Abstract
Purpose
The ability to reproducibly and repeatedly image rodents in non-invasive imaging systems, such as small animal PET and CT, requires a reliable method for anesthetizing, positioning, and heating animals in a simple reproducible manner. In this paper we demonstrate that mice and rats can be reproducibly and repeatedly imaged using an imaging chamber designed to be rigidly mounted on multiple imaging systems.
Procedures
Mouse and rat imaging chambers were made of acrylic plastic and aluminum. MicroCT scans were used to evaluate the positioning reproducibility of the chambers in multi-modality and longitudinal imaging studies. The ability of the chambers to maintain mouse and rat body temperatures while anesthetized with gas anesthesia was also evaluated.
Results
Both the mouse and rat imaging chambers were able to reproducibly position the animals in the imaging systems with a small degree of error. Placement of the mouse in the mouse imaging chamber resulted in a mean distance of 0.23 mm per reference point in multimodality studies, whereas for longitudinal studies the mean difference was 1.11 mm. The rat chamber resulted in a mean difference of 0.46 mm in multimodality studies, and a mean difference of 4.31 mm in longitudinal studies per reference point. The chambers maintained rodent body temperatures at the set point temperature of 38°C.
Conclusions
The rodent imaging chambers were able to reproducibly position rodents in tomographs with a small degree of variability, and were compatible with routine use. The embedded anesthetic line and heating system was capable of maintaining the rodent’s temperature and anesthetic state, thereby enhancing rodent health and improving data collection reliability.
List keywords or phrases: Rodent imaging chamber, Multimodality imaging, Reproducible rodent imaging, Immunocompromised imaging, Gas anesthesia
Introduction
The relatively low maintenance cost and ability to genetically manipulate murine models has greatly expanded their use in biomedical research, particularly in the field of oncology1. One of the primary uses of small animal imaging systems is for longitudinal pre-clinical research which involves repeatedly scanning rodents over weeks or months. With this increasing interest in the longitudinal imaging, it is essential to ensure that robust, comparable and reproducible data are generated. To achieve this, it is important to standardize animal handling, anesthesia, and body temperature during these studies.2
At our institution we designed rat and mouse imaging chambers that can be rigidly and reproducibly mounted on both microPET and microCT tomographs. These imaging chambers made of acrylic and aluminum were designed to anesthetize, position, warm, and provide a pathogen barrier for rodents while minimizing attenuation of imaging information. The use of a reproducibly mounted mouse imaging chamber and the methodologies for imaging volume co-registration has been reported before.3–6 While some past rodent imaging solutions involved just stereotaxic placement of the head,7 we often need to image the entire rodent, which requires a whole body approach to reproducible imaging. Using a rigidly mounted imaging chamber greatly decreases variability between imaging sessions due to human repositioning error, and significantly decreases intra-animal variability.
Developments in molecular imaging probe technology have yielded probes with highly targeted uptake, creating a need to pair functional images with anatomical information from CT or MR.8 The lack of anatomical information in these highly specific molecular images implies that there is insufficient information for co-registration based on image content. By registering the imaging volumes of two or more imaging systems, a fixed translational offset may be all that is necessary to create co-registered images. This simple method of fixed translational offset avoids the need for image content based registration; a process that can be difficult, time consuming, and error prone. Reproducible image positioning also aids in subsequent image analysis when defining regions of interest (ROI).
In the past several years we have seen a large increase in the number of immunocompromised animals used for molecular imaging studies, in particular SCID and Nude mice. These animals have varying immunodeficiencies, thus they must be kept within a protective barrier environment to avoid potential pathogens.9 One of the primary goals of the imaging chambers was to provide barrier conditions when operated under a specific protocol in conjunction with anesthesia induction boxes and biosafety cabinets.
