult brain. These findings were confirmed by conditional depletion of DCX-positive cells in transgenic mice carrying herpes simplex virus thymidine kinase (HSV-TK) under the control of the DCX promoter. In addition, the activated form of Notch1 (Notch intracellular domain, NICD) and its downstream transcriptional targets, Hes1 and sonic hedgehog (Shh), were also expressed in SVZ cells. Increased activation of Notch1 signaling increased SVZ cell proliferation, whereas inhibiting Notch1 signaling resulted in a reduction of proliferating cells in the SVZ. Levels of NICD, Hes1, and Shh were increased in the SVZ at 4 and 24 h after focal cerebral ischemia. Finally, ischemia-induced cell proliferation in the SVZ was blocked by inhibition of the Notch1 signaling pathway, suggesting that Notch1 signaling may have a key role in normal adult and ischemia-induced neurogenesis.
Keywords: doublecortin, ischemia, Jagged1, neurogenesis, Notch1, subventricular zone
Introduction
Neurogenesis occurs in the subventricular zone (SVZ) of the lateral ventricles and in the subgranular zone (SGZ) of the hippocampal dentate gyrus of the adult rodent, nonhuman primate, and human brains (Eriksson et al, 1998; Jin et al, 2001; McDermott and Lantos, 1991; Sanai et al, 2004; Yoshimura et al, 2001). Newly generated cells in the adult brain can differentiate into functional mature neurons and integrate into neuronal networks (Song et al, 2002; van Praag et al, 2002), including those involved in cognitive function (Saxe et al, 2006; Shors et al, 2001; Zhang et al, 2008). We and others have shown that experimental stroke stimulates the proliferation of neuronal stem/progenitor cells (NSCs) located in the SVZ and SGZ of the adult rodent brain. The resulting newborn cells migrate into ischemically damaged brain regions (Arvidsson et al, 2002; Jin et al, 2003; Teramoto et al, 2003), where they differentiate into mature neuronal cells (Arvidsson et al, 2002; Teramoto et al, 2003). We found recently that newborn neurons are also found in the cerebral cortex after ischemic stroke (Jin et al, 2006) and intracerebral hemorrhage (Shen et al, 2008) in humans. However, the capacity of these cells to affect brain repair seems insufficient for a complete recovery after stroke in most cases. Improved recovery might be possible with pharmacological manipulation of neurogenesis, but is likely to require a better understanding of the mechanisms that control NSC proliferation, migration, and differentiation after stroke.
Notch signaling defines a fundamental pathway controlling cell fate acquisition (Artavanis-Tsakonas et al, 1999). Notch signaling pathways have critical roles in the maintenance, proliferation, and differentiation of NSCs in the developing brain, but their roles in the adult brain are less well understood. Recent studies have shown that Notch1 signaling may be conserved in the regulation of adult neurogenesis. For example, Notch1 signaling components, including the Notch1 ligands, Jagged1 and Delta1, are expressed in the SVZ and SGZ of the postnatal brain (Chojnacki et al, 2003; Stump et al, 2002). In addition, disruption of Notch1 signaling using antisense oligonucleotides or a γ-secretase inhibitor interferes with the maintenance and proliferation of NSCs (Chojnacki et al, 2003). The astroglial response of the SVZ to injury is also accompanied by activation of the Notch pathway (Givogri et al, 2006).
This study was undertaken to investigate the role of Notch1 signaling in NSCs located in the SVZ of the normal and ischemic adult brain in vivo. A recent study implicated Notch signaling in cultured SVZ cells isolated from ischemic rat brain and grown in neurosphere cultures (Wang et al, 2009). In our study, we found that Notch1, the activated form of Notch1 (Notch intracellular domain, or NICD), and its downstream target Hes1 were predominantly expressed in doublecortin (DCX)-positive (newborn neuronal) cells, but that the Notch1 ligand, Jagged1, was expressed mainly in glial fibrillary acidic protein (GFAP)-positive (astroglial) cells. Disruption or activation of Notch1 signaling altered the number of proliferating cells labeled by bromodeoxyuridine (BrdU) in the SVZ. Activation of Notch1 signaling in the SVZ was increased after focal cerebral ischemia, and ischemia-induced cell proliferation in the SVZ was partially blocked by a Notch1 signaling inhibitor. Our findings suggest that Notch1 signaling may have an important role in the regulation of cell proliferation in the SVZ of the normal and ischemic brain.
Materials and methods
Focal Cerebral Ischemia
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and every effort was made to minimize suffering and to reduce the number of animals used. Transient focal cerebral ischemia was induced using the suture occlusion technique as previously described (Jin et al, 2001). Male Sprague–Dawley rats weighing 280 to 310 g were anesthetized with 4% isoflurane in 70% N2O and 30% O2 using a mask. After a midline incision in the neck, the right external carotid artery was carefully exposed and dissected. A 19-mm, 3-0 monofilament nylon suture was inserted from the external carotid artery into the right internal carotid artery to occlude the origin of right middle cerebral artery (MCA). After 90 mins of occlusion, the thread was removed to allow reperfusion. The external carotid artery was ligated, and the wound was closed. Sham-operated rats underwent an identical surgery, except that the suture was not inserted. Rectal temperature was maintained at 37.0°C±0.5°C using a heating pad and a heating lamp. At various time points of reperfusion, rats were anesthetized and perfused through the heart with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4), the brains were removed, and cellular morphology was examined with cresyl violet staining.
