Abstract
Background
The 30-item Disabilities Arm Shoulder and Hand (DASH) questionnaire was introduced to facilitate assessment of upper limb functional limitations. To improve practicality and eliminate item redundancy a modified instrument was needed. The 11-item QuickDASH was developed to fulfil these requirements and translated into several languages. However, prospective investigations of psychometric and practical characteristics are limited. No published study investigated readability or used concurrent validation with a standardized upper limb criterion measure. The validity of the QuickDASH has been questioned as the results for factor structure are conflicting, and the English-language version has not yet had factor structure reported. A shortened 9-item version, the QuickDASH-9, that addresses these issues is proposed.
Methods
This two-stage observational study assessed the psychometric and practical characteristics of the QuickDASH and the extracted QuickDASH-9. The Upper Limb Functional Index (ULFI) was the criterion standard in both stages. Stage 1, calibration, reanalyzed extracted QuickDASH and QuickDASH-9 responses from a previous prospective study, by the authors, of the 30-item DASH (n = 137). Stage 2, prospective validation, investigated the QuickDASH through repeated measures in consecutive upper limb musculoskeletal participants' consulting for physical therapy in Australia (n = 67). The QuickDASH and extracted QuickDASH-9 data from both stages was analyzed and compared for psychometric properties, practical characteristics and factor structure.
Results
The proposed QuickDASH-9 had a unidimensional structure, high reliability (ICC 2:1, r = 0.92), internal consistency (alpha = 0.93) and responsiveness (ES = 1.05). It correlated highly with both the DASH (r = 0.97), QuickDASH (r = 0.99) and ULFI criterion (r = 0.85). QuickDASH-9 missing responses reduced to 3.5% from 26% in the QuickDASH. Completion and scoring time was 134 ± 56 seconds and required a computational aid. The QuickDASH demonstrated a bidimensional structure making it invalid. The QuickDASH-9 summary performance was measured on the 'Measurement of Outcome Measures' at 88% and on the 'Bot' clinimetric scale at 75%.
Conclusions
The proposed QuickDASH-9 had a unidimensional structure and similar psychometric precision to the full-length DASH with improved practicality and completion time. The QuickDASH was invalid as its bidimensional structure made a single summated score inappropriate. The QuickDASH-9 offers a future direction for ongoing use of the QuickDASH concept.
Background
The assessment process in both the clinical and research setting has progressively incorporated patient-reported outcome (PRO) measures and upper limb assessment is no exception. Regional and condition specific PROs enable the quantification of patient impairment [1]. Doward and McKenna have described this as a '...needs based approach' [2]. This assists the clinical decision-making process [3,4] and facilitates compliance with the protocols within professional organizations [5], government agencies [6,7] and insurer groups [8]. There are limited upper limb PROs developed specifically for the region as a single kinetic chain [9] that accommodate the requirements of both the clinician and researcher in an efficient and effective manner [10,11].
The 30-item Disabilities Arm Shoulder and Hand (DASH) [9] is reported to fulfil these criteria. It was validated for a variety of disorders [1,9,12-16] and its availability in different languages has increased rapidly [17-19]. The shorter 11-item QuickDASH was developed to reduce respondent and administrative burden and eliminate item redundancy. This improved compliance [20], item redundancy and scale width for higher impairment conditions [21]. Consequently, there is an impetus for the QuickDASH to replace the DASH [22] and be advocated as a criterion standard for upper limb measurement [23,24]. However, the validity of the QuickDASH has been questioned as a consequence of conflicting findings on the factor structure [25,26]. A single factor structure is an essential property of all PROs that provide a single summated score [27]. A PRO must exhibit a single predominant theme or factor, such as upper limb function, that is common to all item-questions. The factor structure must be unidimensional when analyzed. The most appropriate method is Maximum Likelihood extraction (MLE) [28].
A literature search (PubMed, Medline, CINAHL, Embase, Cochrane and Google Scholar) found five prospective studies that investigated the QuickDASH. They considered the psychometric and practical characteristics in general populations [24,25,29], burns patients [30] and as a work injury prediction tool [31]. The original validation [20] and several subsequent studies reanalyzed data with the eleven items extracted from existing 30-item DASH responses [21,26,32]. Only two studies investigated the QuickDASH factor structure. There was a unidimensional structure in the prospective study on the Japanese-language version [25] but a bidimensional structure in reanalyzed extracted data of the French-language version [26]. Both authors used principal component analysis which is considered inappropriate for PROs [28]. Factor structure in the English-language version has not been reported. Consequently, the factor structure must be clarified and determined prospectively with appropriate item-extraction methodology in a general upper limb population.
The primary aim of this study was to determine the factor structure of the QuickDASH and QuickDASH-9. If unidimensional and valid, the next step was to calibrate and validate the psychometric properties and practical characteristics in independent general upper limb populations. Finally these characteristics were compared and correlated with the original full-length DASH and a validated criterion standard, the Upper Limb Functional Index (ULFI) [11,33].
