Abstract
In mammalian testes, extensive junction restructuring takes place in the seminiferous epithelium at the Sertoli–Sertoli and Sertoli–germ cell interface to facilitate the different cellular events of spermatogenesis, such as mitosis, meiosis, spermiogenesis, and spermiation. Recent studies in the field have shown that Rho GTPases and polarity proteins play significant roles in the events of cell–cell interactions. Furthermore, Rho GTPases, such as Cdc42, are working in concert with polarity proteins in regulating cell polarization and cell adhesion at both the blood–testis barrier (BTB) and apical ectoplasmic specialization (apical ES) in the testis of adult rats. In this chapter, we briefly summarize recent findings on the latest status of research and development regarding Cdc42 and polarity proteins and how they affect cell–cell interactions in the testis and other epithelia. More importantly, we provide a new model in which how Cdc42 and components of the polarity protein complexes work in concert with laminin fragments, cytokines, and testosterone to regulate the events of cell–cell interactions in the seminiferous epithelium via a local autocrine-based regulatory loop known as the apical ES—BTB—basement membrane axis. This new functional axis coordinates various cellular events during different stages of the seminiferous epithelium cycle of spermatogenesis.
Keywords: Testis, Spermatogenesis, Sertoli cells, Germ cells, Blood–testis barrier, Adherens junction, Tight junction, Ectoplasmic specialization, Seminiferous epithelial cycle
1. Introduction
In mammalian testes, spermatogenesis takes place in the seminiferous tubule, which is the functional unit that produces spermatozoa (haploid, 1n) from spermatogonia (diploid, 2n) under the influence of the pituitary hormone follicle stimulating hormone (FSH) (Cheng and Mruk, 2002; de Kretser and Kerr, 1988; Sharpe, 1994; Walker and Cheng, 2005). The process of spermatogenesis, however, is also supported by Leydig cells in the interstitium, which produce testosterone to maintain Sertoli and germ cell function and to regulate germ cell maturation (Page et al., 2008; Walker and Cheng, 2005), such as cell-cycle progression (Lie et al., 2009), under the influence of pituitary hormone lutenizing hormone (LH). Additionally, estrogen (e.g., estradiol-17β) produced by Leydig cells in the interstitium, and Sertoli and germ cells in the seminiferous epithelium, is also critical to germ cell development such as apoptosis as demonstrated by studies reported in recent years (Carreau et al., 2008; Hess, 2003; Shaha, 2008).
Morphologically, the seminiferous epithelium is segregated into the basal and the apical (or adluminal) compartments by the blood–testis barrier (BTB) which is a testis-specific ultrastructure between adjacent Sertoli cells located near to the basement membrane (Fig. 7.1). Besides conferring cell polarity, the BTB also provides the “gate” function so that water, electrolytes, nutrients, and biomolecules cannot freely diffuse paracellularly from the interstitium and basal compartment to the apical compartments (Mruk and Cheng, 2008; Mruk et al., 2008). Also, the BTB confers the immunological barrier in the testis (Mruk et al., 2008), which restricts the access of drugs, environmental toxicants, and ions to the developing spermatids behind the BTB. In turn, drugs and ions utilize different drug transporters and ion channels to enter the apical compartment (Mruk and Cheng, 2008).
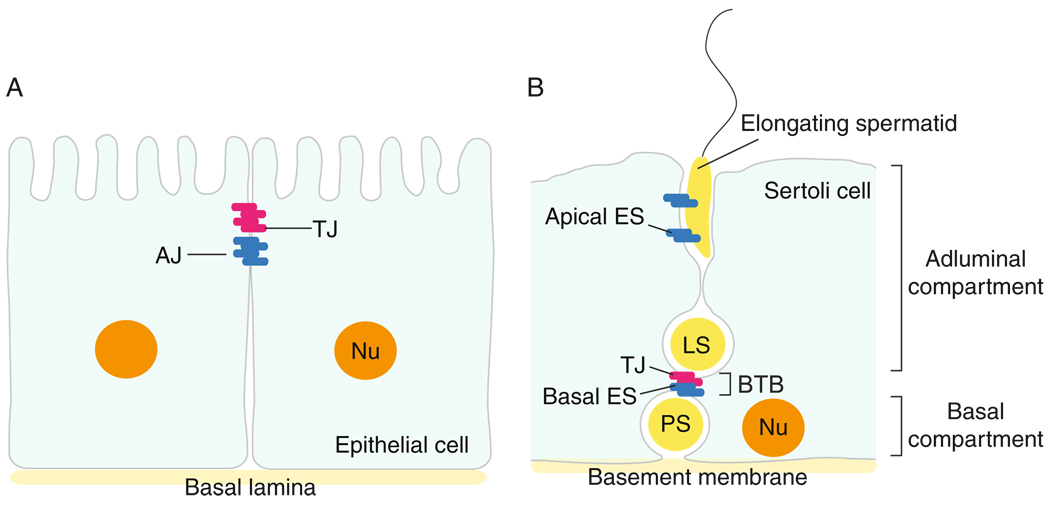
Figure 7.1.
Differences in the spatial arrangement of tight junction (TJ) and adherens junction (AJ) in epithelia versus the seminiferous epithelium in adult mammalian testes. (A) In most epithelia, TJ is restricted to the apical region of adjacent epithelial cells, underneath of which lies AJ, a cell–cell actin-based anchoring junction type. Furthest away from TJ is the basal lamina, a form of extracellular matrix (ECM). In other blood–tissue barrier, the TJ alone confers the barrier function, such as in the blood–brain barrier and the blood–retina barrier. (B) In mammalian testes, such as rats, the blood–testis barrier (BTB) is closest to the basement membrane (a modified form of ECM) instead of the apical region of the Sertoli cell. Also, the BTB is composed of coexisting TJ and basal ES (an atypical AJ type in the testis). The apical ES, however, is restricted to the Sertoli cell–elongating spermatid interface. The presence of the BTB also segregates the seminiferous epithelium into the basal and adluminal compartments.
Spermatogenesis is a highly complex biochemical process which is composed of several discrete cellular events that take place in the seminiferous epithelium of the testis, namely mitosis, meiosis, spermiogenesis, and spermiation, during the seminiferous epithelial cycle (Fig. 7.2). In adult rat testes, the seminiferous epithelial cycle can be divided into 14 stages (12 stages in mice and 6 stages in humans) (de Kretser and Kerr, 1988), which is typified by the unique association between Sertoli cells and the developing spermatids in the seminiferous epithelium (Hess and de Franca, 2008; Leblond and Clermont, 1952; Parvinen, 1982) as shown in Fig. 7.2. During the initial phase of spermatogenesis, spermatogonia undergo mitosis to replenish the most primitive germ cells such as type A spermatogonia, some of which will differentiate into type B spermatogonia. It is noted that some type B spermatogonia, regulated by a yet-to-be-defined mechanism(s), commit to differentiate into primary spermatocytes. These are the germ cells that will enter the cell-cycle progression events and differentiate into preleptotene spermatocytes. Once formed, preleptotene spermatocytes begin to traverse the BTB at stages VIII–IX of the epithelial cycle while differentiating into leptotene and zygotene spermatocytes (Russell, 1977). Concomitant with these events, condensation and replication of the chromatin materials take place during the transit of primary spermatocytes at the BTB, so that diplotene spermatocytes (tetraploid, 4n) can undergo diakinesis, to be followed by meiosis I and then meiosis II that take place at stage XIV of the epithelial cycle in rat testes to generate round spermatids (or step 1 spermatid) (haploid, 1n) (Lie et al., 2009) (Fig. 7.2). Recent studies have shown that the transit of primary spermatocytes at the BTB that takes place at stages VIII–IX of the epithelial cycle is modulated, at least in part, by mitogen-activated protein kinase (MAPK) (e.g., p38 MAPK and extracellular signal-regulated kinase 1/2, ERK1/2) (Li et al., 2006; Lui et al., 2003d; Wong et al., 2004; Xia et al., 2006), which also regulates the “opening” and “closing” of the BTB downstream of cytokines (e.g., TGF-β3 and TNFα) by determining the steady-state level of integral membrane proteins at the BTB (Lui et al., 2001; Siu et al., 2003). ERK1/2 that found in pachytene spermatocytes were also shown to regulate chromatin condensation (Di Agostino et al., 2002; Inselman and Handel, 2004). Collectively, these findings illustrate that the events of primary spermatocytes in transit at the BTB and the preparation of spermatocytes for cell-cycle progression (e.g., chromatin condensation) can be coordinated by MAPK found in Sertoli and germ cells in the microenvironment of the seminiferous epithelium at or near the BTB. These findings also suggest that the cellular events that take place locally in the seminiferous epithelium are highly coordinated, such as by MAPK.
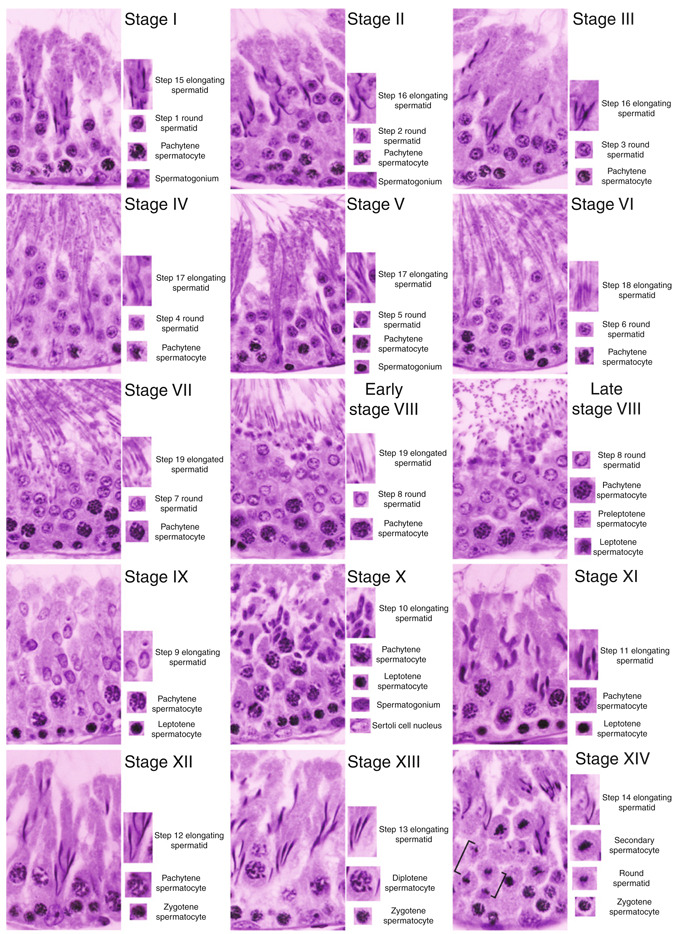
Figure 7.2.
Different stages of the seminiferous epithelial cycle of spermatogenesis in adult rat testes. Stages I through XIV of the epithelial cycle in adult rat testes are shown. The typical germ cells at different stages of their development that are found in specific stage of the epithelial cycle are shown on the right panel of the corresponding micrographs. For instance, both meiosis I and II take place in Stage XIV in rat testes, so that secondary spermatocytes following meiosis I and round spermatids (step 1) frommeiosis II following telephase II (see square brackets) are clearly visible.
Once meiosis completes, spermatids (haploid, 1n) undergo a series of well-defined morphological changes from step 1 through step 19 spermatids in rat testes in a process known as spermiogenesis, which is typified by the condensation of the genetic material into spermatid nucleus in the head region, development of the acrosome above the sperm head, packaging of the mitochondria into the mitochondrial sheath that constitutes the spermatid midpiece and elongation of the tail, as well as formation of the residual body to be phagocytosed by the Sertoli cell at spermiation (Mruk et al., 2008). At present, the biochemical and/or molecular mechanism(s) that regulate spermiogenesis and/or spermiation remain obscure. Recent studies, however, have shown that gonadotropins (e.g., FSH) are involved in spermiation in men (Matthiesson et al., 2006). Once the fully developed elongated spermatids (step 19) line up at the adluminal edge of the epithelium at stage VIII of the epithelial cycle, spermiation takes place in which the adhesion protein complex between step 19 spermatids and the Sertoli cell is disrupted, so that spermatozoa can enter the seminiferous tubule lumen and be transported to the epididymis for their subsequent maturation. During spermiogenesis and spermiation, a cellular phenomenon pertinent to these events that takes place in the seminiferous epithelium has been largely unexplored for decades: the proper orientation of developing spermatids. It is noted that the heads of developing spermatids are pointing toward the basement membrane in all stages of the epithelial cycle from steps 1 through 19 spermatids. It has been postulated that apical ectoplasmic specialization (ES), a testis-specific atypical adherens junction (AJ) type, confers spermatid orientation during spermiogenesis (Vogl et al., 1993, 2008; Wong et al., 2008a). However, other studies have shown that tight junction (TJ) is the cell junction type that confers cell polarity and/or orientation in epithelial cells (Cereijido et al., 2004, 2008). Thus, it is not known how ES confers spermatid polarity in the testis since apical ES at the Sertoli–spermatid interface is a putative anchoring junction type and the only anchoring device between Sertoli cells and spermatids (step 8 through step 19 spermatids). Furthermore, spermiation that takes place at stage VIII of the epithelial cycle is concomitant with the event of BTB restructuring to facilitate the transit of primary spermatocytes across the BTB and the preparation of these germ cells for metaphase I. Interestingly, these three cellular events, namely (i) spermiation, (ii) BTB restructuring, and (iii) germ cell-cycle progression, take place at the opposite ends of the Sertoli cell epithelium. Thus, it seems logical to speculate that there is a local regulatory loop in the seminiferous epithelium to coordinate these cellular events during the epithelial cycle.
In this chapter, we discuss recent findings in the field regarding the role of polarity proteins, such as partitioning-defective3/partitioning-defective6/ atypical protein kinase C (Par3/Par6/aPKC) protein complex, which are the integrated components of the apical ES in conferring spermatid polarity during spermiogenesis besides its involvement in regulating spermatid adhesion. In addition, the small Rho GTPase Cdc42 (Cell division cycle 42), which was first identified as an essential gene for the asymmetric cell division in budding yeast, is found to interact with the Par3/Par6/aPKC protein complex to regulate polarity in mammalian cells. Besides its regulation via the Par6-based protein complex, Cdc42 regulates cell adhesion and junction function through regulating filopodia formation, protein trafficking, and actin cytoskeleton. Furthermore, some of the polarity proteins that are integrated components of the BTB were shown to regulate Sertoli cell adhesion at the BTB via their effects on the kinetics of endocytic vesicle-mediated protein endocytosis, and served as molecular switches to coordinate spermiation and BTB restructuring. Additionally, there are recent findings in the field that illustrate proteins in the basement membrane, such as at the Sertoli cell–extracellular matrix (ECM) interface (e.g., integrins at the hemidesmosome), can also provide a feedback regulatory mechanism to modulate the BTB integrity. In addition, it is apparent that proteins released at spermiation (such as fragments of the laminin–integrin-based adhesion complex at the apical ES) are working in concert with polarity proteins to regulate BTB directly and indirectly via hemidesmosome. In short, based on recently published findings in the field, we report herein the presence of a local apical ES-BTB-basement membrane functional axis that is mediated by polarity proteins to coordinate the events of junction restructuring in the seminiferous epithelium during spermatogenesis. While much work is needed to design functional experiments to tackle the precise roles of this functional axis, we provide a working biochemical model for investigators in the field which serves as a framework for future investigation in the next decade.