Administration of Notch Signaling Activator and Inhibitor and γ-Secretase Inhibitor
Male Sprague–Dawley rats were anesthetized and implanted with an osmotic minipump (Alzet 1003D; Alza Corporation, Mountain View, CA, USA). The cannula was placed in the right lateral ventricle, 4.0 mm deep into the pial surface, –0.8 mm anteroposterior relative to the bregma, and 1.3 mm lateral to the midline. Jagged-1Fc (10 μg; R&D systems, Minneapolis, MN, USA) was incubated for 1 h on ice with anti-human Fc antibody (5 μg; Sigma, St Louis, MO, USA) at a 2:1 stoichiometric ratio and a final concentration of Jagged1-Fc of 50 μg/mL. For Notch1 activation and inhibition studies, each rat was infused for 3 days with 0.5 μL/h of either (1) Notch1-activating antibody (hybridoma clone 8G10, Upstate Biotechnology, Billerica, MA, USA) in artificial cerebrospinal fluid (128 mmol/L NaCl, 2.5 mmol/L KCl, 0.95 mmol/L CaCl2, 1.99 mmol/L MgCl2), (2) the Jagged-Fc–anti-Fc complex, (3) the anti-Fc antibody alone, or (4) artificial cerebrospinal fluid alone (n = 5 to 6 each). For γ-secretase inhibition studies, rats were administered 50 μmol/L of DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]S-phenylglycine t-butyl ester) (Sigma-Aldrich, St Louis, MO, USA) at 50 μg/mL through the intracerebroventricular route by an osmotic minipump for 3 days. Bromodeoxyuridine (50 mg/kg; Sigma-Aldrich) was dissolved in saline and injected intraperitoneally, twice daily for 3 days at 8-h intervals, and rats were killed on day 4.
Transgenic Mice
Mouse genomic DNA was isolated from embryonic brain and PCR was carried out using DCX promoter-specific primers (sense: 5′-CTTTTGTCTCTCTCAGCCTC-3′; antisense: 5′-AGAAAAGGGTGGAGATAAGG-3′), which were designed on the basis of the GeneBank data base sequence (access number: AY590498). The herpes simplex virus thymidine kinase (HSV-TK) gene was excised with BamHI from pBS mcs1mCMVTKpA vector (a gift from Lisa Ellerby, Buck Institute for Age Research) and cloned in a plasmid containing the DCX promoter. The pDCX-TK transgenic vector, which was verified by sequencing, was injected into CD1 embryos in the Buck Institute Transgenic Core. Identification and characterization of transgenic mouse lines was performed by PCR using HSV-TK-specific primers (HSVTK-1, 5′-CCACCACGCAACTGCTGGTG-3′; HSVTK-2, 5′-CGAGGCGGTGTTGTGTGGTGT-3′). Western blots on lysates from F1 and F2 transgenic animals showed that TK protein was detected in the neurogenic regions (SVZ and SGZ) of three out of the seven founder lines obtained. Transgenic pDCX-TK mice were viable, fertile, and normal in size, and did not show any gross physical or behavioral abnormalities. To deplete DCX-positive cells in the brain, GCV (ganciclovir) (Roche, Nutley, NJ, USA) was diluted in a sterile physiologic saline solution and administered intraperitoneally at a rate of 50 mg/kg per day for 14 days.
Immunohistochemistry
Rat brains embedded in paraffin were cut into 6-μm-thick sections, which were deparaffinized with xylene and rehydrated with ethanol, followed by antigen retrieval using the antigen unmasking solution (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. To detect BrdU-labeled cells in brain sections, sections were incubated in methanol at –20°C for 10 mins and in 2 mol/L of HCl at 37°C for 50 mins, and rinsed in 0.1 mol/L of boric acid (pH 8.5). Endogenous peroxidase activity was blocked by incubation for 30 mins at room temperature in 1% H2O2. After several washes with PBS, the sections were incubated in blocking solution (2% goat serum, 0.1% Triton X-100, and 1% bovine serum albumin in PBS) for 1 h at room temperature. Primary antibodies used were (1) goat polyclonal anti-Notch1 (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100); (2) rabbit polyclonal anti-NICD (Abcam, Cambridge, MA, USA; 1:500); (3) goat polyclonal anti-Jagged1 (Santa Cruz Biotechnology; 1:100); (4) rabbit polyclonal anti-Hes-1 (Chemicon, Temecula, CA, USA; 1:500); (5) goat polyclonal anti-DCX (Santa Cruz Biotechnology; 1:100); (6) mouse monoclonal anti-BrdU (Roche; 2 g/mL); and (7) rabbit polyclonal anti-Tuj1 (βIII tubulin; Covance, Princeton, NJ, USA; 1:600). Primary antibodies were added in the blocking buffer and incubated overnight with sections at 4 °C. Sections were then washed with PBS and incubated for 1 h at room temperature with biotinylated goat anti-rabbit or anti-goat antibody (1:200) (for polyclonal antibodies) or biotinylated horse anti-mouse antibody (1:200) (for monoclonal antibodies). Avidin–biotin complex (Vector Elite; Vector Laboratories) and a diaminobenzidine or nickel solution (Vector Laboratories) were used to obtain a visible reaction product. Controls included preabsorption and coincubation of the antibodies with the corresponding antigens. Sections were dehydrated, sealed, and coverslipped. A Nikon microscope and a Nikon digital color camera (DXM1200F; Nikon, Melville, NY, USA) were used to examine and photograph the slides, respectively.
