Abstract
A biodegradable microsphere/scaffold composite based on the synthetic polymer poly(propylene fumarate) (PPF) holds promise as a scaffold for cell growth and sustained delivery vehicle for growth factors for bone regeneration. The objective of the current work was to investigate the in vitro release and in vivo bone forming capacity of this microsphere/scaffold composite containing bone morphogenetic protein-2 (BMP-2) in combination with autologous bone marrow stromal cells (BMSCs) in a goat ectopic implantation model. Three composites consisting of 0, 0.08, or 8 μg BMP-2 per mg of poly(lactic-co-glycolic acid) microspheres, embedded in a porous PPF scaffold, were combined with either plasma (no cells) or culture-expanded BMSCs. PPF scaffolds impregnated with a BMP-2 solution and combined with BMSCs as well as empty PPF scaffolds were also tested. The eight different composites were implanted subcutaneously in the dorsal thoracolumbar area of goats. Incorporation of BMP-2–loaded microspheres in the PPF scaffold resulted in a more sustained in vitro release with a lower burst phase, as compared to BMP-2–impregnated scaffolds. Histological analysis after 9 weeks of implantation showed bone formation in the pores of 11/16 composites containing 8 μg/mg BMP-2–loaded microspheres with no significant difference between composites with or without BMSCs (6/8 and 5/8, respectively). Bone formation was also observed in 1/8 of the BMP-2–impregnated scaffolds. No bone formation was observed in the other conditions. Overall, this study shows the feasibility of bone induction by BMP-2 release from microspheres/scaffold composites.
Introduction
Bone tissue engineering is a challenging field that strives to create alternative methods for current autograft and allograft treatments to restore bone defects or reinforce existing malfunctioning bone. Current strategies are mainly based on three components: (1) scaffolds, (2) (progenitor) cells, and (3) growth and differentiation factors. A three-dimensional biodegradable scaffold is the starting point for most regenerative strategies, providing initial mechanical strength and a framework for attachment and proliferation of cells. The cells are responsible for the matrix deposition that precedes ossification and can be locally recruited after implantation or seeded before implantation. Growth factors can be added to the scaffold to induce cell differentiation toward the osteogenic lineage. These tissue-engineering strategies have proven to be successful in many studies; however, upscaling bone tissue engineering toward clinical applications remains challenging.
The ideal biomaterial for bone regeneration should have good mechanical properties, support cell attachment and differentiation, and allow controlled release of bioactive factors for the modulation of cellular function. Further, the scaffold must biodegrade into nontoxic products to permit natural bone formation and remodeling. Poly(propylene fumarate) (PPF)–based materials are promising as biodegradable scaffolds for filling skeletal defects.1,2 Crosslinking of the linear polyester PPF through double bonds along its polymer backbone results in a biocompatible, biodegradable scaffold with mechanical properties similar to human trabecular bone.3–5 The injectable nature of the material makes it easy to shape the scaffold into a desired interconnected porous structure.6,7 Crosslinked PPF scaffolds have shown to be suitable substrates for the in vitro proliferation and osteoblastic differentiation of bone marrow stromal cells (BMSCs).8,9 Although these material characteristics are favorable for bone regeneration, PPF scaffolds are not osteoinductive. Making PPF scaffolds osteoinductive by adding the appropriate growth factors would be an improvement.
Bone morphogenetic protein-2 (BMP-2) is a very potent growth factor capable of inducing bone formation in both orthotopic and ectopic implantation sites.10–13 However, due to its short in vivo half-life and localized actions, BMP-2 requires a delivery vehicle to sustain its release at the implantation site. Biodegradable poly(lactic-co-glycolic acid) (PLGA) microspheres are ideal candidates for such a sustained release of BMP-2.1 Previous studies have shown that BMP-2 can be successfully encapsulated into PLGA microspheres with retention of its bioactivity.14 Further, the PLGA microspheres could be incorporated into PPF scaffolds to form microsphere/scaffold composites with a decreased burst release of loaded molecules.1
The purpose of this study was to investigate the effect of the incorporation of BMP-2–loaded microspheres in PPF scaffolds in a goat ectopic implantation model. To test whether the addition of progenitor cells would influence the bone regeneration capacity of the composite, some of the scaffolds were combined with autologous BMSCs before implantation.
Materials and Methods
Experimental design
Five different porous PPF scaffolds were prepared consisting of scaffolds alone (blank), scaffolds impregnated with a solution containing 50 μg BMP-2 (BMPimpregnated), and scaffolds with incorporated PLGA microspheres containing 0, 0.08, or 8.0 μg BMP-2/mg PLGA (Mpsempty, Mps-BMPlow, and Mps-BMPhigh, respectively; Table 1). A 50 μg dose of BMP-2 was used for the BMPimpregnated scaffolds since this was approximately the initial amount used for the fabrication of the Mps-BMPhigh scaffolds. The in vitro BMP-2 release profile from the scaffolds was determined in phosphate-buffered saline (PBS). The composites were combined with autologous plasma containing either no cells or autologous BMSCs, and implanted ectopically in goats. After 9 weeks, the composites were harvested and characterized for tissue ingrowth, bone formation, and porosity by histology and histomorphometry.