Methods
Development of the QuickDASH-9
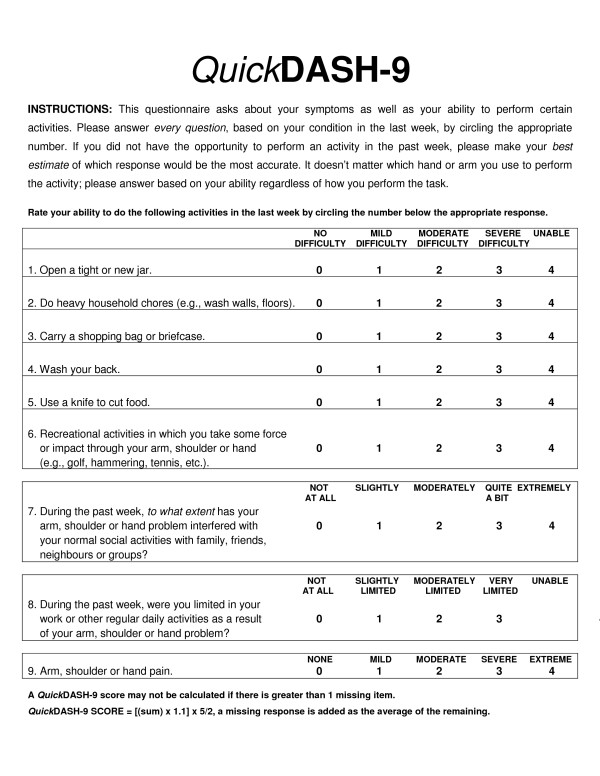
The concept-retention methodology used to reduce the full-length DASH to the QuickDASH [20,34] was employed to produce the QuickDASH-9 (Figure 1). The authors used consensus agreement following feedback from a practicality focus-group composed of 20 patients and five therapists. This ensured face and content validity would be consistent with the QuickDASH. Items #10 (Pins and needles) and #11 (Sleep) were removed as neither are an activity of daily living. It was hypothesised these changes would enable the QuickDASH-9 to exhibit a unidimensional factor structure. The scoring system was also modified from the existing 1-5 scale to a 0-4 scale and the calculation for scoring adjusted accordingly.
Figure 1.
QuickDASH-9.
Design
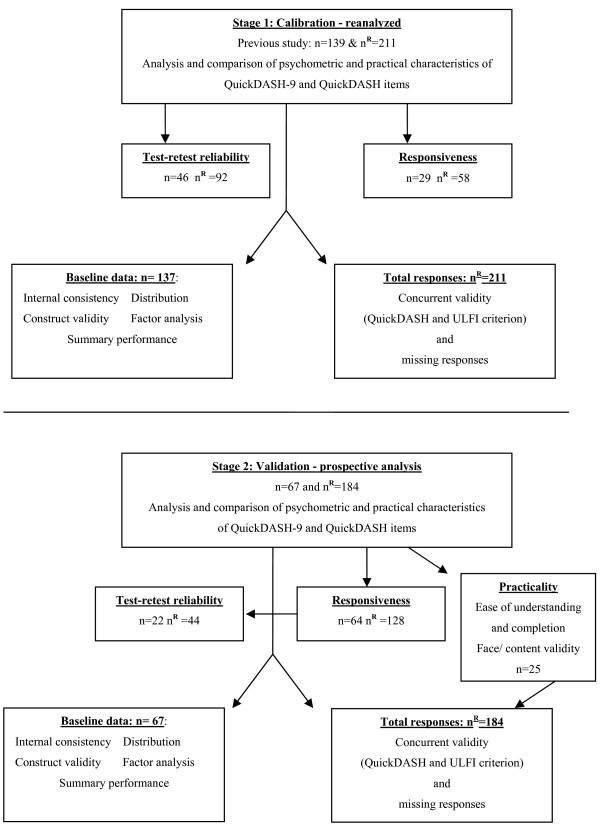
A two stage observational study was used. Stage 1, calibration, extracted the items from the DASH responses in a previous study [11] to form the QuickDASH-9 and QuickDASH. Stage 2, prospective validation, concurrently measured the QuickDASH and ULFI. The QuickDASH-9 scores were determined from extracted QuickDASH responses (Figure 2).
Figure 2.
Flow chart of calibration from stage 1 and validation stage 2. All QuickDASH-9 data was extracted from the QuickDASH; n = total number of participants; nR = total number of responses; practicality n = 25 composed 20 patients and 5 therapists.
Assessment Questionnaires
The DASH is a four-page 30-item PRO on a 5-point Likert scale (1-5). Subsequent raw scores range from 30 to 150 and are converted to a percentage, 0 (no disability) to 100 (most severe disability) [9]. It has two optional sport or music and work scales, not used in this study. Up to three missing responses are permitted [35]. The QuickDASH is a single-page PRO with eleven items extracted from the DASH [20]. It uses the DASH scale and scoring method and allows for one missing response [9].
The QuickDASH-9 is a single-page PRO with nine items extracted from the QuickDASH and DASH. It uses the DASH scoring method on a 0-4 Likert scale and allows for one missing response (Figure 1).
The ULFI is a single-page 25-item PRO on a 3-point Likert scale. Subsequent raw scores range from 0-25 and are multiplied by four to provide a percentage scale, 0 (normal) to 100 (maximum impairment). Up to two missing responses are permitted [11,33]. It has an 11-point global 'Numeric Rating Scale' (NRS) to assess overall status with anchors of 0 ('normal or pre-injury') to 10 ('worst possible'). Two optional components provide a qualitative 'Patient Specific Index' and a self-assessed ranking of duties that can be used to calculate a 'Global Assessment of Body And Limbs' score [36].