2. Rho GTPases and Cell–Cell Interactions in the Testis: Cdc42
Cdc42 (~22 kDa) is one of the best characterized members of the Rho GTPases which belong to the Ras-related small GTPases superfamily (Boureux et al., 2007; Jaffe and Hall, 2005; Wennerberg and Der, 2004). Rho GTPases comprise a family of at least 25 members (Wennerberg and Der, 2004) which are known to regulate a diverse array of cellular processes including cell adhesion and cell movement in epithelia and/or endothelia including the seminiferous epithelium (Huveneers and Danen, 2009; Lui et al., 2003a; Samarin and Nusrat, 2009; Takai et al., 2001). While there are studies in the literature illustrating the role of other Ras superfamily members such as Rab GTPases (e.g., Rab4A, Rab8B) in regulating protein–protein interactions in the testis (Lau and Mruk, 2003; Mruk et al., 2007), most functional studies were performed on Rho GTPases (Hall et al., 1993; Leung et al., 1993; Lui et al., 2003b, 2005; Toure et al., 1998, 2001) in particular Cdc42 which was shown to control multiple cellular functions in different epithelial cells as illustrated in Table 7.1. Thus, we focus our discussion on Cdc42 herein since a survey on other GTPases pertinent to cell–cell interactions in the testis can be found in recent reviews in the field (Mruk and Cheng, 2004; Mruk et al., 2008).
Table 7.1.
Phenotypes of altering Cdc42 activity in different cell and model systems
| Cell/model system | Method of altering dc42 activitya |
Phenotypes | References |
|---|---|---|---|
| MDCKb | CA | ↑Actin, E-cadherin, and β-catenin at cell–cell adhesion site | Kodama et al. (1999) |
| Cells are tightly contacted with each other at the lateral surface | |||
| Protects cells from hepatocyte growth factor (HGF) and 12-O- tetradecanoylphorbol-13-acetate (TPA)-induced junction disruption |
|||
| Caco-2 | DN | Disrupts E-cadherin and F-actin after calcium switch | Otani et al. (2006) |
| MDCK | NWASP–CRIB is overexpressed to inhibit Cdc42 |
↓Afadin, E-cadherin, and claudin-1 at the cell–cell adhesion sites after calcium switch |
Fukuhara et al.(2003) |
| ↑ Afadin, E-cadherin, and claudin-1 at the cell–cell adhesion sites after calcium switch |
|||
| CA | Induces formation of filopodia | ||
| HeLa | CA | Induces cell–cell contact rich in actin, β-catenin, and N-cadherin |
Stoffler et al. (1998) |
| MDCK | DA, DN | Apical localization of transmembrane basolateral markers vesicular stomatitis virus G protein (VSVG) and low-density lipoprotein receptor (LDLR) |
Cohen et al. (2001) |
| DA | Basolateral membrane protein NaK-ATPase found at the apical surface |
||
| MDCK | CA, DN | ↑ Exit of p75 (apical) from trans-Golgi network (TGN) | Musch et al. (2001) |
| ↓Exit of LDLR and neuronal-cell adhesion molecule (NCAM) (basolateral) |
|||
| CA | ↓Perinuclear/cytoplasmic actin but ↑ cortical actin | ||
| MDCK | CA | ↓Endocytic and biosynthetic traffic | Rojas et al. (2001) |
| Occludin found at lateral membrane | |||
| Extended distribution of junctions | |||
| ↑ Cortical actin | |||
| ↓Transepithelial electrical resistance (TER) | |||
| ↑ Paracellular diffusion of radiolabeled inulin, IgA, and transferrin |
|||
| DN | ↑ Cortical actin | ||
| ↓TER | |||
| ↓Apical endocytosis and transcytosis | |||
| ↑ Biosynthetic traffic | |||
| MDCK | DN | Mistargeting of basolateral proteins VSVG and gp58 | Kroschewski et al. (1999) |
| Disrupts recycling of gp58 | |||
| CA | Disrupts ZO-1 distribution at cell–cell interface | ||
| Cell free endocytosis assay system |
Addition of recombinant Rho GDI to inactivate Cdc42 |
↑ Endocytosis of E-cadherin | Izumi et al. (2004) |
| CHO | DN | Disrupts perinuclear clustering of endocytic recyling compartment |
Balklava et al. (2007) |
| HeLa | DN | ↓Recycling of clathrin-independent cargo | |
| ↓Endocytosis of clathrin-dependent cargo | |||
| MCF-7 | CA | ↑ Ubiquitination and degradation of E-cadherin after depletion of calcium |
Shen et al. (2008b) |
| ↑ Filopodia formation | |||
| Cdc42 si | Blocks E-cadherin degradation induced by the removal of calcium |
||
| Sertoli cells | DN | Inhibits TGF-β3-mediated enhancement in protein endocytosis | Wong et al. (2009b) |
Overexpression of constitutively active (CA) or dominant negative (DN) mutant of Cdc42 is commonly used to manipulate Cdc42 activity. In addition, overexpression of Cdc42 and Rac interactive binding (CRIB) domain of neural Wiskott–Aldrich syndrome protein (NWASP) or silencing of Cdc42 (Cdc42 si) is also being used to study the function of Cdc42. ↑, stimulation; ↓, inhibition.
MDCK cells, Madin-Darby Canine Kidney epithelial cells; CHO cells, Chinese Hamster Ovary cells; HeLa cells, cervical cancer cells derived from Henrietta Lacks.
Classical Rho GTPases such as RhoA, Rac1, and Cdc42 contain a Rho-type GTPase-like domain which allows them to shuttle between an active GTP-bound state and inactive GDP-bound state ( Jaffe and Hall, 2005; Valencia et al., 1991; Wennerberg and Der, 2004) (Fig. 7.3). Three classes of proteins are present to regulate the activity of classical Rho GTPases including Cdc42: (1) guanine nucleotide exchange factors (GEFs) which promote the exchange of GDP for GTP to activate Rho GTPases (Garcia-Mata and Burridge, 2007; Rossman et al., 2005); (2) GTPase-activating proteins (GAPs) which enhance the intrinsic GTPase activity of Rho GTPases to inactivate them (Moon and Zheng, 2003; Tcherkezian and Lamarche-Vane, 2007); and (3) guanine nucleotide dissociation inhibitors (GDIs) which functions to prevent the dissociation of GDP from Rho GTPases and sequester them from effector targets (DerMardirossian and Bokoch, 2005) (Fig. 7.3). Upon activation, Rho GTPases undergo conformational changes resulting in an increased affinity for downstream effector proteins which stimulate various cellular processes such as actin dynamics, gene expression, cell-cycle progression, cell migration, and cell adhesion (Heasman and Ridley, 2008; Jaffe and Hall, 2005; Vetter and Wittinghofer, 2001). For instance, it was illustrated that the dynamic interactions between Cdc42, its effector IQ motif containing GTPase activating protein 1 (IQGAP1) and β-catenin played a crucial role in conferring the N-cadherin-based cell adhesion function between Sertoli and germ cells in the testis (Lui et al., 2005). In Sertoli–germ cell cocultures, it was shown that the assembly of stable anchoring junctions between these cells was associated with an increase in Cdc42–IQGAP1 interaction. However, a loss of Sertoli–germ cell anchoring junction adhesion induced by the depletion of calcium in the culture media was shown to cause a loss of Cdc42–IQGAP1 association. Instead, IQGAP1 associates more with catenins, decreasing the pool of catenins associating with the actin-based cell-cell AJ. Thus, this leads to a loss of cadherin-based germ cell adhesion to Sertoli cells (Lui et al., 2005).
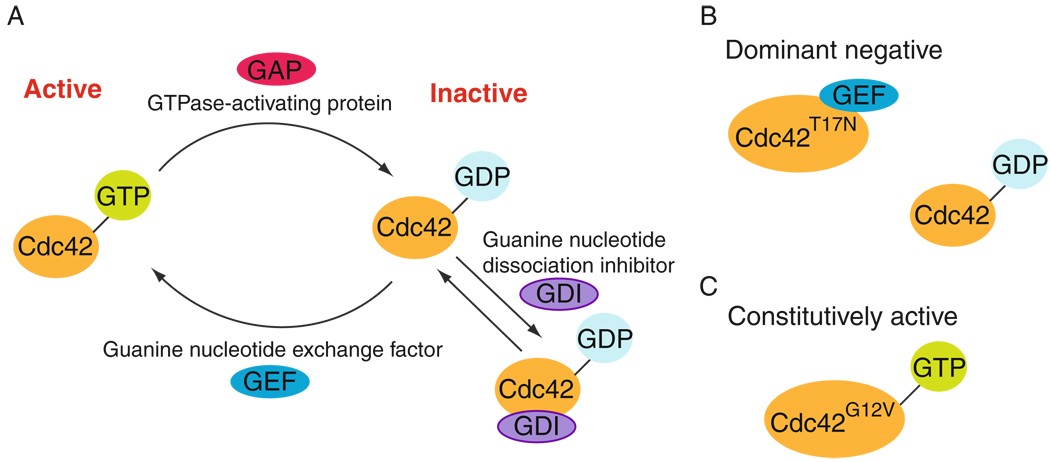
Figure 7.3.
The cycling of Cdc42 GTPase between GTP- (active) and GDP-bound (inactive) state. (A) The activation of Cdc42 GTPase involves the exchange of GDP for GTP via phosphorylation, which is stimulated by GEF (guanine nucleotide exchange factor), leading to an increase in affinity of activated Cdc42 for its effector to stimulate downstream signaling functions. Activated Cdc42 GTPase can be inactivated by its binding with GTPase-activating protein (GAP), leading to dephosphorylation, and a shutdown of the signaling function. The release of GDP from the Cdc42 GTPase is blocked by guanine nucleotide dissociation inhibitor (GDI). The GDI-bound Cdc42 GTPase is sequestered in the cytosol. (B) This illustrates a dominant-negative form of Cdc42 in which the threonine (Thr, T) in residue 17 from the N-terminus is mutated to asparagine (Asn, N), which allows binding of GEF but not effectors. Thus, GEF is sequestered by the dominant-negative form and this prevents endogenous Cdc42 from activating by GEF (Bollag and McCormick, 1991; Heasman and Ridley, 2008). (C) This is the constitutively active form of Cdc42 wherein the glycine (Gly, G) in residue 12 from the N-terminus is mutated to valine (Val, V), which is defective in GTPase activity, thus it cannot be dephosphorylated (i.e., inactivated) but remains phosphorylated (activated). Other common constitutively active mutants include mutation at residue 18 from phenylalanine (Phe, F) to leucine (Leu, L) and mutation at residue 61 from glutamine (Gln, Q) to leucine (Leu, L) (Bollag and McCormick, 1991; Heasman and Ridley, 2008).
Besides switching between GTP- and GDP-bound forms, Rho GTPases can also be regulated by ubiquitination (Asanuma et al., 2006) and phosphorylation (Loirand et al., 2006; Tu et al., 2003). On the other hand, nonclassical/atypical Rho GTPases such as RhoH is constitutively bound to GTP but lacking GTPase activity (Chardin, 2006; Jaffe and Hall, 2005; Wennerberg and Der, 2004). Thus, this subgroup of Rho GTPases is not regulated by GAPs, GEFs, or GDIs. Instead, they are regulated through gene expression, phosphorylation, and ubiquitin/proteasome-mediated degradation (Chardin, 2006). The roles of these atypical Rho GTPases in cell–cell interactions in the testis remain to be investigated. In the following sections, we discuss some of the biological effects of Cdc42 on regulating cell–cell interaction and adhesion, in the seminiferous epithelium and other epithelia, highlighting areas of research that deserve attention in future studies.
2.1. Mediating the action of transforming growth factor-βs (e.g., TGF-β3) in the seminiferous epithelium of adult rat testes
Recent studies have illustrated the crucial role of Cdc42 in mediating the action of transforming growth factor-β (TGF-β) in the testis regarding its effects on junction dynamics in the seminiferous epithelium during spermatogenesis via cross talk with other signaling molecules (Fig. 7.4). Figure 7.4 depicts the three signaling pathways downstream of TGF-βs following the activation of TGF-β2 or TGF-β3 with their receptors at the Sertoli cell membrane. For instance, using the cadmium model to study cell-cell interactions in the testis which is known to induce cell adhesion disruption in the seminiferous epithelium in particular at the Sertoli-Sertoli (e.g., the BTB) and Sertoli-germ cell (e.g., the apical ES) interface (Wong et al., 2004); it has been shown that treatment of rats with cadmium chloride can induce TGF-β3 (Wong et al., 2005). It also activates the Cdc42/JNK pathway downstream of TGF-β3 which leads to an increase in the steady-state level of α2-macroglobulin (a non-specific protease inhibitor) in the seminiferous epithelium, limiting unwanted proteolysis following a surge of protease activities (e.g., cathepsin l) (Wong et al., 2005). This pathway perhaps is also needed to regulate phagocytosis of residual bodies and/or apoptotic germ cells by the Sertoli cell to limit unwanted proteolysis in the seminiferous epithelium, illustrating the significance of Cdc42 in these events. It was also reported that an activation of ERK1/2 via the action of TGF-β3 would limit the anchoring junction restructuring at the Sertoli–germ cell interface, inducing changes in Sertoli–germ cell interactions, without affecting the BTB integrity (Xia and Cheng, 2005; Xia et al., 2006). But since there is cross talk between Cdc42 and Ras (Fig. 7.4) (for a review, see Boutros et al., 2008), Cdc42 can thus mediate the TGF-β3-induced ERK1/2 activation to affect germ cell adhesion in the seminiferous epithelium. Additionally, TGF-β3 was also shown to regulate BTB dynamics and germ cell adhesion via an activation of p38 MAPK (Fig. 7.4) and via the cross talk between Cdc42/Rac, Ras, and MEKK1–4 (Boutros et al., 2008) (Fig. 7.4), Cdc42 thus also plays a role in regulating BTB and anchoring junction dynamics (Lui et al., 2003c; Xia et al., 2006). Taken collectively, the findings summarized in Fig. 7.4 illustrate the critical role of Cdc42 in mediating TGF-β-based actions and other cellular events in the testis via its direct effects on JNK and/or indirect effects on ERK1/2 and p38 MAPK via cross talk with upstream MEKK1–4, MEK1/2, and MKK3/6 (Fig. 7.4) (Boutros et al., 2008).
