Abstract
Biochemical and mechanical signals enabling cardiac regeneration can be elucidated using in vitro tissue-engineering models. We hypothesized that insulin-like growth factor-I (IGF) and slow, bi-directional perfusion could act independently and interactively to enhance the survival, differentiation, and contractile performance of tissue-engineered cardiac grafts. Heart cells were cultured on three-dimensional porous scaffolds in medium with or without supplemental IGF and in the presence or absence of slow, bi-directional perfusion that enhanced transport and provided shear stress. Structural, molecular, and electrophysiologic properties of the resulting grafts were quantified on culture day 8. IGF had independent, beneficial effects on apoptosis (p < 0.01), cellular viability (p < 0.01), contractile amplitude (p < 0.01), and excitation threshold (p < 0.01). Perfusion independently affected the four aforementioned parameters and also increased amounts of cardiac troponin-I (p < 0.01), connexin-43 (p < 0.05), and total protein (p < 0.01) in the grafts. Interactive effects of IGF and perfusion on apoptosis were also present (p < 0.01). Myofibrillogenesis and spontaneous contractility were present only in grafts cultured with perfusion, although contractility was inducible by electrical field stimulation of grafts from all groups. Our findings demonstrate that multi-factorial stimulation of tissue-engineered cardiac grafts using IGF and perfusion resulted in independent and interactive effects on heart cell survival, differentiation, and contractility.
Introduction
Cell-based cardiac repair has emerged as a promising approach to regenerate congenital and acquired lesions of the heart.1 Feasibility has been demonstrated in vivo2–5 and in experimental therapeutic paradigms.6 However, inadequate cell survival and differentiation, insufficient contractile force generation, and lack of knowledge concerning the mechanisms underlying functional improvement limit widespread clinical translation of cell-based therapies.6,7 Strategies to enhance and study the properties of tissue-engineered cardiac grafts in vitro include biochemical and physical signaling (e.g., with regulatory molecules,8 mechanical stretch,9,10 electrical stimulation,11 hydrodynamic shear,12–14 uni-directional perfusion,15–18 and pre-vascularization of porous three-dimensional (3D) scaffolds (e.g., with co-cultured endothelial cells and stem cells).19 In a recent in vivo study, exogenous heart cells were embedded in Matrigel and implanted near an arteriovenous loop to generate vascularized, contractile cardiac tissue.5 However, this approach did not permit elucidation of underlying mechanisms, because the observed sequelae resulted from complex and undefined combinations of transplanted and host cells, compounded by the cascade of regulatory molecules thereby secreted in an uncontrolled mechanical environment. In contrast, in vitro models (i.e., heart cells cultured on biomaterial scaffolds in bioreactors) can achieve the levels of control required for systematic studies of biochemical and mechanical signaling that can in turn enable the functional assembly of 3D tissue-engineered cardiac constructs.
In the present study, insulin-like growth factor-I (IGF) was selected to promote cell survival and growth, and slow, bi-directional perfusion was used to enhance mass transport and provide fluid shear stress. One hundred ng/mL of IGF was selected because this factor and concentration improved the contractile properties of 3D cardiac constructs and increased the viability of the component heart cells, whereas 1 and 10 ng/mL of IGF did not produce significant effects.8 Slow perfusion at a linear flow velocity of 0.2 mm/s was selected because similar flow velocities (0.2 to 0.7 mm/s) enhanced cell survival and differentiation in 3D cardiac constructs.12,16–18 Flow velocities at the low end of those previously tested were selected because velocities higher than 0.523 mm/s were associated with activation of the p38 cell death signal in 3D cardiac constructs.17,18 Bi-directional flow was selected to perfuse the construct with fresh medium via its top and bottom surfaces, thereby enhancing cellular access to oxygen, nutrients, and growth factors; in contrast, uni-directional flow at low velocity, like static culture, can result in spatial concentration gradients.20–22 In the present study, bi-directional flow was achieved by creating relative motion between a construct and its culture medium using simple oscillation of a closed-loop chamber in which the specimen was immobilized. We tested the hypothesis that IGF and slow, bi-directional perfusion could act independently and interactively to enhance the survival, differentiation, and contractile performance of tissue-engineered cardiac grafts.