Setting and Participants
Participants with upper limb musculoskeletal conditions under referral from a medical practitioner were recruited consecutively or successively from primary care physical therapy outpatient clinics. These conditions included soft tissue injury, post surgery, lymphoedema, fractures, chronic regional pain and trauma. Exclusion criteria were <18 years of age, difficulty with English language comprehension and cognitive impairment. Symptom duration ranged from one week to eight years with a mean of 38.7 ± 41.6 weeks. Removal of one outlier at eight years reduced the mean duration to 20.3 ± 25.2 weeks.
Participants receiving ongoing treatment during both the calibration and validation stages were measured at baseline, then at two weekly intervals for six weeks, then four weekly thereafter until discharge. Status was classified as: acute - injured within the previous six weeks; subacute - six to twelve weeks; and chronic - greater than twelve weeks [37].
Study Stages
Stage 1, calibration
Existing data was reanalyzed. This included 211 responses from 137 participants from nine physical therapy outpatient centres in three different Australian states. The methodology is described in a previous publication by the authors [11]. Demographic details are presented in Table 1.
Table 1.
Participant demographics
| Demographics | Stage 1 Calibration (Gabel et al 2006) |
Stage 2 Validation ULFI, QuickDASH |
|---|---|---|
| Participants (n) | 137 | 67 |
| Responses (n) | 211 | 184 |
| Age (years) | 48.4 ± 15.6 | 48.3 ± 18.6 |
| Gender: Female (%) | 54 | 35 |
| Dominance: Right (%) | 77 | 93 |
| Injury: Duration (weeks) | 24.5 ± 28.8 | 11.7 ± 17.8 |
| Time range (weeks) | 1 - 433 | 1 - 80 |
| Work status: Employed (%) | 61 | 57 |
| Retired (%) | 0 | 36 |
| Unemployed (%) | 39 | 7 |
| Injured at work (%) | 40 | 23 |
| On work cover (%) | 30 | 23 |
Stage 2, validation
A prospective investigation examined 184 responses from 67 participants, recruited from six physical therapy outpatient centres in one Australian state. Demographic details are presented in Table 1. Repeated measures were made for subgroups of responsiveness (n = 64) and reliability (n = 22). This provided prospective investigation of the concurrently completed QuickDASH and ULFI to determine psychometric and practical characteristics (Figure 2). All QuickDASH-9 responses were extracted from the QuickDASH.
Analysis - Methodological Characteristics
Test-retest reliability
The ICC (2:1) [38] was used at 72 hours from baseline during a period of non-treatment with the NRS as an external reference [3,9,11].
Responsiveness
Effect size (ES) and standard response mean (SRM) were used [39]. The NRS provided an external reference standard. Two compared measures were taken. The first at baseline, with the repeated measures made following a period of anticipated change due to natural healing and therapist intervention. These periods were consequently a partial duration of the injury classification being: two weeks for acute participants, four weeks for subacute and six weeks for chronic [9,11,40].
Measurement error
The minimal detectable change was taken at the 90% level (MDC90) [41].
Validity
Face and content validity were determined from the development studies [11,20,21] and supported in this study by the practicality focus-group (Figure 2). Criterion or concurrent validity was assessed using a Pearson correlation coefficient. Construct validity was demonstrated by a standard t-test that verified change between the baseline and the repeated measures [11,20].
Internal consistency
Distribution and normality
This was determined through inspection of the histograms and the one-sample Kolmogorov-Smirnov (KS) test [44].
Factor analysis
The MLE method was used [28] with varimax rotation if two or more factors were determined and coefficient suppression was set at 0.5 [44,45]. Factor extraction was determined a-priori by: the scree-plot curve point of inflection [46]; an eigenvalue cut-off of 1.0 [47]; and that ≥ 10% of total explained variance was accounted for where average communality after extraction was ≥ 0.6 [45].
Sample size
To ensure sufficient sample power to provide an 80% confidence level in determining actual change above 10.5%, the MDC90 for the DASH, the Dawson and Trapp method was used [48] where required, sample size (n) is:
(U1-U0) = clinically important difference between the means; SD = standard deviation in the population. Za = two tailed and Zb = lower tail as defined from Tables of significance levels.
Analysis - Practical Characteristics
Missing responses
These were noted as a percentage of total responses.
Completion and scoring time
These were calculated from the average of three separate tests in the practicality focus group.
Readability
The Flesch-Kincaid reading scale was used to determine ease of comprehension and readability [49,50] and calculated from the grammar function from within the word processing program.
Summary performance
Two clinimetric scales were used. The 25-item 'Measurement of Outcome Measures' that considered a measures characteristics under four categories: methodological, practical, distributional and general. The total was summated and multiplied by four to provide scores from 0 to 100% [11]. The 12-item 'Bot scale' considered twelve individual practical and methodological characteristics of a measure and is scored on a 0-12 scale that can be converted to a percentage [16].
Statistical analysis
The Statistical Package for Social Sciences version 14.0 (SPSS Inc, Chicago, IL) was used to analyze the data on an intention-to-treat principle. Statistical sigificance was accepted at the p < 0.05 level. Pooled samples of each questionnaire enabled determination of distribution, missing responses, internal consistency and factor analysis.
Ethics
Ethics approval was given by the University of the Sunshine Coast Human Research Ethics Committee.
Results
Factor Structure
The QuickDASH-9, DASH and the ULFI each had a unidimensional structure determined for their factor matrix in both stages, so no varimax rotation occurred. The QuickDASH had a bidimensional structure, invalidating any single summated score and precluding any further valid analysis of its psychometric properties.
