Abstract
Biochemical, histological, and physiological evidence suggest strongly that astrocytes may either defend or damage brain tissue, depending on the brain carbohydrate content preceding global ischemia (28,43). This paper will first review the concept of acidosis in ischemia and the possible role of severe, compartmentalized astrocytic acidosis in pan necrosis. Results are then presented demonstrating that astrocytes are also capable of maintaining an alkaline intracellular pH (pHi) during normoglycemic global ischemia. Mechanisms underlying depolarization-dependent astroglial alkalosis are then reviewed. Recent experiments indicate that bicarbonate (HCO3−) transport is a major mechanism by which astroglia not only alkalinize their interior but also acidify the interstitium. Maintenance of alkalosis during normoglycemic ischemia supports the hypothesis that astroglial HCO3− transport might ultimately protect neurons from excitotoxicity in ischemia without infarction (17). Inhibition of astroglial HCO3− transport may be a critical and requisite event, ultimately leading to compartmentalized astroglial acidosis and irreversible injury to all cell types.
Keywords: Acidosis, Alkalosis, Excitotoxicity, Infarction, Na+/HCO3−-co-transport, Reactive astrocytosis, Selective neuronal vulnerability
ASTROGLIAL ACIDOSIS IN HYPERGLYCEMIC GLOBAL ISCHEMIA
A Brief History of Acidosis in Ischemia
Over the last 25 years, several investigators have measured tissue pH during brain ischemia under various metabolic circumstances in an attempt to establish a link between acidosis and tissue injury. The general hypothesis driving most experiments was that the deprivation of oxygen and the arrest of aerobic metabolism lead to enhanced glycolysis in order to meet energy demands. Increased lactate production, combined with ongoing ATP hydrolysis, produces an accumulation of protons that ultimately exceeds brain cell buffering capacity. This hypothesis motivated Ljunggren et al.’s (33) experiments demonstrating that tissue pH drops and lactate concentration rises during cerebral ischemia. Ljunggren et al. (33) and others (18,36,49,58) showed further that animals, made hyperglycemic before ischemia, attained higher concentrations of lactate than euglycemic controls. Siemkowicz and Hansen (53) added support for these observations by directly measuring interstitial pH during cerebral ischemia with pH-sensitive micro-electrodes and found that brains of animals pretreated with glucose were considerably more acidotic (pH 6.1) than saline-treated controls (pH 6.6).
Parallel investigations demonstrated a strong correlation between the carbohydrate level preceding severe ischemia and the extent of brain infarction (death of all cellular elements), suggesting further that glycolytic acidosis may be injurious to the brain, Myers and Yamaguchi (37) provided the first direct in vivo evidence of the harmful effects of ischemic lactate. In cardiac arrest in young monkeys, animals infused with glucose before ischemia suffered far more brain damage than either fasted animals or animals infused with saline. In a rat global ischemia model, Pulsinelli and co-workers (48) convincingly demonstrated that pre-ischemic hyperglycemia could shift the pathological outcome from selective neuronal death to infarction. This relationship between pre-ischemic carbohydrate levels and pathological outcome also seemed to hold clinically. In patients, relatively hyperglycemic prior to a stroke, clinical outcome was significantly worse than in normoglycemic patients (47).
Neuroglial Acidosis and Infarction
Friede and Van Houten (13) were the first to suggest a relationship between brain injury and neuroglial acidosis. In their experiments with rat brain slices, they observed enhanced tissue pathology when anoxic slices were incubated with elevated concentrations of glucose. Cellular pathology was most notable in regions of high neuroglial density. In the presence of high glucose, anoxic glial cells demonstrated marked edema and klasmatodendrosis, a pathological transformation involving the shortening, swelling, and ultimate disintegration of cell processes (41). When these investigators added agents that blocked glycolysis, however, glial pathological changes and tissue acidosis were dramatically attenuated. These observations led Friede and Van Houten to speculate that glycolytic acidosis during anoxia causes tissue injury in brain, and that the source of hydrogen ions as well as the primary target of acid-induced injury may, in both cases, be neuroglial cells.
Some 20 years later, Plum reintroduced and developed the concept that glial cells (and astrocytes in particular) might be the glycolytic engines generating harmful levels of lactic acidosis, and that astrocytes may in turn be uniquely susceptible to this acidosis. Drawing on the work outlined above and others, Plum proposed: ‘The presence or absence of lactate damage to astrocytes determines whether brain ischemia confines its damage to selectively distributed neurons or produces regional necrosis in all cellular elements, with greater immediate and subsequent injury to the tissue’(43).
Plum made a clear distinction between two different pathological outcomes following global ischemia: one in which only selectively vulnerable neurons die, and the other in which all brain cells die. Thus, with moderate- to low levels of lactate, astrocytes are spared and ischemia damages select neurons “by a process independent of pH or osmolal change” (43). High levels of lactate, on the other hand, are associated with an excessive acidosis to which astrocytes are most sensitive. The reasoning behind this astroglial hypothesis was as follows. Biochemical and histological evidence suggest that astrocytes contain more glycogen than other cells. During anoxia, this store is plundered to drive glycolysis, such that protons and osmols increase in astrocytes more than other cells. When brain lactate levels rise above a critical threshold—that is, one associated with the transition from selective neuronal death to infarction (44)—acidosis and other unknown factors irreversibly damage anoxic astrocytes and endothelial cells. As a result, astroglial nutritional support and ion homeostatic mechanisms fail, and the blood-brain barrier breaks down, leading to generalized necrosis and progressive edema.
Astroglial Acid Compartmentation in Hyperglycemic Ischemia
The evidence and arguments detailed above strongly suggested that acidotic damage to astrocytes is a necessary prerequisite for infarction. What was missing from these investigations were mechanistic data demonstrating how astrocytes might be particularly sensitive to excessive acidosis, and specifically, to excessive anaerobic glycolysis. The first clues finally came from a group of experiments examining brain pH, PCO2, HCO3−, and lactate levels together during complete ischemia with and without the association of high brain glucose levels. Above a critical tissue lactate level of ~17 mmol/kg, levels attained in hyperglycemic animals, interstitial pH remained fixed (28). This implied that a separate compartmented system, perhaps a population of cells, continued to produce increasingly higher lactate concentrations observed in the whole tissue, and yet retain for itself the protons co-generated in a 1:1 stoichiometric ratio with lactate. In addition, and perhaps more importantly, tissue PCO2 remained fixed, suggesting that this acidic compartment had lost its HCO3− content (30).
Estimates of postischemic total brain and interstitial [HCO3−] revealed that the level of this anion also remained unchanged in spite of a progressive rise in lactate, providing further evidence of an acidotic, relatively HCO3−-free compartment. At lactate values of 19 mmol/kg or higher, the pH in this HCO3−-free compartment must have been 5.2 or less. An estimate of the size of the HCO3−-exhausted space suggested that the acidotic compartment represented approximately 36% of total brain volume, a value that is close to that estimated by Hertz and Schousboe (21) for glial cell space in the neocortex.