Materials and Methods
Cells
All studies involving experimental animals were performed according to a protocol approved by an Institute Committee on Animal Care. Heart cells were obtained from 2-day-old neonatal Sprague Dawley rats (3 studies totaling 74 rat pups). In brief, the ventricles were harvested, minced into 1-mm3 pieces, and incubated for 16 h at 4°C in 0.06% (w/v) trypsin in Hank's balanced salt solution (HBSS). The partially digested tissue was subjected to a series of digestions (each for 5 min at 37°C and 50 rpm) in a solution of 0.1% (w/v) type II collagenase (Worthington, Lakewood, NJ) in HBSS. The freshly dissociated heart cells were plated in T-flasks, the cells that rapidly attached to the flasks were discarded, and the cells that remained unattached after 1 h of pre-plating were used to prepare constructs.8 Immediately before construct preparation, the cells were centrifuged (1,000 rpm, 10 min) and resuspended at high density in Matrigel (1.2% w/v, Becton-Dickinson, Franklin Lakes, NJ), working at 4°C to maintain the cell–Matrigel mixture in a liquid state. Under these conditions, cells used for construct preparation can be expected to consist of a mixed population of approximately 42% cardiomyocytes, 40% cardiac fibroblasts, and minor fractions of unidentified cell types.23 Likewise, the constructs generated over an in vitro culture period of 5 to 11 days can be expected to consist of approximately 40% to 50% cardiomyocytes.23
Construct preparation and cultivation
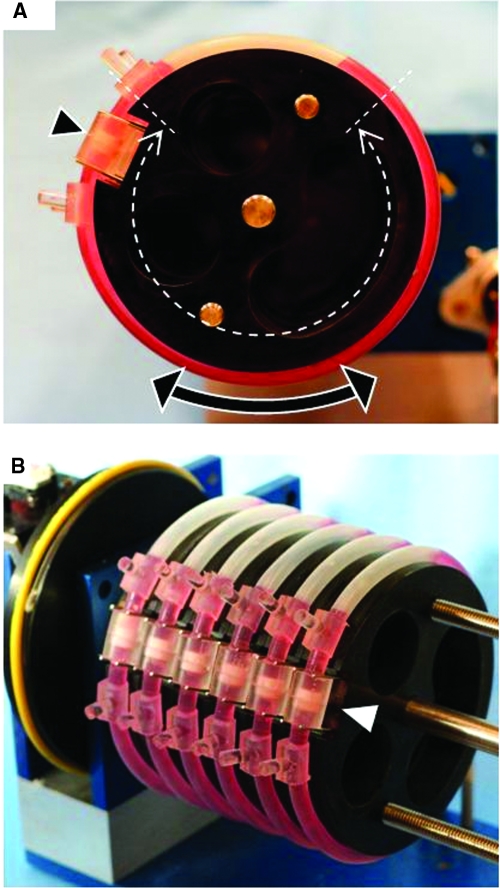
The scaffold was a water-insoluble collagen sponge with interconnected pores fabricated from collagen derived from a partial hydrochloric acid extraction of purified dermal (corium) collagen (Ultrafoam, Davol Inc., Providence, RI).8,11,16,24 Scaffolds were prepared as die-punched discs that were approximately 8 mm in diameter by 3 mm thick when dry and contracted to approximately 7 mm in diameter by 1.5 mm thick within 24 h after wetting. For bioreactor studies, a silicone rubber molding kit (Sylgard 184, Ellsworth Adhesives, Germantown, WI) was used to fabricate specimen holders and affix them to gas-permeable silicone tubing (1/32" wall Tygon 3350, Cole Parmer, Vernon Hills, IL). In brief, each scaffold was press-fitted into the base of a specimen holder and seeded with 6 million cells mixed in 40 μL of Matrigel. The mixture was delivered uniformly to the top surface of the scaffold using a pipet, wherein gelation of the Matrigel entrapped the cells within the scaffold.24 Immediately thereafter, the specimen holder was closed, resulting in a 3D construct immobilized in a 6.35-mm-diameter by 8-mm-long chamber within a loop of tubing (Fig. 1). This closed-loop chamber was mounted on a 12.5-cm-diameter supporting disc, and 10 mL of culture medium was added through an inlet port (Fig. 1).
FIG. 1.
Oscillatory perfused bioreactor. (A) The tissue culture vessel is a closed loop of gas-permeable silicone tubing connected to a chamber (arrowhead) within which a single disc-shaped construct is fixed in place such that fluid cannot flow around its edges. The design principle is to apply bi-directional direct perfusion to the full thickness of the construct by oscillating the vessel about its central axis in a pendulum-like motion. (B) The prototype system included a set of 6 to 12 closed loops, each containing a single construct and 10 mL of culture medium and mounted on an incubator-compatible motorized base. Color images available online at www.liebertonline.com/ten.
Multiple closed-loop chambers (6 to 12) were mounted on an incubator-compatible base that slowly oscillated the chambers about their central axes in a pendulum-like motion (Fig. 1). Closure of limit switches positioned on either side of the device triggered oscillation of the closed-loop chamber. Limit switch closure toggled the state of a J-K flip-flop, which toggled the state of a double pull-double throw relay, which reversed the polarity of the direct current power to the motor, thereby reversing its direction of rotation. In the present study, the arc of oscillation ranged from 180° to 270°, and the oscillatory speed was 0.05 revolutions per min. This corresponded to a linear flow velocity of 0.2 mm/s, as estimated through simple geometrical calculations based on actual device dimensions. Interstitial fluid flow was ensured by fixing the construct within the specimen chamber such that fluid could not flow around the construct. This bioreactor system considered features of significance not only to tissue culture, but also to clinical translation (asepsis, scale-up, automation, and ease of use).25
In addition to constructs cultured in bioreactors, otherwise-identical constructs were cultured in static Petri dishes (1 construct and 10 mL of medium per 35-mm well). A statically cultured construct is expected to encounter diffusional limitations, in part due to its position on the bottom of the dish and in part due to absence of convective mixing. Culture media were supplemented or not with 100 ng/mL of IGF (Peprotech, Rocky Hill, NJ). Four experimental groups were established: (i, ii) static cultures in control or IGF-supplemented medium (static CTL, static + IGF) and (iii, iv) bioreactor cultures in control or IGF-supplemented medium (bioreactor CTL, bioreactor + IGF). Media were completely replaced on day 4, and constructs were harvested on day 8, consistent with our recent study.8
Apoptosis, cell viability, metabolic activity, and deoxyribonucleic acid content
Apoptosis was assessed in constructs and native neonatal rat ventricles using the terminal deoxynucleotidyl transferase biotin 2'-deoxyuridine 5'-triphosphate nick end labeling (TUNEL) assay.8 In brief, an observer blinded to the identity of the specimen counted apoptotic and total cells, identified using TUNEL and staining with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI), respectively, for four randomly selected regions of duplicate specimens from each group, and then the apoptotic cells were expressed as a percentage of the total cells. Cell viability was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.8 A glucose-lactate analyzer (Model 2300 Stat Plus Yellow Springs Instruments, Yellow Springs, OH) was used to measure media concentrations of glucose and lactate, and the molar ratio of lactate produced to glucose consumed was calculated as an index of aerobicity, with aerobic and anaerobic metabolism indicated by values of 1.0 and 2.0, respectively.8 Construct amounts of deoxyribonucleic acid (DNA) was assessed for five constructs per group using the Quant-iT Picogreen double strand DNA assay kit (Invitrogen, Carlsbad, CA).