Factor loadings for all items in both the QuickDASH and QuickDASH-9 exceeded the 0.50 suppression level. In the calibration stage the QuickDASH-9 and QuickDASH had primary eigenvalues of 5.4 and 5.7 respectively which accounted for 54% and 62% of variances. In the validation stage these increased respectively to eigenvalues of 6.1 and 6.5 which accounted for 61% and 59% of variance. The QuickDASH-9 factor order was consistent apart from question-item #5 'Use knife' which loaded sixth in the calibration and first in the validation stage (Table 2). The QuickDASH demonstrated identical factor order in both stages (Table 3); however, in addition to the invalid bidimensional structure, one item 'Limited in work' changed factors and another 'Socialize' had cross-loading in the validation stage.
Table 2.
QuickDASH-9 factor matrix
| Stage 1 Calibration (n = 137) | Factor | Stage 2 Validation (n = 67) | |||
|---|---|---|---|---|---|
| Question # | Item | 1 | 1 | Item | Question # |
| 2 | Heavy chores | .796 | .850 | Use knife | 5 |
| 3 | Carry bag | .744 | .828 | Heavy chores | 2 |
| 8 | Limited in work | .743 | .790 | Limited in work | 8 |
| 1 | Open jar | .704 | .782 | Open jar | 1 |
| 6 | Forceful recreation | .701 | .769 | Carry bag | 3 |
| 5 | Use knife | .696 | .749 | Wash back | 4 |
| 7 | Socialize | .667 | .718 | Forceful recreation | 6 |
| 4 | Wash back | .645 | .709 | Socialize | 7 |
| 9 | Pain intensity | .565 | .648 | Pain intensity | 9 |
Maximum likelihood extraction with suppression at 0.50. No varimax rotation as only 1-factor is extracted.
Table 3.
QuickDASH rotated factor matrix
| Stage 1 Calibration (n = 137) | Stage 1 Factors | Stage 2 Factors | Stage 2 Validation (n = 67) | ||||
|---|---|---|---|---|---|---|---|
| Question # | Item | 1 | 2 | 1 | 2 | Item | Question # |
| 2 | Heavy chores | .823 | .906 | Heavy chores | 2 | ||
| 1 | Open jar | .709 | .888 | Open jar | 1 | ||
| 5 | Use knife | .661 | .701 | Use knife | 5 | ||
| 3 | Carry bag | .642 | .684 | Carry bag | 3 | ||
| 6 | Forceful recreation | .618 | .621 | Forceful recreation | 6 | ||
| 4 | Wash back | .616 | .567 | Wash back | 4 | ||
| 8 | Limited in Work | .589 | .859 | Limited in work | 8 | ||
| 9 | Pain intensity | .894 | .808 | Pain intensity | 9 | ||
| 11 | Sleep | .736 | .775 | Sleep | 11 | ||
| 10 | Pins and needles | .539 | .680 | Pins and needles | 10 | ||
| 7 | Socialize | .512 | .543 | .615 | Socialize | 7 | |
Maximum likelihood extraction and varimax rotation with suppression at 0.50.
Psychometric properties
These are presented for each PRO in Table 4 with the construct validity in Table 5. The values for the QuickDASH are invalid but are provided as a comparison to the other PROs and to the findings of previous QuickDASH studies.
Table 4.
Methodological characteristics of QuickDASH-9, QuickDASH, DASH and ULFI
| Reliability | Internal consistency | Error score | Responsiveness | Missing responses | ||||
|---|---|---|---|---|---|---|---|---|
| Stage | Rxx (ICC) | Alpha | SEM | MDC90 | SD100 | ES | SRM | Percentage |
| Calibration | (n = 46) | (n = 139) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 29) | (n = 137) |
| QuickDASH-9 | 0.94 | 0.89 | 4.82 | 11.22% | 20.05% | 1.19 | 1.65 | 11% |
| QuickDASH | 0.94 | 0.92 | 4.98 | 11.58% | 20.71% | 1.21 | 1.75 | 12.5% |
| DASH | 0.98 | 0.96 | 2.84% | 6.63% | 19.67% | 1.41 | 2.18 | 34%% |
| ULFI | 0.96 | 0.89 | 4.50% | 10.50% | 21.61% | 1.28 | 1.87 | <0.5% |
| Validation | (n = 22) | (n = 67) | (n = 64) | (n = 64) | (n = 64) | (n = 64) | (n = 64) | (n = 67) |
| QuickDASH-9 | 0.94 | 0.925 | 7.38% | 17.18% | 26.07% | 1.05 | 1.21 | 3.5% |
| QuickDASH | 0.91 | 0.92 | 6.73% | 15.66% | 23.20% | 1.05 | 1.23 | 26.5% |
| ULFI | 0.98 | 0.92 | 3.41% | 7.93% | 24.16% | 0.93 | 1.25 | <0.5% |
The QuickDASH psychometric properties are invalid. They are provided as a comparison to the other PROs and to the findings of previous QuickDASH studies.
SD100: Standard deviation at baseline (100% scale); Rxx: Test-retest reliability coefficient; ICC: Intra-class Correlation Coefficient for test-retest reliability; SEM: Standard Error of the Measurement; MDC90: Minimal Detectable Change (90% CI); ES: Effect Size; SRM: Standard Response Mean; Alpha: Cronbach's Alpha.
Table 5.