The ability to easily provide gas anesthesia and maintain the animal’s anesthetic state was another major motivating factor in designing these chambers. One of the main advantages of gas anesthesia is that it provides a constant level of anesthesia over long scanning periods, which is difficult to do with injectable drugs like ketamine and xylazine. By providing a constant level of anesthesia, movements during image acquisition can be eliminated. Motion during image acquisition creates artifacts and errors in quantitation, especially with high resolution modalities such as microCT and microMR.10
The chamber was also designed with a stable reproducible heating system. This prevents hypothermia and maintains a normal physiological environment which is needed for several reasons. First, rodents have a large surface area to body weight ratio making it difficult for them to maintain their core body temperature while anesthetized.11 Second, imaging facilities are often kept at cooler ambient temperatures (20–22°C) and tend to have significant airflow to counteract the large amount of heat generated by the imaging devices.4 Third, recent work has shown that the uptake of radiolabeled compounds, especially in peripheral tumors, is highly dependent on rodent body temperature.2
The rigid mounting of the imaging chamber on a microPET or a microCT system allows easy co-registration of images. Functional imaging modalities such as PET and SPECT lack detailed anatomical content important for accurate interpretation.12 To make up for this, investigators need to co-register functional and anatomical data; primarily PET and CT data sets of the same subject. To this end many manufacturers are now developing combined functional and anatomical tomographs (PET/CT, SPECT/CT, PET/MRI), where there is no need to move the rodent between modalities.13 Most institutions do not have multimodality tomographs and even at sites that do, the ability to reproducibly place the subject in a fixed position for longitudinal studies is still needed. Also, the built-in heating and gas anesthesia delivery capabilities of the chambers are still highly desired.
Therefore, our motivations and requirements for developing rodent imaging chambers included; (a) to provide a pathogen barrier for immunocompromised rodents, (b) to be easily disinfected between uses, (c) to be compatible with multiple imaging systems, (d) to provide gas anesthesia, (e) to maintain the rodent’s body temperature, (f) to have fixed positioning between tomographs, (g) to provide reproducible animal positioning for longitudinal studies, and (h) to help facilitate high throughput in the imaging facility.
In this work we report on the reproducibility, repeatability, and temperature maintaining ability of the mouse and rat multi-modality imaging chambers. We examined the temperature regulation, mounting accuracy, and the accuracy of animal positioning in longitudinal studies using animal skeletal markers and rigidly fixed chamber markers. We also report on an anesthesia system with manifold delivery and heated induction boxes to support imaging chamber use.
Materials and Methods
Micro-CT
All microCT data was acquired on a MicroCAT II tomograph (Siemens Preclinical Solutions; Knoxville, TN). Exposure settings were 70 kVp and 2 mm Al filtration for a total of 90 mAs (360 degree rotation in 1 degree steps). Total imaging time was seven minutes, with an axial FOV of 10 cm. Images were reconstructed using a Feldkamp algorithm to a cubic voxel size of 0.20 mm for mice, and 0.40 mm for rats. Two axial bed positions were used to cover the rat’s body length and merged together using an in house developed software program. Mice were fully covered in one bed position.
Anesthesia System
The anesthesia system consisted of dual vaporizers that deliver isoflurane gas to a manifold distribution system to multiple locations. Individual anesthetic lines were connected to the imaging devices, induction boxes, and inside the bio-safety cabinet. The vaporizers were interlocked so that only one was operable at a time, allowing for servicing and replenishing of one vaporizer while the other remains in use. Oxygen was delivered to the vaporizers at a constant pressure of 6 PSI. The vaporizers were calibrated to provide isoflurane at the necessary percentages (0 – 5%) for any flow demand. Individual orifices were located downstream from the vaporizers and were set to deliver gas at either 2 L/min for rat imaging chambers and anesthesia induction boxes or 0.5 L/min for the mouse imaging chambers. The system’s maximum flow rate was greater than 17 L/min, to accommodate multiple induction boxes and imaging chambers in use at the same time. At the end of each line, a small plastic ball valve was used to turn flow on or off creating point of use control over the gas delivery. With this arrangement, multiple locations could use gas anesthesia with constant flow. (This is stated in the discussion) Waste anesthetic gases were collected using a separate manifold system, piped to the room return air ducts, and ejected outside of the building after dilution. [Figure 1A]
Figure 1.