Cardiac marker and total protein contents
Construct amounts of total protein, cardiac troponin-I (Tn-I), and connexin-43 (Cx-43) were assessed after homogenization of constructs in 1 N ammonium hydroxide/2% Triton X-100 buffer.8 Total protein was determined for five constructs per group using a Bio-Rad DC assay kit (Hercules, CA). Amounts of cardiac Tn-I and Cx-43 were determined using Western blot using triplicate constructs from each group and control specimens of native neonatal rat heart tissue.8 In brief, homogenates of construct or native heart, each containing 20 μg of total protein, were loaded onto a gel, electroblotted, blocked for nonspecific antibodies, and incubated with a primary antibody (anti-cardiac Tn-I (clone 23C6, Biodesign, Saco, ME) or anti-Cx-43 (C6219, Sigma, St. Louis, MO)), an appropriate secondary antibody, and immunocomplexes were developed using enhanced horseradish peroxidase–luminol chemiluminescence (Amersham, Buckinghamshire, UK) and detected using photographic film. The intensity of the entire resulting band was quantified using Image J software. Band intensity was measured for blotted samples containing equal amounts of total protein and expressed as a percentage of native neonatal rat heart tissue, as in previous studies of these and other marker proteins in 3D cardiac constructs.8,11,13,14
Structural assessments
Cell morphology in 8-day constructs was assessed after immunohistochemical staining for cardiac Tn-I. In brief, two or three specimens per group were incubated with anti-cardiac Tn-I, an appropriate secondary antibody, stained with a Standard Elite ABC kit (Vector, Peterborough, UK), and counterstained with hematoxylin.8 Cell distribution, elongation, and differentiation were assessed at magnifications of 100X and 1000X. Overall tissue morphology was assessed using image analysis as follows. Digital photomicrographs obtained from two specimens per group were calibrated (μm/pixel) and subjected to intensity thresholding to differentiate lighter pores from the surrounding, darker background, and pore areas were measured using SigmaScan Pro (SPSS Inc., Chicago, IL). The minimum, maximum, and average effective (circular) pore radii were calculated for each pore as the square root of (area/π); 645 individual pores were quantified.
Electrophysiologic assessments
Contractile properties were assessed in an environmentally controlled test chamber8 in which there were two ¼-inch-diameter carbon rod electrodes (Ladd Research, Williston, VT) separated by 1.5 cm and connected to platinum wire leads. The leads were attached to a computer-controlled electrical pulse generator based on a LabPro sensor interface (Vernier Software and Technology, Beaverton, OR). If present, spontaneous contraction rate was recorded. Electrically induced contractions were then elicited using electric field stimulation applied as a 1-Hz biphasic square voltage waveform. The pulse width was half of the wavelength (i.e., 0.5 sec). Excitation threshold was determined by incrementally increasing the voltage until a synchronous contraction of the construct followed each stimulus. Contractile amplitude was assessed usng automated analysis of the change in construct cross-sectional area during one contractile cycle, using digitized videos of constructs paced at 1 Hz and a voltage 1.5 times as high as threshold.8
Statistical analyses
Data were calculated as means ± standard errors and analyzed using two-way analysis of variance in conjunction with Tukey's post hoc test using Statistica (Tulsa, OK).