Construct validity comparison between baseline and repeated measures
| Stage | Baseline Mean |
Repeat Test * Mean |
Paired t-Stat ** |
|---|---|---|---|
|
Calibration Sample n = 31 |
|||
| QuickDASH-9 | 54.9 ± 20.5 | 46.9 ± 26.6 | 5.2 |
| QuickDASH | 58.1 ± 20.8 | 40.6 ± 23.1 | 5.7 |
| DASH | 51.6 ± 20.5 | 34.7 ± 22.2 | 6.4 |
| ULFI | 58.1 ± 23.0 | 41.3 ± 26.6 | 5.6 |
|
Validation Sample n = 64 |
|||
| QuickDASH-9 | 47.0 ± 26.1 | 23.2 ± 18.6 | 4.1 |
| QuickDASH | 44.4 ± 23.2 | 20.0 ± 16.1 | 4.2 |
| ULFI | 41.5 ± 24.6 | 19.8 ± 19.1 | 7.6 |
Distribution
The impairment range of 0-100% was shown for all PROs with the number of 5% histogram increments for the total score being QuickDASH-9 = 19, QuickDASH = 18 and DASH = 17 whilst the ULFI had values in all 20 increments.
Practical Characteristics
Missing responses
These are detailed in Table 4.
Completion and scoring times
The QuickDASH-9 and QuickDASH were respectively 134 ± 56 seconds and 155 ± 64 seconds and both required a computational aid. The ULFI was 132 ± 51 seconds.
Readability
This was found at grade twelve for the QuickDASH-9 and at grade seven for the ULFI.
Summary performance
The 'Measurement of Outcome Measures' score for the QuickDASH-9 was 88%, the DASH was 72% and the ULFI was 96%. On the 12-point Bot scale the score for the QuickDASH-9 was nine (75%), the DASH was seven (58%) and the ULFI was twelve (100%). The QuickDASH was invalid with respective clinimetric scores of 44% and three (25%).
Discussion
This study proposes the QuickDASH-9, with its valid unidimensional structure, as a way to overcome the existing shortcomings of the QuickDASH. This will enable the concept to continue. The modifications that produce the QuickDASH-9 fulfil the original aims of the QuickDASH [20]: a shortened version of the full-length DASH with comparable or preferable psychometric properties, improved practicality and the elimination of item redundancy [9,11]. In attempting to achieve these aims the QuickDASH produced a bidimensional factor structure. Its validity as a single summated score cannot be supported.
Our findings propose the DASH scoring scale of 1-5 be modified to 0-4 in the QuickDASH-9. This uses the established format of a 0 based anchor rather than a 1 [51]. This should facilitate practicality and ensure consistency of scoring with other PROs.
The bidimensional structure of the QuickDASH, demonstrated in this study using MLE, is consistent with previous findings by Fayad [26] but conflicts with the unidimensional structure found by Imaeda [25]. However, both previous researchers used principal component analysis which is not recommended [28]. In this study the QuickDASH bidimensional structure demonstrated two factors that can be broadly divided into 'activity' and 'non-activity' items which supports previous findings [21,26]. The original DASH has a unidimensional structure [33,52-54]. This means the reductive process of concept-retention methodology, that reduces the DASH's 30 items to eleven in the QuickDASH, causes a fundamental change in the factor structure [55]. It is critical that a PRO exhibits a unidimensional structure if it is to accurately reflect the measured region with a single summated score [27].
There is a distinct lack of prospective studies of the QuickDASH and no English versions were found that investigated factor structure. Furthermore, reporting of psychometric properties is incomplete if the factor structure is not stated [24,29-31], and consequently misleading and the results invalid if the structure is not unidimensional.
The use of extracted items from the DASH as the sole method to validate the QuickDASH without prospective testing [20,21,26,32], should only be investigatory. This methodology risks shared measurement error and does not account for part or whole correlation [56] which can lead to type I errors [43]. By completing the prospective aspect of this study on a general upper limb population with a consistent regional reference standard, the ULFI, these error concerns are alleviated for the QuickDASH. However, for the QuickDASH-9 the same criticism applies as it is investigatory research only.
Should the findings of this study be supported by further research, then the QuickDASH-9 would be appropriate to replace the QuickDASH and also the original DASH. Similar proposals are already in place in other body regions. The Neck Disability Index, an advocated PRO, was recently shown to be invalid due to its bidimensional structure [57,58]. It is proposed that a shortened unidimensional version, the NDI-8 replaces the original [58].
The reliability and responsiveness are lower in the QuickDASH-9 compared to the DASH. This is anticipated and consistent with previous QuickDASH findings [20,21,26,32] as the reduction in items from 30 to nine is substantial.
The QuickDASH-9 mean percentage scores were found to be higher than those of the DASH. This supports previous findings that a shortened tool with improved internal consistency will show greater scale width, particularly for higher impairment conditions [21]. The choice of eleven items for the QuickDASH is based on the a-priori assumption drawn from the 'Spearman-Brown prophesy'. Specifically, that a minimum of eleven items is required to produce an internal consistency within the clinically accepted range of 0.90 to 0.95 [20]. This study has shown that in a shortened 9-item version, the internal consistency can remain within this range and provide a valid instrument with significant gains in practicality. However, a computational scoring aid is still required.