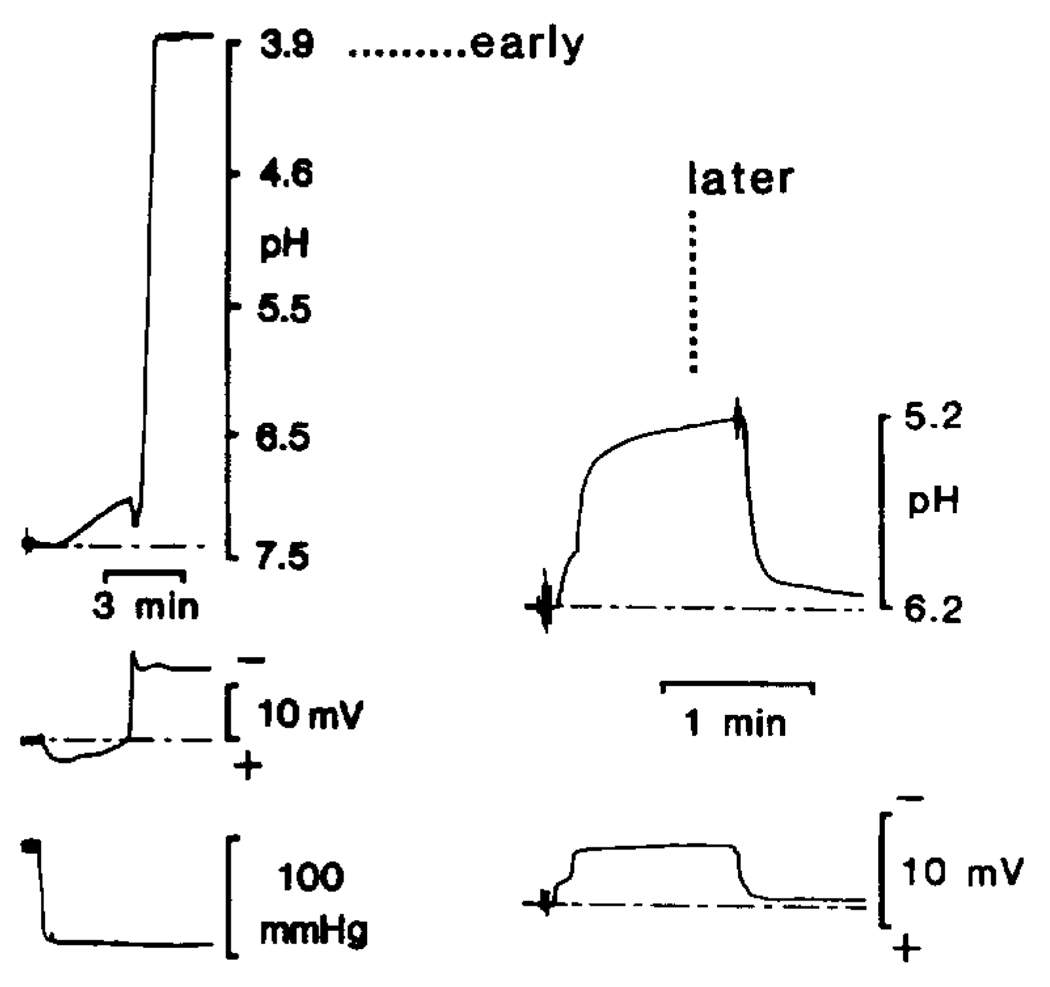
Although other experiments questioned the validity of acid compartmentation in global ischemia (23), direct measurements of cellular pHi, using pH-sensitive microelectrodes finally provided direct evidence that a separate, relatively acidic compartment existed during hyperglycemic and complete ischemia (25) (Fig. 1). Two foci more acidic than the interstitial space (pH 6.7–6.20) were found. The first was only occasionally seen at the onset of ischemia and reached pH 3.82–4.89, while the second was seen 5–6 min after the onset of ischemia and reached pH 4.46–5.93. A small direct current potential and massive increase in cell input resistance supported the conclusion that these acidic foci are in intracellular space. Horse-radish peroxidase injections via the reference barrels of pH-sensitive microelectrodes provided evidence that these extreme levels of acidosis occurred in astrocytes.
FIG. 1.
pH-sensitive microelectrode (pH-ISM) measurements in presumed astrocytes during hyperglycemic and complete ischemia. pH ISMs are positioned approximately 400–800 µm below the parietal surface. Following cardiac arrest, systolic blood pressure rapidly falls to a minimum, as indicated in the lower left-hand record. The middle trace at the left indicates the negative shift in the DC potential accompanying cell entry. It is believed that the pH-ISM spontaneously enters a glial cell as it swells with depolarization. The trace at the top left shows first a slow interstitial acid shift, and then, at the onset of anoxic depolarization, a second pH shift orders of magnitude more acidic than the interstitial space. This suggests entry into an extremely acidic environment. ISMs could be withdrawn back into the interstitium, as indicated by positive shifts (~5–10 mV) in DC potential and a return to an interstitial pH of 6.3 (data not shown; see 24), expected values of potential and pH in the interstitial space of hyperglycemic and completely ischemic brain (25). Such extreme levels of acidosis were seen only at the beginning of experiments. On the right, the top trace demonstrates a more typical pH change seen 30 min after the onset of complete hyperglycemic ischemia. Note that [H+] reached a maximum of about 5.2 pH here. Later in experiments, maximum levels of acidity typically remained one to two orders of magnitude higher than the interstitial space.
Although the above results demonstrate that these presumed astrocytes can generate and probably succumb to excessive acidosis, the precise molecular mechanisms of acid-induced injury and death remain to be defined. Pulsinelli et al. (46) suggested a free radial mechanism for injury. Since bicarbonate is essential for the sequestration of iron within transferrin, free iron in an excessively acidic (and very low HCO3−) environment may catalyze the generation of free radicals with subsequent irreversible injury to membranes by lipid peroxidation (46,51). Other injurious mechanisms most likely also interact in an acidic milieu to destroy astrocytes (26,92). Nonetheless, in vivo- as well as recent tissue culture data (15,52,57) now clearly demonstrate that astrocytes are uniquely susceptible to acidotic injury. Thus, this acute sensitivity may be a major determinant of brain infarction following hyperglycemic global ischemia.