Results
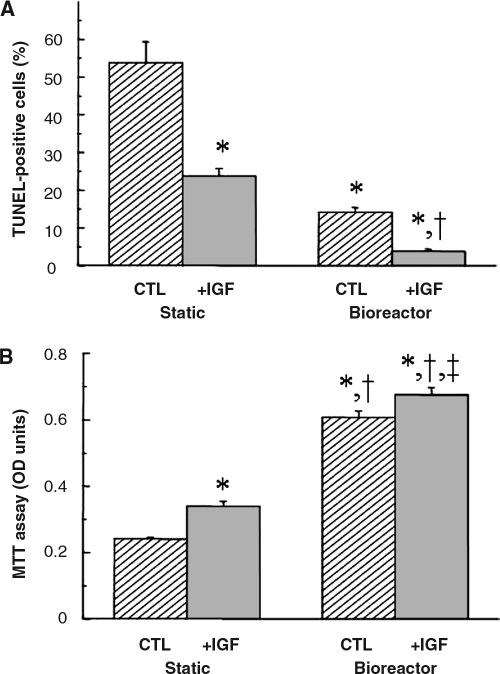
IGF and also by perfusion increased the total amount of DNA per construct to some degree, but these effects did not reach statistical significance (Table 1). The number of apoptotic (TUNEL-positive) cells expressed as a percentage of total (DAPI-positive) cells showed significant reductions due to IGF (p < 0.01) and perfusion (p < 0.01), and there was an interactive effect between these two factors (p < 0.01) (Fig. 2A, Table 1). Apoptotic cells were readily observed in constructs from the static CTL group and were progressively less prevalent in the static + IGF, bioreactor CTL, and bioreactor + IGF groups (Supplemental Fig. 1, available online at www.liebertonline.com/ten). IGF (p < 0.01) and perfusion (p < 0.01) significantly increased cell viability measured using MTT assay (Fig. 2B, Table 1). Moreover, perfusion significantly lowered the molar ratio of lactate produced to glucose consumed, indicating more aerobic cell metabolism in bioreactor cultures than static controls (1.38 ± 0.03 vs 1.62 ± 0.02, p < 0.01).
Table 1.
Individual and Interactive Effects of Experimental Parameters on Construct Properties
| |
Culture vessel (culture medium) |
|
|
|
|||
|---|---|---|---|---|---|---|---|
| Eight-day construct property | Static (CTL) | Static (IGF) | Bioreactor (CTL) | Bioreactor (IGF) | Individual effect of bioreactor | Individual effect of IGF | Interactive effect of bioreactor and IGF |
| TUNEL-positive cells (%, n = 3) | 53.8 ± 5.6 | 23.8 ± 1.9a | 14.1 ± 1.3a | 3.83 ± 0.6a,b | p < 0.01 | p < 0.01 | p < 0.01 |
| MTT (OD units/construct, n = 5) | 0.24 ± 0.005 | 0.34 ± 0.014a | 0.61 ± 0.019a,b | 0.68 ± 0.022a–c | p < 0.01 | p < 0.01 | NS |
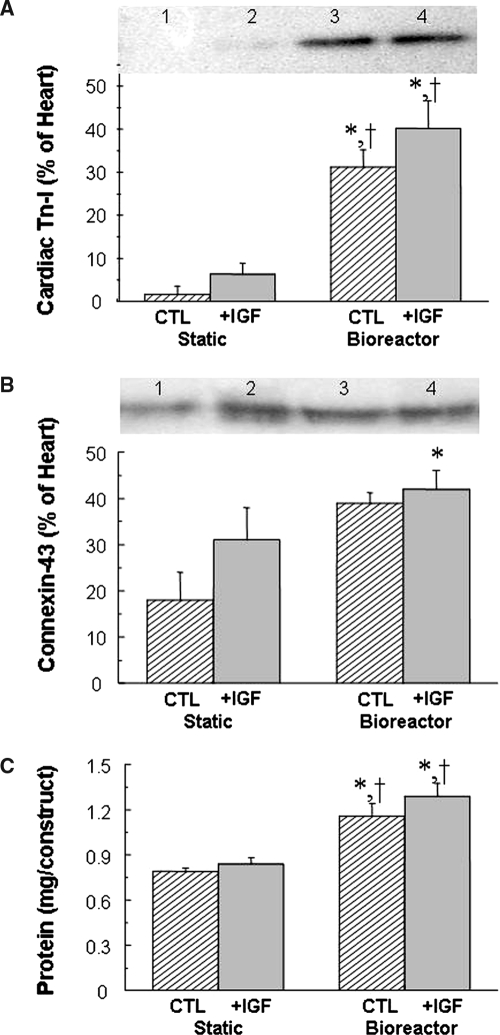
| Cardiac troponin-I (% of native, n = 3) | 1.7 ± 1.7 | 6.4 ± 2.5 | 31 ± 4.02a,b | 40 ± 6.4a,b | p < 0.01 | NS | NS |
| Connexin-43 (% of native, n = 3) | 18 ± 6.48 | 31 ± 7.04 | 39 ± 2.3 | 42 ± 4.04a | p < 0.05 | NS | NS |
| Total protein (mg/construct, n = 5) | 0.79 ± 0.02 | 0.84 ± 0.04 | 1.16 ± 0.08a,b | 1.29 ± 0.09a,b | p < 0.01 | NS | NS |
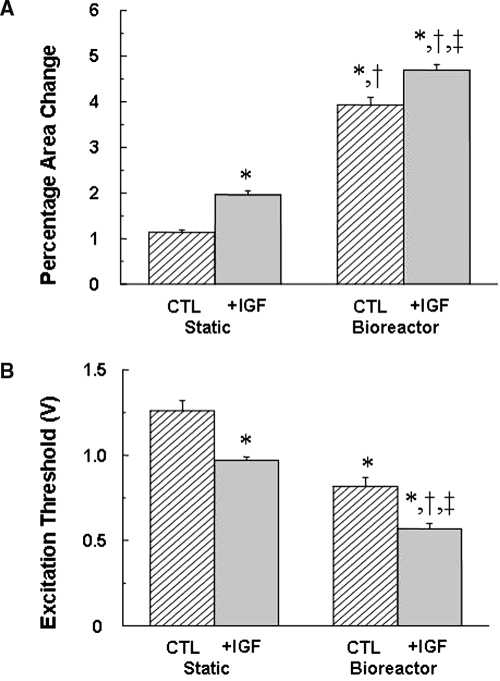
| Contractile amplitude (% area change, n = 12) | 1.14 ± 0.05 | 1.96 ± 0.09a | 3.93 ± 0.17a,b | 4.701 ± 0.12a–c | p < 0.01 | p < 0.01 | NS |
| Excitation threshold (volts, n = 12) | 1.26 ± 0.06 | 0.97 ± 0.02a | 0.82 ± 0.05a | 0.57 ± 0.03a–c | p < 0.01 | p < 0.01 | NS |
| Construct wet weight (mg, n = 5) | 42.6 ± 1.6 | 43.8 ± 1.9 | 51.4 ± 4.8 | 50.6 ± 3.8 | p < 0.05 | NS | NS |
| DNA (μg/construct, n = 5) | 11.4 ± 1.56 | 12.7 ± 0.94 | 13.8 ± 1.32 | 14.7 ± 0.7 | NS | NS | NS |
Data represent the mean ± SEM of n = 3–12 independent samples.