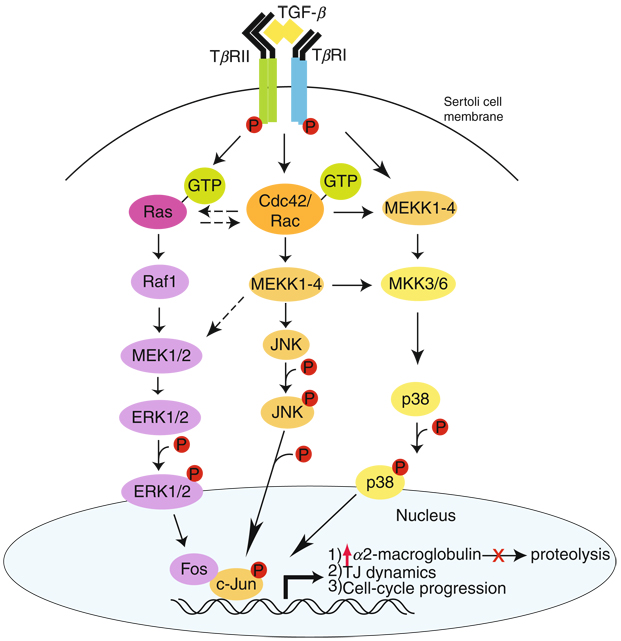
Figure 7.4.
The TGF-β-mediated signaling function in epithelia including the seminiferous epithelium of adult rat testes. Based on recent studies in the field, TGF-βs are known to regulate different cellular functions in the testis following activation of their receptors via ligand-receptor binding, such as for cell cycle progression in germ cells, TJ dynamics, and α2-macroglobulin production, via ERK, p38 MAP or JNK (black arrows). This schematic diagram illustrates the significance of Cdc42 in TGF-βs-mediated signaling function since this GTPase regulates not only the JNK signaling pathway downstream, also the ERK1/2 and p38 MAPK pathways via its cross talk with Ras and MEK1/2 (hatched arrows) or its direct effects on MEKK1–4 and MKK3/6 (black arrows).
2.2. Regulation of filopodia formation
Among the numerous cellular processes that Cdc42 regulates, actin cytoskeleton dynamic and filopodia formation are the best documented. Filopodia are thin, finger-like cytoplasmic protrusions which contain parallel bundles of filamentous-(F-) actin. They are found at the leading edge of migrating cells such as fibroblasts and tumor cells and have important implication in wound healing and formation of cell–cell contact (Chhabra and Higgs, 2007; Gupton and Gertler, 2007; Mattila and Lappalainen, 2008). Activated Cdc42 stimulates the initiation and elongation of actin filament and causes the extension of filopodia. These protruded filopodia, in turn, align opposing cells and adhere them together, leading to the formation of cell junctions (Chhabra and Higgs, 2007; Gupton and Gertler, 2007; Mattila and Lappalainen, 2008; Vasioukhin et al., 2000). This suggests that activation of Cdc42 might enhance cell–cell adhesion via increasing the number of filopodia. Consistent with this postulation, several studies have shown that overexpression of a constitutively active mutant of Cdc42 induces cell–cell contact and strengthens cell–cell adhesion (Kodama et al., 1999; Rojas et al., 2001; Stoffler et al., 1998). In addition, by using electron microscopy, these cells have been shown to have extended cell junctions with the cell membrane fused along the lateral borders (Kodama et al., 1999; Rojas et al., 2001). It has been suggested that Cdc42 affects the rate of AJ and TJ formation based on studies using the calcium switch model (Fukuhara et al., 2003). Induced activation of Cdc42 increases the velocities of junction formation after calcium switch. Conversely, specific inhibition of Cdc42 by overexpressing the Cdc42 and Rac interactive binding (CRIB) domain of neural Wiskott–Aldrich syndrome protein (NWASP) (NWASP–CRIB) (Takenawa and Miki, 2001) delays AJ and TJ formation (Fukuhara et al., 2003). Besides, activation of Cdc42 protects cells from hepatocyte growth factor (HGF) and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced junction disruption (Kodama et al., 1999). Collectively, these results show that activation of Cdc42 facilitates the formation of cell–cell adhesion. Interestingly, it has been reported that higher amount of activated Cdc42 is required for the assembly of claudin-based TJ than that of E-cadherin-based AJ (Fukuhara et al., 2003). A possible explanation is that low level of activated Cdc42 is able to recruit AJ effectors, which has a lower affinity for activated Cdc42 (Garrard et al., 2003). Subsequently, as mature AJ forms, more Cdc42 is activated by AJ proteins such as nectins (Honda et al., 2003; Kawakatsu et al., 2002) which is required to recruit TJ effectors for TJ assembly (Fukuhara et al., 2003). In the seminiferous epithelium of rat testes, filopodia per se are not visible in Sertoli cells even though isolated Sertoli cells in culture are highly motile cells and are capable of migrating across the pores of the bicameral units (Mruk et al., 1997, 2003). They also have “finger-like” structures which resemble filopodia when Sertoli cells are cultured in vitro (Lee and Cheng, 2003; Siu et al., 2005). Nevertheless, Sertoli cells in vivo form “finger-like” cytoplasmic processes and/or structures which “hold” up to 30–50 germ cells to support their development at various stages (Weber et al., 1983). It is highly likely that Cdc42 regulates the formation of these “finger-like” structures in the seminiferous epithelium in vivo which are similar to filopodia in other epithelia. This postulation is supported by recent studies using immunohistochemistry which shows that Cdc42 is localized in the entire seminiferous epithelium illustrating its possible involvement in new junction assembly between Sertoli cells and developing germ cells (Wong et al., 2009b). However, Cdc42 is predominantly localized at the BTB in virtually all stages of the epithelial cycle except at stage VIII when BTB undergoes restructuring to facilitate the transit of primary preleptotene spermatocytes (Wong et al., 2009b). Work is needed in future studies to assess if component proteins of the filopodia, such as enabled/vasodilator-stimulated phosphoprotein (ENA/VASP) family proteins (Mattila and Lappalainen, 2008), are found in the seminiferous epithelium, and if Cdc42 regulates their function.
2.3. Cdc42 in protein trafficking
Epithelial cells, including Sertoli cells in the testes, are polarized cells with a differential distribution of plasma membrane proteins and macromolecules, which, in turn, are separated into the apical and basolateral domains by the TJs. The presence of TJs acts as a barrier to prevent the free diffusion of the plasma membrane components between the apical and basolateral domains (Shin et al., 2006). Therefore, the establishment and maintenance of cell polarity and cell junctions are intimately related. On the other hand, polarized distribution of plasma membrane components is highly regulated by the biosynthetic, endocytic, recycling, and transcytotic mechanisms in cells (Duffield et al., 2008; Mellman and Nelson, 2008; Zahraoui et al., 2000). The involvement of Cdc42 in cell polarity and protein trafficking was initially reported in yeast. For instance, Cdc42 mutants are defective in budding. Instead, they continue to grow into large spherical cells which failed to display polarized protein secretion and asymmetric distribution of actin cytoskeleton which are necessary for budding (Adams et al., 1990). Subsequent studies in mammalian cells revealed that Cdc42 also plays a central role in establishing cell polarity and directed protein trafficking (Cerione, 2004; Etienne-Manneville, 2004; Jaffe and Hall, 2005). Membranous or secretory proteins are budded into secretory vesicles from the trans-Golgi network (TGN). Initial protein sorting is carried out in the TGN and common recycling endosomes (Ang et al., 2004; Rodriguez-Boulan et al., 2005). Proteins which are targeted to the apical or basolateral domains are exocytosed to the cell surface. Exocyst complex, which is composed of eight evolutionarily conserved proteins, is present to tether, dock, and fuse the post-Golgi secretory vesicles at the specific sites in the plasma membrane (Wu et al., 2008). Transcytosis also plays a significant role in mediating the transport of proteins between different compartments of the cell (Leyt et al., 2007; Polishchuk et al., 2004; Tuma and Hubbard, 2003). At the same time, proteins are continuously endocytosed and recycled back to the plasma membrane (Doherty and McMahon, 2009).
In 1999, Mellman and colleagues reported that Cdc42 is functionally linked to protein trafficking in mammalian epithelia cells. In particular, it is essential to target newly synthesized proteins to the basolateral region of cells. At the same time, recycling of basolateral proteins is also controlled by Cdc42 (Kroschewski et al., 1999). Subsequent studies confirmed Cdc42’s role in regulating the basolateral protein trafficking. It was found that constitutive activation or inactivation of Cdc42 leads to the mislocalization of basolateral membrane markers vesicular stomatitis virus G protein (VSVG) and low-density lipoprotein receptor (LDLR) to the apical membrane. However, the apical distribution of p75 membrane marker remains unchanged. Interestingly, activation state of Cdc42 seems to have no effect on the targeting of secretory proteins to apical (gp80/glucosaminoglycans) or basolateral (laminin/ heparan sulfate proteoglycan) domains (Cohen et al., 2001). Further investigation demonstrates that Cdc42 differentially affects the rate of release of apical and basolateral proteins from the TGN. For instance, exit of apical protein p75 from the TGN is increased while the release of basolateral proteins LDLR or neuronal-cell adhesion molecule (N-CAM) is inhibited after the overexpression of dominant-active or -inactive Cdc42. These observations are possibly due to the reorganization of perinuclear/cytoplasmic actin to the cortical region of the cells, mediated by the activation of Cdc42 (Musch et al., 2001). Cdc42 is also involved in spatiotemporal activation of the exocyst complex which is required for the docking and fusion of vesicles to the plasma membrane (Wu et al., 2008). It was shown that GTP-bound Cdc42 together with phosphatidylinositol 4,5-bisphosphate recruited Sec3, one of the protein subunits of the exocyst complex, to the site of polarized growth in yeast. Recruitment of Sec3 to the bud site, in turn, activates the exocyst complex to increase the rate of polarized secretion of proteins for budding (Zhang et al., 2001, 2008). A recent paper by Sakurai-Yageta et al. (2008) demonstrates that activation of Cdc42 induces the interaction between its downstream effector IQGAP1 and Sec3/Sec8 exocyst subunits in breast adenocarcinoma cells. This interaction is necessary for the exocytosis of membrane type 1-metalloproteinase (MT1-MMP) to degrade ECM which promotes the invasive characteristic of tumor cells.
Endocytosis is another event of vesicular trafficking which is tightly regulated by Cdc42. In Drosophila neuroectodermal epithelium, Cdc42 regulates vesicular trafficking by inhibiting the endocytosis of apical proteins from the plasma membrane. Overexpression of dominant-negative Cdc42 results in an enhanced endocytic uptake of AJ proteins such as Drosophila epithelial-cadherin (DE-cadherin) and Armadillo (Drosophila β-catenin, Arm) as well as apical polarity proteins such as Crumbs (Crb). The endocytosed apical proteins are found accumulated in enlarged endosomal compartments, illustrating that inhibition of Cdc42 activity also blocks the transport of apical proteins from early to late endosomes (Harris and Tepass, 2008). Interestingly, regulation of endocytosis by Cdc42 is reversed in the Drosophila dorsal thorax epithelium where Cdc42 promotes endocytosis (Georgiou et al., 2008; Leibfried et al., 2008). Both studies show that deletion of Cdc42 blocks endocytosis. Disruption in the localization of E-cadherin and Arm was observed (Georgiou et al., 2008; Leibfried et al., 2008) but not basolateral marker lethal giant larvae (Lgl) (Leibfried et al., 2008). Studies by electron and fluorescent microscopy revealed the presence of elongated tubular extensions from the plasma membrane. Furthermore, when stained with an antibody against the extracellular domain of E-cadherin in fixed but nonpermeabilized cells, these tubular structures were shown to contain cell surface E-cadherin, indicating a defect in vesicle scission from the plasma membrane during endocytosis (Georgiou et al., 2008; Leibfried et al., 2008). The observed discrepancies in endocytosis regulation might be ascribed to the fact that Cdc42 works together with Bazooka (Drosophila Par3) to regulate endocytosis in neuroectoderm (Harris and Tepass, 2008) while this regulation is independent of Bazooka in dorsal thorax epithelium (Georgiou et al., 2008; Leibfried et al., 2008).
In a genome-wide search for genes regulating endocytosis, Cdc42 as well as Par6 are found to be two conserved endocytic regulators in Caenorhabditis elegans and mammalian cells. Further analysis shows that the blockade in endocytosis by expressing dominant-negative mutants of Par6 or Cdc42 is likely due to the disruption of recycling endosomes. In addition, Par6 and Cdc42 differentially regulate the uptake and recycling of clathrin-independent or clathrin-dependent cargo proteins, illustrating that clathrin also actively participates in this Par6/Cdc42-mediated endocytosis (Balklava et al., 2007). By using a cell-free endocytosis assay system, it has been reported that trans-interaction of E-cadherin activates Cdc42 which, in turn, inhibits the endocytosis of trans-interacting E-cadherin via the F-actin linking activity of IQGAP1 (Izumi et al., 2004). AJ-enriched membrane fraction from liver is used in the cell-free assay system to study endocytosis, instead of the traditionally used biotinylation of cell surface proteins (Le et al., 1999) or labeling of cell surface protein by using antibody which targets the extracellular domain of protein (Georgiou et al., 2008). Although it was found that TJ proteins such as claudin-1 and occludin are not endocytosed in the cell-free assay system, which is in contrast to recently published reports which show that TJ proteins are continuously endocytosed (Matsuda et al., 2004; Shen et al., 2008a), this assay still is a valuable tool which provides easy manipulation to characterize individual factors which are involved in endocytosis. A recent report has also demonstrated the role of Cdc42 in mediating cytokine-induced acceleration in protein endocytosis at the Sertoli cell BTB (Wong et al., 2009b). For instance, overexpression of dominant-negative Cdc42 in Sertoli cells with an established TJ-permeability barrier was shown to abolish the TGF-β3-mediated acceleration of protein endocytosis, such as occludin (Wong et al., 2009b).