Table 1.
Experimental Groups and Implant Compositions
| PPF scaffold | Cells | Initial mps loading (μg BMP/mg PLGA) | Composite (Mps/PPF/porogen) (%) | BMP/scaffold (μg) | Number of scaffolds | |
|---|---|---|---|---|---|---|
| 1 | Blank | No cells | No mps | 0/30/70 | 0 | 4 |
| 2 | BMPimpregnated | BMSCs | No mps | 0/30/70 | 50 | 8 |
| 3 | Mpsempty | No cells | 0 | 6/24/70 | 0 | 8 |
| 4 | Mpsempty | BMSCs | 0 | 6/24/70 | 0 | 8 |
| 5 | Mps-BMPlow | No cells | 0.08 | 6/24/70 | 0.04–0.38 | 8 |
| 6 | Mps-BMPlow | BMSCs | 0.08 | 6/24/70 | 0.04–0.38 | 8 |
| 7 | Mps-BMPhigh | No cells | 8.0 | 6/24/70 | 39 | 8 |
| 8 | Mps-BMPhigh | BMSCs | 8.0 | 624/70 | 39 | 8 |
Mps, microparticles; PLGA, poly(lactic-co-glycolic acid); BMP, bone morphogenetic protein-2; PPF, poly(propylene fumarate); BMSCs, bone marrow stromal cells.
Materials
PLGA (75:25 lactic-to-glycolic ratio, Mw = 62 kDa, Medisorb®; Lakeshore Biomaterials, Birmingham, AL), poly(vinyl alcohol) (PVA, 87–89% mole hydrolyzed, Mw = 13–23 kDa; Sigma-Aldrich, St. Louis, MO), and isopropanol (IPA; Sigma-Aldrich) were used for the microsphere preparation. PPF with a molecular weight of 3100 and a polydispersity index of 2.7 was synthesized by a two-step reaction process as previously described.2 N-vinylpyrrolidinone (NVP; Acros, Pittsburgh, PA) and bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO; Ciba Specialty Chemicals, Tarrytown, NY) were used for the scaffold fabrication. BMP-2 (kindly provided by Wyeth Pharmaceuticals, Madison, NJ) was concentrated by centrifuging at 5000 g in a Centricon-10 filter unit (Amicon, Beverly, MA) and reconstituted to the appropriate concentrations in an aqueous buffer (pH 4.5) consisting of 5 mM glutamate, 5 mM NaCl, 0.5% sucrose, 2.5% glycine, and 0.01% polysorbate 80 (all from Sigma-Aldrich). An enzyme-linked immunosorbent assay (ELISA, Quantikine BMP-2 Immunoassay®; R&D Systems, Minneapolis, MN) was used for the entrapment efficiency and release assay.
Microsphere fabrication
A water-in-oil-in-water (W1-O-W2) double-emulsion-solvent-extraction technique was used for microsphere preparation.14 Briefly, 50 μL of a 0, 0.40, or 40 mg/mL BMP-2 solution was emulsified in a solution of 250 mg PLGA in 1 mL dichloromethane using a vortexer at 3050 rpm. The entire mixture was re-emulsified for 30 s in 2 mL of 1% w/v aqueous PVA solution to create the double emulsion. The content was then added to 100 mL of 0.3% w/v aqueous PVA solution and 100 mL of 2% w/v aqueous IPA solution with stirring for 1 h. The extraction of the dichloromethane to the external alcohol phase resulted in precipitation of the dissolved polymers and subsequently the formation of microspheres. The microspheres were collected by centrifugation, washed twice with ddH2O, and finally vacuum dried. The resulting powder was stored at −20°C prior to use.
Scaffold fabrication
Porous PPF scaffolds were fabricated using a salt leaching technique. Sodium chloride particles that were sieved to a size range of 300–500 μm were used as porogen. Briefly, 60 μL of an initiator solution (100 mg/mL of BAPO in dichloromethane) was added to a solution of 0.9 g of PPF in 0.27 mL NVP and mixed well with a spatula. The PPF/ NVP/BAPO paste was then combined with the appropriate microsphere formulation (0 or 6% w/w) and 70% w/w NaCl particles as indicated in Table 1. The mixture was forced into glass cylindrical vials with a diameter of 6 mm and placed under UV light (PS135; Matcon, Middenbeemster, The Netherlands) for 30 min for photocrosslinking. The cylindrical scaffolds were then cut into 3-mm-thick disks and sterilized by ethanol evaporation. Finally, the disks were placed in PBS for 12 h to leach out the NaCl particles. Eight blank PPF scaffolds were impregnated with 19 μL of a solution containing 50 μg BMP-2. All implants were cryopreserved at −20°C until use.