Double or Triple Immunostaining
Double or triple immunostaining was performed on brain sections as previously described (Jin et al, 2003). The primary antibodies used, in addition to those listed above, were mouse monoclonal anti-nestin (Chemicon; 1:200), mouse monoclonal anti-vimentin (Sigma; 1:500), and mouse monoclonal anti-GFAP (Sigma; 1:500). The secondary antibodies were Alexa Fluor 488-, 594-, or 647-conjugated donkey anti-mouse, anti-goat, or anti-rabbit IgG (immunoglobulin G) (1:200 to 500; Molecular Probes, Carlsbad, CA, USA). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole) using the proLong Gold antifade reagent (Molecular Probes). Fluorescence signals were detected using an LSM 510 NLO Confocal Scanning System mounted on an Axiovert 200 inverted microscope (Carl Zeiss Ltd., Chester, VA, USA) equipped with a two-photon Chameleon laser (Coherent, Santa Clara, CA, USA), and images were acquired using the LSM 510 Imaging Software (Carl Zeiss Ltd). Two-, three-, or four-color images were scanned using argon, 543 HeNe, 633 HeNe, and Chameleon lasers. Selected images were viewed at high magnification, and three-dimensional images were constructed using Imars software (Beverly Hills, CA, USA). Controls included omitting either the primary or secondary antibody or preabsorbing the primary antibody.
Quantification of Immunoreactive Cells
Bromodeoxyuridine-labeled cells in the SVZ were counted blindly in five to seven 50-μm-thick coronal sections per animal, spaced 140 μm apart; this protocol avoids counting single cells twice because of the thickness of sections and provides accurate estimates of cell numbers. Tuj1-positive cells in the SVZ were counted blindly in four coronal sections per animal (n = 4 to 5 per group). Cells were counted under high power (× 200) on a Nikon E300 microscope (Nikon) with a Magnifier digital camera, and the image was displayed on a computer monitor. Results were expressed as the average number of BrdU-positive cells per section or as the percentage of Tuj1-positive cells in each condition.
Western Blotting
The SVZ was dissected from rat brains and cell lysates extracted in PBS containing 0.05% Nonidet P-40 (Roche, Nutley, NJ, USA), 0.25% sodium deoxycholate, 50 mmol/L Tris-HCl (pH 8.5), 100 mmol/L NaCl, 1 mmol/L EDTA (ethylenediaminetetraacetic acid) (pH 8.0), 1 μg/mL aprotinin, and 100 μg/mL phenylmethylsulfonyl fluoride. Protein (50 μg, determined using a Bio-Rad assay, Bio-Rad, Hercules, CA, USA) was boiled at 100 °C in the SDS sample buffer for 5 mins, electrophoresed on SDS/12% PAGE gels, and transferred into polyvinyldifluoridine membranes, which were incubated overnight at 4 °C using one of the following primary antibodies: (1) rabbit anti-NICD (Abcam; 1:500); (2) rabbit anti-Hes1 (Chemicon; 1:1,000); (3) rabbit anti-sonic hedgehog (Shh) (Santa Cruz Biotechnology; 1:1,000); or (4) mouse monoclonal anti-actin (Oncogene Science, Cambridge, MA, USA; 1:20,000). Membranes were washed with PBS/0.1% Tween 20, incubated at room temperature for 60 mins with horseradish peroxidase conjugated anti-mouse, anti-rabbit, or anti-goat secondary antibody (Santa Cruz Biotechnology; 1:3,000), and thereafter washed thrice for 15 mins with PBS/Tween 20. Peroxidase activity was visualized by chemiluminescence (NEN Life Science Products, Boston, MA, USA). Antibodies were removed with stripping buffer (100 mmol/L 2-mercaptoethanol/2% SDS/62.5 mmol/L Tris-HCl, pH 6.7) at 50°C for 30 mins, followed by washing with PBS/Tween 20, and membranes were reprobed.
Measurement of γ-Secretase Activity
The activity of γ-secretase in rat brain SVZ (n = 6) was determined using a γ-secretase activity kit (R&D Systems) according to the manufacturer's instructions. Data were expressed as fold increase in fluorescence over that of background controls (reactions in the absence of tissue or cell lysates).
Statistical Analysis
Quantitative results were expressed as the mean±s.e.m. The statistical significance of differences between means was evaluated using one-way analysis of variance (ANOVA). P < 0.05 was regarded as statistically significant.