Figure 1A: Dual isoflurane vaporizers with a manifold distribution system distributing gas anesthetic to all imaging devices including heated induction boxes. (Blue lines are the scavenging system.)
Figure 1B: Biosafety cabinet with anesthetic manifold system distributing anesthesia to heated induction boxes.
Induction Boxes
Animals were transferred from their cages inside the biosafety cabinet to heated plastic induction boxes to induce anesthesia. These boxes had resistive wire heating elements attached to an aluminum heat diffusion plate attached to the bottom of each box. A small electronics box containing the power supply, control electronics, and temperature readout was located in the biosafety cabinet and connected to the induction boxes. The controllers were set to maintain the induction boxes at 36°C. [Figure 1B]
Imaging Chambers
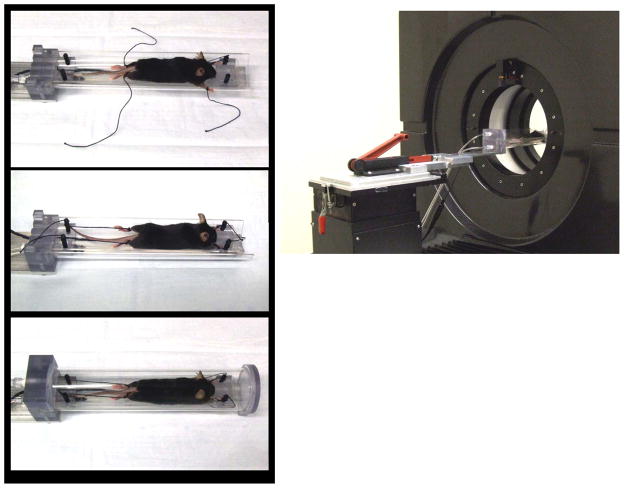
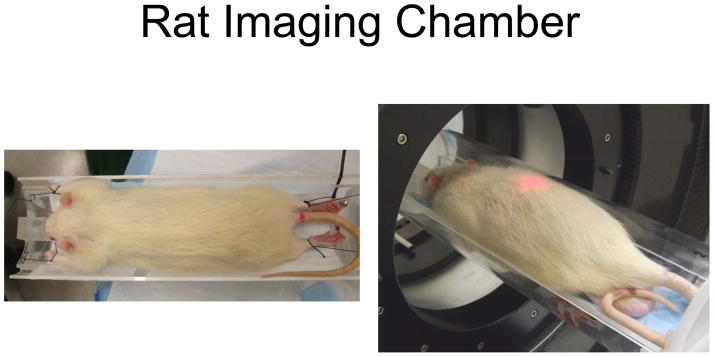
Sharing the same basic concept, two sizes of imaging chambers were designed to accommodate rats or mice. The imaging chambers were comprised of three main parts. First there was an aluminum mounting plate that could be mounted on either the microPET or microCT imaging devices. Attached to this main mounting plate was an aluminum adapter plate that held the imaging chamber. The imaging chamber was composed of an acrylic plastic cylinder split in half lengthwise to form a base and cover. There was also a removable inner sled holding the animal with four corner posts for securing the limbs. This sled was curved to accommodate an average rodent (different for a mouse and rat), and had a heating strip and a nose cone for anesthesia delivery. The sled curvature helped ensure that the animal was located in the center of the imaging chamber. Anesthetic gas was carried into the chamber through tubing and was delivered to the animal through the nose cone. The exhaust port was located at the rear of the chamber, allowing the entire chamber to fill with anesthetic gasses. The temperature of the mouse and rat sleds are controlled by a Minco CT-198 sensorless controller (Minco Products, Inc. Minneapolis, MN) that utilized the resistance temperature coefficient of the heating element to sense its temperature. [Figures 2 and 3]
Figure 2.
Mouse imaging chamber showing assembly of the mouse in the imaging chamber and chamber with aluminum adapter plate mounted on a microPET. Anesthesia is delivered via the built in nose cone and limbs are tied to the posts, heat is supplied by the heating strip mounted to the bottom of the sled.