Microsphere and scaffold characterization
The entrapment efficiency of BMP-2 in the PLGA microspheres was determined by normalizing the actual amount of entrapped BMP-2 to the starting amount. Approximately 10 mg of microspheres was dissolved in 0.75 mL of dichloromethane and 0.75 mL of a strong desorption buffer consisting of 0.5 M arginine, 0.5 M NaCl, and 50 mM K2HPO4. The BMP-2 was extracted over a period of 48 h with a buffer change after 24 h. The concentration of the extracted BMP-2 was analyzed by ELISA following the manufacturer's instruction. To determine the in vitro BMP-2 release profile, the scaffolds were placed in microcentrifuge tubes containing 1.0 mL of pH 7.4 PBS and maintained at 37°C on an orbital shaker set at 100 rpm to ensure continuous mixing. At days 0.5, 1, 2, 3, 5, 7, 9, 13, 17, 21, and 24, the supernatant was collected, stored at −20°C, and replaced with fresh PBS. The samples were assayed for BMP-2 concentration using the BMP-2 ELISA.
BMSC culture and seeding conditions
The animal experiments were approved by the Institutional Animal Care and Use Committee. Ten adult Dutch milk goats were obtained from a professional stockbreeder at least 4 weeks prior to surgery. Autologous BMSCs were derived and expanded 3 weeks preoperatively. Briefly, bone marrow aspirates were taken from both iliac wings under general anesthesia. The BMSCs in the aspirates were culture expanded in standard culture medium containing 15% fetal bovine serum (FBS; Gibco, Paisly, Scotland) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Gibco) with media changes every 3 days. The proportion of BMSCs in the bone marrow aspirates was determined by a colony-forming efficiency (CFU) assay in which nucleated cells from the aspirate were plated in two 25 cm2 flasks at a density of 1 × 105 cells/cm2. After 8 or 9 days, the colonies were washed with PBS, fixed in 8% formalin, stained with methylene blue, and counted under an inverted microscope. The rest of the expanded cells were cryopreserved after passage 2 in medium containing 30% FBS and 10% dimethylsulfoxide (DMSO; Sigma-Aldrich) in aliquots of 1 × 107 cells/mL until use.
On the day of surgery, aliquots of cryopreserved cells were thawed on ice, washed with autologous serum, and resuspended in serum at a concentration of 1 × 106 cells/mL. The cell viability was determined by trypan blue exclusion. The BMSCs were transported to the operating room on ice in aliquots of 5.5 mL. During surgery, plasma was obtained by centrifuging 10 mL of venous blood at 1200 g in a plastic tube. The BMSCs aliquots were centrifuged at 300 g, the medium was decanted, and the cells were resuspended in autologous plasma. Plasma with no cells or with BMSCs (0.5 × 106 cells/scaffold) was combined with the prewetted scaffolds and allowed to clot before implantation.
Surgical procedure
Each goat received six implants in the thoracolumbar area according to a randomized scheme (Table 2). Prior to surgery, the goats were sedated by an intravenous injection of detomidine, and general inhalation anesthesia was provided by a halothane gas mixture. Six subcutaneous pockets were created in the dorsal thoracolumbar area by blunt dissection and filled with one of the implants according to a randomized scheme. To increase the statistical power to determine an effect of BMSCs, groups 5 and 6, and groups 7 and 8 (Tables 1 and 2) were implanted pairwise. The pockets were closed with a nonresorbable suture to indicate their location postmortem. In addition to the subcutaneous implantations, in the course of separate studies, the goats also received ceramic implants intramuscularly and in osteoconduction chambers on the transverse processes of L3 and L5.15,16 Postoperative pain relief was achieved by buprenorphine. To monitor the dynamics of calcification, the fluorochrome markers calcein green (10 mg/kg intravenously; Sigma-Aldrich), oxytetracyclin (32 mg/kg intramuscularly according to the manufacturer's instructions; Mycofarm, Boxmeer, The Netherlands), and xylenol orange (80 mg/kg, intravenously; Sigma-Aldrich) were administered after 3, 5, and 7 weeks, respectively. At 9 weeks postimplantation, euthanasia was performed by an overdose of pentobarbital (Organon, Oss, The Netherlands).
Table 2.
Implantation Scheme and Location of the Experimental Groups
| |
Pocket contents |
|||||
|---|---|---|---|---|---|---|
| Goat | 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | Mem + C | Mem | Blo + C | Blo | Bhi + C | Bhi |
| 2 | Bim + C | Bl | Mem + C | Mem | Blo + C | Blo |
| 3 | Bhi + C | Bhi | Bim + C | Bl | Mem + C | Mem |
| 4 | Blo + C | Blo | Bhi + C | Bhi | Bim + C | Mem |
| 5 | Mem | Mem + C | Blo | Blo + C | Bhi | Bhi + C |
| 6 | Bl | Bim + C | Mem | Mem + C | Blo | Blo + C |
| 7 | Bhi + C | Bhi | Mem | Bim + C | Bl | Mem + C |
| 8 | Blo + C | Blo | Bhi + C | Bhi | Mem | Bim + C |
| 9 | Mem + C | Bim + C | Blo + C | Blo | Bhi + C | Bhi |
| 10 | Bhi | Bhi + C | Mem + C | Bim + C | Blo + C | Blo |
|
Pocket location | ||
|---|---|---|
| Cranial | ||
| 1 2 3 |
S p i n e |
4 5 6 |
| Caudal | ||
Bim, BMPimpregnated; Mem, Mpsempty; Mlo, Mps-BMPlow; Mhi, Mps-BMPhigh; Bl, blank; C, autologous bone marrow stromal cells.
Mps, microparticles; BMP, bone morphogenetic protein-2.