Significantly different (p < 0.05 by Tukey's test) from corresponding constructs in the static control group.
Significantly different (p < 0.05 by Tukey's test) from corresponding constructs in the static + IGF group.
Significantly different (p < 0.05 by Tukey's test) from corresponding constructs in the bioreactor control group.
CTL, control medium; IGF, medium supplemented with insulin-like growth factor I; TUNEL, terminal deoxynucleotidyl transferase biotin-2′-deoxyuridine 5′-triphosphate nick end labeling; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetraxolium bromide; OD, optical density; DNA, deoxyribonucleic acid; NS, not statistically significant.
FIG. 2.
Cell viability in 8-day cardiac constructs from static and bioreactor cultures in control (CTL) and insulinlike growth factor (IGF)-supplemented (+IGF) media. (A) Apoptotic (terminal deoxynucleotidyl transferase biotin-20-deoxyuridine 50-triphosphate nick end labeling–positive) cells expressed as a fraction of total (4',6-diamidino-2-phenylindole dihydrochloride–stained) cells, representative images of which are shown in Supplemental Figure 1 (available online at www.liebertonline.com/ten), (B) Cell viability, assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Data are the means ±standard errors of the mean of three to six measurements. *Significantly different from static control; †significantly different from static + IGF; ‡significantly different from bioreactor control.
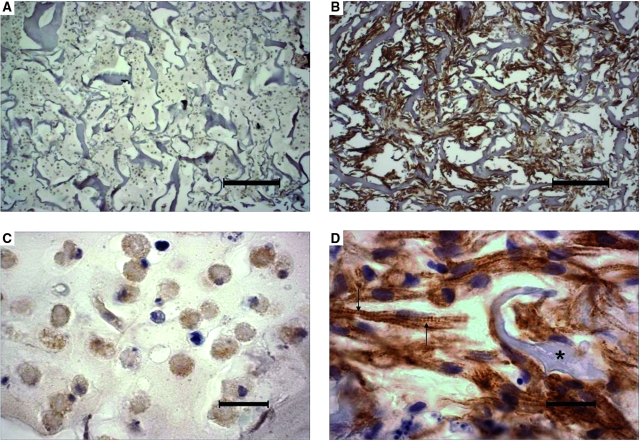
In all groups, the 8-day constructs were approximately 1 mm thick and resembled a loose network of interconnected cells, scaffold remnants, and open pores (Fig. 3 A, B). Cell distributions were spatially uniform, without any sign of core necrosis. To estimate fluid shear stress (τ) due to a mean linear flow velocity (v) of 0.2 mm/s, the following set of simplifying assumptions were made. The pores, which actually exhibited highly variable morphology, were assumed to be circular to facilitate calculation of an effective pore radius (R) by image analysis. Flow, which would actually exhibit preferential distribution through the larger pores, was assumed to be uniformly distributed to estimate fluid shear stress at the wall of the pore according to the Hagen-Poiseuille equation: τ = 4μv/R.26 Viscosity (μ) of the culture medium was assumed to be equal to that of water at 37°C (0.0076 dyn-s/cm2).27 The minimum, maximum, and average effective pore radii in 8-day constructs were 4, 126, and 20 μm, respectively, and the corresponding range of estimated shear stresses was 0.05 to 1.5 dyn/cm2, with an average value of 0.3 dyn/cm2.
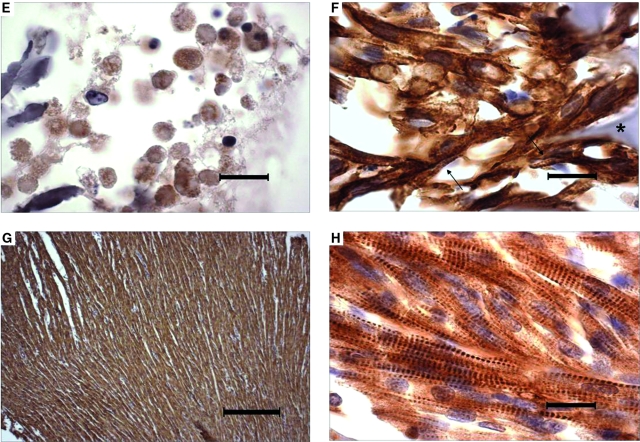
FIG. 3.