Figure 3.
Rat imaging chamber
To assemble each chamber for an imaging session, the removable sled was first placed in a disinfectant (Virkon®, DuPont, Wilmington, DE) for 10 minutes. This procedure should be performed once per cage of animals. The sled was connected to the temperature controller to provide heating and the anesthetic line was connected to supply the nose cone. Next, the anesthetized animal was removed from the induction box and placed on the sled with the nose positioned in the nose cone. With anesthetic gas delivered through the nose cone, the investigator could take as much time as necessary to position the mouse and assemble the chamber. Short lengths of suture material were used to create small loops to facilitate securing the limbs to the four posts. Care was taken to only put a small amount of tension on the line: only enough to extend the limb slightly without cutting off circulation. When positioning reproducibility was not essential, limbs could be quickly secured using low strength adhesive paper tape. Once all four limbs were secure, the sled could be returned to the imaging chamber bottom, the anesthesia line switched over to the chamber connection, and the chamber top secured over the mouse or rat. The animal was then encapsulated in a positive pressure chamber that could be moved to or between imaging devices. The gas could be disconnected for the few seconds it takes to transfer the chamber between the biosafety cabinet and imaging systems without the animal waking up, since the entire chamber was filled with anesthetic gas. Once imaging was completed, the rodent was removed from the chamber and returned to its cage within the biosafety cabinet, thus maintaining barrier conditions throughout the entire imaging process. During animal recovery, cages were kept on a heated plate behind a lead brick enclosure for radiation shielding.
Chamber Positioning Tests
Multimodality test
An adult female C57BL/6J mouse (26 g) was anesthetized with isoflurane and placed in the imaging chamber with limbs secured to the posts. To effectively simulate PET-CT studies, the chamber was first imaged using CT, then attached to the PET bed, then placed back on the CT system, and imaged again. This process was repeated 5 times to assess the changes in bed locations due to use in separate imaging systems. The CT system has higher spatial resolution than the PET system, so any variability with bed repositioning would be best assessed using repeated CT scans.
An adult male Sprague Dawley rat (275 g) was anesthetized with isoflurane and positioned in the imaging chamber and imaged with 2 bed positions in the microCT. Between the scans, the imaging chamber was taken out of the tomograph, placed on the PET system and then repositioned back in the CT imaging system, in the same manner described above for the mouse (n=5).
Longitudinal repositioning test
The mouse was placed in the imaging chamber using limb ties and chamber posts. The chamber was then imaged using the microCT, removed from the scanner, and placed in the biosafety cabinet. Inside the biosafety cabinet, the mouse was removed from the chamber, and repositioned back into the chamber, reattaching the limb ties to the posts and imaged again (n=5). This was done to effectively simulate long term studies.
The rat was placed in the imaging chamber, the limbs were restrained using limb ties and chamber posts and scans of two bed positions were performed. Subsequently, the chamber was removed from the tomograph, the rat was taken out of the chamber, repositioned back into the chamber, limb ties were reattached, and imaged again using microCT (n=5).
Temperature Testing
A Nude mouse weighing 28 grams was anesthetized for one hour using 1.5% isoflurane. The mouse was mounted in the mouse imaging chamber using the limb ties and chamber posts. A rectal thermometer was inserted, and a temperature probe was attached to the bottom of the sled (Physitemp Model BAT-12; Mouse Rectal Probe; Physitemp Instruments Inc. Clifton, NJ) The temperature of the sled was set to 37°C for the first 40 minutes and 38°C for the following 20 minutes to determine the optimal setting. For the rat chamber a 250 gram Sprague Dawley rat was used to take measurements in a similar manner to the mouse. The sled was set to 38°C for the whole recording period in the rat experiment.