Results
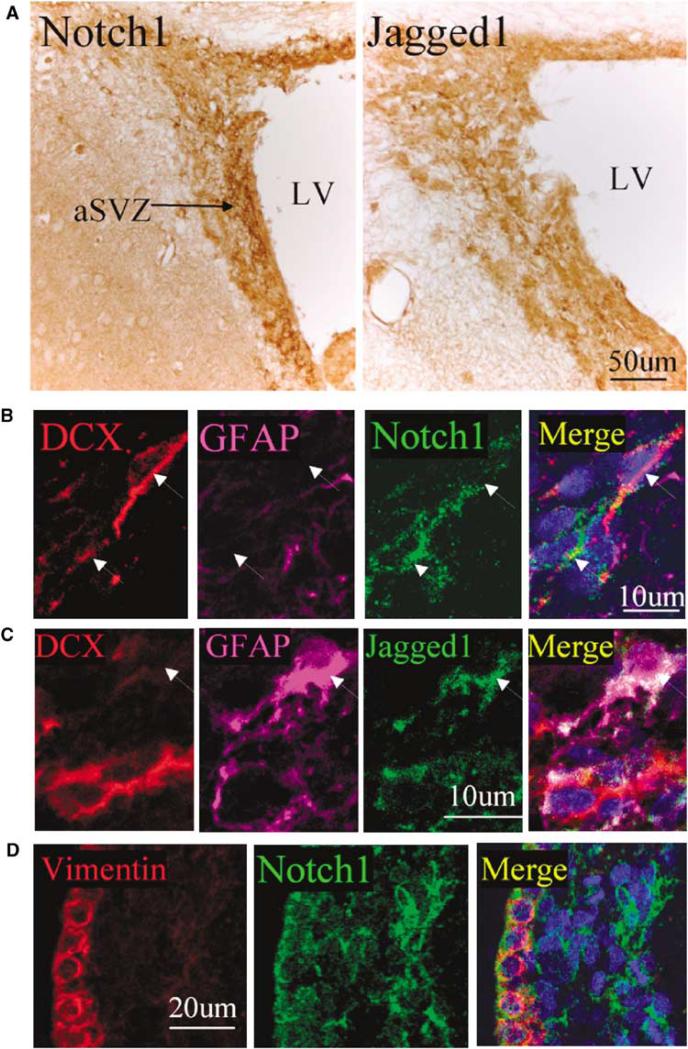
The activity of the Notch1 signaling pathway regulates neural cell fate during embryonic development of the central nervous system. Hence, we first inquired whether Notch signaling also has a pivotal role in the regulation of neurogenesis in the adult brain. By immunohistochemical analysis, we confirmed that Notch1 and the Notch1 ligand, Jagged1, were expressed in the SVZ of the normal adult rat brain (Figure 1A). High-magnification views showed that both Notch1 and Jagged1 were located mainly in both the cell membrane and cytoplasm of SVZ cells (insets in Figure 1A). Triple-label immunostaining showed that Notch1 immunoreactivity was largely colocalized with the neuronal marker DCX (Figure 1B), whereas Jagged1-positive cells also expressed the astroglial marker GFAP (Figure 1C). In addition, Notch1-positive cells coexpressed vimentin (Figure 1D), a protein marker for ependymal cells.
Figure 1.
Expression of Notch1 and Jagged1 in the normal adult rat brain SVZ. (A) Notch1 (left panel) and Jagged 1 (right panel) immunoreactivity (brown) SVZ. The lateral ventricle is at the right. Insets show Notch1- and Jagged1-immunopositive cells at a higher magnification. (B, C) Triple immunolabeling shows coexpression of DCX (red) and Notch1 (green), but not GFAP (purple), in presumptive NPCs, and of Jagged1 (green) and GFAP (purple), but not DCX (red), in presumptive astrocytes. (D) Notch1 (green) was expressed in vimentin-positive (red) cells. DAPI (blue) was used to counterstain nuclei.
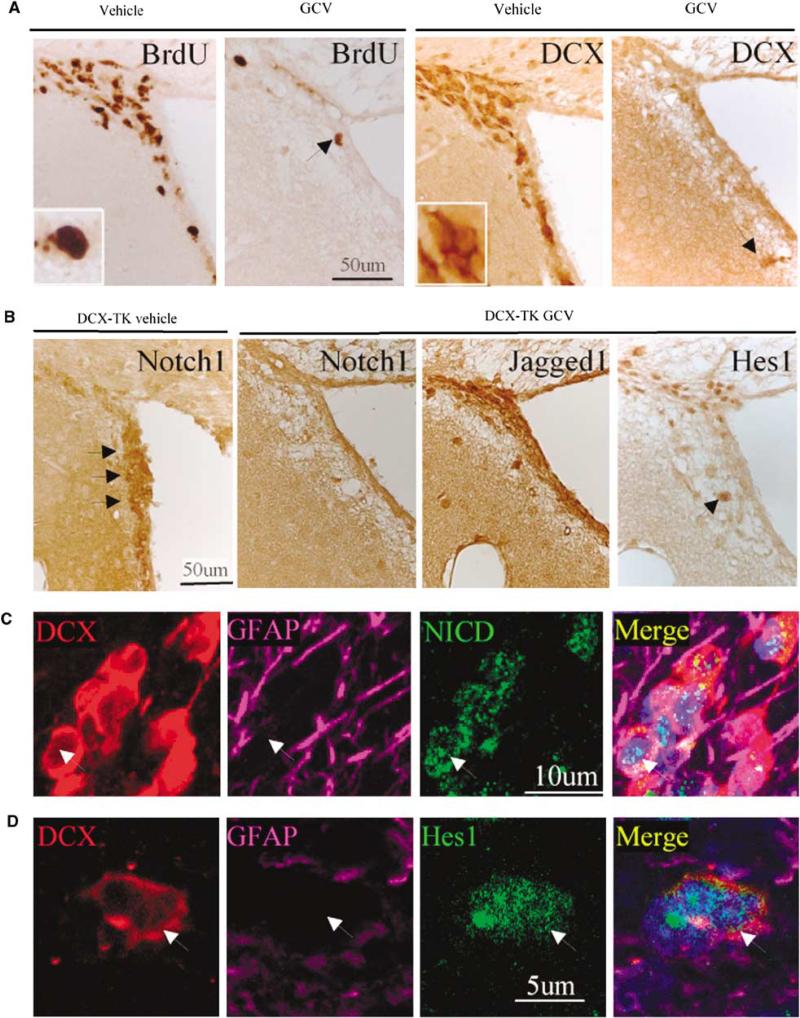
To confirm the phenotypes of Notch1- and Jagged1-positive cells, we developed transgenic mice that carried HSV-TK under the control of the DCX promoter. As shown in Figure 2A, DCX- and BrdU-positive cells were depleted from the SVZ of DCX-TK transgenic mice treated by an intraventricular administration of GCV for 14 days, consistent with the loss of newborn neurons. Notch1 was similarly depleted from the SVZ cells of these mice, confirming its localization to new neurons. In contrast, Jagged1, expression was unaffected in GCV-treated DCX-TK mice (Figure 2B). Expression of the Notch1 signaling mediator, Hes1, was also reduced in the SVZ of GCV-treated DCX-TK mice. Finally, triple-label immunostaining showed that NICD and Hes1 were expressed mainly in DCX-positive cells in the SVZ (Figures 2C and 2D), confirming the presence of Notch1 signaling pathways in DCX-positive cells.