ASTROGLIAL ALKALOSIS IN NORMOGLYCEMIC ISCHEMIA
During and following normoglycemic global ischemia, astrocytes seem to be highly resistant to injury (42). Only selectively vulnerable neuronal populations succumb (44). These observations led Plum to hypothesize that, under circumstances in which ischemia does not injure glia, astrocytes instead may function to defend brain tissue (43). In the light of this speculation, our laboratory has measured astroglial pHi in the context of normoglycemic global ischemia (27). Our preliminary results indicate that ischemic astrocytes not only maintain pHi in the face of mild (e.g. ~6.8) interstitial acidosis, but may even alkalinize relative to their own resting baseline pH during global ischemia under normoglycemic conditions. If the mechanisms underlying astroglial alkalosis are the same as those giving rise to depolarization-dependent alkalosis and, reciprocally, interstitial acidosis during cortical stimulation and spreading depression, astrocytes may, in the short term, produce a mildly acidic interstitial environment that protects selectively vulnerable neurons from excitotoxic injury, while simultaneously buffering an intraglial acid load.
Methods
Normoglycemic global ischemia was carried out according to methods detailed previously (45). Briefly, 15 male Wistar rats were fasted for 12–24 h before ischemia, but were provided free access to water (27). Animals were anesthetized with halothane. Blood gas variables and blood glucose levels were normalized prior to pH/electrical recordings and constantly monitored throughout experiments. Intracellular pH-sensitive microelectrodes were fabricated as previously described (25). To record interstitial pH (pHo), the tips of intracellular electrodes were broken to a tip diameter of ~2–4 µM. Blood flow was monitored by a surface, laser/Doppler technique. All signals were stored on videotape.
During ischemia, normal Ringer’s superfusate was replaced by one containing only 20 mM of bicarbonate and an elevated carbon dioxide pressure so that superfusate pH was 6.80, a value near that of interstitial space during normoglycemic and complete ischemia (28). In this way, superfusate could continue to be used as a calibration point for pH recordings without creating significant changes in the acid-base status of superficial exposed brain. Furthermore, all electrical recordings were made >500 µm below the pial surface, a depth not affected by surface superfusion.
Results
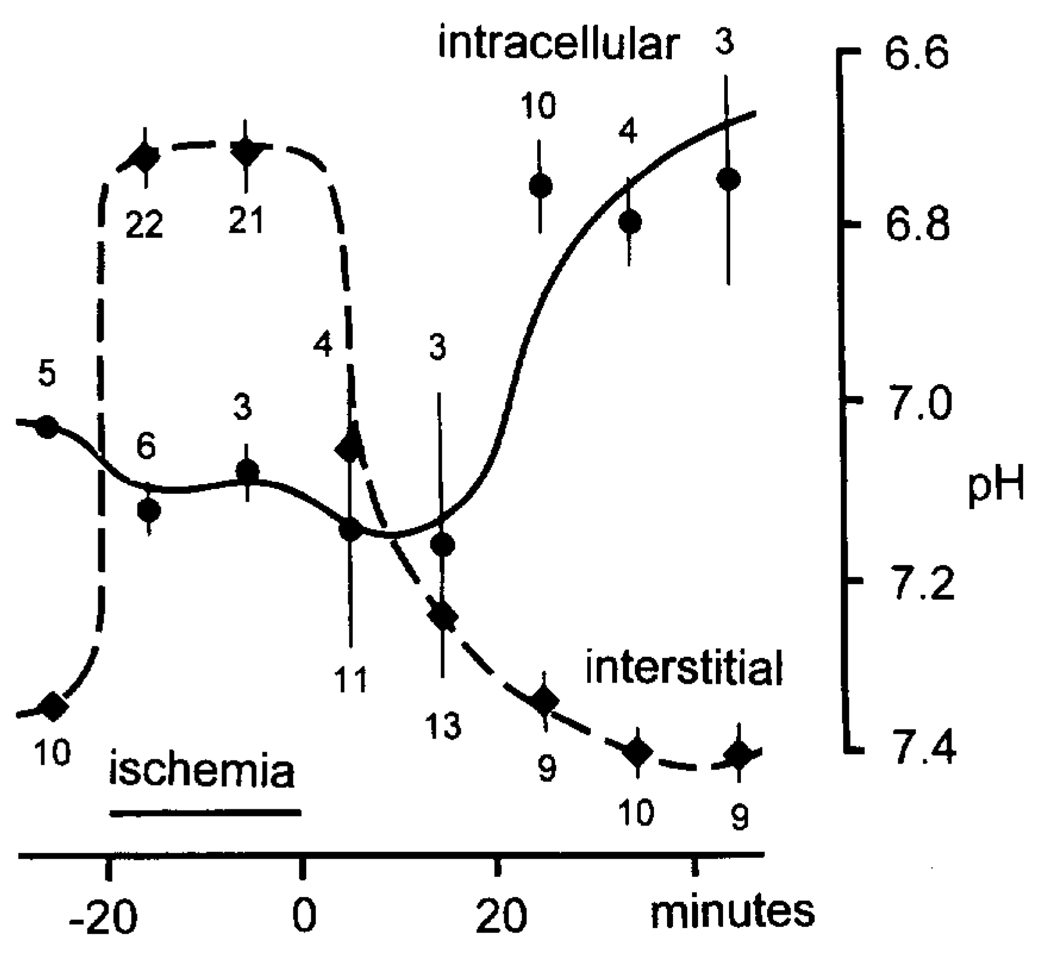
A summary of both intraglial and pHo values is shown in Fig. 2. All animals were normothermic and normotensive and had blood gas values and hematocrits within normal ranges. Before ischemia the blood glucose was 4.8 ± 0.2 mM (n = 15; range 3.3–6.2), pHo was 7.34 ± 0.01 (n = 10; range 7.28–7.40), and astrocytic pH was 7.03 ± 0.01 (n = 5; range 7.00–7.06). During ischemia, the pHo reached a peak of 6.72 ± 0.03 (n = 43; range 6.45–6.92), whereas glial pH shifted more alkaline to 7.11 ± 0.04 (n = 9; range 7.03–7.22).
FIG. 2.
Astroglial alkalosis during normoglycemic, nearly complete ischemia. pH-ISMs were used to measure pHi in presumed glia prior to, during, and several minutes after global ischemia. Before and after ischemic depolarization, cells were identified as astrocytes by their high membrane potential, low membrane impedance, and absence of synaptic or injury discharges. A microelectrode taper that selectively penetrates astrocytes under normal conditions was chosen for recordings during anoxic depolarization. Horseradish peroxidase injections using this taper have consistently stained astrocytes (24). Triangles refer to interstitial recordings; dots indicate intracellular recordings. Recordings were grouped into 10-min intervals and averaged. The vertical bars through points represent standard error of means. Interstitial numbers below triangles, and intracellular numbers above dots, represent the number of recordings for a particular 10-min interval. Note that, during ischemia, astrocytes were alkaline as the interstitium acidified. The measurements also suggest that astrocytes may become slightly more alkaline relative to their own normal baseline pH.