2.4. Effects on actin cytoskeleton
Cdc42 is a well-known actin regulator. Cdc42 regulates actin polymerization via: (i) WASPs/actin-related protein2/3(Arp2/3), (ii) formins/mammalian diaphanous (mDia), and (iii) LIM kinase (LIMK)/Rho kinase (ROCK)/cofilin ( Jaffe and Hall, 2005; Ridley, 2006). Since several recent reviews and/or reports are in the literature including studies in the testes which discuss the regulation of actin by Cdc42 via these protein complexes (Heasman and Ridley, 2008; Jaffe and Hall, 2005; Lui et al., 2003a,b; Ridley, 2006; Witte and Bradke, 2008), we focus on highlighting how Cdc42 affects cell junctions and vesicular trafficking via its effects on actin dynamics.
Actin cytoskeleton is involved in regulating multiple events in vesicular formation and transport. It is involved in the budding and scission of vesicles from both the TGN (exocytic vesicles) and plasma membrane (endocytic vesicles) (Merrifield et al., 2002). It also facilitates the docking and fusion of secretory vesicles to the plasma membrane, especially to the basolateral membrane (Rodriguez-Boulan et al., 2005). Consistent with this functional role, Cdc42 is known to regulate exocytosis to the basolateral region (Cohen et al., 2001; Kroschewski et al., 1999). Finally, it also serves as the track for myosin-driven vesicles to move within the cell. In addition, it is known that Cdc42 regulates endocytosis in the dorsal thorax epithelium of Drosophila pupae via the WASP/Arp2/3 and dynamin (Georgiou et al., 2008; Leibfried et al., 2008). Collectively, these studies illustrate how Cdc42 regulates vesicle scission and trafficking via its effects on actin dynamics.
Interestingly, it was found that Cdc42 regulates cell tension and cell shape by altering the distribution of actin (Musch et al., 2001; Otani et al., 2006) and E-cadherin (Otani et al., 2006). Activation of Cdc42 causes redistribution of actin from the perinuclear/cytoplasmic region to the cortical region, leading to the rounding of cells (Musch et al., 2001). Similarly, Tuba, a Cdc42-specific GEF, also helps in maintaining the normal tension in cells by activating Cdc42 which, in turn, regulates N-WASP to control the distribution of actin and E-cadherin (Otani et al., 2006).
2.5. Ubiquitination of junction proteins
Ubiquitination is an important mechanism to control the homeostasis of transmembrane proteins in epithelia by regulating protein endocytosis and degradation in the lysosomes (d’Azzo et al., 2005; Lui and Cheng, 2007; Reyes-Turcu et al., 2009). Through a three-step enzymatic reaction, which is carried out by (1) ubiquitin-activating enzyme (E1), (2) ubiquitinconjugating enzyme (E2), and (3) ubiquitin ligase (E3), ubiquitin, a small globular protein is added onto the target protein (monoubiquitination). More ubiquitin proteins can be conjugated onto existing ubiquitin to form a polyubiquitin chains on the target protein (polyubiquitination). Apart from degradation of misfolded proteins, recent studies have shown that ubiquitination, particularly monoubiquitination, is also involved in regulating the homeostasis of normal cellular proteins via protein trafficking of endocytosed proteins since ubiquitinated proteins can be recycled back to cell surface via the endosome-mediated sorting mechanism (Berthouze et al., 2009; d’Azzo et al., 2005;Haugsten et al., 2008;Huang et al., 2009). Ubiquitinated proteins can be recognized by downstream effector proteins containing ubiquitin-binding domains which, in turn, activate protein endocytosis and degradation (Chen and Sun, 2009; Hicke and Dunn, 2003; Pickart and Fushman, 2004). For instance, it is known that Hakai, a c-Cbl like E3 ubiquitin ligase, ubiquitinates E-cadherin and leads to its endocytosis and degradation (Fujita et al., 2002). Subsequent study in MDCK cells reveals that Cdc42 is one of the upstream signaling molecules regulating the ubiquitination of E-cadherin via Hakai (Shen et al., 2008b). By depleting the calcium level in the culture environment, it causes the endocytosis and degradation of E-cadherin. It was found that Cdc42 was first activated after calcium depletion. Cdc42 activation, in turn, stimulated the epidermal growth factor receptor (EGFR) signaling pathway, which phosphorylated Src kinase and E-cadherin, leading to the binding of Hakai. Instead of recycling back to cell surface, E-cadherin was shown to be targeted to the lysosomes for degradation (Shen et al., 2008b). Since recent studies have shown that cytokine-induced transient BTB disruption, such as by TGF-β2, TGF-β3, and TNFα, is mediated via enhanced endocytosis of integral membrane proteins at the BTB (Xia et al., 2009; Yan et al., 2008a), it remains to be determined if these endocytosed proteins are ubiquitinated, perhaps mediated by Cdc42, so that they can be targeted to late endosome for intracellular degradation, thereby compromising the BTB integrity.
2.6. Assembly and maintenance of epithelial apico-basal cell polarity
The roles of Rho GTPases, in particular Cdc42, in the formation and maintenance of apico-basal cell polarity in epithelia by working in concert with the Par-based polarity protein complex has been intensively investigated in recent years (Iden and Collard, 2008; Yamada and Nelson, 2007). Mammalian Par3/Par6/aPKC complex binds to activated Cdc42 via the semi-CRIB domain in Par6 ( Joberty et al., 2000; Lin et al., 2000). Binding of activated Cdc42 to Par6 induces a conformational change in the C-terminal postsynaptic density-95/disks large/zonula occludens-1 (PDZ) domain of Par6, increasing its affinity for downstream mediators (Garrard et al., 2003; Peterson et al., 2004). For instance, binding of GTP-bound Cdc42 to Par6 enhances the kinase activity of aPKC (Yamanaka et al., 2001). This event can activate the Crumbs- and Scribble-based polarity complexes, recruiting them to the TJ site and also phosphorylating downstream target proteins, whose identities remain unknown to date (Iden and Collard, 2008). This process, however, leads to polarization and maturation of the epithelium into fully polarized epithelium. It remains to be determined the target proteins downstream of the Cdc42/Par3/Par6/aPKC protein complex in the Sertoli cell that help to cause cell polarization in the seminiferous epithelium. However, recent studies have shown that JAMs and Src kinases are likely involved in this event (Wong et al., 2008b).
3. Polarity Proteins and Cell–Cell Interactions in the Testis
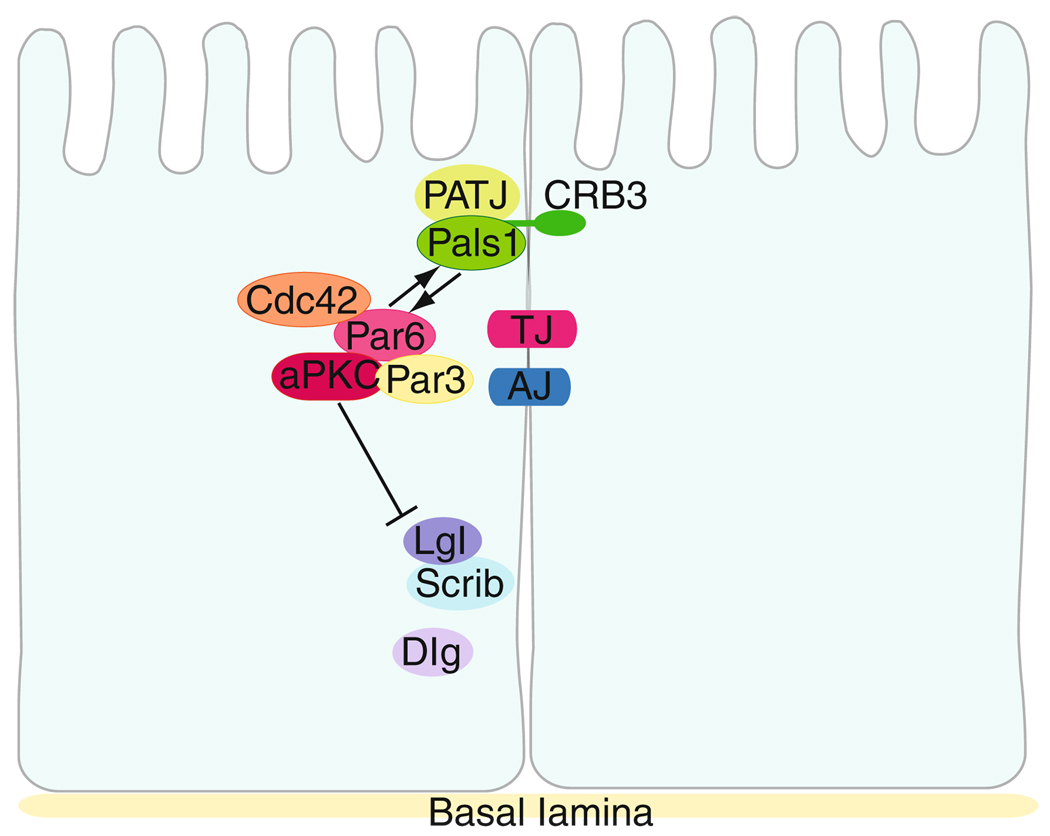
In epithelia, including the seminiferous epithelium of adult mammalian testes, the differential distribution of cellular proteins and macromolecules along the apical and basolateral membrane domains, which is maintained by the “fence function” conferred by TJ, causes the establishment of the apical and basal polarity between adjacent epithelial cells (Mruk and Cheng, 2004; Shin et al., 2006; Yeaman et al., 1999). Earlier genetic and biochemical analyses based on studies in Drosophila melanogaster and C. elegans have identified three protein complexes that are known to be involved in determining cell polarity (Assemat et al., 2008), and subsequent studies have also confirmed the roles of these highly conserved proteins in conferring polarity in mammalian cells: (1) the Crumbs (CRB) protein complex (Bazellieres et al., 2009; Makarova et al., 2003; Wong et al., 2008a), (2) the partitioning-defective (Par) protein complex (Assemat et al., 2008; Goldstein and Macara, 2007; Wong et al., 2008a), and (3) the Scribble complex (Nakagawa and Huibregtse, 2000; Santoni et al., 2002) (Fig. 7.5) (Table 7.2).
Figure 7.5.
The three highly conserved polarity protein complexes: the partitioning defective (PAR), Crumbs (CRB) and Scribble complexes, that are found in multiple epithelia including the seminiferous epithelium in rat testes. Many components of these proteins are also found in germ cells in the seminiferous epithelium. Interaction between Par6 and Pals1 provides cross talk between the CRB and Par complexes (black arrows). aPKC is a crucial component in the polarity protein complexes which provides cross talk between the three conserved polarity complexes. Phosphorylation of Lgl by aPKC maintains the Scribble complex at the basolateral domain (solid line bars).
Table 7.2.
Components of the Crumbs (CRB), partitioning-defective (PAR), and Scribble complexes in mammalian cells
| Polarity complex |
Component proteins |
Protein type | Apparent molecular weight (kDa)a |
References |
|---|---|---|---|---|
| CRB | CRB3b | Transmembrane | 24 | Makarova et al. (2003), Wong et al. (2008b) |
| Pals1 | Cytoplasmic | 77 | Wong et al. (2008b) | |
| PATJ | Cytoplasmic | 55, 100 | Wong et al. 2008b) | |
| Par | Par3 | Cytoplasmic | 100, 150, 180 | Fujita et al. (2007), Lin et al. (2000), Wong et al. (2008b) |
| Par6 | Cytoplasmic | 37, 45, 60 |
Cline and Nelson (2007), Wong et al. (2008b),Yamanaka et al. (2003) |
|
| aPKC | Cytoplasmic | 80 | Wong et al. (2008b) | |
| Cdc42 | Cytoplasmic | 22 | Gliki et al. (2004), Lui et al. (2003b, 2005) | |
| 14-3-3 | Cytoplasmic | 30 | Chaudhary and Skinner (2000), Perego and Berruti (1997), Wong et al. (2009a) | |
| Scribble | Scrib | Cytoplasmic | 175 | Assemat et al. (2008) |
| Lgl1/2 | Cytoplasmic | 113, 115 | Assemat et al. (2008) | |
| Dlg1-4 | Cytoplasmic | 80–200 | Assemat et al. 2008) |
Apparent molecular weight denoted here is mostly based on studies in the testis (Wong et al., 2008b, 2009a). It was found that the molecular weight of PATJ is different from some of the published results which is 75–230 kDa (Lemmers et al., 2002). However, it should be noted that in the testis, a smaller mRNA transcript of 4.1 kb is detected besides the 7 kb transcript which is detected in other tissues such as small intestine and heart (Lemmers et al., 2002). Thus, it is possible that PATJ protein of smaller molecular weight is detected in the testis. Molecular weight of components of the Scribble complex is based on studies in other epithelia since not much research has been carried out in the testis.
CRB3, Crumbs3; Pals1, protein associated with Lin-7 1; PATJ, Pals1 associated tight junction protein; Par3, partitioning-defective3; Par6, partitioning-defective6; aPKC, atypical protein kinase C; Cdc42, cell cycle division 42; Scrib, Scribble; Lgl1/2, lethal giant larvae1/2; Dlg1-5, discs large1–5.
3.1. The Crumbs (CRB) protein complex
The CRB3/protein associated with Lin-7 1 (Pals1)/Pals1 associated tight junction protein (PATJ) polarity protein complex in mammalian cells, including Sertoli and germ cells in the testis, is the homologue of the Drosophila CRB/Stardust/DmPATJ complex (Wong et al., 2008a) (Table 7.2). There are three isoforms of integral membrane protein CRB in mammals, known as CRB1, CRB2, and CRB3, with CRB3 expressed predominantly in epithelial cells (Makarova et al., 2003). Interestingly, the expression of CRB3 in germ cells is higher than that in Sertoli cells in rat testes (Wong et al., 2008b). Pals1 is a membrane-associated guanylate kinase (MAGUK) protein. Similar to zonula occludens-1 (ZO-1, an adaptor at TJ ), Pals1 possesses a guanylate kinase (GUK) domain but it has no intrinsic catalytic activity; however, it interacts with a number of peripheral proteins via its PDZ domain, including CRB3 and Par6 (Makarova et al., 2003; Roh et al., 2002b). The interaction between Pals1 and Par6 also provides cross talk between the Par and CRB complexes (Hurd et al., 2003b; Wang et al., 2004). PATJ is a scaffolding protein with multiple PDZ domains, as such it is capable of interacting with several proteins at the TJ, including claudin-1 and ZO-3 (Roh et al., 2002a). Both Pals1 and PATJ are found in Sertoli and germ cells in rat testes with their expression more predominant in germ cells than Sertoli cells, analogous to CRB3 (Wong et al., 2008b). These findings illustrate that the CRB polarity protein complex is present in the seminiferous epithelium and it is an integrated complex of both Sertoli and germ cells (Fig. 7.5). In addition, cross talk between the CRB and Par complexes is crucial in regulating the adhesion of germ cells onto the Sertoli cell in the seminiferous epithelium (Wong et al., 2008b). Similar to Par6 and Cdc42, members of the CRB complex have been implicated in the protein trafficking process. Knockdown of Pals1 leads to defect in AJ formation, seemingly due to a disruption in E-cadherin exocytosis after the depletion of Pals1 (Wang et al., 2007). Furthermore, Pals1 and PATJ are found interacting with Rich1/angiomotin complex, a complex which was thought to be important in targeting membrane proteins to TJ sites (Wells et al., 2006).