Figure 2.
Cellular compartmentation of Notch1 signaling in the normal adult rat brain SVZ. (A) pDCX-TK transgenic mice were treated with vehicle or GCV, and immunostaining was performed using antibodies against BrdU or DCX. Compared with vehicle treatment, GCV depleted BrdU- and DCX-immunopositive cells from the SVZ. (B) GCV also depleted Notch1 and Hes1 (compare with Figure 4C, left), but not Jagged1 (compare with Figure 1A, right), consistent with expression of Notch1 and Hes1 in NPCs. Triple immunostaining shows extensive colocalization of DCX (red), but not GFAP (purple) with (C) NICD and (D) Hes1 (both green). DAPI (blue) was used to counterstain nuclei.
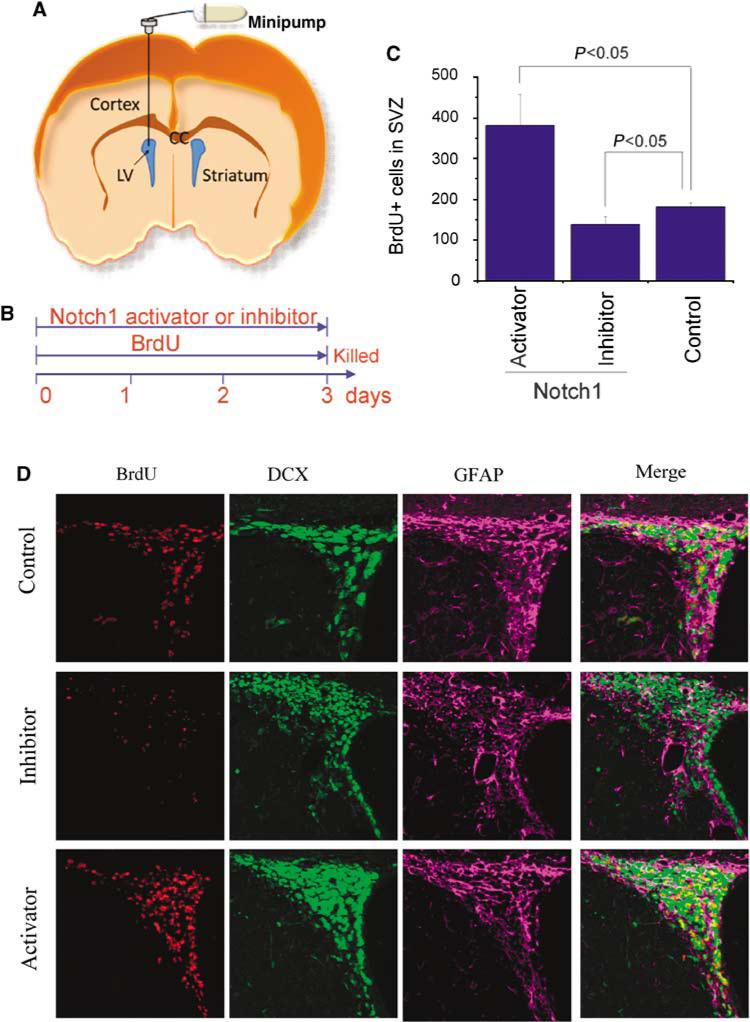
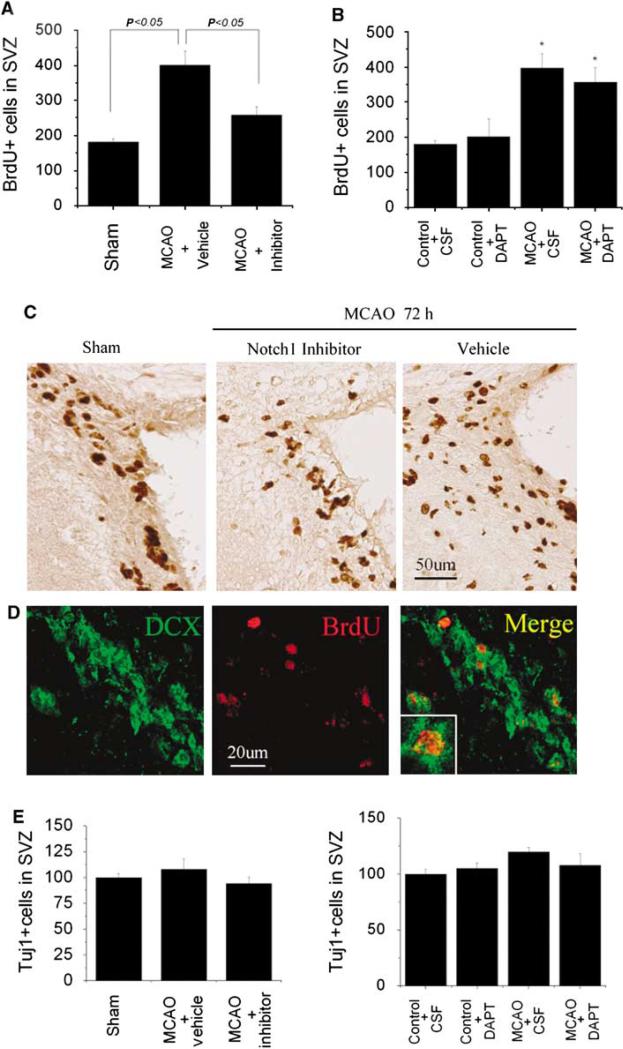
To further test the significance of Notch1 signaling in the proliferation of SVZ cells in the adult rat brain, we used an antibody directed against the extracellular domain of Notch1 to activate Notch1 signaling (Conboy et al, 2003) and a soluble Jagged1-Fc fusion protein to block Notch1 activation (Hicks et al, 2002) (Figures 3A and 3B). As shown in Figures 3C and 3D, the numbers of both BrdU- and DCX-positive cells in the SVZ were increased 3 days after intracerebral administration of the Notch1 signaling activator and reduced 3 days after intracerebral infusion of the Notch1 signaling inhibitor. Triple immunolabeling showed that the inhibition or activation of Notch1 signaling had less marked effects on the number of GFAP-positive than of BrdU- or DCX-positive cells (Figure 3D).