Histology and histomorphometry
After explantation, the implants were fixed in a 4% phosphate-buffered formaldehyde solution (pH 7.4). The implants were dehydrated in graded series of alcohol and embedded in methylmethacrylate. After polymerization, sections were cut using a sawing microtome (Leica SP1600; Leica Microsystems, Nussloch, Germany) and stained with methylene blue/basic fuchsin or with hematoxylin and eosin for routine histology and histomorphometry. An additional unstained section was sawn for fluorescence microscopy. Using light and fluorescence microscopy, the general tissue response, bone formation, and fluorochrome labels were evaluated. For histomorphometry, high-resolution (300 dpi), low-magnification (40 × ) digital micrographs covering the complete implant were made of blinded sections. The areas of interest were pseudocolored, and the colorized pixels were measured to calculate the percentage of scaffold porosity and bone area relative to the available pore space.
Statistical analysis
Both in vitro (n = 4) and in vivo (n = 4 or 8) results are reported as means ± standard deviations. Analysis of variance (ANOVA) with Bonferroni-corrected post hoc tests were used to analyze differences in porosity between the experimental groups. A two-tailed paired Student's t-test was used to determine the effect of cells on the amount of newly formed bone in the Mps-BMPhigh scaffolds (groups 7 and 8). All tests were performed by SPSS (version 13.0; SPSS, Chicago, IL), and the level of significance was set at p = 0.05.
Results
Scaffold characterization
The entrapment efficiency of BMP-2 in the microspheres loaded at 8.0 μg/mg PLGA was 82 ± 3.6% (n = 4), which resulted in a scaffold loading of approximately 39 μg BMP-2 per Mps-BMPhigh implant. Despite the use of the strong desorption buffer and repetition of the extraction procedure, it was difficult to estimate the entrapment efficiency of the 0.08 μg/mg loaded microspheres. The entrapment efficiency of these microspheres varied between 8.1% and 78% (n = 9). Based on these encapsulation yields, the amount of BMP-2 in the Mps-BMPlow scaffolds was estimated between 0.04 and 0.38 μg BMP-2 per implant. Since the BMP-2 loss during the impregnation process of the blank scaffolds was limited, the BMPimpregnated scaffolds were loaded with 50 μg of BMP-2. This was also approximately the starting amount for the fabrication of the Mps-BMPhigh scaffolds.
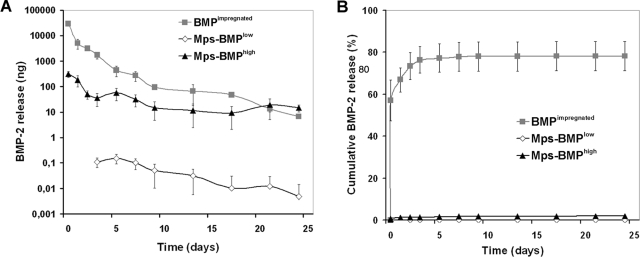
The BMP-2 release profiles from the microsphere/scaffold composites and scaffolds impregnated with a BMP-2 solution are shown in Figure 1. The BMPimpregnated scaffolds showed a considerable initial burst release of 34 ± 2.9 μg in the first day, followed by a rapid decline of the released amounts. The Mps-BMPhigh scaffolds showed a much lower burst of 0.49 ± 0.07 μg in the first day, followed by a prolonged sustained release. The Mps-BMPlow scaffolds showed no measurable in vitro BMP-2 release during the first 2 days with a low sustained release for the rest of the experiment.
FIG. 1.
BMP-2 release profiles expressed as (A) amount of released protein (in ng) and (B) normalized release (as % of initial loading) from different composites in PBS at 37°C.
BMSC characterization
The bone marrow aspirates contained 5.6 ± 1.5 × 106 nucle-ated cells/mL, which showed a CFU of 1.9 ± 0.6 colonies per 1.0 × 105 cells. The doubling time of the proliferating BMSCs was 1.2 ± 0.6 days, resulting in cryopreservation of 60–100 million cells within 3 weeks after obtaining the aspirates. Trypan blue exclusion indicated 95% cell viability after thawing.
Animals
During the experiments, all goats remained in good health and did not show any complications. After 9 weeks of implantation, all implants were easily identified and retrieved.
Histology
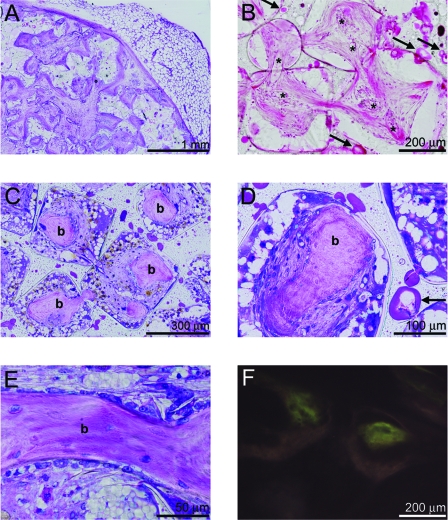
Microscopic evaluation of transverse sections showed that all composites maintained their original shape. All scaffolds were surrounded by fibrous tissue from which well-vascularized connective tissue grew into the pores of the scaffold (Fig. 2A, B). Microfragmentation of PLGA was seen in the intact PPF network, indicating degradation of the microspheres (Fig. 2B). No signs of PPF degradation could be observed. The tissue response to the PLGA-PPF materials was relatively uniform. There was a mild foreign body reaction to the implanted materials as indicated by some inflammatory cells inside the pores of the scaffolds, including macrophages and giant cells at the polymer–tissue interface.