Cell density, distribution, and differentiation in 8-day cardiac constructs from static and bioreactor cultures in control (CTL) and insulinlike growth factor (IGF)-supplemented (+IGF) media and in native neonatal ventricular tissue. (A, B) Low-magnification images from the (A) static CTL and (B) bioreactor + IGF groups. Scale bars: 200 μm. (C-F) High-magnification images from the (C) static CTL, (D) bioreactor CTL, (E) static + IGF, and (F) bioreactor + IGF groups. Scale bars: 20 μm. (G–H) Native neonatal rat ventricle. Scale bars: (G) 200 and (H) 20 μm. Representative sections were immunostained for cardiac troponin-I. Cross-striations and scaffold remnants are indicated by arrows and asterisks, respectively. Color images available online at www.liebertonline.com/ten.
The majority of cells in 8-day constructs from all groups exhibited strong positive immunostaining for cardiac Tn-I, a cardiac-specific marker protein (Fig. 3 C-F). In the presence of perfusion (Fig. 3 D, F), most of the cells appeared elongated and contained centrally positioned elongated nuclei, suggestive of early differentiation. In contrast, most of the cells in static cultures appeared rounded (Fig. 3 C, E). Cross-striations characteristic of native myocardium were observed only in the presence of perfusion. Under the conditions tested in the present study, supplemental IGF did not cause any morphological changes that could be detected using immunostaining for cardiac Tn-I. Native neonatal rat ventricular tissue was comprised of higher-density and more-oriented cells than tissue engineered cardiac grafts (Fig. 3 G, H). Perfusion significantly increased the amounts of cardiac Tn-I (p < 0.01, Fig. 4A, Table 1), connexin-43 (p < 0.05, Fig. 4B, Table 1), and total protein (p < 0.01, Fig. 4C, Table 1) in homogenized constructs. Supplemental IGF did not significantly affect cardiomyocyte differentiation, as assessed according to the amounts of cardiac markers and total protein measured in homogenized constructs.
FIG. 4.
Cardiac marker and total proteins in 8-day cardiac constructs from static and bioreactor cultures in control (CTL) and insulin-like growth factor (IGF)-supplemented (+IGF) media. (A, B) Representative images and Western blot data for two marker proteins: (A) cardiac troponin-I and (B) connexin-43. Lanes 1, 2, 3, and 4 indicate static CTL, static + IGF, bioreactor CTL, and bioreactor + IGF, respectively. (C) Total protein. Data are the means ± standard errors of the mean of three to six measurements. *Significantly different from static CTL; †significantly different from static + IGF.
Robust spontaneous contractility at a rate of approximately 1.0 Hz was readily apparent in 8-day constructs cultured with perfusion (the bioreactor CTL and bioreactor + IGF groups) but not in static cultures (the static CTL and static + IGF groups). The slow rate of spontaneous beating compared with the normal neonatal rat heart rate can be attributed to separation of atria from ventricles during heart cell isolation.5 In all experimental groups, electrical field stimulation readily induced synchronous contraction.IGF (p < 0.01, Fig. 5A, Table 1) and perfusion (p < 0.01, Fig. 5A, Table 1) significantly increased contractile amplitude, assessed according to percentage area change. Moreover, IGF (p < 0.01) and perfusion (p < 0.01) significantly lowered excitation thresholds (Fig. 5B, Table 1). The bioreactor + IGF group exhibited the highest contractile amplitude (412% higher than the static CTL group) and the lowest excitation threshold (45% as high as the static CTL group).
FIG. 5.
Electrophysiologic assessment of 8-day cardiac constructs from static and bioreactor culture in control (CTL) and insulin-like growth factor (IGF)-supplemented (+IGF) media. (A) Percentage area change, an index of construct contractile amplitude and (B) excitation threshold. Data are the means ± standard errors of the mean of 12 to 28 measurements. *Significantly different from static CTL; †significantly different from static + IGF; ‡significantly different from bioreactor CTL.
Discussion
One novelty of the present study was that multi-factorial stimulation with IGF and perfusion independently and interactively reduced apoptosis and enhanced viability of heart cells in tissue-engineered cardiac grafts (Fig. 2, Table 1). The overall rate of cell death in the static CTL group was similar to values we and others previously reported for statically cultured 3D cardiac constructs.8,16,17,28,29 IGF-mediated cardioprotection was consistent with our previous study8 and with previous studies that implicated the Akt signaling pathway.4,30–32 The perfusion-mediated increase in cell viability, also consistent with previous studies,15–18,20 was presumably due to enhanced oxygen transport, because hypoxia is a powerful inducer of apoptosis.33 In the present study, the entire culture vessel was made of highly gas-permeable silicone rubber; hence the entire device served as an oxygenator from the moment heart cells were placed therein.