3.2. The partitioning-defective (Par) protein complex
The Par3/Par6 proteins were first identified in C. elegans which regulates anterior–posterior polarity in zygote (Kemphues et al., 1988). The Par3/ Par6 proteins form a conserved complex with GTP-Cdc42 and atypical protein kinase C (aPKC) (Assemat et al., 2008; Wong et al., 2008a) (Fig. 7.5) and several other protein components (Table 7.2). In mammals, Par6 serves as a crucial adaptor, recruiting Par3, active Cdc42/Rac1 and aPKC to facilitate TJ assembly ( Joberty et al., 2000; Lin et al., 2000). aPKC, besides activating Par3 via phosphorylation, also activates CRB3 in the CRB protein complex and Scribble in the Scribble complex, illustrating that it plays a crucial role to maintain cross talk between the three polarity protein complexes in different epithelia (Iden and Collard, 2008). In the testis, Par3, Par6, Cdc42, and aPKC have been identified in Sertoli and germ cells (Fujita et al., 2007; Gliki et al., 2004; Lui et al., 2003b; Wong et al., 2008b). Using immunohistochemistry and dual-labeled immunofluorescence analysis, Par6 is localized both at the basal ES/TJ at the BTB and apical ES at the spermatid–Sertoli cell interface, colocalizing with occludin, N-cadherin, and γ-catenin at the BTB and with nectin-3 at the apical ES. Besides, its expression is greatly diminished at both BTB and apical ES at stage VIII of the epithelial cycle during BTB restructuring and spermiation (Wong et al., 2008b). More importantly, the loss of Par6 at the apical ES was shown to associate with a loss of orientation of the elongating spermatids in the seminiferous epithelium (Wong et al., 2008b). A study by coimmunoprecipitation has demonstrated that the Par6-based polarity complex plays a crucial role to regulate adhesion of elongating/elongated spermatids to the Sertoli cell in the seminiferous epithelium via a novel mechanism. Par6/Pals1 forms a complex with JAM-C in both Sertoli cells and spermatids to allow the homotypic interaction between JAM-C to confer adhesion of spermatids in the seminiferous epithelium in all epithelial stages except at stage VIII (Wong et al., 2008b). At the late stage VIII or when spermatids are induced to leave the seminiferous epithelium by adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide], the Par6/Pals1 complex becomes tightly associated with Src kinase, “pulling” the Par6/Pals1 complex away from JAM-C, thus destabilizing the JAM-C-based adhesion function. This, in turn, disrupts the apical ES to facilitate spermiation (Wong et al., 2008b). Additionally, the knockdown of Par6 or Par3 leads to a redistribution of JAM-A and α-catenin at the Sertoli—Sertoli cell interface, destabilizing the TJ-permeability barrier function, leading to transient disruption of the BTB integrity (Wong et al., 2008b). These findings thus demonstrate unequivocally the significance of Par6 in conferring adhesion and polarity function at the BTB and apical ES. Furthermore, these data also illustrate that the Par6-based polarity complex serves as the molecular “switch” which coordinates the events of spermiation and BTB restructuring at stage VIII of the epithelial cycle (see Section 4).
3.3. The Scribble protein complex
The mammalian Scribble complex, which consists of Scribble (Scrib, also known as Vartul), disks large (Dlg1–5) and lethal giant larvae (Lgl1/2), are localized at the basolateral domain of epithelial cells (Assemat et al., 2008; Yamanaka and Ohno, 2008) (Table 7.2) (Fig. 7.5). Initial studies in D. melanogaster revealed that they are tumor suppressor genes as their deletions lead to overproliferation and outgrowth of tissue. Subsequent studies in mammalian cells showed that the Scribble complex is associated with tumorigenesis in mammals, possibly via their interaction with tumor suppressor genes such as adenomatous polyposis coli (APC) (Etienne-Manneville et al., 2005; Matsumine et al., 1996). Binding of Lgl and Par3 to Par6/aPKC complex is mutually exclusive. Phosphorylation of Lgl by aPKC segregates it from Par6/aPKC complex, thus allowing the binding of Par3 and localization of Par3/Par6/aPKC at the apical domain; at the same time, basolateral localization of Lgl is also maintained. Thus, kinase activity of aPKC plays an essential role in establishing and maintaining the antagonistic interactions between the apical Par complex and basolateral Scribble complex (Yamanaka et al., 2003). In MDCK cells, Scrib was shown to be involved in regulating E-cadherin-mediated cell–cell adhesion, via stabilizing interaction between E-cadherin and α-catenin (Qin et al., 2005). While members of the Scribble complex are shown to express in the testis, such as Lgl2 (Assemat et al., 2008), however, their functional roles in spermatogenesis remain to be clarified.
3.4. 14-3-3 proteins
As briefly discussed earlier, there are three major polarity protein complexes in epithelia, two of which, namely the CRB- and Par-based polarity complexes, have been identified and functionally studied in mammalian testes (Fujita et al., 2007; Gliki et al., 2004; Lui et al., 2003b; Wong et al., 2008b). Furthermore, recent functional studies have illustrated the significance of some of their component proteins in cell adhesion and BTB function in the testis (Wong et al., 2008a,b). Herein, we switch our focus on 14-3-3 proteins, which are the homologues of C. elegans Par5 in mammalian cells (Morton et al., 2002), since recent studies have illustrated the importance of 14-3-3 proteins in spermatogenesis (Wong et al., 2009a).
14-3-3 proteins are a family of small (~30 kDa) acidic proteins which are expressed in all eukaryotic cells (Aitken, 2006; Morrison, 2009; Sun et al., 2009) including Sertoli and germ cells (Chaudhary and Skinner, 2000; Perego and Berruti, 1997;Wong et al., 2009a). The name “14-3-3” is used to describe the elution andmigration pattern of this group of proteins on two-dimensional DEAE-cellulose chromatography and starch gel electrophoresis from which they were initially isolated from mammalian brain (Aitken, 2006; Morrison, 2009; Sun et al., 2009). To date, seven isoforms of 14-3-3 are found in mammals namely α, ε, η, γ, τ/θ, ζ, and σ(Aitken, 2006; Bridges and Moorhead, 2005;Muslin and Lau, 2005). Binding of 14-3-3s to target proteins is mostly phosphorylation dependent, where they recognize conserved phosphoserine/ phosphothreonine containing motifs in target proteins (Aitken, 2006; Bridges and Moorhead, 2005). However, 14-3-3s can also bind to unphosphorylated domains in target proteins (Waterman et al., 1998).
Through binding to over 200 target proteins, 14-3-3s are involved in diverse cellular processes such as cell-cycle control, protein transcription, protein trafficking, and DNA repair ( Jin et al., 2004; Pozuelo Rubio et al., 2004). 14-3-3s are thought to be regulated by phosphorylation to form homo- or heterodimers (Aitken, 2006; Woodcock et al., 2003). They function as scaffolding proteins to colocalize target proteins in close proximity to facilitate phosphorylation or enzymatic activity to occur. Functional domains on target proteins can be masked upon 14-3-3s binding to prevent interaction with other effector proteins. The binding of 14-3-3s onto their target proteins can also induce changes in protein conformation, thereby modulating their activities (Bridges and Moorhead, 2005).
Besides binding to the conserved phosphoserine/phosphothreonine containing motifs, 14-3-3s were found to recognize a dibasic motif in several cell surface channel proteins (Mrowiec and Schwappach, 2006; Shikano et al., 2006). For instance, KCNK3 (potassium channel, subfamily K, member 3 which is a member of the superfamily of potassium channel proteins) was found to contain two trafficking signals: one for β-COP, a component of coat protein complex I (COPI) and one for 14-3-3β. Binding of β-COP and 14-3-3β to KCNK3 is mutually exclusive. Phosphorylation of KCNK3 favors the binding of 14-3-3β which overwrites the retention signal of β-COP to maintain channel in endoplasmic reticulum (ER). As a result, KCNK3 are transported to the plasma membrane (O’Kelly et al., 2002). This mechanism also helps to ensure multimeric membrane proteins are fully assembled before they are transported to the cell surface (Heusser et al., 2006; Yuan et al., 2003). Along with this line of evidence, recent studies have shown that 14-3-3 regulates the kinetics of protein trafficking in Sertoli cells with an established TJ-permeability barrier (Wong et al., 2009a). It was shown that multiple isoforms of 14-3-3 are found in both Sertoli and germ cells including 14-3-3α, β, θ, γ, δ, and ζ(Chaudhary and Skinner, 2000; Perego and Berruti, 1997; Wong et al., 2009a), with germ cells express relatively more 14-3-3 than Sertoli cells (Wong et al., 2009a). Knockdown of 14-3-3θ by RNAi leads to a loss of cell adhesion function at the Sertoli cell BTB, which is resulted from a mislocalization of N-cadherin and ZO-1, but not α- and β-catenins, at the Sertoli–Sertoli cell interface. Such changes in protein localization were shown to be mediated via changes in the kinetics of protein endocytosis by enhancing the internalization of JAM-A and N-cadherin in Sertoli cells with an established TJ-permeability barrier (Wong et al., 2009a). Studies by immunohistochemistry and duallabeled immunofluorescence analysis have demonstrated the localization of 14-3-3θ at the apical ES and BTB in adult rat testes (Wong et al., 2009a). Furthermore, a considerable loss of 14-3-3θ at the apical ES was detected prior to spermiation, illustrating that 14-3-3θ is likely to be involved in the maintenance of the apical ES function (Wong et al., 2009a). In short, these findings illustrate the crucial regulatory role of 14-3-3 in the testis during spermatogenesis at both the apical ES and BTB. Thus we speculate that similar to Par6, 14-3-3 perhaps also serves as a molecular “switch” to coordinate the events of spermiation and BTB restructuring that take place simultaneously at the opposite ends of the seminiferous epithelium at stage VIII of the epithelial cycle.
Phosphorylation of integral membrane proteins and their peripheral adaptors is an important biochemical process to controlTJ and AJ functions, such as by regulating the subcellular localization of TJ and AJ proteins (Gonzalez-Mariscal et al., 2008;Nelson, 2008; Sallee et al., 2006). 14-3-3s are involved in this process by interacting with Raf, the central downstream effector of Ras GTPases (Hekman et al., 2004; Light et al., 2002). Activation ofRas by growth factors such as epidermal growth factor (EGF) phosphorylates C-Raf, causing its translocation from cytosol to the plasma membrane. Binding of 14-3-3 to C-Raf counteracts this activation, eventually leading to the concentration of cadherins and β-catenin at the cell–cell interface (Rajalingam and Rudel, 2005; Rajalingam et al., 2005). Apart from regulating the localization of TJ and AJ proteins, 14-3-3 also regulates cell junction function by controlling the degradation of junction proteins by the proteasome. Human immunodeficiency virus-1 (HIV-1) crosses the blood–brain barrier (BBB) by downregulating the levels of TJ proteins of endothelial cells that constitute the BBB via neurotoxic viral proteins gp120 and Tat (Andras et al., 2003; Kanmogne et al., 2005). Further investigation shows that HIV-1 gp120 enhances the degradation of TJ proteins mediated by the proteasome pathway. Interestingly, silencing of 14-3-3τ (also termed θ) accelerates the gp120-mediated TJ proteins degradation, indicating that the presence of 14-3-3τ protects the endothelial cells by maintaining the integrity of the TJ (Nakamuta et al., 2008). To this end, it is not known if the disappearance of 14-3-3θ at the apical ES prior to spermiation or when 14-3-3θ is knocked down in Sertoli cells by RNAi affects the degradation of TJ or AJ proteins besides protein endocytosis. Further analysis will be needed to resolve this issue.
Several studies suggest that 14-3-3s are central mediators involved in integrin-regulated cell adhesion/migration and cytoskeleton dynamics. In a yeast two-hybrid screen, 14-3-3β was found to bind to the β subunit of integrin in a phosphorylation-independent manner (Han et al., 2001). On the other hand, 14-3-3s also bind to phosphorylated cytoplasmic domain of β2-integrin (Fagerholm et al., 2002). Overexpression of 14-3-3β increases integrin-mediated cell spreading and migration when cells were plated on fibronectin (Han et al., 2001). Conversely, by making use of the interaction between 14-3-3 and another adhesion receptor glycoprotein Ib to sequester endogenous 14-3-3ζ (Du et al., 1994, 1996), it was found that integrin-mediated cell spreading was delayed. Inhibition in cell spreading is due to a block in integrin-induced Cdc42 and Rac activation, indicating that 14-3-3 may serve as mediator to transduce signal downstream of integrin (Bialkowska et al., 2003). 14-3-3 may also help to localize activated Rac to membrane ruffles (Chahdi and Sorokin, 2008; Somanath and Byzova, 2009). An interesting question raised is that overexpression of 14-3-3β does not result in tyrosine phosphorylation of focal adhesion kinase (FAK), p130cas and paxillin, three downstream signaling molecules of integrin (Han et al., 2001), despite 14-3-3ζ is known to bind to phosphorylated p130cas (Garcia-Guzman et al., 1999). It is likely that 14-3-3 acts down-stream of p130cas. Furthermore, different isoforms of 14-3-3 may exhibit a distinct binding pattern and hence cellular activities. Since α6β1-integrin–laminin-333 is the major cell adhesion protein complex at the apical ES at the elongating/elongated spermatid–Sertoli cell interface in the seminiferous epithelium (Mulholland et al., 2001; Palombi et al., 1992; Salanova et al., 1995; Siu and Cheng, 2004; Yan and Cheng, 2006), and β1-integrin is also a component of the hemidesmosome in the testis (Yan et al., 2008b), it remains to be determined if 14-3-3 plays a critical role in mediating the integrin-based signaling function at these sites during spermatogenesis, such as the breakdown of the integrin–laminin protein complex at the apical ES during spermiation, and the cross talk between hemidesmosome and BTB during BTB restructuring at stages VIII–XI of the epithelial cycle. While 14-3-3 regulates cell adhesion and spreading via integrin, it also exhibits a direct effect on actin cytoskeleton which affects the formation of membrane protrusion. 14-3-3 specifically binds to phosphorylated cofilin at Ser-3, a phosphorylation site that inactivates cofilin activity and hence actin severing and depolymerization. Binding of 14-3-3 protects phosphorylated cofilin from dephosphorylation and maintains a pool of inactive cofilin in the cells (Gohla and Bokoch, 2002).