FIG. 2.
Methylene blue-basic fuchsine (A, C, D, E) and hematoxylin and eosin (B) stained or unstained (F) histological sections of (A, B) Mps-BMPlow and (C–F) Mps-BMPhigh composites after 9 weeks of subcutaneous implantation in goats. (A) Overview and (B) detailed view of an Mps-BMPlow implant showing ingrowth of well-vascularized fibrous tissue into the pores of the composite (vessels indicated by asterisks) and microfragmentation of PLGA in the intact PPF network (indicated by the central excavation and uneven staining of the microspheres at arrows). (C) Bone formation (indicated by letter b) was seen in the center of the pores of the Mps-BMPhigh implants. The bone had a woven (D) or lamellar (E) appearance, with osteocytes inside the matrix and osteoblasts on the surface. (F) Despite the little autofluorescence of the tissue, no fluorochromes could be detected in the newly formed bone. Color images available online at www.liebertonline.com/ten.
Bone formation was observed in the pores of 11/16 of the Mps-BMPhigh scaffolds and 1/8 of the BMPimpregnated scaffolds. No bone was formed in the blank, Mpsempty, or Mps-BMPlow scaffolds. The newly formed bone was found throughout the implants, mainly clustered in the center of the pores with little contact between the bone and scaffold (Fig. 2C, D). The bone had a woven or lamellar appearance, with osteocytes inside the matrix and osteoblasts lining the bone surfaces (Fig. 2D, E). One of the BMPimpregnated scaffolds loaded with BMSCs showed trabecular bone in the fibrous capsule of the implant. Unfortunately, the excision margin of the other BMPimpregnated scaffolds was not large enough to investigate the bone formation outside the scaffold borders. Despite little autofluorescence of the tissue, no fluorochromes could be detected in the bone that had formed in the polymer scaffolds (Fig. 2F). Fluorescence microscopy did show all fluorochromes in scaffolds implanted in the same animals in the course of another study. This indicates the onset of bone mineralization was between 7 and 9 weeks, after the administration of the final fluorochrome.
Histomorphometry
Table 3 summarizes the measurements of scaffold porosity and bone formation for the different implant conditions. Bone formation was observed inside the pores of 1/8 of the BMPimpregnated scaffolds, 5/8 of the Mps-BMPhigh scaffolds without cells, and 6/8 of the Mps-BMPhigh scaffolds with BMSCs. The porosity of the blank/BMPimpregnated and Mps-BMPhigh scaffolds was significantly higher compared to the Mpsempty and Mps-BMPlow scaffolds (p < 0.05). The bone formation in the Mps-BMPhigh implants was 2.9 ± 3.0% (varying from 0% to 7.2%, n = 8) and 2.7 ± 2.4% (varying from 0% to 5.6%, n = 8) of the available pore space for the scaffolds without cells and with BMSCs, respectively. BMSC seeding had no effect on bone formation. In the one-PPF implant impregnated with BMP solution, 0.2% of the pore space was filled by newly formed bone.
Table 3.
Histomorphometry Results of the Implants
| Scaffold | Cells | Porosity (%) | Scaffolds (with bone/total) | Bone (% of pore space) | |
|---|---|---|---|---|---|
| 1 | Blank | No cells | 77.4 ± 4.5a | 0/4 | 0 |
| 2 | BMPimpregnated | BMSCs | 1/8 | 0.0 ± 0.1 | |
| 3 | Mpsempty | No cells | 71.7 ± 1.7 | 0/8 | 0 |
| 4 | Mpsempty | BMSCs | 0/8 | 0 | |
| 5 | Mps-BMPlow | No cells | 73.1 ± 2.4 | 0/8 | 0 |
| 6 | Mps-BMPlow | BMSCs | 0/8 | 0 | |
| 7 | Mps-BMPhigh | No cells | 75.3 ± 2.2a | 5/8 | 2.9 ± 3.0 |
| 8 | Mps-BMPhigh | BMSCs | 6/8 | 2.7 ± 2.4 |
Scaffold porosity (mean ± SD), incidence of bone formation, and the percentage of bone area (mean ± SD) of the available pore space.
Significantly higher than Mpsempty and Mps-BMPlow (p < 0.05, ANOVA).
Mps, microparticles; BMP, bone morphogenetic protein-2; BMSCs, bone marrow stromal cells.
Discussion
This study demonstrates that PPF scaffolds can be rendered osteoinductive by incorporation of BMP-2–loaded PLGA microspheres. Compared to BMP-2–impregnated scaffolds, microsphere incorporation resulted in a lower burst and a more sustained in vitro release over a prolonged period of time. In contrast to the high burst release of the BMP-2–impregnated scaffolds, the sustained BMP-2 release from the PLGA microspheres resulted in a higher amount of ectopic bone in the scaffold pores after 9 weeks of implantation in goats. In the current experimental setup, the addition of autologous BMSCs before implantation had no significant effect on the osteoinductive capacity of the construct.