In both stages of this study the QuickDASH-9 showed inferior psychometric properties to the DASH and ULFI, particularly for reliability and error scores. In relation to the DASH this is outweighed by the gains in practicality and internal consistency, but not in comparison to the ULFI. These findings are reflected in the summary scores of the 'Measurement of Outcome Measures' and the 'Bot scale' that supports the preference of the QuickDASH-9 over the DASH. However, both tools remained notably lower than the ULFI on both scales which scores as the preferred instrument for both clinical and research purposes due to its practicality and lower missing responses.
Limitations
The study investigated only outpatients presenting to primary care physical therapy practices and further research is required to clarify these findings in an inpatient setting. The findings are general and extrapolation to specific conditions must be made with caution till such conditions are individually investigated. There was a consistent difference in the QuickDASH-9 order of factor loading between the calibration and validation stages. This is most likely from differences in the samples due to the diverse range of diagnoses and duration times used in each stage.
Strengths
The findings have broad implications for use in the general population as they are not specific to one condition or population group as participants were from general outpatient populations. Two independent population samples are used for data extraction to examine the QuickDASH-9 characteristics. The use of a consistent reference criterion, the ULFI, supports the similarity of findings in the two samples.
Implications for Practice
The QuickDASH-9 as a valid shortened form of the DASH provides a practical approach to measurement of the upper limb. This enhanced practicality reduces the burden to both the patient and clinician, optimizing clinical practice without compromising the accuracy and error measurement capacity of the instrument.
Implications for Research
A prospective validation of the QuickDASH-9 is required in an independent sample using an established criterion, such as the ULFI. Further investigation of the psychometric properties in samples of specific populations and conditions is also required. This could initially be investigative through extraction of responses from existing DASH and QuickDASH studies, with prospective investigation to follow. However, with the summary performance of all forms of the DASH concept shown to be lower than the ULFI, the adoption of the ULFI as a single preferred standard may be preferable.
Conclusions
The unidimensional structure found in the proposed QuickDASH-9 is valid and consistent with the full-length DASH. This achieves the original aim of the QuickDASH, to be a shortened version with comparable or preferable psychometric properties, no item redundancy and higher practicality. The QuickDASH, with a bidimensional structure, is invalid for the production of a summated score. This shortcoming is overcome by the QuickDASH-9. Furthermore, the QuickDASH-9 eliminates item redundancy found in the DASH, improves internal consistency, completion and scoring times and enhances practicality. The QuickDASH-9 offers a viable future option for the DASH concept.
Abbreviations
DASH: Disabilities of Arm; Shoulder and Hand questionnaire; ES: Effect size; ICC: Intraclass correlation coefficient; KS: Kolmogorov-Smirnov test for normality; n: number of participants; nR: number of responses; MDC: Minimal detectable change; MLE: Maximum likelihood extraction; NDI: Neck Disability Index; NRS: Numeric Rating Scale; PRO: Patient Reported Outcome; QuickDASH: Shortened 11-item version of Disabilities of Arm, Shoulder and Hand questionnaire; QuickDASH-9: Shortened 9-item version of Disabilities of Arm, Shoulder and Hand questionnaire; SD: Standard deviation; SRM: Standard response mean; ULFI: Upper Limb Functional Index.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CPG is the principal investigator. He designed the study and is responsible for the protocol. CPG is also responsible for data acquisition and analysis. Together with CPG, BB and MY developed the key ideas underlying this study, interpreted the data, wrote and revised the manuscript. MM has been involved in interpretation of the data and revising the manuscript critically for shortcomings of the original QuickDASH and for the validation of the QuickDASH-9. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
C Philip Gabel, Email: cp.gabel@bigpond.com.
Michael Yelland, Email: m.yelland@griffith.edu.au.
Markus Melloh, Email: markus.melloh@otago.ac.nz.
Brendan Burkett, Email: bburkett@usc.edu.au.
Acknowledgements
We gratefully acknowledge the Griffith University School of Medicine for its support through a writing bursary to the principle author. Furthermore, we would like to thank all participating patients, General Practitioners and therapists for their time and effort.