As mentioned earlier, C. elegans Par5 was identified as a 14-3-3 protein. In addition, Par5/14-3-3 is thought to control the asymmetric localization of other Par proteins (Morton et al., 2002). Similar to its role in C. elegans, 14-3-3 is found to work together with Par proteins, which, in turn, controls cell polarity and cell adhesion in mammalian cells. 14-3-3 regulates the activity of the Par3/Par6/aPKC on cell polarity via a phosphorylation-dependent interaction with Par3. Interestingly, the interaction between 14-3-3 and Par3 does not depend on the phosphorylation of Par3 by aPKC(Hurd et al., 2003a). This result is strengthened by the observation that Par3β or Par3L, a splice variant of Par3 which lacks aPKC binding domain (Gao et al., 2002; Kohjima et al., 2002), interacts with 14-3-3(Izaki et al., 2005). On the other hand, protein phosphatase 1α (PP1α) dephosphorylates Par3, in turn, controlling the binding between 14-3-3, Par3, and aPKC, which subsequently regulates TJ assembly (Traweger et al., 2008).
4. The Apical ES-BTB-Basement Membrane Functional Axis in the Seminiferous Epithelium that Coordinates the Cellular Events of Spermiation and BTB Restructuring During the Seminiferous Epithelial Cycle of Spermatogenesis: The Role of Polarity Proteins in Mediating the Apical ES-BTB-Basement Membrane Axis
The apical ES, once formed between step 8 spermatids and Sertoli cells, is the only anchorage device that persists through step 19 spermatids in the rat testis until spermiation (Mruk et al., 2008; Russell, 1993). Recent studies have shown that the Par polarity proteins, such as Par6 and 14-3-3θ (Par5) are found at the apical ES, likely to be used to confer spermatid orientation (Wong et al., 2008b, 2009a) even though this is a putative anchoring junction type. This conclusion was reached based on the observations that a loss of spermatid orientation, such as by treatment of rats with adjudin to induce spermatid loss from the epithelium, is associated with a significant decline in the expression of Par6 and 14-3-3θ at the apical ES (Wong et al., 2008b, 2009a). The loss of Par6 is also associated with defragmentation of actin filament bundles at the apical ES and the loss of adhesion function at the site, illustrating polarity proteins are integrated components of the apical ES and may take part in conferring cell adhesion (Wong et al., 2008b).
A recent study has shown that biologically active laminin β3 and/or γ3 chains formed at the apical ES at spermiation can destabilize the BTB (Yan et al., 2008b). In this context, it is of interest to note that matrix metallo-protease-2 (MMP-2) (Siu and Cheng, 2004), a protease that is able to cleave laminin and is activated by membrane type 1-matrix metalloprotease (MT1-MMP), is a putative component of the apical ES that appears in ~stage VI–VIII of the epithelial cycle (but considerably diminished in late stage VIII when spermiation takes place), colocalizing with the laminin γ3 chain (Siu and Cheng, 2004). We speculate that the presence of Par6 protein (Wong et al., 2008b, 2009a) or 14-3-3 is involved in targeting MT1-MMP to the apical ES, similar to the role ascribed to Cdc42 (Sakurai-Yageta et al., 2008), to activate and increase the secretion of MMP-2 such as at stage VIII of the seminiferous epithelial cycle just before spermiation to facilitate the cleavage of the laminin chains. Besides disrupting BTB integrity, it was shown that the biologically active laminin fragments generated at the apical ES also modulated BTB integrity indirectly via their effects on the hemidesmosome by reducing the steady-state level of β1-integrin at the hemidesmosome (Yan et al., 2008b). Thus, Par-based polarity proteins may play a role in coordinating these events at the apical ES, hemidesmosome, and the BTB.
On the other hand, polarity proteins such as 14-3-3 (Par5) are known to regulate protein endocytosis since a loss of 14-3-3 protein function by RNAi leads to a redistribution of TJ and basal ES proteins from the Sertoli–Sertoli cell interface, thereby destabilizing the BTB integrity (Wong et al., 2008b). However, it remains to be determined if Par6 and/ or 14-3-3θ also regulate protein endocytosis at the apical ES since ultra-structurally, apical ES and basal ES are almost identical except that ultra-structures pertinent to apical ES are found on both sides of the Sertoli cells in basal ES (Wong et al., 2008b, 2009a).
Collectively, these findings have prompted us to propose a biochemical model shown in Fig. 7.6 regarding the presence of a functional apical ES-BTB-basement membrane functional axis that coordinates the events of spermiation and BTB restructuring that occur simultaneously at stage VIII of the epithelial cycle. It is likely that the biologically active laminin fragments are working in concert with the Par-based polarity complexes and perhaps other protein kinases (e.g., FAK and Src) that transmit signals between these sites to coordinate these events during spermatogenesis. For instance, recent studies have shown that FAK and/or Src is an integrated component of the apical ES and BTB in rat testes (Siu et al., 2003; Yan and Cheng, 2006). In short, Par-based polarity proteins serve as molecular switches whereas laminin fragments act as autocrine factors to coordinate the events of apical ES and BTB restructuring that occur at the opposite ends of the seminiferous epithelium at stage VIII of the epithelial cycle.
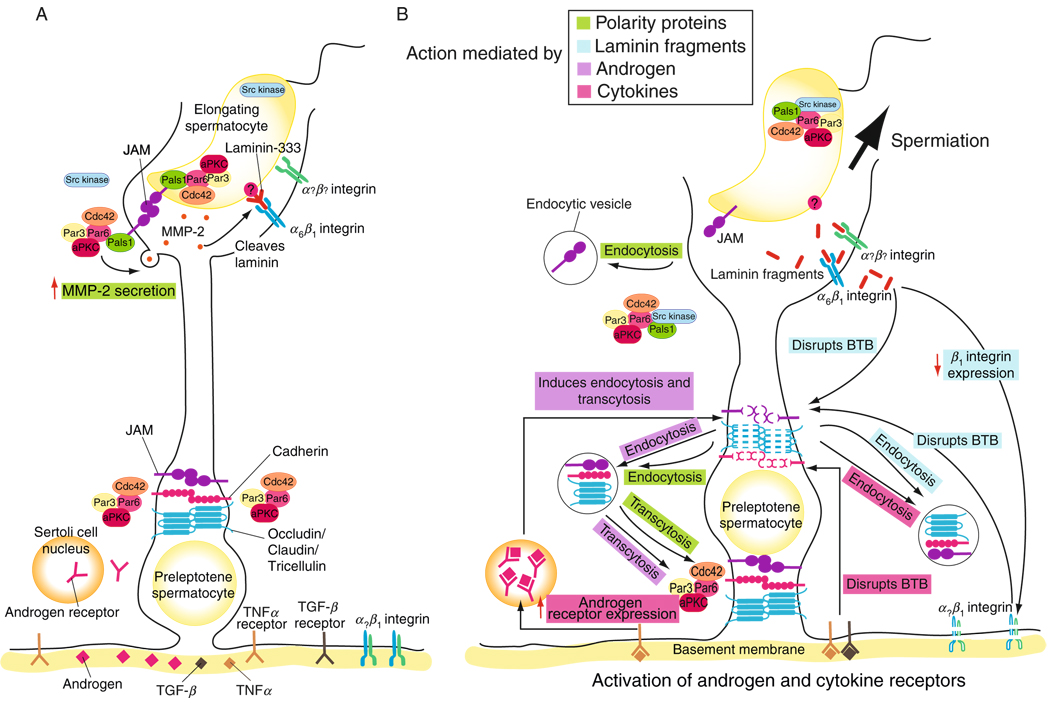
Figure 7.6.
Schematic drawing illustrating the involvement of cytokines, testosterone, biologically active fragments of laminin chains, hemidesmosome, and polarity proteins in regulating spermiation and BTB restructuring during the seminiferous epithelial cycle of spermatogenesis. This schematic drawing was prepared based on recent findings in the field as detailed in the text. (A) In this panel, the known protein complexes at the apical ES, namely the JAM-C-based protein complex and the α6-β1-integrin/ laminin-333-based protein complex are shown. The cell adhesion at the BTB is conferred by the JAM-A-based, cadherin-based, and the occludin-, claudin-, and tricellulin- based protein complexes. Just prior to spermiation, the polarity protein complex, Cdc42/Par3/Par6/Pals1/aPKC, remains associated with JAM-C. The presence of the polarity complex is likely involved in targeting and activating MMP-2 at the apical ES, which apparently is being used to cleave the laminin chains to generate the biologically active fragments. (B) During spermiation at the apical ES, Src kinase was shown to associate more tightly with Par6 and Pals1, causing the dissociation of the Par-based polarity complex from the JAM-C-based protein complex. JAM-C may also be internalized via endocytosis due to the absence of Par6 at the apical ES at spermiation, further destabilizing the JAM-C-based adhesion and facilitating spermiation at stage VIII of the epithelial cycle. Laminin fragments generated at the apical ES site were shown to perturb the BTB integrity directly or indirectly, acting as autocrine factors, via their effects on a yet-to-be identified integrin receptor at the BTB, and β1-integrin at the hemidesmosome. At the BTB, the biologically active laminin fragments apparently accelerate endocytic-vesicle-mediated endocytosis of integral membrane proteins, destabilizing the “old” TJ-fibrils above a primary preleptotene spermatocyte in transit at the BTB at stage VIII of the epithelial cycle. Cytokines, such as TGF-β2 and TGF-β3, are also likely to be involved in “destabilizing” the “old” BTB by accelerating endocytosis of BTB integral membrane proteins above the primary spermatocytes in transit. However, the combined effects of testosterone and TNFα-induced androgen receptor expression may accelerate the production (e.g., de novo synthesis of occludin, claudins, and JAMs) and assembly of “new” TJ-fibrils behind a primary spermatocyte in transit, and by transcytosing junction proteins from the “old” barrier to new site behind the spermatocyte. The processes of protein endocytosis and recycling, and perhaps transcytosis, are facilitated by polarity protein components, such as Par3, Par6, and 14-3-3. The polarity protein complex may serve as initial spatial cue to direct endocytosed proteins for forming “new” barrier behind the primary spermatocyte. Through the combined and concerted efforts of cytokines, testosterone, and biologically active laminin fragments, and with the participation of polarity proteins via their actions on protein endocytosis, recycling, and transcytosis, “new” TJ-fibrils can be formed behind a primary spermatocyte in transit prior to the dissolution of the “old” TJ-fibrils. Thus, the BTB is being restructured to facilitate the transit of spermatocytes while the immunological barrier can be maintained during spermatogenesis.
5. Roles of Polarity Proteins in Coordinating the Opposing Effects of Cytokines and Testosterone in Primary Preleptotene Spermatocyte Transit at the BTB
Cytokines are known regulators of TJ-permeability barrier in many epithelia, such as in small intestine and kidney, in studies using different cell lines (Al-Sadi et al., 2009; Walsh et al., 2000). The initial reports describing the disruptive effects of cytokines (e.g., TGF-β3 and TNFα) on the Sertoli cell TJ-permeability function appeared only in the early 2000s (Lui et al., 2001, 2003c; Siu et al., 2003). Since then, however, much progress has been made in the field, which includes the identification of the p38MAPK and ERK1/2 as the two putative signaling pathways utilized byTGF-β3 in the testis to regulate BTB dynamics in vivo either alone or in combination with anchoring junction restructuring in the seminiferous epithelium (Li et al., 2006; Lui et al., 2003d; Wong et al., 2004; Xia et al., 2006; Yan et al., 2008b). These findings are important because they illustrate that the BTB permeability function can be manipulated by using specific inhibitors against these two MAPKs (Lui et al., 2003c,d; Xia and Cheng, 2005) (for a review, see Li et al., 2009). For instance, the cadmium-induced BTB disruption can be delayed by blocking the activation of p38 MAPK (Lui et al., 2003d; Wong et al., 2004), illustrating the environmental toxicant-induced BTB damage can possibly be therapeutically “protected” via the use of specific inhibitors against p38 MAPK (Siu et al., 2009; Li et al., 2009). On the other hand, it is of interest that testosterone promotes the integrity of the BTB (Meng et al., 2005) and maintains the junctional complex integrity in the seminiferous epithelium (Wang et al., 2006) via classical (i.e., involving androgen receptor) and perhaps nonclassical androgen action (i.e., involving ERK1/2 and c-Src nonreceptor protein kinases) (Walker, 2009). These opposing effects of cytokines (e.g., TGF-β3 and TNFα) and testosterone that disrupt and promotes the BTB integrity, respectively, seemingly suggest that if these molecules are working in concert, they can provide a novel mechanism to facilitate the migration of primary spermatocytes across the BTB while maintaining the immunological barrier. For instance, testosterone can promote the assembly of “new” TJ-fibrils below a primary preleptotene spermatocyte in transit while cytokines promote the dissolution of “old” TJ-fibrils above the spermatocyte in transit. Recent findings seem to support this novel mechanism in the testis. It was shown that both cytokines (e.g., TGF-β2, TGF-β3) (Xia et al., 2009; Yan et al., 2008a) and testosterone (Yan et al., 2008a) enhance endocytosis of integrated proteins at the BTB utilizing the clathrin-dependent pathway. However, endocytosed proteins following treatment of Sertoli cells with cytokines were shown to be targeted for degradation, whereas testosterone promoted recycling, perhaps transcytosis, of the endocytosed proteins back to the cell surface (Yan et al., 2008a). In this context, it is of interest to note that TNFα was shown to stimulate androgen receptor expression by Sertoli cells (Chuang et al., 2007) via upregulation of NFκB, which binds to several enhancer motifs in the androgen receptor promoter, thereby promoting androgen receptor expression (Delfino et al., 2003). Thus, TNFα can have a dual-effect on the Sertoli cell TJ-barrier by promoting the assembly of “new” TJ-fibrils behind a primary spermatocyte in transit while disrupting the “old” TJ-fibrils above the migrating spermatocyte. As such, the immunological barrier can be maintained during the transit of spermatocytes (Fig. 7.6). Nonetheless, this postulate must be further validated in future functional studies to examine the opposing effects of cytokines and testosterone on junction restructuring.