This behaviour of astroglial pH was somewhat unexpected. Given the massive acidosis seen during hyperglycemic ischemia, it was not unreasonable to expect some intracellular acidosis during normoglycemic ischemia that merely intensified with hyperglycemia and excessive anaerobic glycolysis. That astrocytes maintained an alkaline pH relative to interstitial pH, and perhaps a more alkaline pH than their own normal resting baseline, strongly suggests that, during normoglycemic ischemia, astrocytes maintain plasma membrane integrity and function, including active transport processes moving proton equivalents. As suggested above, astroglial pH behaviour during normoglycemic ischemia may arise from the same mechanisms responsible for depolarization-induced alkalosis in astrocytes during normal brain activity and spreading depression. Inhibition of these mechanisms may be a necessary event leading to the dramatic transition from alkalosis to massive, compartmentalized acidosis observed during hyperglycemic ischemia.
ASTROGLIAL ALKALOSIS AND NA+/HCO3− CO-TRANSPORT
Historical Perspective
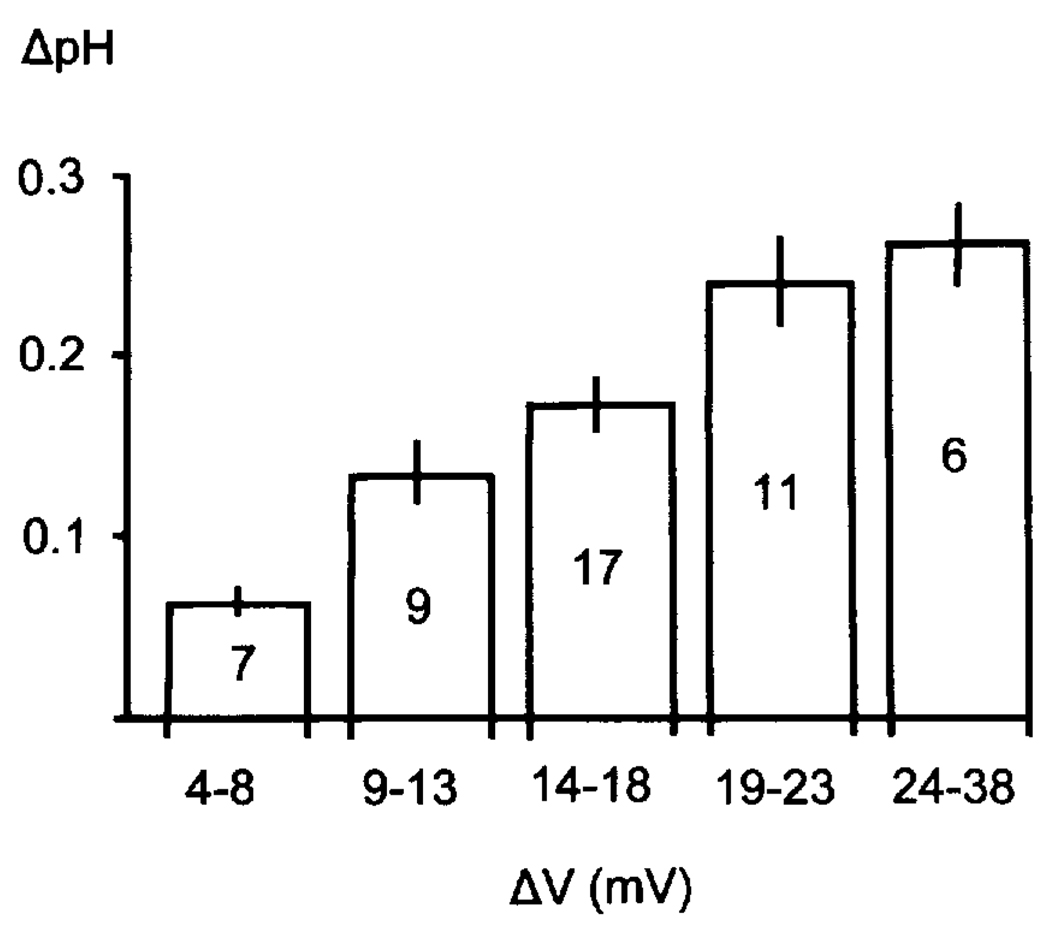
The evidence for depolarization-linked alkalosis in astrocytes first came from intracellular pH (pHi) measurements of rat cortical astrocytes in vivo (9). These experiments used pH-sensitive microelectrodes to uncover a profound alkalosis with cell depolarization, despite a well-documented concomitant rise in brain lactate, CO2 production, and predominant interstitial acidosis. This seemingly “independent” pH behaviour demonstrated that astrocytes need not simply respond passively and in parallel to pH changes in the interstitium (34). Subsequent measurements indicated that the magnitude of glial alkalotic shifts was directly correlated with the degree of cell depolarization. Cortical stimulation of various intensities produced a graded rise in glial pHi (~pH 0.1–0.3) (Fig. 3). During spreading depression, when the membrane potential collapses to nearly 0 mV, astrocyte pH increased by as much as 0.80 pH (Fig. 4). In the presence of interstitial Ba2+, which blocks glial K+ conductances, astrocytes hyperpolarized during neuronal activity (9), and either no pH shift or a small acidification was seen. The conclusion was that depolarization per se was required to elicit the glial alkaline shift.
FIG. 3.
Depolarization-dependent astroglial alkalosis. Fine-tipped pH-ISMs were used to record from astrocytes in rat frontal cortex in vivo. With cortical stimulation, glia depolarized and became more alkaline by 0.05–0.40 pH (9). This is in contrast to a predominant acidification of the interstitial space during stimulation or spreading depression (9). This figure represents the pooled results of astroglial alkalotic shifts occurring at different cortical stimulus frequencies. Greater alkaline shifts were seen with progressively larger membrane depolarizations, indicating that the extrusion of protons or proton equivalents from astrocytes may be directly dependent on cell membrane potential.