Dvir et al. found that cell death in 3D cardiac constructs subjected to pulsatile, uni-directional flow was two to three times as high at shear stresses of 2.4 and 5.4 dyn/cm2 as at a lower shear stress of 0.6 dyn/cm2, as determined according to activation of the p38 cell death signal and the MTT and trypan blue exclusion assays.18 In the present study, in which shear stresses ranged from 0.05 to 1.5 dyn/cm2, one could speculate that the observed interactive effects of IGF and perfusion on apoptosis may be due to IGF-mediated rescue of flow-mediated apoptosis via the superposition of two distinct mechanisms. As an alternative or complementary explanation, shear stress may have enhanced expression and release of IGF and its binding proteins by a subset of the seeded heart cells as reported for vascular cells.34,35
In the present study, slow, bi-directional perfusion of porous constructs at a low flow velocity of 0.2 mm/s yielded spontaneously contractile 8-day grafts, in contrast to uni-directional flow regimes16–18 and higher velocities of 0.4 mm/s16 and 0.5 mm/s17,18 used previously during heart cell culture on the same16 and different17,18 scaffolds, which did not yield spontaneously contractile grafts. These findings suggest that interstitial flow conditions may mediate spontaneous contractility of cardiac grafts exposed to slow, bi-directional perfusion. In support of this possibility, other studies of heart cells in monolayer cultures showed that low rates of fluid shear increased spontaneous beating in association with integrin-dependent and β-adrenergic signaling36 and that fluid jet pulses triggered action potentials.37 Another study estimated that interstitial fluid shear present in vivo, in native myocardium was low (0.05 dyn/cm2) using a model of parallel plates representing heart cell in sheets that were separated by a distance of 10 μm and moved at a relative velocity of 50 μm/s during systole.36
Perfusion enhanced cardiomyocyte differentiation to a far greater degree than did supplemental IGF with respect to cell elongation and myofibrillogenesis (Fig. 3) and construct amounts of contractile, gap junctional, and total proteins (Fig. 4, Table 1). Dvir et al.18 found that uni-directional interstitial fluid flow increased contractile and gap junctional proteins in engineered cardiac grafts in association with activation of a known inducer of cardiomyocyte hypertrophy, ERK1/2. The investigators speculated that the AT1 receptor may have responded to shear stress exerted by the interstitial fluid flow.18 Other studies have demonstrated that mechanically active bioreactors,12–15 mechanical stretch,9,38 and electrical stimulation11 induced cell differentiation in tissue-engineered cardiac grafts.
In accordance with their effects on heart cell viability, spontaneous contractility, and differentiation, IGF and perfusion improved the functional performance of tissue-engineered cardiac grafts by increasing contractile amplitude and lowering the excitation threshold (Fig. 5, Table 1). IGF receptor–mediated AKT signaling may explain the effects of IGF on contractile properties,31 which is consistent with results of previous in vitro8,9 and in vivo4 reports. It is likely that mechanotransduction explains the effects of perfusion on contractile properties.39 Consistent with our previous studies, perfusion culture yielded a viable 3D network of interconnected cardiomyocytes that were uniformly distributed within the 1-mm-thick graft (Fig. 3B), whereas in non-perfused cultures, the viable heart cells were mainly present in an approximately 100-μm-thick zone at the surfaces of 1-mm-thick grafts.13–16,20 These findings indicate that, although further improvements in graft size and cellularity will be required to address the in vivo reconstruction of full-thickness myocardium, tissue-engineered cardiac constructs can already provide a high-fidelity in vitro model in which to test the efficacy and safety of drugs.
In conclusion, the present study used a bioreactor to show independent and interactive effects of IGF and slow, bi-directional perfusion on the survival, differentiation, and contractile performance of 3D cardiac constructs. The results demonstrated the value of a multi-factorial approach that can potentially encompass emerging gene, stem cell, and vascularization strategies for basic cardiac tissue-engineering research and the design of cell-based grafts for myocardial repair.
Supplementary Material
Acknowledgments
We are indebted to J. Bales for help with design and implementation of the electrical circuit to control bioreactor oscillation, A. Gallant and P. Morley for help with bioreactor fabrication, R. Langer for general advice, and S. Kangiser for help with manuscript preparation. This work was supported by grants from the National Aeronautics and Space Administration (NNJ04HC72G to LEF), the National Institutes of Health (1F32HL084968-01 to GCE), and the Progetto Roberto Rocca Collaboration.