How can the endocytosed proteins be transcytosed from the apical to the basal region of a primary preleptotene spermatocyte in transit? Indeed a recent study shed light on such possibility. Coureuil et al. (2009) found that Neisseria meningitides, the bacteria that cause cerebrospinal meningitis in humans, forms an “ectopic early junction-like domain” between their adhesion sites on microvascular endothelial cells. Through activating Cdc42, Par3/Par6/aPKC complex is recruited to the ectopic early junction- like domain which subsequently targets TJ (e.g., claudin-5) and AJ (VE-cadherin) proteins to the site. Interestingly, immunofluorescence microcopy shows that TJ and AJ proteins found at the ectopic early junction-like domain are recruited from the intercellular junctions of microvascular endothelial cells, leading to an increase in permeability of the endothelial cell layer to facilitate the entry of N. meningitides. It is likely that primary preleptotene spermatocytes utilize the Cdc42/Par3/Par6/ aPKC complex to transcytosed junction proteins between two Sertoli cells, from the apical to the basal region of the migrating preleptotene spermatocyte. In this way, AJ and TJ are disrupted to allow the entry of preleptotene spermatocytes into the adluminal compartment of the seminiferous epithelium. At the same time, BTB integrity is maintained as new junctions are formed at the base of the migrating cells. This speculation is supported by several recent reports. First, we demonstrated that the silencing of Par3 or Par6 perturbed the Sertoli cell BTB by causing mislocalization of integral membrane proteins and/or their adaptors at the BTB site, such as JAM-A, N-cadherin, α-catenin, and/or ZO-1, thereby disrupting the BTB integrity (Wong et al., 2008b). This shows that, similar to other epithelial cells, Par polarity complex is necessary to target and maintain junction proteins at the cell–cell interface. Second, it is increasingly clear regarding the role of 14-3-3 and Cdc42 in regulating protein endocytosis in Sertoli cells with an established TJ-permeability barrier that mimics the BTB in vivo (Wong et al., 2009a,b). More importantly, recent findings have demonstrated unequivocally that Cdc42, besides facilitating protein endocytosis at the Sertoli cell BTB, is also crucial to the TGF-β3-mediated acceleration of protein endocytosis (Wong et al., 2009b). For instance, overexpression of a dominant negative Cdc42 renders the loss of responsiveness of the Sertoli cells to TGF-β3-induced enhancement in protein endocytosis (Wong et al., 2009b). These findings are important since they illustrate that Cdc42 is working in concert with the Par3/Par6-based polarity complex to regulate cytokine-mediated Sertoli cell BTB integrity via protein endocytosis. However, several important questions will be needed to address in future studies: What are the possible extrinsic or intrinsic cues that activate and localize the Cdc42/Par3/Par6/aPKC polarity complex to the basal region of a migrating preleptotene spermatocyte? How are these extrinsic or intrinsic cues be asymmetrical initiated? We speculate that cytokines, such as TGF-β-3, are likely one of the extrinsic cues that regulates junction dynamics via Par proteins (Bose and Wrana, 2006; Ozdamar et al., 2005; Wang et al., 2008a). Recent studies have found that Wnt family proteins, which are involved in regulating planar cell polarity (Seifert and Mlodzik, 2007), are also involved in activating downstream Par polarity complex (Schlessinger et al., 2007; Zhang et al., 2007). Lastly, what is the role of Cdc42, Par3 or Par6 in the clathrin-mediated endocytosis? Does Hrs also play a role in cytokine-mediated endocytic trafficking in light of its function in early endosome sorting mechanism (Huang et al., 2009).
6. Concluding Remarks and Future Perspectives
Herein we provide an updated discussion based on the latest findings in the field on the role of Rho GTPases (e.g., Cdc42) and components of the mammalian polarity protein complexes (e.g., Par6, Par3, and 14-3-3) on cell–cell interactions in the testis at the Sertoli–Sertoli cell interface (e.g., the BTB) and the Sertoli–germ cell interface (e.g., the apical ES). More importantly, we have provided an integrated model as depicted in Fig. 7.6 on how the different cellular events pertinent to cell–cell interactions in the seminiferous epithelium of adult rat testes are coordinated via the crucial actions of polarity proteins, biologically active laminin fragments, and hemidesmosome on BTB dynamics and spermiation. Figure 7.6 also illustrates the concerted effects of cytokines (e.g., TNFα), androgens (e.g., testosterone), and polarity proteins (e.g., Par6) to regulate the transit of primary preleptotene spermatocytes at the BTB during stage VIII of the seminiferous epithelial cycle of spermatogenesis. However, there are many open questions remain to be addressed in future studies. For instance, are the components of the Scribble protein complex present in the seminiferous epithelium? If they are, how does this protein complex interact with the CRB- and Par-based polarity protein complexes to affect cell adhesion and cell polarity at the BTB and apical ES in the testis? What are the target proteins downstream following activation of aPKC by Cdc42 in the Par3/Par6 protein complex that elicit the assembly of cell polarity, such as spermatid orientation and Sertoli cell polarity, in the seminiferous epithelium? Many of these questions can now be tackled based on the model depicted in Fig. 7.6.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034; R03 HD051512; U54 HD029990, Project 5) to CYC.
REFERENCES
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A. 14-3-3 proteins: a historic overview. Semin. Cancer Biol. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J. Neurosci. Res. 2003;74:255–265. doi: 10.1002/jnr.10762. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat. Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- Bazellieres E, Assemat E, Arsanto JP, Le Bivic A, Massey-Harroche D. Crumbs proteins in epithelial morphogenesis. Front Biosci. 2009;14:2149–2169. doi: 10.2741/3368. [DOI] [PubMed] [Google Scholar]
- Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialkowska K, Zaffran Y, Meyer SC, Fox JE. 14-3-3 zeta mediates integrin-induced activation of Cdc42 and Rac. Platelet glycoprotein Ib-IX regulates integrin-induced signaling by sequestering 14-3-3 zeta. J. Biol. Chem. 2003;278:33342–33350. doi: 10.1074/jbc.M301217200. [DOI] [PubMed] [Google Scholar]
- Bollag G, McCormick F. Regulators and effectors of Ras proteins. Annu. Rev. Cell Biol. 1991;7:601–632. doi: 10.1146/annurev.cb.07.110191.003125. [DOI] [PubMed] [Google Scholar]
- Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr. Opin. Cell Biol. 2006;18:206–212. doi: 10.1016/j.ceb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of Ras-like GTPases in eukaryotes. Mol. Biol. Evol. 2007;24:203–216. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol. Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci. STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- Carreau S, de Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Adv. Med. Sci. 2008;53:139–144. doi: 10.2478/v10039-008-0022-z. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L. Cell adhesion, polarity, and epithelia in the dawn of metazoans. Physiol. Rev. 2004;84:1229–1262. doi: 10.1152/physrev.00001.2004. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim. Biophys. Acta. 2008;1778:770–793. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Cerione RA. Cdc42: new roads to travel. Trends Cell. Biol. 2004;14:127–132. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Chahdi A, Sorokin A. Protein kinase A-dependent phosphorylation modulates beta1Pix guanine nucleotide exchange factor activity through 14-3-3beta binding. Mol. Cell. Biol. 2008;28:1679–1687. doi: 10.1128/MCB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P. Function and regulation of Rnd proteins. Nat. Rev. Mol. Cell Biol. 2006;7:54–62. doi: 10.1038/nrm1788. [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Skinner MK. Characterization of a novel transcript of 14-3-3 theta in Sertoli cells. J. Androl. 2000;21:730–738. [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Chuang CK, Lee KH, Fan CT, Su YS. FSH-sensitive murine Sertoli cell lines immortalized by human telomerase gene hTERT express the androgen receptor in response to TNF-alpha stimulation. Biosci. Rep. 2007;27:403–411. doi: 10.1007/s10540-007-9063-y. [DOI] [PubMed] [Google Scholar]
- Cline EG, Nelson WJ. Characterization of mammalian Par 6 as a dual-location protein. Mol. Cell. Biol. 2007;27:4431–4443. doi: 10.1128/MCB.02235-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- de Kretser D, Kerr J. The cytology of the testis. In: Knobil E, Neill J, Ewing L, Greenwald C, Markert C, Pfaff D, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- Delfino FJ, Boustead JN, Fix C, Walker WH. NF-kappaB and TNF-alpha stimulate androgen receptor expression in Sertoli cells. Mol. Cell. Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Di Agostino S, Rossi P, Geremia R, Sette C. The MAPK pathway triggers activation of Nek2 during chromosome condensation in mouse spermatocytes. Development. 2002;129:1715–1727. doi: 10.1242/dev.129.7.1715. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Du X, Harris SJ, Tetaz TJ, Ginsberg MH, Berndt MC. Association of a phospholipase A2 (14-3-3 protein) with the platelet glycoprotein Ib-IX complex. J. Biol. Chem. 1994;269:18287–18290. [PubMed] [Google Scholar]
- Du X, Fox JE, Pei S. Identification of a binding sequence for the 14-3-3 protein within the cytoplasmic domain of the adhesion receptor, platelet glycoprotein Ib alpha. J. Biol. Chem. 1996;271:7362–7367. doi: 10.1074/jbc.271.13.7362. [DOI] [PubMed] [Google Scholar]
- Duffield A, Caplan MJ, Muth TR. Protein trafficking in polarized cells. Int. Rev. Cell Mol. Biol. 2008;270:145–179. doi: 10.1016/S1937-6448(08)01404-4. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42—the centre of polarity. J. Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J. Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerholm S, Morrice N, Gahmberg CG, Cohen P. Phosphorylation of the cytoplasmic domain of the integrin CD18 chain by protein kinase C isoforms in leukocytes. J. Biol. Chem. 2002;277:1728–1738. doi: 10.1074/jbc.M106856200. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Fujita E, Tanabe Y, Hirose T, Aurrand-Lions M, Kasahara T, Imhof BA, et al. Loss of partitioning-defective-3/isotype-specific interacting protein (par-3/ASIP) in the elongating spermatid of RA175 (IGSF4A/SynCAM)-deficient mice. Am. J. Pathol. 2007;171:1800–1810. doi: 10.2353/ajpath.2007.070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara A, Shimizu K, Kawakatsu T, Fukuhara T, Takai Y. Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 2003;278:51885–51893. doi: 10.1074/jbc.M308015200. [DOI] [PubMed] [Google Scholar]
- Gao L, Macara IG, Joberty G. Multiple splice variants of Par3 and of a novel related gene, Par3L, produce proteins with different binding properties. Gene. 2002;294:99–107. doi: 10.1016/s0378-1119(02)00681-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Guzman M, Dolfi F, Russello M, Vuori K. Cell adhesion regulates the interaction between the docking protein p130(Cas) and the 14-3-3 proteins. J. Biol. Chem. 1999;274:5762–5768. doi: 10.1074/jbc.274.9.5762. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J. 2003;22:1125–1133. doi: 10.1093/emboj/cdg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr. Biol. 2008;18:1631–1638. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr. Biol. 2002;12:1704–1710. doi: 10.1016/s0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci. STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Hall C, Sin WC, Teo M, Michael GJ, Smith P, Dong JM, et al. α2-chimerin, an SH2-containing GTPase-activating protein for the Ras-related protein p21rac derived by alternate splicing of the human n-chimerin gene, is selectively expressed in brain regions and testes. Mol. Cell. Biol. 1993;13:4986–4998. doi: 10.1128/mcb.13.8.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DC, Rodriguez LG, Guan JL. Identification of a novel interaction between integrin beta1 and 14-3-3beta. Oncogene. 2001;20:346–357. doi: 10.1038/sj.onc.1204068. [DOI] [PubMed] [Google Scholar]
- Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugsten EM, Malecki J, Bjorklund SM, Olsnes S, Wesche J. Ubiquitination of fibroblast growth factor receptor 1 is required for its intracellular sorting but not for its endocytosis. Mol. Biol. Cell. 2008;19:3390–3403. doi: 10.1091/mbc.E07-12-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hekman M, Wiese S, Metz R, Albert S, Troppmair J, Nickel J, et al. Dynamic changes in C-Raf phosphorylation and 14-3-3 protein binding in response to growth factor stimulation: differential roles of 14-3-3 protein binding sites. J. Biol. Chem. 2004;279:14074–14086. doi: 10.1074/jbc.M309620200. [DOI] [PubMed] [Google Scholar]
- Hess RA. Estrogen in the adult male reproductive tract: a review. Reprod. Biol. Endocrinol. 2003;1:52. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science+Business Media, LLC; 2008. pp. 1–15. [Google Scholar]
- Heusser K, Yuan H, Neagoe I, Tarasov AI, Ashcroft FM, Schwappach B. Scavenging of 14-3-3 proteins reveals their involvement in the cell-surface transport of ATP-sensitive K+ channels. J. Cell Sci. 2006;119:4353–4363. doi: 10.1242/jcs.03196. [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- Honda T, Shimizu K, Kawakatsu T, Fukuhara A, Irie K, Nakamura T, et al. Cdc42 and Rac small G proteins activated by trans-interactions of nectins are involved in activation of c-Jun N-terminal kinase, but not in association of nectins and cadherin to form adherens junctions, in fibroblasts. Genes Cells. 2003;8:481–491. doi: 10.1046/j.1365-2443.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- Huang SH, Zhao L, Sun ZP, Li XZ, Geng Z, Zhang KD, et al. Essential role of Hrs in endocytic recycling of full-length TrkB receptor but not its isoform TrkB. T1. J. Biol. Chem. 2009;284:15126–15136. doi: 10.1074/jbc.M809763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd TW, Fan S, Liu CJ, Kweon HK, Hakansson K, Margolis B. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 2003a;13:2082–2090. doi: 10.1016/j.cub.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 2003b;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat. Rev. Mol. Cell Biol. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- Inselman A, Handel MA. Mitogen-activated protein kinase dynamics during the meiotic G2/MI transition of mouse spermatocytes. Biol. Reprod. 2004;71:570–578. doi: 10.1095/biolreprod.104.027938. [DOI] [PubMed] [Google Scholar]
- Izaki T, Kamakura S, Kohjima M, Sumimoto H. Phosphorylation-dependent binding of 14-3-3 to Par3beta, a human Par3-related cell polarity protein. Biochem. Biophys. Res. Commun. 2005;329:211–218. doi: 10.1016/j.bbrc.2005.01.115. [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J. Cell Biol. 2004;166:237–248. doi: 10.1083/jcb.200401078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr. Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Kanmogne GD, Primeaux C, Grammas P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: implications for the pathogenesis of HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 2002;277:50749–50755. doi: 10.1074/jbc.M209846200. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kodama A, Takaishi K, Nakano K, Nishioka H, Takai Y. Involvement of Cdc42 small G protein in cell-cell adhesion, migration, and morphology of MDCK cells. Oncogene. 1999;18:3996–4006. doi: 10.1038/sj.onc.1202773. [DOI] [PubMed] [Google Scholar]
- Kohjima M, Noda Y, Takeya R, Saito N, Takeuchi K, Sumimoto H. PAR3beta, a novel homologue of the cell polarity protein PAR3, localizes to tight junctions. Biochem. Biophys. Res. Commun. 2002;299:641–646. doi: 10.1016/s0006-291x(02)02698-0. [DOI] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat. Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Lau AS, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann. N. Y. Acad. Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lee NP, Cheng CY. Regulation of Sertoli cell tight junction dynamics in the rat testis via the nitric oxide synthase/soluble guanylate cyclase/3′, 5′-cyclic guanosine monophosphate/protein kinase G signaling pathway: an in vitro study. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr. Biol. 2008;18:1639–1648. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- Lemmers C, Medina E, Delgrossi MH, Michel D, Arsanto JP, Le Bivic A. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J. Biol. Chem. 2002;277:25408–25415. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- Leung T, How BE, Manser E, Lim L. Germ cell beta-chimaerin, a new GTPase-activating protein for p21rac, is specifically expressed during the acrosomal assembly stage in rat testis. J. Biol. Chem. 1993;268:3813–3816. [PubMed] [Google Scholar]