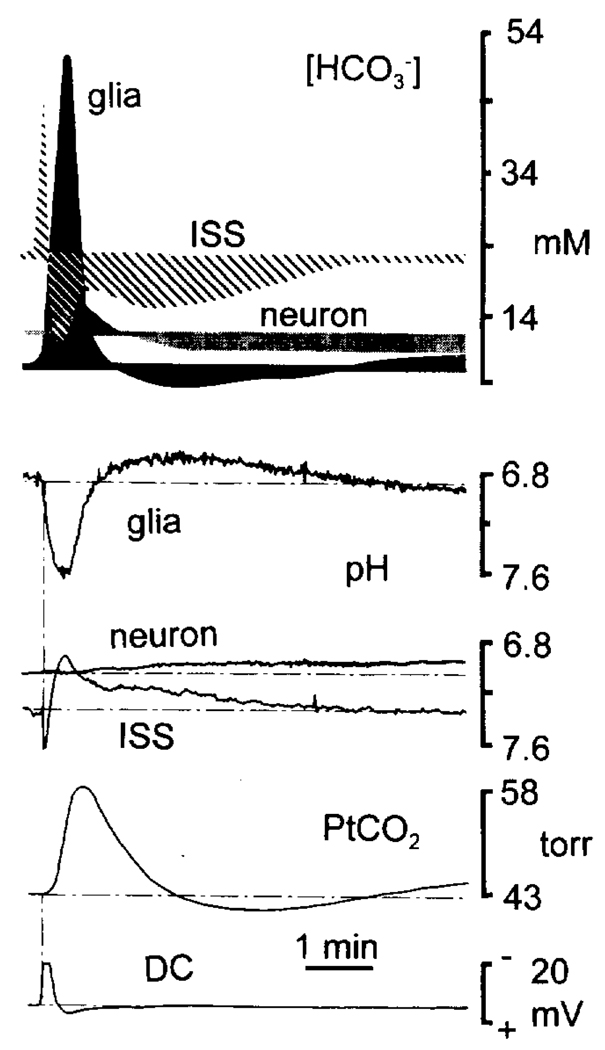
FIG. 4.
Acid-base and calculated [HCO3−] changes during spreading depression (SD). The bottom four traces are from different experiments but represent typical recordings during spreading depression. The top figure is a composite of [HCO3−] in all three brain compartments, calculated via the Henderson-Hasselbach equation from the data shown below it. The DC (bottom trace) and interstitial (ISS) pH changes were recorded simultaneously. The glial and ISS pH changes show that the peak interstitial acid shift (pH 6.93) was correlated in time with the peak alkaline glial transient (pH 7.61). Neuronal pH (7.08) was comparatively unchanged during SD, but became slightly acidic (6.92) with cell repolarization. Calculated [HCO3−] values were based on the assumption that carbon dioxide and the first ionization equilibrium constant of carbonic acid (6.13) were equivalent in all brain compartments. Glial (solid area) [HCO3−] was 7 mM before SD and reached a peak of 51 mM during SD and maximum depolarization. Interstitial [HCO3−] (hatched area), in contrast, predominantly fell to <11 mM, before progressively returning to baseline. After increasing slightly from 12 to 16 mM during SD, neuronal [HCO3−] (stippled area) eventually dropped to <8 mM.
Passive efflux of acid (or influx of base), e.g. via a H+ or HCO3− ion channel, as a mechanism for astrocytic alkalinization was excluded on thermodynamic grounds. Given a pHi and pHo of 7.0 and 7.3, respectively, the equilibrium potential for H+ or an acid–base equivalent (OH−, HCO3−, NH4+, etc.) is −18 mV. Since astrocyte membrane potentials are far negative of this value throughout cortical stimulation, either some form of active transport across the membrane and/or the internal consumption of protons is necessary. Intracellular consumption of protons could occur via the hydrolysis of phosphocreatinine (31), but this is highly unlikely because the magnitude of the astrocytic acid-base perturbation would require millimolar amounts of substrate to be utilized within seconds. Moreover, concomitant ATP hydrolysis should generate protons. Finally, alkaline shifts did not occur in the presence of Ba2+, despite the likely hydrolysis of phosphocreatinine reserves during electrogenic Na+/K+ ATPase-driven hyperpolarization (2). Therefore, a more likely prospect is the active transport of some acid-base equivalent across the glial membrane. Based on intracellular measurements of strong ions (Na+, K+ and Cl−) in glial cells of olfactory slices (2), depolarization produces an astrocytic anion deficit that can be filled by HCO3−. Calculations of intracellular and interstitial [HCO3−] indicate that astroglial HCO3− may rise by an order of magnitude during spreading depression (Fig. 4) (24). At any given depolarization, both the anion deficit and measured alkalosis in the above experiments can be accounted for by the same rise in [HCO3−].
Electrogenic Na+/HCO3− Co-transport in Astrocytes
Data obtained from non-mammalian glial preparations (10), mammalian brain slices (16), and cultured rat astrocytes (7,50) suggest the presence of an electrogenic Na+-dependent carrier that transports HCO3− and/or CO32− into astrocytes with cell depolarization. First described in the basolateral membrane of renal tubule cells, electrogenic bicarbonate transport involves the net transmembrane flux of one Na+ ion coupled to the flux of at least two HCO3− ions, one CO32− or some combination of both anions (5). Electrogenicity arises from the net transfer of at least one negative charge (as either HCO3− or CO32−) moving in the same direction as Na+. The basic properties of electrogenic HCO3− transport are: (1) an absolute requirement for the presence of Na+ and HCO3−; (2) no requirement for Cl− ions; (3) net transfer of negative charge with each transport cycle; and (4) pharmacological inhibition by stilbene derivatives SITS and DIDS. Since the original work in the renal tubule cell, electrogenic bicarbonate transport has been observed in a number of other cell types, both epithelial and non-epithelial. Although some deviations from the renal cotransporter have been observed (e.g. insensitivity to stilbene inhibitors DIDS and SITS), the basic ionic properties of this transport are retained, whether in smooth muscle, corneal epithelium, or leech glial cells (5).
Evidence for a glial electrogenic Na+/HCO3− co-transport was first found in a series of studies on pH regulation in single glial cells of the invertebrate leech (10). Deitmer and Schlue noted that replacement of HEPES-buffered media with one that was HCO3−-buffered caused a rapid rise in pHi and a membrane hyperpolarization. The HCO3−-stimulated effects were blocked by DIDS and Na+-free solutions, but were unaffected by Cl− -free media, amiloride or furosemide. In experiments using a two-electrode voltage clamp, Munsch and Deitmer measured a cell current that is Na+ and HCO3−-dependent, Cl−-independent, and blocked by DIDS and SITS (35). The reversal potential for this current indicated a transport stoichiometry of two HCO3− ions for each Na+ transported. Given the concentrations of intra- and interstitial HCO3− in the leech, the reversal potential was nearly equivalent to the glial cell’s resting membrane potential. This means that any depolarization from rest would be sufficient to drive HCO3− into the cell.
Work in glial cells of the mudpuppy optic nerve also demonstrated an electrogenic process that was Na+/HCO3−-dependent, SITS-sensitive, and Cl−-independent (1). In addition, Newman demonstrated a Na+/HCO3− co-transport current using the whole cell voltage clamp technique with salamander Muller cells (39). The current was Na+- and HCO3−-dependent, Cl−-independent, and blocked by stilbenes and harmaline. Newman also observed a binding stoichiometry of approximately three HCO3− anions for every Na+ cation, different from the ratio of 2:1 observed in both leech and mudpuppy glial cells.