Analysis
To test the positioning reproducibility of placing the mouse and rat body in the tomograph, seven bone landmarks were used. An experienced investigator identified these locations using the microCT images. The skeletal landmark locations chosen were; top of skull, top of spine, left and right shoulder joints, left and right hip joints, and the most distal joint of the sternum. For the mouse imaging chamber, two fixed positions on the sled were also used for chamber reproducibility testing. The pixel locations were determined for each coordinate and the mean value was used as the reference location.
The average, minimum, and maximum distances to the mean values are reported. The formula used was distance= ((xm−x)2+(ym−y)2+(zm−z)2)½, where m=mean. Analysis was done using AMIDE (http://amide.sourceforge.net/) and Microsoft Excel (Microsoft Office Professional Edition, 2003, Microsoft Corporation, Redmond, WA.).
Results
For mouse multimodality imaging testing, the average distance between the mean and any marker location was 0.23 mm (± 0.13 mm). The maximum distance found was 0.57 mm and the minimum distance found was 0.04 mm. For mouse longitudinal testing, the average distance between the mean and any marker was 1.11 mm (± 0.89 mm). The maximum distance found was 3.38 mm and the minimum distance found was 0.04 mm. [Table 1]
Table 1.
Mouse and rat reproducibility summary chart. Difference is defined as the distance to the mean from the point measured.
| Average difference | Max. difference | Min. difference | |
|---|---|---|---|
| Mouse multimodality measurements | 0.23 mm (0.13) | 0.57 mm | 0.04 mm |
| Mouse longitudinal measurements | 1.11 mm (0.89) | 3.38 mm | 0.04 mm |
| Rat multimodality measurements | 0.46 mm (0.25) | 0.97 mm | 0.11 mm |
| Rat longitudinal measurements | 4.31 mm (2.11) | 9.09 mm | 0.52 mm |
| Average difference | Maximum difference | Minimum difference | |
|---|---|---|---|
| Mouse multimodality measurements | 0.23 mm (0.13) | 0.57 mm | 0.04 mm |
| Mouse longitudinal measurements | 1.11 mm (0.89) | 3.38 mm | 0.04 mm |
| Rat multimodality measurements | 0.46 mm (0.25) | 0.97 mm | 0.11 mm |
| Rat longitudinal measurements | 4.31 mm (2.11) | 9.09 mm | 0.52 mm |
For rat multimodality imaging; the average distance between the mean and any marker location was 0.46 mm (± 0.25 mm). The maximum difference was 0.97 mm and the minimum distance found was 0.11 mm. For rat longitudinal testing, the average distance was 4.31 mm (± 2.11 mm) with a maximum distance of 9.09 mm and a minimum distance of 0.52 mm. [Table 1]
The chamber positioning tests reveal that errors introduced by the chamber movement are small, on average less than one pixel in the microCT images created with 400 micron voxels.
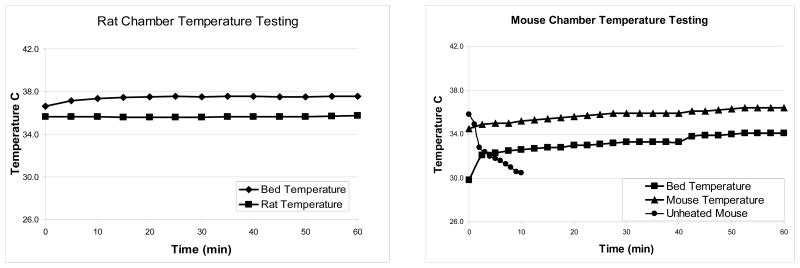
The chamber’s ability to maintain temperature was demonstrated by raising the mouse’s body temperature initially at 34.5°C to 35.9°C after 25 minutes and maintaining it at that temperature with the controller set to 37°C. When the heating controller was set at 38°C, the mouse temperature correspondingly rose from 35.9°C to 36.4°C. The mouse temperature remained quite stable over the measurement duration. Similar results were observed for the rat chamber, where the heater was able to maintain the rat at 36°C when set at 38°C [Figure 4]
Figure 4.