References
- Angst F, Goldhahn J, Drerup S, Aeschlimann A, Schwyzer H, R S. Responsiveness of six outcome assessment instruments in total shoulder arthroplasty. Arthritis Care Res. 2008;59(3):391–398. doi: 10.1002/art.23318. [DOI] [PubMed] [Google Scholar]
- Doward LC, McKenna SP. Defining Patient-Reported Outcomes. Value Health. 2004;7(S1):S4–S8. doi: 10.1111/j.1524-4733.2004.7s102.x. [DOI] [PubMed] [Google Scholar]
- Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89(1):69–74. doi: 10.1016/j.apmr.2007.08.126. [DOI] [PubMed] [Google Scholar]
- Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumat. 2002;14(2):109–114. doi: 10.1097/00002281-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Policy on outcome measures and treatment justification. http://www.tac.vic.gov.au/upload/TAC+Physio+Chart.pdf
- Management of Soft Tissue Injuries. http://www.workcover.nsw.gov.au/Documents/Publications/InjuryManagementRTW/RehabilitationProviders/overview_improving_outcomes_5364.pdf
- Website for the Canadian Institute for Work Health. http://www.iwh.on.ca
- The Clinical Framework for the Delivery of Health Services. http://www.tac.vic.gov.au/upload/clinical-framework-single.pdf
- Beaton DE, Katz NK, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole of the parts? Validity, reliability, and responsiveness of Disabilities of the Arm Shoulder and Hand outcome measure in different regions of the upper limb. J Hand Ther. 2001;14:128–146. [PubMed] [Google Scholar]
- Michener LA, Leggins BG. A review of self-report scales for the assessment of functional limitation and disability of the shoulder. J Hand Ther. 2001;14:68–76. doi: 10.1016/s0894-1130(01)80036-3. [DOI] [PubMed] [Google Scholar]
- Gabel CP, Michener L, Burkett B, Neller A. The Upper Limb Functional Index (ULFI): Development and Determination of Reliability, Validity and Responsiveness. J Hand Ther. 2006;19(3):328–349. doi: 10.1197/j.jht.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Hudak PL, Amadio PC, Bombardier C. (UECG) TUECG. Development of an upper extremity outcome measure: The DASH (Disabilities of the Arm, Shoulder, and Head) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Davis AM, Beaton DE, Hudak P, Amadio P, Bombardier C, Cole C, Hawker G, Katz JN, Makela M, Marx RG. Measuring disability of the upper extremity: a rationale supporting the use of a regional outcome measure. J Hand Ther. 1999;12:269–274. doi: 10.1016/s0894-1130(99)80063-5. [DOI] [PubMed] [Google Scholar]
- MacDermid JC, Tottenham V. Responsiveness of the disability of the arm, shoulder, and hand (DASH) and patient-rated wrist/hand evaluation (PRWHE) in evaluating change after hand therapy. J Hand Ther. 2004;17(1):18–23. doi: 10.1197/j.jht.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Bialocerkowski A. Disabilities of the arms, shoulder and hand questionnaire. Aust J Physiother: Clinimetrics. 2007;53(2):135. doi: 10.1016/s0004-9514(07)70050-8. [DOI] [PubMed] [Google Scholar]
- Bot SD, Terwee CB, Windt DA van der, Bouter LM, Dekker J, de Vet HC. Clinimetric evaluation of shoulder disability questionnaires: a systematic review of the literature. Ann Rheum Dis. 2004;63(4):335–341. doi: 10.1136/ard.2003.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SJ, Parnianpour M, Abedi M, Askary-Ashtiani A, Karimi A, Khorsandi A, Mehdian H. Cultural adaptation and validation of the Persian version of the Disabilities of the Arm, Shoulder and Hand (DASH) outcome measure. Clin Rehabil. 2008;22(8):749–757. doi: 10.1177/0269215508085821. [DOI] [PubMed] [Google Scholar]
- Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4(11) doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Development and testing of the DASH and Quick-DASH Outcome Measure Instruments and the DASH User's Manual. http://www.dash.iwh.on.ca
- Beaton DE, Wright JG, Katz JN, Group UEC. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–1046. doi: 10.2106/JBJS.D.02060. [DOI] [PubMed] [Google Scholar]
- Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7(4) doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt MC, Veale GA. Early return to work following open carpal tunnel decompression in lamb freezing workers. J Hand Surg Eur Vol. 2008;33(4):440–444. doi: 10.1177/1753193408090145. [DOI] [PubMed] [Google Scholar]
- Atroshi I, Lyrén PE, Gummesson C. The 6-item CTS symptoms scale: a brief outcomes measure for carpal tunnel syndrome. Qual Life Res. 2009;18(3):347–358. doi: 10.1007/s11136-009-9449-3. [DOI] [PubMed] [Google Scholar]
- Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18(6):920–6. doi: 10.1016/j.jse.2008.12.015. Epub 2009 Mar 17. [DOI] [PubMed] [Google Scholar]
- Imaeda T, Toh S, Wada T, Uchiyama S, Okinaga S, Kusunose K, Sawaizumi T. Impairment Evaluation Committee, Japanese Society for Surgery of the Hand. Validation of the Japanese Society for Surgery of the Hand Version of the Quick Disability of the Arm, Shoulder, and Hand (QuickDASH-JSSH) questionnaire. J Orthop Sci. 2006;11(3):248–253. doi: 10.1007/s00776-006-1013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayad F, Lefevre-Colau MM, Gautheron V, Macé Y, Fermanian J, Mayoux-Benhamou A, Roren A, Rannou F, Roby-Brami A, Revel M. Reliability, validity and responsiveness of the French version of the questionnaire Quick Disability of the Arm, Shoulder and Hand in shoulder disorders. Man Ther. 2009;14(6):206–12. doi: 10.1016/j.math.2008.01.013. Epub 2008, Apr 23. [DOI] [PubMed] [Google Scholar]
- Meads D, Doward L, McKenna S, Fisk J, Twiss J, Eckert B. The development and validation of the Unidimensional Fatigue Impact Scale (U-FIS) Mult Scler. 2009. in press . [DOI] [PubMed]
- Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4(272-299) [Google Scholar]