Indirect evidence for electrogenic Na+/HCO3− co-transport in mammalian astrocytes comes from studies in hippocampal slices and in rat astrocyte cultures (6,50,56). Voipa and Kaila measured interstitial acid transients and a rise in interstitial PCO2 that appear to result from the titration of a HCO3 -induced alkaline load in astrocytes within acute brain slices (56). In cultures, where pHi was measured using the pH-sensitive fluorescent dye BCECF, Boyarsky et al. (6) demonstrated a potent HCO3− and Na+ dependent alkalosis in astrocytes, and Shrode and Putnum (50) observed a depolarization-induced alkalinization seen only in the presence of CO2/HCO3− and Na+. Recently, some of the basic electrophysiological and pharmacological properties of electrogenic Na+/HCO3− co-transport have been identified in cultured rat hippocampal and cerebellar astrocytes (10,40).
Evidence that Na+/HCO3− Co-transport Acidifies the Interstitium
Measurements of interstitial and astrocytic pHi in mammalian hippocampal slices have provided direct evidence that astrocytes secrete acid through a depolarization-linked mechanism that is Na+ and HCO3−-dependent (8,17). In both normal and gliotic hippocampal slices, Grichtchenko and Chesler measured depolarization-induced interstitial acid shifts that are abolished in Na+-free media, and greatly diminished in HEPES-buffered, HCO3−-free solutions (17). Moreover, these acid shifts are unaffected by amiloride or its analogs, stilbenes, zero Cl− media, zero or elevated glucose, lactate transport inhibitors, zero Ca2+ or Cd2+. Notably, this process is not pharmacologically inhibited by either DIDS, SITS or harmaline, which are effective blockers of Na+-dependent HCO3− transport elsewhere. This lack of pharmacological block is not inconsistent with other studies of non-epithelial electrogenic Na+/HCO3− transport, however, which also demonstrate a lack of sensitivity to stilbenes (5). Further studies in this same hippocampal slice preparation also examined depolarization-dependent alkalosis in astrocytes (16). It was shown that the determinants of a major portion of this alkalosis were equivalent to those producing the interstitial acidosis in the above studies: that is, the alkalosis was dependent on depolarization, Na+ and HCO3−, and showed no dependence on Cl−. Thus, these data are also consistent with an astroglial electrogenic, Na+-dependent HCO3−/CO32− transport mechanism.
ASTROGLIAL ALKALOSIS MAY PROTECT BRAIN CELLS DURING GLOBAL ISCHEMIA
Astrocytes are ion homeostatic machines that run their extensive energy-dependent ion and substrate transport systems primarily on glycolytic ATP (12). With ischemic depolarization, ATP hydrolysis is maximally stimulated as astroglial membrane pumps and transporter systems futilely attempt to re-establish their ionic environment and that of the interstitial space. ATP hydrolysis produces a massive intracellular acid load in astrocytes. As detailed above, astrocytes can buffer this acid load by coupling extracellular HCO3− to the driving force of Na+ and transporting the anion in against its concentration gradient. Thus Na+/HCO3− co-transport may protect astrocytes during normoglycemic ischemia from the potentially damaging effects of massive acidosis that might otherwise occur without access to titratable base.
It also follows that HCO3− transport into astrocytes may be a major mechanism producing interstitial acidosis during ischemia. Since ischemic protons primarily arise from ATP hydrolysis and not lactate ions per se, astrocytes are faced with the choice of either expelling their protons or transporting in base. According to the data detailed above, and especially the recent work in brain slices (16,17), it appears that astrocytes have chosen the latter solution. If this is true, astrocytes may then protect vulnerable neurons from excitotoxic injury primarily through interstitial acidification via the transport of HCO3−. Acid interstitial pH dramatically reduced the open channel probability of NMDA (54) and voltage-gated Ca2+ (3) channels, both of which appear to be major neuronal entry pathways for Ca2+ influxes associated with excitotoxicity. Experiments in tissue culture (14) and brain slices (55) have extended these observations by demonstrating a neuroprotective effect of mild acidosis against hypoxic and excitotoxic injury.
FAILURE OF HCO3− TRANSPORT MAY CONTRIBUTE TO ACID COMPARTMENTATION
During hyperglycemic and complete ischemia in rats, interstitial [K+] remains within a physiological range twice as long as during normoglycemic ischemia (19). This suggests that energy-dependent homeostatic mechanisms and ATP hydrolysis also occur for this extended period. As a consequence, prolonged ATP hydrolysis produces a greater astroglial intracellular acid load. We propose that excessive acidosis from enhanced ATP hydrolysis leads to greater astroglial HCO3− transport and acid secretion, which in turn ultimately inhibits HCO3− transport. Although this may at first seem circular, it is perhaps not unexpected when viewed in light of data suggesting that most channels and transporters are significantly inhibited by acidic pH (11,22,32). Moreover, precedence for this concept already exists in barnacle muscle experiments showing that acid extrusion falls almost three orders of magnitude when bath pH drops to 6.8 (4). Indeed, HCO3− transport into astrocytes, through either anion channels or transporters, must be at least mostly if not completely inhibited in hyperglycemic and complete ischemia, since interstitial HCO3−, pH, and PCO2 remain fixed, despite the progressive rise in tissue lactate (29). If HCO3− were being transported into astrocytes under these conditions, one would expect at least some change in these pH-related parameters. Finally, inhibition of electrogenic HCO3− transport into astrocytes should be reflected more generally as an increase in cell input resistance, which is precisely what it is seen in vivo with hyperglycemic and complete ischemia (25) and in cultured astrocytes following metabolic injury (20). Therefore, astroglial acid compartmentation may not only be associated with inhibition of HCO3− transport but may in fact arise because of it when inhibition is coupled to excessive acid production in intact cells (26).
CONCLUSIONS
With normal brain activity, spreading depression, and normoglycemic ischemia, Na+/HCO3− co-transport functions to move titrable HCO3− against its concentration gradient into astrocytes. Na+/HCO3− co-influx may give rise to astroglial alkalosis and interstitial acidosis, acid-base shifts that serve to protect neurons and astrocytes from excitotoxicity and acidotoxicity, respectively. With excessive ATP hydrolysis and greater acid loads during hyper-glycemic ischemia, however, HCO3− transport from the interstitial to intraglial space may be inhibited if electrogenic Na+/HCO3− co-transport is sensitive to acidic pH in the same fashion as most ion channels and transporters. Without HCO3− transport mechanisms, excessive protons may not be buffered as astrocytes succumb to severe, compartmentalized acidosis. Thus functional Na+/HCO3− co-transport may be a critical determinant of astroglial viability during ischemia. Failure of this HCO3− transport system may ultimately lead to astroglial death and brain infarction.