Figure 3.
Modulation of Notch1 signaling significantly alters cell proliferation in normal adult rat brain SVZ. (A) Notch1 activator (receptor-activating antibody) and inhibitor (Jagged-Fc) were administered by an osmotic pump into the lateral ventricle. (B) Time course of administration of Notch 1 activator or inhibitor and of BrdU. (C, D) Immunostaining shows that the Notch1 activator increased and the Notch1 inhibitor decreased the number of BrdU-positive (red) and Dcx-positive (green), but not GFAP-positive (purple), cells in the SVZ. Data in panel C are means±s.d. (n = 4 to 6 per group).
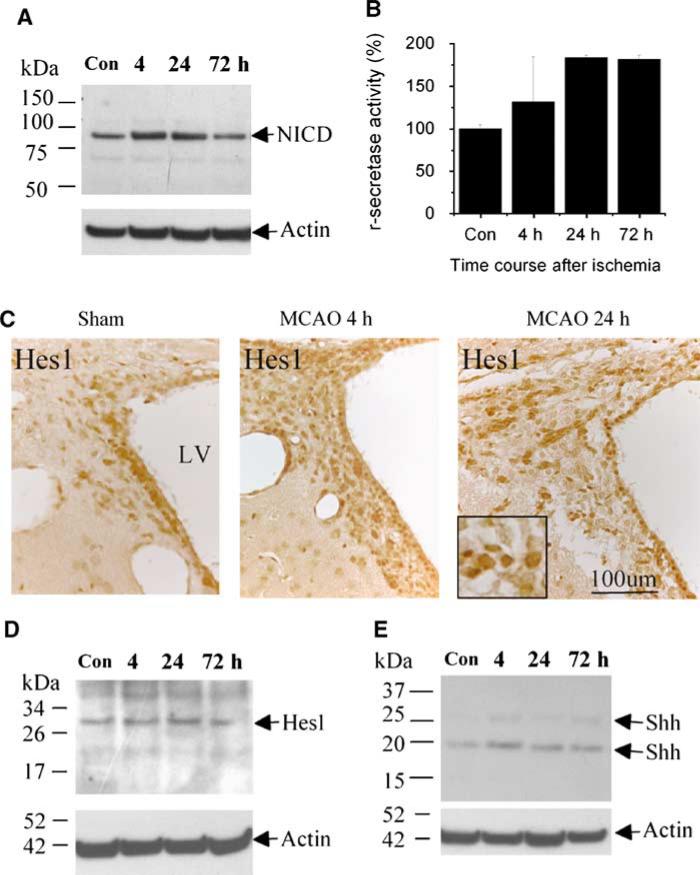
To determine whether Notch1 signaling is also involved in the proliferation of SVZ cells induced by focal cerebral ischemia, the SVZ was dissected from rat brains after 4, 24, and 72 h of reperfusion after MCA occlusion (MCAO), and western blotting was performed using an antibody against the activated form of Notch1, NICD. As shown in Figure 4A, NICD was expressed under control (sham-operated) conditions, expression increased at 4 and 24 h, and basal expression returned by 72 h after MCAO. The phenotype of cells expressing Notch1 signaling molecules in the SVZ of rat brain was not altered significantly after focal ischemia. Cleavage of Notch1 to generate NICD is accomplished by the action of γ-secretase, which is followed by the translocation of NICD to the nucleus, where it regulates transcription (De Strooper et al, 1999). Accordingly, we measured γ-secretase activity in the SVZ, and found that it was increased after MCAO (Figure 4B). The expression of Hes1 (which is transcriptionally induced by activation of Notch1 signaling; Androutsellis-Theotokis et al (2006)) and that of Shh (another downstream target of Notch1 signaling (Androutsellis-Theotokis et al, 2006), which independently regulates Hes1 (Wall et al, 2009)) was also increased in the SVZ cells after focal ischemia. (Figures 4C and 4D).