Previous in vitro studies have shown that PLGA microspheres are effective vehicles for sustained delivery of BMP-2.14,17–19 The BMP-2 retention is based on physical entrapment as well as protein–polymer interactions as a result of ionic, hydrophobic, and/or hydrogen bonding.17,18 During the fabrication process, the encapsulation and interactions lead to a reasonable encapsulation efficiency of the Mps-BMPhigh microspheres. Probably due to the strong protein–polymer interactions, only a fraction of BMP-2 could be extracted from the Mps-BMPlow microspheres resulting in an underestimation of their entrapment efficiency. An alternative to the extraction/ELISA method for the entrapment efficiency measurements could be radioactive labeling of the BMP-2 prior to incorporation its into the microspheres. This method would obviate protein extraction and could also be used for the in vitro and in vivo release measurements.20–26
The PLGA microspheres were incorporated into the PPF scaffold in an attempt to better maintain local in vivo concentrations at osteoinductive levels for sufficient time. The impregnation of PPF scaffolds with a high BMP-2 dose is inherent to minimal retention and failed to consistently produce bone inside the scaffolds. In contrast, BMP-2 incorporation into PLGA microspheres resulted in a gradual in vitro release from the PPF composites. This gradual BMP-2 release was probably responsible for bone induction in the higher-loaded microsphere/scaffold composites. Previous ectopic studies in rodents reported that BMP-2–induced bone formation was related to the dosage.27–31 Apparently, the local BMP-2 concentrations in the composites with a lower loading were not sufficient to induce bone formation. Although the amounts released by the higher-loaded scaffolds were sufficient to induce bone formation, both microsphere formulations released < 2.5% of the incorporated BMP-2 in the first 24 days. Therefore, further optimization of the BMP-2 pharmacokinetics is required to obtain a release profile that coincides better with the normal rate of bone formation.
Surprisingly, the preoperative seeding of autologous cryopreserved BMSCs on the composite formulations in this study did not enhance ectopic bone formation. Although the mechanism of bone induction in ceramics is completely different, the osteogenic potential of autologous BMSCs in goats was shown in ceramic scaffolds where they clearly enhanced ectopic bone formation.15,32,33 Preoperative seeding of cryopreserved BMSCs resulted in similar amounts of newly formed bone in ceramic scaffolds as in precultured constructs.33 Therefore, this seeding method was also employed for this study to overcome complicated logistics for the preparation of preoperatively cultured constructs. Although the plasma polymerization during the preoperative seeding method resulted in a 100% cell loading efficiency and retention during implantation procedure, the behavior of the cells in vivo on the scaffolds applied in this study is unknown.
The absence of bone formation in the cell-seeded composites without BMP-2 was as expected, since these synthetic polymers do not possess osteoinductive characteristics in contrast to ceramics. Therefore, the initiation of the BMSC osteogenesis in the PPF scaffolds is likely to depend on BMP-2 signaling. However, the absence of an effect of autologous BMSCs on bone formation in BMP-2–loaded scaffolds is less clear. In vitro studies have shown that the osteogenic capacity of BMSCs in the presence of BMP-2 is dose dependent.34,35 Since the histological results show large amounts of nonresorbed PLGA in the PPF matrix after 9 weeks of follow-up, the degradation of the PLGA microspheres and the subsequent release of the BMP-2 from the microspheres might have been too slow. This could have resulted in insufficient BMP-2 concentrations in the early days of implantation to induce differentiation of the seeded cells, since BMSC-derived osteogenesis normally starts within 3 weeks after implantation.15 In the absence of such an osteoinductive stimulus, the undifferentiated BMSCs could have differentiated to the fibrous tissue in the microsphere/scaffold composite. The bone formation and late ossification by the BMP-2 released at a later time point are probably derived from host cells that were locally recruited from for example the circulation.36
In conclusion, this study shows that sustained release of BMP-2 from microspheres contained in PPF scaffolds can induce ectopic bone formation in these scaffolds that are otherwise nonosteoinductive. In contrast to the enhanced ectopic bone formation on ceramic scaffolds by BMSCs, the addition of stem cells to the scaffolds in the current study did not further enhance bone formation, possibly due to a slow rise of the local BMP concentration and a critical delay before the osteoinductive threshold dose is reached. Future studies should be aimed at improving the timing and rate of BMP-2 release to further stimulate the extent and rate of bone formation, either alone or in combination with mesenchymal stem cells.
Acknowledgments
The authors gratefully acknowledge the Stichting Annafonds, the National Institutes of Health (R01 AR45871 and R01 EB03060), and The Netherlands Organization for Health Research and Development (ZonMW) for financial support. The authors thank Dr. Esmaiel Jabbari and Mr. James Greutzmacher from the Tissue Engineering and Biomaterials Laboratory at the Mayo Clinic for their assistance with polymer synthesis.