- Leyt J, Melamed-Book N, Vaerman JP, Cohen S, Weiss AM, Aroeti B. Cholesterol-sensitive modulation of transcytosis. Mol. Biol. Cell. 2007;18:2057–2071. doi: 10.1091/mbc.E06-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Xia W, Mruk DD, Wang CQ, Yan HH, Siu MK, et al. Tumor necrosis factor α reversibly disrupts the blood–testis barrier and impairs Sertoli–germ cell adhesion in the seminiferous epithelium of adult rat testes. J. Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Cheng CY. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 2009;15:159–168. doi: 10.1016/j.molmed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PP, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem. Sci. 2009;34:366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light Y, Paterson H, Marais R. 14-3-3 antagonizes Ras-mediated Raf-1 recruitment to the plasma membrane to maintain signaling fidelity. Mol. Cell. Biol. 2002;22:4984–4996. doi: 10.1128/MCB.22.14.4984-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Loirand G, Guilluy C, Pacaud P. Regulation of Rho proteins by phosphorylation in the cardiovascular system. Trends Cardiovasc. Med. 2006;16:199–204. doi: 10.1016/j.tcm.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis: a molecular and biochemical perspective. Cytokine Growth Factor Rev. 2007;18:299–311. doi: 10.1016/j.cytogfr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Rho GTPases and spermatogenesis. Biochim. Biophys. Acta. 2003a;1593:121–129. doi: 10.1016/s0167-4889(02)00348-8. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol. Reprod. 2003b;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor β-3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol. Reprod. 2003c;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003d;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Lui WY, Mruk DD, Cheng CY. Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli–germ cell adherens junction dynamics in the testis. J. Cell Physiol. 2005;202:49–66. doi: 10.1002/jcp.20098. [DOI] [PubMed] [Google Scholar]
- Makarova O, Roh MH, Liu CJ, Laurinec S, Margolis B. Mammalian crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1) Gene. 2003;302:21–29. doi: 10.1016/s0378111902010843. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- Matthiesson KL, McLachlan RI, O’Donnell L, Frydenberg M, Robertson DM, Stanton PG, et al. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J. Clin. Endocrinol. Metab. 2006;91:3962–3969. doi: 10.1210/jc.2006-1145. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting, and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc. Natl. Acad. Sci. USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- Moon SY, Zheng Y. Rho GTPase-activating proteins in cell regulation. Trends Cell. Biol. 2003;13:13–22. doi: 10.1016/s0962-8924(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DG, Shakes DC, Nugent S, Dichoso D, Wang W, Golden A, et al. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Dev. Biol. 2002;241:47–58. doi: 10.1006/dbio.2001.0489. [DOI] [PubMed] [Google Scholar]
- Mrowiec T, Schwappach B. 14-3-3 proteins in membrane protein transport. Biol. Chem. 2006;387:1227–1236. doi: 10.1515/BC.2006.152. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Delivering non-hormonal contraceptives to men: advances and obstacles. Trends Biotechnol. 2008;26:90–99. doi: 10.1016/j.tibtech.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D, Zhu LJ, Silvestrini B, Lee WM, Cheng CY. Interactions of proteases and protease inhibitors in Sertoli-germ cell cocultures preceding the formation of specialized Sertoli-germ cell junctions in vitro. J. Androl. 1997;18:612–622. [PubMed] [Google Scholar]
- Mruk DD, Siu MK, Conway AM, Lee NP, Lau AS, Cheng CY. Role of tissue inhibitor of metalloproteases-1 in junction dynamics in the testis. J. Androl. 2003;24:510–523. doi: 10.1002/j.1939-4640.2003.tb02703.x. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Lau AS, Sarkar O, Xia W. Rab4A GTPase catenin interactions are involved in cell junction dynamics in the testis. J. Androl. 2007;28:742–754. doi: 10.2164/jandrol.106.002204. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol. Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J. 2001;20:2171–2179. doi: 10.1093/emboj/20.9.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muslin AJ, Lau JM. Differential functions of 14-3-3 isoforms in vertebrate development. Curr. Top. Dev. Biol. 2005;65:211–228. doi: 10.1016/S0070-2153(04)65008-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, et al. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J. Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport. 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J. Cell Biol. 2006;175:135–146. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr. Rev. 2008;29:465–493. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of beta 1 integrin subunit in rat seminiferous epithelium. Biol. Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr. Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Perego L, Berruti G. Molecular cloning and tissue-specific expression of the mouse homologue of the rat brain 14-3-3 theta protein: characterization of its cellular and developmental pattern of expression in the male germ line. Mol. Reprod. Dev. 1997;47:370–379. doi: 10.1002/(SICI)1098-2795(199708)47:4<370::AID-MRD3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Peterson FC, Penkert RR, Volkman BF, Prehoda KE. Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol. Cell. 2004;13:665–676. doi: 10.1016/s1097-2765(04)00086-3. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat. Cell Biol. 2004;6:297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, et al. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation, and trafficking. Biochem. J. 2004;379:395–408. doi: 10.1042/BJ20031797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalingam K, Rudel T. Ras-Raf signaling needs prohibitin. Cell Cycle. 2005;4:1503–1505. doi: 10.4161/cc.4.11.2142. [DOI] [PubMed] [Google Scholar]
- Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, et al. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat. Cell Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Roh MH, Liu CJ, Laurinec S, Margolis B. The carboxyl terminus of zona occludens-3 binds and recruits a mammalian homologue of discs lost to tight junctions. J. Biol. Chem. 2002a;277:27501–27509. doi: 10.1074/jbc.M201177200. [DOI] [PubMed] [Google Scholar]
- Roh MH, Makarova O, Liu CJ, Shin K, Lee S, Laurinec S, et al. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 2002b;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Ruiz WG, Leung SM, Jou TS, Apodaca G. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 2001;12:2257–2274. doi: 10.1091/mbc.12.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am. J. Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 365–390. [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, et al. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J. Cell Biol. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor alpha 6 beta 1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol. Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Sallee JL, Wittchen ES, Burridge K. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. J. Biol. Chem. 2006;281:16189–16192. doi: 10.1074/jbc.R600003200. [DOI] [PubMed] [Google Scholar]
- Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci. 2009;14:1129–1142. doi: 10.2741/3298. [DOI] [PubMed] [Google Scholar]
- Santoni MJ, Pontarotti P, Birnbaum D, Borg JP. The LAP family: a phylogenetic point of view. Trends Genet. 2002;18:494–497. doi: 10.1016/s0168-9525(02)02738-5. [DOI] [PubMed] [Google Scholar]
- Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J. Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shaha C. Estrogens and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science+Business Media, LLC; 2008. pp. 42–64. [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008a;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Hirsch DS, Sasiela CA, Wu WJ. Cdc42 regulates E-cadherin ubiquitination and degradation through an epidermal growth factor receptor to Src-mediated pathway. J. Biol. Chem. 2008b;283:5127–5137. doi: 10.1074/jbc.M703300200. [DOI] [PubMed] [Google Scholar]
- Shikano S, Coblitz B, Wu M, Li M. 14-3-3 proteins: regulation of endoplasmic reticulum localization and surface expression of membrane proteins. Trends Cell Biol. 2006;16:370–375. doi: 10.1016/j.tcb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Siu MK, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MK, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli–germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somanath PR, Byzova TV. 14-3-3β-Rac1–p21 activated kinase signaling regulates Akt1-mediated cytoskeletal organization, lamellipodia formation, and fibronectin matrix assembly. J. Cell Physiol. 2009;218:394–404. doi: 10.1002/jcp.21612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffler HE, Honnert U, Bauer CA, Hofer D, Schwarz H, Muller RT, Drenckhahn M, Bahler M. Targeting of the myosin-I myr 3 to intercellular adherens type junctions induced by dominant active Cdc42 in HeLa cells. J. Cell Sci. 1998;111:2779–2788. doi: 10.1242/jcs.111.18.2779. [DOI] [PubMed] [Google Scholar]
- Sun I, Wong E, Li M, Lee W, Cheng CY. 14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis. J. Endocrinol. 2009;202:327–336. doi: 10.1677/JOE-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Tcherkezian J, Lamarche-Vane N. Current knowledge of the large RhoGAP family of proteins. Biol. Cell. 2007;99:67–86. doi: 10.1042/BC20060086. [DOI] [PubMed] [Google Scholar]
- Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, et al. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J. Biol. Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- Toure A, Morin L, Pineau C, Becq F, Dorseuil O, Gacon G. Tat1, a novel sulfate transporter specifically expressed in human male germ cells and potentially linked to rhogtpase signaling. J. Biol. Chem. 2001;276:20309–20315. doi: 10.1074/jbc.M011740200. [DOI] [PubMed] [Google Scholar]
- Traweger A, Wiggin G, Taylor L, Tate SA, Metalnikov P, Pawson T. Protein phosphatase 1 regulates the phosphorylation state of the polarity scaffold Par-3. Proc. Natl. Acad. Sci. USA. 2008;105:10402–10407. doi: 10.1073/pnas.0804102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Wu WJ, Wang J, Cerione RA. Epidermal growth factor-dependent regulation of Cdc42 is mediated by the Src tyrosine kinase. J. Biol. Chem. 2003;278:49293–49300. doi: 10.1074/jbc.M307021200. [DOI] [PubMed] [Google Scholar]
- Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol. Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Valencia A, Chardin P, Wittinghofer A, Sander C. The Ras protein family: evolutionary tree and role of conserved amino acids. Biochemistry. 1991;30:4637–4648. doi: 10.1021/bi00233a001. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- Vogl AW, Pfeiffer DC, Redenbach DM, Grove B. Sertoli cell cytoskeleton. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater: Cache River Press; 1993. pp. 39–86. [Google Scholar]
- Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 186–211. [Google Scholar]
- Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74:602–607. doi: 10.1016/j.steroids.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Walsh SV, Hopkins AM, Nusrat A. Modulation of tight junction structure and function by cytokines. Adv. Drug Deliv. Rev. 2000;41:303–313. doi: 10.1016/s0169-409x(00)00048-x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hurd TW, Margolis B. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J. Biol. Chem. 2004;279:30715–30721. doi: 10.1074/jbc.M401930200. [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen XW, Margolis B. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol. Biol. Cell. 2007;18:874–885. doi: 10.1091/mbc.E06-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, et al. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim. Biophys. Acta. 2008;1782:51–59. doi: 10.1016/j.bbadis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Waterman MJ, Stavridi ES, Waterman JL, Halazonetis TD. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat. Genet. 1998;19:175–178. doi: 10.1038/542. [DOI] [PubMed] [Google Scholar]
- Weber JE, Russell LD, Wong V, Peterson RN. Three-dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli–Sertoli and Sertoli–germ-cell relationships. Am. J. Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Wells CD, Fawcett JP, Traweger A, Yamanaka Y, Goudreault M, Elder K, et al. A Rich1/Amot complex regulates the Cdc42 GTPase and apical-polarity proteins in epithelial cells. Cell. 2006;125:535–548. doi: 10.1016/j.cell.2006.02.045. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Der CJ. Rho-family GTPases: it’s not only Rac and Rho (and I like it) J. Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- Witte H, Bradke F. The role of the cytoskeleton during neuronal polarization. Curr. Opin. Neurobiol. 2008;18:479–487. doi: 10.1016/j.conb.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood–testis barrier dynamics: an in vivo study. J. Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Siu MK, Cheng CY. Blood–testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim. Biophys. Acta. 2008a;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. USA. 2008b;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EW, Sun S, Li MW, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009a doi: 10.1210/en.2009-0427. doi:10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Cdc42 regulates Sertoli cell blood–testis barrier dynamics via its effects on the events of cytokine-induced protein endocytosis. 2009b (submitted) [Google Scholar]
- Woodcock JM, Murphy J, Stomski FC, Berndt MC, Lopez AF. The dimeric versus monomeric status of 14-3-3zeta is controlled by phosphorylation of Ser58 at the dimer interface. J. Biol. Chem. 2003;278:36323–36327. doi: 10.1074/jbc.M304689200. [DOI] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P. The ghost in the machine: small GTPases as spatial regulators of exocytosis. Trends Cell Biol. 2008;18:397–404. doi: 10.1016/j.tcb.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Cheng CY. TGF-β3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: an in vivo study. Dev. Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood–testis barrier and Sertoli–germ cell adhesion. J. Biol. Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- Xia W, Wong EW, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev. Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Synapses: sites of cell recognition, adhesion, and functional specification. Annu. Rev. Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity, and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, et al. PAR-6 regulates aPKC activity in a novel way and mediates cell–cell contact-induced formation of the epithelial junctional complex. Genes Cells. 2001;6:721–731. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Blood–testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008a;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood–testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. USA. 2008b;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yuan H, Michelsen K, Schwappach B. 14-3-3 dimers probe the assembly status of multimeric membrane proteins. Curr. Biol. 2003;13:638–646. doi: 10.1016/s0960-9822(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Zahraoui A, Louvard D, Galli T. Tight junction, a platform for trafficking and signaling protein complexes. J. Cell Biol. 2000;151:F31–F36. doi: 10.1083/jcb.151.5.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bi E, Novick P, Du L, Kozminski KG, Lipschutz JH, et al. Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 2001;276:46745–46750. doi: 10.1074/jbc.M107464200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhu J, Yang GY, Wang QJ, Qian L, Chen YM, et al. Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat. Cell Biol. 2007;9:743–754. doi: 10.1038/ncb1603. [DOI] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]