Rat and Mouse imaging chambers heated over one hour. The mouse chamber was set at 37°C for the first 40 minutes and then set at 38°C for the final 20 minutes. The rat chamber was set at 38°C the whole hour. Additionally one mouse was anesthetized under isoflurane anesthesia and unheated to show how rapidly a mouse’s body temperature can drop if not provided supplemental heat.
Discussion
The use of these imaging chambers helps to standardize and facilitate rodent handling and imaging. Due to significant interest in multi-modality imaging, (PET/CT, SPECT/CT, PET/MR), and longitudinal imaging, the ability to reproducibly scan rodents becomes essential if rodents must be moved between imaging locations. As the field of small animal imaging continues to grow, so will the demand to easily image rodents in high throughput facilities. By using the imaging chamber we were able to reproducibly image rodents in different tomographs and at different times in a highly reproducible manner. This allows simplification of the spatial co-registration process. [Figure 5]
Figure 5.
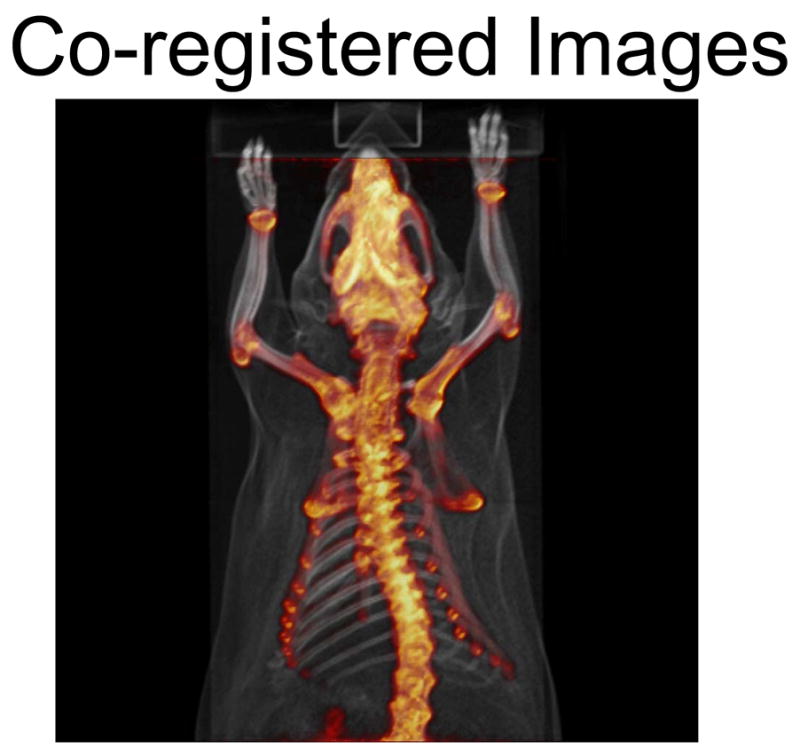
PET and CT images co-registered
Positioning of the animals within the imaging systems can sometimes be a critical consideration, particularly if the radial resolution changes over the field of view (FOV), or if anything located outside the FOV creates an image artifact.14 The use of the curved sled ensures that the rodents are located in the center of the chamber, maximizing the part of the animal that can be imaged.15, 16 The curved shape of the sled allows gravity to keep the animal centered.” Rats pose a difficult challenge, since they vary widely in size and can easily exceed the 10 cm maximum FOV of the microCT system used here.
As expected there was more variability in the repositioning values when the rat and mouse were removed from and replaced in the chamber between images; however, the error is still small and below the image resolution of the microPET system. The spine showed the most variance in both the rat and mouse longitudinal testing. For images where approximately 1 mm is sufficient, the chamber offers a simple, easy hardware offset solution. The rats, due to their larger size, proved more difficult to reposition as accurately, primarily due to their tendency to roll slightly to one side or another. The rat spine has an average mean distance of 6.17mm, whereas the top of the rat skull only had an average mean distance of 3.18mm. It should be noted here that the chamber was not designed for stereotactic brain imaging where sub millimeter positioning might be required. Instead the focus was in ease of use in routine operation. Further refinements are underway to stabilize the head for brain research experiments.