References
- 1.Kempen D.H.R. Kim C.W. Lu L. Dhert W.J.A. Currier B.L. Yaszemski M.J. Controlled release from poly(lactic-co-glycolic acid) microspheres embedded in an injectable, biodegradable scaffold for bone tissue engineering. Mater Sci Forum. 2003;426–432:3151. [Google Scholar]
- 2.Wang S. Lu L. Yaszemski M.J. Bone-tissue-engineering material poly(propylene fumarate): correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules. 2006;7:1976. doi: 10.1021/bm060096a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher J.P. Vehof J.W. Dean D. van der Waerden J.P. Holland T.A. Mikos A.G. Jansen J.A. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59:547. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 4.Hedberg E.L. Kroese-Deutman H.C. Shih C.K. Crowther R.S. Carney D.H. Mikos A.G. Jansen J.A. In vivo degradation of porous poly(propylene fumarate)/poly(DL-lactic-co-glycolic acid) composite scaffolds. Biomaterials. 2005;26:4616. doi: 10.1016/j.biomaterials.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Peter S.J. Suggs L.J. Yaszemski M.J. Engel P.S. Mikos A.G. Synthesis of poly(propylene fumarate) by acylation of propylene glycol in the presence of a proton scavenger. J Biomater Sci Polym Ed. 1999;10:363. doi: 10.1163/156856299x00423. [DOI] [PubMed] [Google Scholar]
- 6.Lee K.W. Wang S. Fox B.C. Ritman E.L. Yaszemski M.J. Lu L. Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules. 2007;8:1077. doi: 10.1021/bm060834v. [DOI] [PubMed] [Google Scholar]
- 7.Lee K.W. Wang S. Lu L. Jabbari E. Currier B.L. Yaszemski M.J. Fabrication and characterization of poly(propylene fumarate) scaffolds with controlled pore structures using 3-dimensional printing and injection molding. Tissue Eng. 2006;12:2801. doi: 10.1089/ten.2006.12.2801. [DOI] [PubMed] [Google Scholar]
- 8.Peter S.J. Lu L. Kim D.J. Mikos A.G. Marrow stromal osteoblast function on a poly(propylene fumarate)/beta-tricalcium phosphate biodegradable orthopaedic composite. Biomaterials. 2000;21:1207. doi: 10.1016/s0142-9612(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 9.Peter S.J. Lu L. Kim D.J. Stamatas G.N. Miller M.J. Yaszemski M.J. Mikos A.G. Effects of transforming growth factor beta1 released from biodegradable polymer microparticles on marrow stromal osteoblasts cultured on poly(propylene fumarate) substrates. J Biomed Mater Res. 2000;50:452. doi: 10.1002/(sici)1097-4636(20000605)50:3<452::aid-jbm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Gerhart T.N. Kirker-Head C.A. Kriz M.J. Holtrop M.E. Hennig G.E. Hipp J. Schelling S.H. Wang E. Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin Orthop Relat Res. 1993;317 [PubMed] [Google Scholar]
- 11.Hollinger J.O. Schmitt J.M. Buck D.C. Shannon R. Joh S.P. Zegzula H.D. Wozney J. Recombinant human bone morphogenetic protein-2 and collagen for bone regeneration. J Biomed Mater Res. 1998;43:356. doi: 10.1002/(sici)1097-4636(199824)43:4<356::aid-jbm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Vehof J.W. Mahmood J. Takita H. van't Hof M.A. Kuboki Y. Spauwen P.H. Jansen J.A. Ectopic bone formation in titanium mesh loaded with bone morphogenetic protein and coated with calcium phosphate. Plast Reconstr Surg. 2001;108:434. doi: 10.1097/00006534-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Weber F.E. Eyrich G. Gratz K.W. Maly F.E. Sailer H.F. Slow and continuous application of human recombinant bone morphogenetic protein via biodegradable poly(lactide-co-glycolide) foamspheres. Int J Oral Maxillofac Surg. 2002;31:60. doi: 10.1054/ijom.2001.0154. [DOI] [PubMed] [Google Scholar]
- 14.Oldham J.B. Lu L. Zhu X. Porter B.D. Hefferan T.E. Larson D.R. Currier B.L. Mikos A.G. Yaszemski M.J. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng. 2000;122:289. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 15.Kruyt M.C. Dhert W.J. Oner F.C. van Blitterswijk C.A. Verbout A.J. de Bruijn J.D. Analysis of ectopic and orthotopic bone formation in cell-based tissue-engineered constructs in goats. Biomaterials. 2007;28:1798. doi: 10.1016/j.biomaterials.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 16.Kruyt M.C. Wilson C.E. de Bruijn J.D. van Blitterswijk C.A. Oner C.F. Verbout A.J. Dhert W.J. The effect of cell-based bone tissue engineering in a goat transverse process model. Biomaterials. 2006;27:5099. doi: 10.1016/j.biomaterials.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Duggirala S.S. Mehta R.C. DeLuca P.P. Interaction of recombinant human bone morphogenetic protein-2 with poly(d,l lactide-co-glycolide) microspheres. Pharm Dev Technol. 1996;1:11. doi: 10.3109/10837459609031413. [DOI] [PubMed] [Google Scholar]