Figure 4.
Notch1 signaling in adult rat brain SVZ is activated after MCAO. (A) The SVZ was dissected up to 72 h after MCAO and western blots were performed. NICD abundance was increased 4 to 24 h after MCAO (top). The blot was reprobed using anti-actin antibody to control for protein loading (bottom). (B) γ-secretase activity was increased 4 to 72 h after MCAO. Data are means±s.d. (n = 4 to 6 per group). (C) Immunohistochemistry shows increased Hes1 expression in the SVZ 4 and 24 h after MCAO. Nuclear protein was isolated from the SVZ and western blotting was performed with anti-Hes1 (D) or anti-Shh (E) antibody. Blots were reprobed with anti-actin.
To confirm the role of Notch1 signaling in ischemia-induced neurogenesis, we treated rats undergoing MCAO with the same soluble Jagged1-Fc fusion protein used previously (Figure 3) to block Notch1 activation in nonischemic rats. This Notch1 inhibitor attenuated the ischemia-induced increase in BrdU labeling (Figures 5A and 5C). Treatment with the γ-secretase inhibitor DAPT (Dovey et al, 2001) also reduced BrdU labeling, albeit to a lesser extent (Figure 5B). Although the number of BrdU-positive cells significantly decreased after blocking Notch1 signaling, the number of Tuj1-positive cells was not significantly changed after inhibiting the Notch1 signaling pathway (Figure 5E). This may be due to fact that Tuj1-positive cells were counted only 3 days after administration of Notch1 signaling inhibitor, and it may take longer to notice a change in the number of mature neurons.
Figure 5.
Inhibition of Notch1 signaling reduces cell proliferation after MCAO in adult rat brain SVZ. BrdU-positive cell counts in SVZ of ischemic brain were reduced by inhibitors of Notch1 (Jagged-Fc) (A)or γ-secretase (DAPT) (B). Data are means±s.d. (n = 4 to 6 per group). (C) Immunostaining shows the decrease in BrdU-positive cells in the SVZ 72 h after MCAO. (D) Double immunostaining shows BrdU labeling of nuclei in DCX-positive cells. (E) Tuj1-positive cell counts in SVZ of ischemic brain were not significantly altered by inhibitors of Notch1 (Jagged-Fc) (left panel) or γ-secretase (DAPT) (right panel).
Discussion
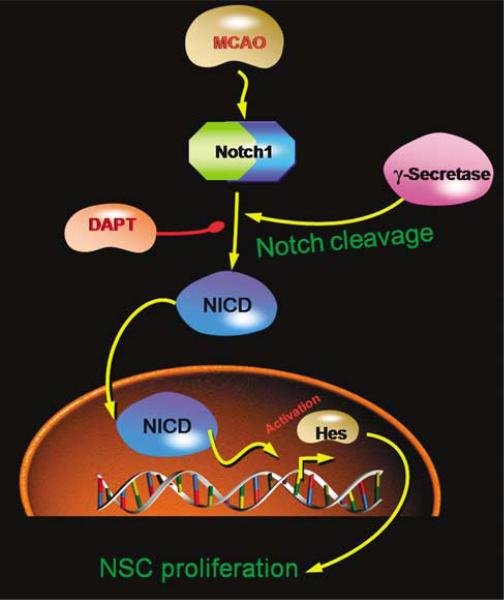
In this study, we show that Notch1 signaling pathways regulate ischemia-induced neurogenesis in the adult rat brain in vivo. We found that Notch1, activated Notch1 (NICD), and a downstream effector of Notch1 signaling (Hes1) are localized to neurons, and that the Notch1 ligand (Jagged1) is expressed in astrocytes, of the SVZ, suggesting intercellular communication between astrocytes and neurons. We also observed that activation of Notch1 signaling increases SVZ neurogenesis, whereas inhibition of Notch1 signaling decreases neurogenesis, consistent with the ability of Notch1 to enhance the proliferation of neuronal (Androutsellis-Theotokis et al, 2006) and nonneuronal (Conboy et al, 2003) stem cells. Next, we studied the effects of manipulating Notch1 signaling on ischemia-induced neurogenesis after MCAO, and found that the same Notch signaling pathways involved in neurogenesis under physiologic conditions also contribute to the neurogenesis-promoting effect of ischemia. The mediators and reagents we used and the pathways they implicate are shown schematically in Figure 6.
Figure 6.
Schematic representation of Notch1 signaling pathway involved in ischemic regulation of cell proliferation in adult rat brain SVZ.
Notch1 has been identified before in neurogenic regions of the postnatal brain, including the SVZ (Givogri et al, 2006; Mizutani et al, 2007), where Notch1 activation contributes to the maintenance and proliferation of NSCs (Chojnacki et al, 2003). In both the SVZ and the rostral migratory stream, through which new neurons migrate from SVZ to reach the olfactory bulb, Notch1 appears to be localized to neurons and Notch ligands to astrocytes (Givogri et al, 2006), which we also observed. Binding of Notch ligands, such as Jagged1, results in the proteolytic cleavage of Notch1, first extracellularly and then in the transmembrane domain. The latter cleavage is accomplished by the γ-secretase enzyme complex, resulting in the release of NICD that translocates to the nucleus, where it regulates transcription (De Strooper et al, 1999). Thus, the finding that NICD is expressed in DCX-positive cells in the SVZ indicates that Notch1 signaling is activated in this cell population, consistent with the demonstration that the siRNA knockdown of Notch1 expression decreases NPC proliferation in cultured neurospheres (Chen et al, 2008).