In rodent imaging there are many potential sources of measurement changes beyond biological variability; primarily from instruments, animals, and people. Kastenmayer and associates recently studied evaluator bias using microCT scans for estimating organ volume and found that the majority of variation occurred when the mouse was repeatedly scanned; they also looked at reproducible mounting.17 Our imaging took place over the course of one day using the same person, so having multiple people involved in the scanning process can add more variability. Extended use over five years has shown that the system provides a very reliable and consistent image alignment.
Another benefit of the chamber is the built-in nose cone and gas anesthesia delivery system. The benefits of using gas anesthesia (isoflurane) over injectable anesthesia, such as ketamine, are many.9 Isoflurane is not a controlled substance and thus relieves investigators from regulatory burdens. With isoflurane, the anesthesia level is easily adjustable and there is rapid recovery of the rodent. This feature is beneficial both for animals frequently imaged and it enables investigators to quickly move on to other activities. At our institute the use of the gas anesthesia system, induction boxes, and imaging chambers is now standard for all animal imaging work with microPET and microCT.
In our facility we use one anesthetic system to serve the microPET/microCT area and one for the optical and surgical area. A single vaporizer system was chosen for each location, with a manifold to deliver isoflurane anesthesia using orifices to meter the flow to all points of use. A previous version of the system using flow meters with adjustable orifices was difficult to use and required constant adjustment of the gas flow as each location was turned on or off. In essence, the current system is a ‘set it and forget it’ arrangement that allows investigators a worry-free anesthesia and heating system.
Another beneficial feature of the imaging chambers and induction boxes is the built-in heating element, providing heat to all areas were rodents are anesthetized. The induction boxes, work surface areas, and chambers were preset to 36°C, so investigators did not need to monitor temperature. This alleviated the need for invasive rectal probe measurements that could harm or cause other problems for rodents. Preset heating where the temperature is controlled is critical because mice can easily become heat stressed at temperatures exceeding 37°C. 11 A properly controlled temperature has minimal overshoot, undershoot, and lag time. [Figure 4]
The ability to image immunocompromised rodents is another advantageous feature of our imaging chamber. The barrier or isolated imaging feature of the chamber, when used with sterile techniques and the biosafety cabinets, helps investigators maintain the health of their immunocompromised models. Due to the ability to easily disinfect the chamber between mice, use of the biosafety cabinet, and the positive pressure inside, it provides a sufficient barrier to maintain the health of immunocompromised rodents. We are currently looking into the possibilities of being able to image rodents infected with biohazardous agents with our chamber as well.
Because the chamber helps to standardize the use of anesthesia, heating, and positioning it minimizes the learning curve of new investigators to the imaging center. It also helps to speed regulatory approval of animal protocols. Coupled with an easy to use disinfectant solution, it is a simple procedure to keep the chamber clean and ready to use within minutes. Another feature is the ability to maintain the health of all the animals imaged in the chamber. The steady gas anesthesia and heated bed has nearly eliminated the loss of animals due to hypothermia or overdose of anesthesia.
Conclusions
Reproducible and easy to use mouse and rat imaging chambers have been devised to facilitate the positioning, anesthesia, heating, and isolation of mice undergoing imaging experiments using microPET and microCT. The anesthesia system together with the imaging chambers has provided a robust and reproducible method for longitudinal research in a high throughput molecular imaging facility.
Acknowledgments
We wish to thank the Crump Imaging Center Staff for their support; Waldemar Ladno, Judy Edwards, and Antonia Lu; Jim Houts of Summit Anesthesia Solutions; Dennis Corey; Marcelo Couto and Tim Lawson of UCLA Division of Laboratory Animal Medicine; and Andy Gustillo and Steve Grubweiser of the UCLA School of Engineering and Applied Sciences. Support for this project was provided by the Biological and Environmental Research Division of the Department of Energy (DOE) #FC03-02ERG3420; the National Cancer Institute (NCI) #R25 CA098010:01; the In Vivo Cellular and Molecular Imaging Center (NIH ICMIC) #R01 EB001943; the Small Animal Imaging Resource Program (SAIRP) #R24 CA92865; the Jonsson Comprehensive Cancer Center UCLA; and the NCI SPORE in Prostate Cancer.