- 18.Schrier J.A. DeLuca P.P. Recombinant human bone morphogenetic protein-2 binding and incorporation in PLGA microsphere delivery systems. Pharm Dev Technol. 1999;4:611. doi: 10.1081/pdt-100101400. [DOI] [PubMed] [Google Scholar]
- 19.Schrier J.A. Fink B.F. Rodgers J.B. Vasconez H.C. DeLuca P.P. Effect of a freeze-dried CMC/PLGA microsphere matrix of rhBMP-2 on bone healing. AAPS PharmSciTech. 2001;2:E18. doi: 10.1208/pt020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kempen D.H.R. Lu L. Hefferan T.E. Creemers L.B. Maran A. Classic K.L. Dhert W.J.A. Yaszemski M.J. Retention of in vitro and in vivo BMP-2 bioactivity in sustained delivery vehicles for bone tissue engineering. Accepted by Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis-Ugbo J. Kim H.S. Boden S.D. Mayr M.T. Li R.C. Seeherman H. D'Augusta D. Blake C. Jiao A. Peckham S. Retention of 125I-labeled recombinant human bone morphogenetic protein-2 by biphasic calcium phosphate or a composite sponge in a rabbit posterolateral spine arthrodesis model. J Orthop Res. 2002;20:1050. doi: 10.1016/S0736-0266(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 22.Uludag H. D'Augusta D. Palmer R. Timony G. Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M. Takahashi Y. Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 24.Yokota S. Uchida T. Kokubo S. Aoyama K. Fukushima S. Nozaki K. Takahashi T. Fujimoto R. Sonohara R. Yoshida M. Higuchi S. Yokohama S. Sonobe T. Release of recombinant human bone morphogenetic protein 2 from a newly developed carrier. Int J Pharm. 2003;251:57. doi: 10.1016/s0378-5173(02)00581-1. [DOI] [PubMed] [Google Scholar]
- 25.Kempen D.H.R. Lu L. Classic K.L. Hefferan T.E. Creemers L.B. Maran A. Dhert W.J.A. Yaszemski M.J. A non-invasive screenings model for simultaneous evaluation of multiple in vivo growth factor release profiles. J Control Release. 2008;130:15. doi: 10.1016/j.jconrel.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruhe P.Q. Boerman O.C. Russel F.G. Mikos A.G. Spauwen P.H. Jansen J.A. In vivo release of rhBMP-2 loaded porous calcium phosphate cement pretreated with albumin. J Mater Sci Mater Med. 2006;17:919. doi: 10.1007/s10856-006-0181-z. [DOI] [PubMed] [Google Scholar]
- 27.Bessho K. Carnes D.L. Cavin R. Ong J.L. Experimental studies on bone induction using low-molecular-weight poly (DL-lactide-co-glycolide) as a carrier for recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2002;61:61. doi: 10.1002/jbm.10169. [DOI] [PubMed] [Google Scholar]
- 28.Fujimura K. Bessho K. Kusumoto K. Konishi Y. Ogawa Y. Iizuka T. Experimental osteoinduction by recombinant human bone morphogenetic protein 2 in tissue with low blood flow: a study in rats. Br J Oral Maxillofac Surg. 2001;39:294. doi: 10.1054/bjom.2001.0647. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita N. Terai H. Okada T. Nozaki K. Inoue H. Miyamoto S. Takaoka K. A new bone-inducing biodegradable porous beta-tricalcium phosphate. J Biomed Mater Res A. 2004;70:450. doi: 10.1002/jbm.a.30102. [DOI] [PubMed] [Google Scholar]
- 30.Saito N. Okada T. Horiuchi H. Murakami N. Takahashi J. Nawata M. Ota H. Nozaki K. Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 31.Wang E.A. Rosen V. D'Alessandro J.S. Bauduy M. Cordes P. Harada T. Israel D.I. Hewick R.M. Kerns K.M. LaPan P. Luxenberg D.P. McQuaid D. Moutsatsos I.K. Nove J. Wozney J.M. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA. 1990;87:2220. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kruyt M.C. de Bruijn J.D. Wilson C.E. Oner F.C. van Blitterswijk C.A. Verbout A.J. Dhert W.J. Viable osteogenic cells are obligatory for tissue-engineered ectopic bone formation in goats. Tissue Eng. 2003;9:327. doi: 10.1089/107632703764664792. [DOI] [PubMed] [Google Scholar]
- 33.Kruyt M.C. de Bruijn J.D. Yuan H. van Blitterswijk C.A. Verbout A.J. Oner F.C. Dhert W.J. Optimization of bone tissue engineering in goats: a peroperative seeding method using cryopreserved cells and localized bone formation in calcium phosphate scaffolds. Transplantation. 2004;77:359. doi: 10.1097/01.TP.0000102550.58160.39. [DOI] [PubMed] [Google Scholar]
- 34.Lecanda F. Avioli L.V. Cheng S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J Cell Biochem. 1997;67:386. [PubMed] [Google Scholar]
- 35.Vehof J.W. de Ruijter A.E. Spauwen P.H. Jansen J.A. Influence of rhBMP-2 on rat bone marrow stromal cells cultured on titanium fiber mesh. Tissue Eng. 2001;7:373. doi: 10.1089/10763270152436436. [DOI] [PubMed] [Google Scholar]
- 36.Otsuru S. Tamai K. Yamazaki T. Yoshikawa H. Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]