ACKNOWLEDGEMENTS
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS-19108), the American Heart Association of Metropolitan Chicago and the Brain Research Foundation of The University of Chicago. C.D.L. was supported by an MD/PhD Training Grant in Growth and Development (HD-07009) from the NICHD and an NRSA (F31-MH11126) from the NIMH.
REFERENCES
- 1.Astion ML, Orkand RK. Electrogenic Na/HCO3 cotransport in neuroglia. Glia. 1988;1(5):355–357. doi: 10.1002/glia.440010508. [DOI] [PubMed] [Google Scholar]
- 2.Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J. Physiol. 1987;273:295–316. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes S, Bui Q. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J. Neurosci. 1991;11(12):4105–4123. doi: 10.1523/JNEUROSCI.11-12-04015.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boron WF, McCormick WC, Roos A. pH regulation in barnacle muscle fibers: dependence on intracellular and extra-cellular pH. Am. J. Physiol. 1979;237:C185–C193. doi: 10.1152/ajpcell.1979.237.3.C185. [DOI] [PubMed] [Google Scholar]
- 5.Boron WF, Boulpaep EL. The electrogenic Na/HCO3 cotransporter. Kidney International. 1989;36:392–402. doi: 10.1038/ki.1989.208. [DOI] [PubMed] [Google Scholar]
- 6.Boyarsky G, Ransom B, Schlue WR, Davis MBE, Boron WF. Intracellular pH regulation in single cultured astrocytes from rat forebrain. Glia. 1993;8:241–248. doi: 10.1002/glia.440080404. [DOI] [PubMed] [Google Scholar]
- 7.Brune T, Fetzer S, Backus KH, Deitmer JW. Evidence for electrogenic sodium-bicarbonate cotransport in cultured rat cerebellar astrocytes. Pflugers Arch. 1994;429:64–71. doi: 10.1007/BF02584031. [DOI] [PubMed] [Google Scholar]
- 8.Chesler M. The regulation and modulation of pH in the nervous system. Progr. Neurobiol. 1990;34:401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 9.Chesler M, Kraig RP. Intracellular pH transients of mammalian astrocytes. J. Neurosci. 1989;9:2011–2019. doi: 10.1523/JNEUROSCI.09-06-02011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deitmer JW, Schlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cell. J. Physiol. 1989;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doering AE, Lederer WJ. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J. Physiol. 1993;466:481–499. [PMC free article] [PubMed] [Google Scholar]
- 12.Erecinska M, Silver IA. ATP and brain function. J. Cereb. Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- 13.Friede RL, Van Houten WH. Relations between post-mortem alterations and glycolytic metabolism in the brain. Exp. Neurol. 1961;4:197–204. doi: 10.1016/0014-4886(61)90041-3. [DOI] [PubMed] [Google Scholar]
- 14.Giffard RG, Monyer H, Choi DW. Selective vulnerability of cultured cortical glia to injury by extracellular acidosis. Brain Res. 1990;530:138–141. doi: 10.1016/0006-8993(90)90670-7. [DOI] [PubMed] [Google Scholar]
- 15.Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F. The effects of extracellular acidosis on neurons and glia in vitro. J. Cereb. Blood Flow Metab. 1989;9:471–477. doi: 10.1038/jcbfm.1989.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994a;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 17.Grichtchenko II, Chesler M. Depolarization-induced acid secretion in gliotic hippocampal slices. Neuroscience. 1994b;62:1057–1070. doi: 10.1016/0306-4522(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 18.Griffith JK, Cordisco BR, Lin CW, LaManna JC. Distribution of intracellular pH in the rat brain cortex after global ischemia as measured by color film histophotometry of neutral red. Brain Res. 1992;573:1–7. doi: 10.1016/0006-8993(92)90108-l. [DOI] [PubMed] [Google Scholar]
- 19.Hansen AJ. Extracellular ion concentrations in cerebral ischemia. In: Zeuthen T, editor. The application of ion-selective microelectrodes. Amsterdam: Elsevier; 1981. pp. 239–253. [Google Scholar]
- 20.Harold DE, Walz W. Metabolic inhibition and electrical properties of type-1-like cortical astrocytes. Neuroscience. 1992;47:203–211. doi: 10.1016/0306-4522(92)90133-m. [DOI] [PubMed] [Google Scholar]
- 21.Hertz L, Schousboe A. Ions and energy metabolism of the brain at the cellular level. In: Pfeiffer CC, Smythies JR, editors. International Review of Neurobiology. Vol 10. New York: Academic Press; 1975. pp. 141–211. [DOI] [PubMed] [Google Scholar]
- 22.Hille B. Ionic channels of excitable membranes. Sunderland: Sinauer Assoc; 1992. [Google Scholar]
- 23.Katsura K, Ekholm A, Asplund B, Siesjo BK. Extracellular pH in the brain during ischemia: relationship to the severity of lactic acidosis. J. Cereb. Blood Flow Metab. 1991;11:597–599. doi: 10.1038/jcbfm.1991.109. [DOI] [PubMed] [Google Scholar]
- 24.Kraig RP, Chesler M. Dynamics of volatile buffers in brain cells during spreading depression. In: Somjen GG, editor. Mechanisms of cerebral hypoxia and stroke. New York: Plenum Press; 1988. pp. 279–289. [Google Scholar]
- 25.Kraig RP, Chesler M. Astrocytic acidosis in hyperglycemic and complete ischemia. J. Cereb. Blood Flow Metab. 1990;10:104–114. doi: 10.1038/jcbfm.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraig RP, Lascola CD, Caggiano A. Glial response to brain ischemia. In: Kettenmann H, Ransom B, editors. Neuroglial cells. New York: Oxford Press; 1995. pp. 965–976. [Google Scholar]
- 27.Kraig RP, Petito CK. Interrelation of proton and volume regulation in astrocytes. In: Ginsberg MD, Dietrich WD, editors. Cerebrovascular disease. New York: Raven Press; 1988. pp. 239–246. [Google Scholar]
- 28.Kraig RP, Pulsinelli WA, Plum F. Hydrogen ion buffering during complete brain ischemia. Brain Res. 1985a;342:281–290. doi: 10.1016/0006-8993(85)91127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraig RP, Pulsinelli WA, Plum F. Heterogeneous distribution of hydrogen and bicarbonate ions during complete brain ischemia. In: Kogure K, Hossmann KA, Siesjo BK, Welsh FA, editors. Progress in Brain Research. vol. 63. 1985b. pp. 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraig RP, Pulsinelli WA, Plum F. Carbonic acid buffer changes during complete brain ischemia. Am. J. Physiol. 1986;250:R348–R357. doi: 10.1152/ajpregu.1986.250.3.R348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krebs HA, Woods HF, Alberti KGMM. Hyperlactemia and lactic acidosis. Essays Med. Biochem. 1975;1:81–103. [Google Scholar]