After NICD translocates to the nucleus and forms a complex with the DNA-binding protein RBP-J (recombination recognition sequence-binding protein at the J-κsite), also known as CSL, RBP-J/CSL becomes a transcriptional activator and induces the expression of downstream components, such as Hes1 and Hes5, which function as transcriptional repressors, and are effectors of Notch1-mediated inhibition of neuronal differentiation in development (Ohtsuka et al, 1999). In Hes1/Hes5 double knockout mice, radial glial cells show reduced proliferation and premature differentiation into neurons. Moreover, Hes1 and Hes5 prevent embryonic progenitor cells from differentiating into neurons (Ishibashi et al, 1994; Ohtsuka et al, 2001). Accordingly, we found that Hes1 was expressed in DCX-positive cells.
At least two previous studies have addressed the role of Notch signaling in the brain's response to ischemia. In one case (Androutsellis-Theotokis et al, 2006), cerebral ischemia was induced in spontaneously hypertensive rats by permanent distal MCAO. Intraventricular administration of the Notch ligand Dll4 increased the number of BrdU-positive cells in the SVZ and, in combination with fibroblast growth factor-2, improved motor deficits up to 45 days after ischemia. In another study (Wang et al, 2009), cells were isolated from rat SVZ 7 days after MCAO (induced by the injection of a fibrin-rich clot) and cultured as neurospheres in vitro. Compared with cultures from control rats, cultures from ischemic rats showed increases in the number and size of neurospheres and the percentage of cells labeled by BrdU or the anti-βIII tubulin antibody TuJ1, as well as enhanced expression of Notch, NICD, and Hes1 mRNA and NICD protein. Addition of either siRNA directed against Notch or a γ-secretase inhibitor to neurosphere cultures blocked these effects.
Our findings are substantially in agreement with, and extend, these previous studies. In essence, we find that the Notch signaling cascade shown by Wang et al (2009) to be activated by ischemia in vitro is also activated in vivo. Whereas Androutsellis-Theotokis et al (2006) used Dll4 to activate Notch signaling in vivo, we used a receptor-activating antibody, and we also showed that ischemia-induced neurogenesis could be blocked by inhibitors of Notch1 signaling, including both a receptor-blocking fusion protein and a γ-secretase inhibitor. Finally, we found evidence for ischemic induction of two downstream targets of Notch1 signaling, Hes1 and Shh, in the SVZ of the ischemic brain in vivo. As noted above, Hes1 is a transcriptional repressor that is induced when NICD complexes with RBP-J/CSL. Hes1 acts during neural development for maintaining the stem-cell state by repressing the transcription of proneural genes, such as Mash1 and Neurogenin2 (Kageyama et al, 2008). Sonic hedgehog (Shh) is another gene that is activated by Notch1 signaling, and which regulates adult neurogenesis (Machold et al, 2003). Interestingly, in the retina, Shh also regulates Hes1, by a Notch1-independent mechanism (Wall et al, 2009). Thus, multiple ischemia-induced signaling pathways seem to converge to enhance the activity of Hes1 in NPCs located in the SVZ.
In summary, our findings provide evidence for the involvement of Notch1 signaling in physiologic and ischemia-induced neurogenesis in the adult brain. Neurogenesis induced by ischemia or other forms of brain injury may represent an endogenous mechanism for limiting the extent of injury or promoting recovery (Kaneko and Sawamoto, 2009). If so, identifying signaling pathways that mediate injury-induced neurogenesis may lead to new therapeutic strategies for neurologic disease.
Acknowledgments
Supported by USPHS Grants AG21980 (to K.J.) and NS44921 (to DAG).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;449:351–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Cui X, Roberts C, Lu M, Chopp M. Atorvastatin promotes presenilin-1 expression and Notch1 activity and increases neural progenitor cell proliferation after stroke. Stroke. 2008;39:220–6. doi: 10.1161/STROKEAHA.107.490946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–41. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–81. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- Hicks C, Ladi E, Lindsell C, Hsieh JJ, Hayward SD, Collazo A, Weinmaster G. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J Neurosci Res. 2002;68:655–67. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 1994;13:1799–805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–89. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008;50(Suppl 1):S97–103. doi: 10.1111/j.1440-169X.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63:155–64. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–50. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- McDermott KW, Lantos PL. Distribution and fine structural analysis of undifferentiated cells in the primate subependymal layer. J Anat. 1991;178:45–63. [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–5. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes Hes1 and Hes5 in expansion of neural stem cells of the developing brain. J Biol Chem. 2001;276:30467–74. doi: 10.1074/jbc.M102420200. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–4. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Xie L, Mao X, Zhou Y, Zhan R, Greenberg DA, Jin K. Neurogenesis after primary intracerebral hemor-rhage in adult human brain. J Cereb Blood Flow Metab. 2008;28:1460–8. doi: 10.1038/jcbfm.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Stump G, Durrer A, Klein AL, Lutolf S, Suter U, Taylor V. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002;114:153–9. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–32. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall DS, Mears AJ, McNeill B, Mazerolle C, Thurig S, Wang Y, Kageyama R, Wallace VA. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J Cell Biol. 2009;184:101–12. doi: 10.1083/jcb.200805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chopp M, Zhang RL, Zhang L, Letourneau Y, Feng YF, Jiang A, Morris DC, Zhang ZG. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience. 2009;158:1356–63. doi: 10.1016/j.neuroscience.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci USA. 2001;98:5874–9. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]