Footnotes
All work completed at UCLA
References
- 1.Croy ACDSJP, Manz M, et al. Mouse Models of Immunodeficiency. In: Fox JG, Barthold SW, Davisson MT, et al., editors. The Mouse in Biomedical Research. Elsevier Press; 2007. pp. 275–289. [Google Scholar]
- 2.Fueger BJ, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 3.Chow PL, Stout DB, Komisopoulou E, Chatziioannou AF. A method of image registration for small animal, multi-modality imaging. Phys Med Biol. 2006;51:379–90. doi: 10.1088/0031-9155/51/2/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stout DB, et al. Small animal imaging center design: the facility at the UCLA Crump Institute for Molecular Imaging. Mol Imaging Biol. 2005;7:393–402. doi: 10.1007/s11307-005-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout D, Lawson TP, Silverman RW, Chatziioannou AF. Immuno-isolated imaging of SCID and nude mice in microPET. Annual Conference of the Academy of Molecular Imaging; 2001. [Google Scholar]
- 6.Bahadur AN, et al. Multimodality Chamber for coregistered anatomical and molecular imaging of small animals. Lab Anim (NY) 2007;36:29–35. doi: 10.1038/laban0907-29. [DOI] [PubMed] [Google Scholar]
- 7.Rubins DJ, Meadors AK, Yee S, Melega WP, Cherry SR. Evaluation of a stereotactic frame for repositioning of the rat brain in serial positron emission tomography imaging studies. J Neurosci Methods. 2001;107:63–70. doi: 10.1016/s0165-0270(01)00352-1. [DOI] [PubMed] [Google Scholar]
- 8.Herschman HR. PET reporter genes for noninvasive imaging of gene therapy, cell tracking and transgenic analysis. Crit Rev Oncol Hematol. 2004;51:191–204. doi: 10.1016/j.critrevonc.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Hendrich H, Horst M. Housing and Maintenance. In: Hendrich H, editor. The Laboratory Mouse. Elevier Academic Press; 2004. pp. 395–408. [Google Scholar]
- 10.Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur J Nucl Med Mol Imaging. 2002;29:98–114. doi: 10.1007/s00259-001-0683-3. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby RO, Fox JG, Davisson M. Biology and Diseases of Mice. In: Fox JG, Anderson LC, Loew FM, et al., editors. Laboratory Animal Medicine. 2. Elsevier Science; 2002. pp. 35–120. [Google Scholar]
- 12.Deroose CM, et al. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J Nucl Med. 2007;48:295–303. [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenaar DJ, Kapusta M, Li J, Patt BE. Rationale for the combination of nuclear medicine with magnetic resonance for pre-clinical imaging. Technol Cancer Res Treat. 2006;5:343–50. doi: 10.1177/153303460600500406. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh J, et al. A novel reconstruction algorithm to extend the CT scan field-of-view. Med Phys. 2004;31:2385–91. doi: 10.1118/1.1776673. [DOI] [PubMed] [Google Scholar]
- 15.Beattie BJ, et al. Multimodality Registration without a Dedicated Multimodality Scanner. Mol Imaging. 2007;6:108–20. [PubMed] [Google Scholar]
- 16.Zanzonico P, et al. Animal-specific positioning molds for registration of repeat imaging studies: comparative microPET imaging of F18-labeled fluoro-deoxyglucose and fluoro-misonidazole in rodent tumors. Nucl Med Biol. 2006;33:65–70. doi: 10.1016/j.nucmedbio.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Kastenmayer RJ, Perdue KA. Variation in organ volumes of matched BALB/c mice by microcomputed tomography analysis. J Am Assoc Lab Anim Sci. 2007;46:7–12. [PubMed] [Google Scholar]