- 32.Lang R, Oberleithner H, Kolb HA, Paulmichl M, Vokl H, Wang W. Interaction of intracellular pH and cell membrane potential. In: Huassinger D, editor. pH mechanisms and control. New York: Academic Press; 1988. pp. 27–42. [Google Scholar]
- 33.Ljunnggren B, Norberg K, Siesjo BK. Influence of tissue acidosis upon restitution of brain energy metabolism following total ischemia. Brain Res. 1974;77:173–183. doi: 10.1016/0006-8993(74)90782-3. [DOI] [PubMed] [Google Scholar]
- 34.Mellergard PE, Ouyang YB, Siesjo BK. The regulation of intracellular pH in cultured astrocytes and neuroblastoma cells and its dependence on extracellular pH in a HCO3-free solution. Can. J. Physiol. Pharmac. 1992;70 suppl.:S293–S300. doi: 10.1139/y92-275. [DOI] [PubMed] [Google Scholar]
- 35.Munsch T, Deitmer JW. Sodium-bicarbonate cotransport current in identified leech glial cells. J. Physiol. 1994;474:43–53. doi: 10.1113/jphysiol.1994.sp020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers RE. Lactic acid accumulation as a cause of brain edema and cerebral necrosis resulting from oxygen deprivation. In: Korobkin R, Guilleminault C, editors. Advances in perinatal neurology. New York: Spectrum Publishing; 1979. pp. 88–114. [Google Scholar]
- 37.Myers R, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Arch. Neurol. 1977;34:65–74. doi: 10.1001/archneur.1977.00500140019003. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-induced death in neurons and glia. J. Neurosci. 1991;11(8):2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newman EA. Sodium-bicarbonate cotransport in retinal Muller (glial) cells of the salamander. J. Neurosci. 1991;11(12):3972–3983. doi: 10.1523/JNEUROSCI.11-12-03972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connor ER, Sontheimer H, Ransom BR. Rat hippocampal astrocytes exhibit electrogenic sodium-bicarbonate co-transport. J. Neurophys. 1994;72:2580–2589. doi: 10.1152/jn.1994.72.6.2580. [DOI] [PubMed] [Google Scholar]
- 41.Penfield W. Neuroglial and microglial—the interstitial tissue of the nervous system. In: Cowdry EV, editor. Special cytology, the form and function of the cell in health and disease. New York: Hoeber; 1928. pp. 1033–1068. [Google Scholar]
- 42.Petito CK, Babiak T. Early proliferative changes in astrocytes in post-ischemic noninfarcted rat brain. Ann. Neurol. 1982;11:510–518. doi: 10.1002/ana.410110511. [DOI] [PubMed] [Google Scholar]
- 43.Plum F. What causes infarction in ischemic brain?: The Robert Wartenberg Lecture. Neurology. 1983;33:222–233. doi: 10.1212/wnl.33.2.222. [DOI] [PubMed] [Google Scholar]
- 44.Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. In: Kogure K, Hossmann KA, Siesjo BK, Welsh FA, editors. Progress in Brain Research. vol. 63. 1985. pp. 29–37. [DOI] [PubMed] [Google Scholar]
- 45.Pulsinelli WA, Duffy TE. Regional energy balance in rat brain after transient forebrain ischemia. J. Neurochem. 1983;40:1500–1503. doi: 10.1111/j.1471-4159.1983.tb13599.x. [DOI] [PubMed] [Google Scholar]
- 46.Pulsinelli WA, Kraig RP, Plum F. Hyperglycemia, cerebral acidosis, and ischemic brain damage. In: Plum F, Pulsinelli W, editors. Cerebrovascular diseases. New York: Raven Press; 1985. pp. 201–205. [Google Scholar]
- 47.Pulsinelli WA, Levy DE, Sigsbee B, Scherer P, Plum F. Increased damage after ischemic stroke in patients with hyperglycemia with or without diabetes mellitus. Am. J. Med. 1983;74:540–544. doi: 10.1016/0002-9343(83)91007-0. [DOI] [PubMed] [Google Scholar]
- 48.Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: A neuropathologic study in the rat. Neurology. 1982;32:1239–1246. doi: 10.1212/wnl.32.11.1239. [DOI] [PubMed] [Google Scholar]
- 49.Rehncrona S, Rosen I, Siesjo B. Brain lactic acidosis and ischemic cell damage. I. Biochemistry and neurophysiology. J. Cereb. Blood Flow Metab. 1981;1:297–311. doi: 10.1038/jcbfm.1981.34. [DOI] [PubMed] [Google Scholar]
- 50.Shrode LD, Putnam RW. Intracellular pH regulation in primary rat astrocytes and C6 glioma cells. Glia. 1994;12:196–210. doi: 10.1002/glia.440120305. [DOI] [PubMed] [Google Scholar]
- 51.Siesjo BK, Bendek G, Koide TY, Westberg E, Weiloch T. Influences of acidosis on lipid peroxidation in brain tissue in vitro. J. Cereb. Blood Flow Metab. 1985;5:253–260. doi: 10.1038/jcbfm.1985.32. [DOI] [PubMed] [Google Scholar]
- 52.Siesjo BK, Katsura KI, Zhao Q, Folbergrova J, Pahlmark K, Siesjo P, Smith ML. Mechanisms of secondary brain damage in global and focal ischemia: a speculative synthesis. J. Neurotrauma. 1995:943–956. doi: 10.1089/neu.1995.12.943. [DOI] [PubMed] [Google Scholar]
- 53.Siemkowicz E, Hansen A. Brain extracellular ion composition and EEG activity following 10 min ischemia in normo- and hypoglycemic rats. Stroke. 1981;12:236–240. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- 54.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-d-aspartate channel by extracellular H+ Proc. Natl. Acad. Sci. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tombaugh GC, Sapolski RM. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990;506:343–345. doi: 10.1016/0006-8993(90)91277-n. [DOI] [PubMed] [Google Scholar]
- 56.Voipio J, Kaila K. Interstitial PCO2 and pH in rat hippocampal slices measured by means of a novel fast CO2H+-sensitive microelectrode based on a PVC-gelled membrane. Pflugers Arch. 1993;423:193–201. doi: 10.1007/BF00374394. [DOI] [PubMed] [Google Scholar]
- 57.Walz W, Wuttke WA. Resistance of astrocyte electrical membrane properties to acidosis changes in the presence of lactate. Brain Res. 1989;504:82–86. doi: 10.1016/0006-8993(89)91600-4. [DOI] [PubMed] [Google Scholar]
- 58.Welsh F, McKee A. Effect of glucose pretreatment on recovery of ATP and CBF following unilateral hypoxia ischemia in mouse brain. J. Cereb. Blood Flow Metab. 1983;3 suppl. 1:377–378. [Google Scholar]