- Matheson LN, Melhorn JM, Mayer TG, Theodore BR, Gatchel RJ. Reliability of a visual analog version of the QuickDASH. J Bone Joint Surg Am. 2006;88(8):1782–1787. doi: 10.2106/JBJS.F.00406. [DOI] [PubMed] [Google Scholar]
- Wu A, Edgar DW, Wood FM. The QuickDASH is an appropriate tool for measuring the quality of recovery after upper limb burn injury. Burns. 2007;33(7):843–849. doi: 10.1016/j.burns.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Stover B, Silverstein B, Wickizer T, Martin DP, Kaufman J. Accuracy of a disability instrument to identify workers likely to develop upper extremity musculoskeletal disorders. J Occ Rehab. 2007;17(2):227–245. doi: 10.1007/s10926-007-9083-2. [DOI] [PubMed] [Google Scholar]
- Abramo A, Kopylov P, Tagil M. Evaluation of a treatment protocol in distal radius fractures: a prospective study in 581 patients using DASH as outcome. Acta Orthop. 2008;79(3):376–385. doi: 10.1080/17453670710015283. [DOI] [PubMed] [Google Scholar]
- Gabel CP, Michener LA, Melloh M, Burkett BB. The Upper Limb Functional Index (ULFI) - Validation with Improved Psychometric and Practical Characteristics Using a Three-Point Likert Scale. J Hand Ther. 2010. http://www.jhandtherapy.org/article/S0894-1130(09)00127-6/abstract [DOI] [PubMed]
- Gabel CP, Burkett B, Yelland M. Short Version Outcome Measures: Balancing Fidelity and Practicality. Phys Ther Rev. 2009;14(4):221–225. doi: 10.1179/174328809X452890. [DOI] [Google Scholar]
- McConnell S, Beaton DE, Bombardier C. The DASH Outcome Measure User's Manual. Toronto, Canada: Institute for Work and Health; 1999. [Google Scholar]
- Gabel P, Barden L, Burkett B, Neller L. Integrating Injury Screening with Measurement and Monitoring: A Conceptual Approach Using A Patient Global Assessment of Body and Limbs Scale. South African Journal of Physiotherapy. 2006;62(4):2–8. [Google Scholar]
- O'Halloran J, Miller GC, Britt H. Defining chronic conditions for primary care with ICPC-2. Fam Pract. 2004;21(4):381–386. doi: 10.1093/fampra/cmh407. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Liang MH. Evaluating measurement responsiveness. J Rheumatol. 1995;22:1191–1192. [PubMed] [Google Scholar]
- Liang MH. Longitudinal construct validity: establishment of clinical meaning in patient evaluative instruments. Med Care. 2000;38(9 Suppl):II84–90. [PubMed] [Google Scholar]
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: description, application and alternatives. J Consult Clin Psychol. 1999;67:300–307. doi: 10.1037/0022-006X.67.3.300. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi: 10.1007/BF02310555. [DOI] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw-Hill; 1994. [Google Scholar]
- Fields A. Discovering Statistics using SPSS. 2. London: SAGE Publications Ltd; 2005. [Google Scholar]
- Stevens JP. Applied multivariate statistics for the Social Sciences. 2. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioural Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20:141–151. doi: 10.1177/001316446002000116. [DOI] [Google Scholar]
- Dawson B, Trapp R. Basic and Clinical Biostatistics. 2. Sydney: Mcgraw Hill; 2001. [Google Scholar]
- Doak CC, Doak LG, Root JH. Teaching patients with low literacy skills. 2. Philadelphia: J.B. Lippincott; 1996. [Google Scholar]
- Paasche-Orlow MK, Taylor HA, Brancati FL. Readability Standards for Informed-Consent Forms as Compared with Actual Readability. N Engl J Med. 2003;348(8):721–726. doi: 10.1056/NEJMsa021212. [DOI] [PubMed] [Google Scholar]
- De Vet HC, Ostelo RW, Terwee CB, Roer N van der, Knol DL, Beckerman H, Boers M, Bouter LM. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131–142. doi: 10.1007/s11136-006-9109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EW, Chung MM, Li AP, Lo SK. Construct validity of the Chinese version of the disabilities of the arm, shoulder and hand questionnaire (DASH-HKPWH) J Hand Surg [Br] 2005;30(1):29–34. doi: 10.1016/j.jhsb.2004.09.010. [DOI] [PubMed] [Google Scholar]
- MacDermid JC, Wessel J, Humphrey R, Ross D, Roth JH. Validity of self-report measures of pain and disability for persons who have undergone arthroplasty for osteoarthritis of the carpometacarpal joint of the hand. Osteoarthritis Cartilage. 2007;15(5):524–530. doi: 10.1016/j.joca.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Themistocleous GS, Goudelis G, Kyrou I, Chloros GD, Krokos A, Galanos A, Gerostathopoulos NE, Soucacos PN. Translation into Greek, cross-cultural adaptation and validation of the Disabilities of the Arm, Shoulder, and Hand Questionnaire (DASH) J Hand Ther. 2006;19(3):350–357. doi: 10.1197/j.jht.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Reeve B, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, Thissen D, Revicki D, Weiss DJ, Hambleton RK. Psychometric Evaluation and Calibration of Health-Related Quality of Life Item Banks: Plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45(5 (Suppl 1)):S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. The Patient-Reported Outcomes Measurement Information System (PROMIS) Overview and Developmental Work) [DOI] [PubMed] [Google Scholar]
- Coste J, Guillemin F, Pouchot J, Fermanian J. Methodological approaches to shortening composite measurement scales. J Clin Epidemiol. 1997;50(3):247–252. doi: 10.1016/S0895-4356(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Young SB, Aprill C, Braswell J, Ogard W, Richards JS, McCarthy JP. Psychological Factors and Domains of Neck Pain Disability. Pain Med. 2009;10(2):310–318. doi: 10.1111/j.1526-4637.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- Velde G van der, Beaton D, Hogg-Johnston S, Hurwitz E, Tennant A. Rasch analysis provides new insights into the measurement properties of the neck disability index. Arthritis Rheum. 2009;61(4):544–551. doi: 10.1002/art.24399. [DOI] [PubMed] [Google Scholar]