References
- 1.Laflamme M.A. Murry C.E. Regenerating the heart. Nat Biotechnol. 2005;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann W.H. Melnychenko I. Wasmeier G. Didie M. Naito H. Nixdorff U. Hess A. Budinsky L. Brune K. Michaelis B. Dhein S. Schwoerer A. Ehmke H. Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu T. Sekine H. Yang J. Isoi Y. Yamato M. Kikuchi A. Kobayashi E. Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006;20:708. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 4.Davis M.E. Hsieh P.C. Takahashi T. Song Q. Zhang S. Kamm R.D. Grodzinsky A.J. Anversa P. Lee R.T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morritt A.N. Bortolotto S.K. Dilley R.J. Han X. Kompa A.R. McCombe D. Wright C.E. Itescu S. Angus J.A. Morrison W.A. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- 6.Murry C.E. Field L.J. Menasche P. Cell-based cardiac repair: reflections at the 10-year point. Circulation. 2005;112:3174. doi: 10.1161/CIRCULATIONAHA.105.546218. [DOI] [PubMed] [Google Scholar]
- 7.Eschenhagen T. Zimmermann W.H. Kleber A.G. Electrical coupling of cardiac myocyte cell sheets to the heart. Circ Res. 2006;98:573. doi: 10.1161/01.RES.0000215627.13049.5d. [DOI] [PubMed] [Google Scholar]
- 8.Cheng M.Y. Park H. Engelmayr G.C. Moretti M. Freed L.E. Effects of regulatory factors on engineered cardiac tissue in vitro. Tissue Eng. 2007;13:2709. doi: 10.1089/ten.2006.0414. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann W.H. Schneiderbanger K. Schubert P. Didie M. Munzel F. Heubach J.F. Kostin S. Nehuber W.L. Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 10.Tobita K. Liu L.J. Janczewski A.M. Tinney J.P. Nonemaker J.M. Augustine S. Stolz D.B. Shroff S.G. Keller B.B. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- 11.Radisic M. Park H. Shing H. Consi T. Schoen F.J. Langer R. Freed L.E. Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrier R.L. Papadaki M. Rupnick M. Schoen F.J. Bursac N. Langer R. Freed L.E. Vunjak-Novakovic G. Cardiac tissue engineering: cell seeding, cultivation parameters and tissue construct characterization. Biotechnol Bioeng. 1999;64:580. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Papadaki M. Bursac N. Langer R. Merok J. Vunjak-Novakovic G. Freed L.E. Tissue engineering of functional cardiac muscle: molecular, structural and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 14.Bursac N. Papadaki M. White J.A. Eisenberg S.R. Vunjak-Novakovic G. Freed L.E. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 15.Carrier R.L. Rupnick M. Langer R. Schoen F.J. Freed L.E. Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 16.Radisic M. Yang L. Boublik J. Cohen R.J. Langer R. Freed L.E. Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 17.Dvir T. Benishti N. Shachar M. Cohen S. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Eng. 2006;12:2843. doi: 10.1089/ten.2006.12.2843. [DOI] [PubMed] [Google Scholar]
- 18.Dvir T. Levy O. Shachar M. Granot Y. Cohen S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007;13:2185. doi: 10.1089/ten.2006.0364. [DOI] [PubMed] [Google Scholar]
- 19.Caspi O. Lesman A. Basevitch Y. Gepstein A. Arbel G. Habib M. Gepstein L. Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 20.Carrier R.L. Rupnick M. Langer R. Schoen F.J. Freed L.E. Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol Bioeng. 2002;78:617. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- 21.Martin Y. Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481. doi: 10.1016/j.biomaterials.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 22.Muschler G.F. Nakamoto C. Griffith L.G. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A:1541. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M. Park H. Martens T.P. Salazar-Lazaro J.E. Geng W. Wang Y. Langer R. Freed L.E. Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res. 2008;86A:713. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radisic M. Euloth M. Yang L. Langer R. Freed L.E. Vunjak-Novakovic G. High density seeding of myocyte cells for tissue engineering. Biotechnol Bioeng. 2003;82:403. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 25.Moretti M.G. Cheng M.Y. Nichol J.W. Freed L.E. 2nd Annual Conference on Methods in Bioengineering. Cambridge, MA: 2007. An oscillatory perfused bioreactor for cell and tissue culture. [Google Scholar]
- 26.White F.M. New York: McGraw-Hill; 1979. Fluid Mechanics. [Google Scholar]
- 27.Green D.W. New York: McGraw-Hill Professional; 1997. Perry's Chemical Engineers' Handbook. [Google Scholar]
- 28.Radisic M. Malda J. Epping E. Geng W. Langer R. Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzkopf R. Shachar M. Dvir T. Dayan Y. Holbova R. Leor J. Cohen S. Autospecies and post-myocardial infarction sera enhance the viability, proliferation, and maturation of 3D cardiac cell culture. Tissue Eng. 2006;12:3467. doi: 10.1089/ten.2006.12.3467. [DOI] [PubMed] [Google Scholar]
- 30.Fujio Y. Nguyen T. Wencker D. Kitsis R.N. Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latronico M.V. Costinean S. Lavitrano M.L. Peschle C. Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015:250. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 32.Torella D. Rota M. Nurzynska D. Musso E. Monsen A. Shiraishi I. Zias E. Walsh K. Rosenzweig A. Sussman M.A. Urbanek K. Nadal-Ginard B. Kajstura J. Anversa P. Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 33.Kang P.M. Haunstetter A. Aoki H. Usheva A. Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 34.Passerini A.G. Milsted A. Rittgers S.E. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J Vasc Surg. 2003;37:182. doi: 10.1067/mva.2003.66. [DOI] [PubMed] [Google Scholar]
- 35.Elhadj S. Akers R.M. Forsten-Williams K. Chronic pulsatile shear stress alters insulin-like growth factor-I (IGF-I) binding protein release in vitro. Ann Biomed Eng. 2003;31:163. doi: 10.1114/1.1540637. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzen-Schmidt I. Schmid-Schonbein G.W. Giles W.R. McCulloch A.D. Chien S. Omens J.H. Chronotropic response of cultured neonatal rat ventricular myocytes to short-term fluid shear. Cell Biochem Biophys. 2006;46:113. doi: 10.1385/CBB:46:2:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong C.R. Bursac N. Tung L. Mechanoelectrical excitation by fluid jets in monolayers of cultured cardiac myocytes. J Appl Physiol. 2005;98:2328. doi: 10.1152/japplphysiol.01084.2004. [DOI] [PubMed] [Google Scholar]
- 38.Fink C. Ergun S. Kralisch D. Remmers U. Weil J. Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 39.Lammerding J. Kamm R.D. Lee R.T. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.