Abstract
Sudden cardiac and cerebral events are most common in the morning. A fundamental question is whether these events are triggered by the increase in physical activity after waking, and/or a result of circadian variation in the responses of circulatory function to exercise. Although signaling pathways from the master circadian clock in the suprachiasmatic nuclei to sites of circulatory control are not yet understood, it is known that cerebral blood flow, autoregulation and cerebrovascular reactivity to changes in CO2 are impaired in the morning and, therefore, could explain the increased risk of cerebrovascular events. Blood pressure (BP) and the rate pressure product (RPP) show marked ‘morning surges’ when people are studied in free-living conditions, making the rupture of a fragile atherosclerotic plaque and sudden cardiac event more likely. Since cerebral autoregulation is reduced in the morning, this surge in BP may also exacerbate the risk of hemorrhagic and ischemic strokes in the presence of other acute and chronic risk factors. Increased sympathetic activity, decreased endothelial function, and increased platelet aggregability could also be important in explaining the morning peak in cardiac and cerebral events but how these factors respond to exercise at different times of day is unclear. Evidence is emerging that the exercise-related responses of BP and RPP are increased in the morning when prior sleep is controlled. We recommend that such ‘semi-constant routine’ protocols are employed to examine the relative influence of the body clock and exogenous factors on the 24-h variation in other circulatory factors.
Keywords: Blood pressure, Blood flow, Hemostasis, Endothelial function, Suprachiasmatic nuclei, Exercise, Thrombosis, Stroke
Introduction
Chronobiology is the study of cyclic variations in living organisms (Dunlap et al. 2004). Biological rhythms can be found in many time domains, ranging from milliseconds to years. In fact, it is difficult to find a physiological function which does not vary over time in a cyclical manner. Since humans are diurnally active animals, it is not surprising that biological rhythms that fluctuate over a 24-h period have the most fundamental effects on physiological functioning. Such fluctuations are termed circadian rhythms. The related term of ‘diurnal variation’ refers to changes in physiological outcomes solely during the typical hours of daylight, e.g. morning versus early evening.
It is important from a mechanisms perspective to establish the extent of endogenous control of a circadian rhythm, i.e. to what extent the 24-h variation in a physiological function is controlled by a self-sustaining body clock. Unfortunately, most circadian rhythms are influenced or ‘masked’ strongly by sleep, physical activity as well as flucuations in the environment. These influences can be controlled with a ‘constant routine’ protocol, in which subjects are essentially bed-rested in a stable environment for at least 24 h (Minors and Waterhouse 1984). For example, when the resting blood pressure (BP) of normal healthy volunteers is measured in a constant routine, the endogenous body clock is thought to account for only 10–15% of the daily variation in BP measured with ambulatory monitors during ‘normal’ daily living (Atkinson et al. 2006). Nevertheless, such a protocol does not allow one to identify whether the responses of physiological variables to various stressors, e.g. exercise, show circadian variation. Such knowledge would be useful in explaining whether the circadian variations in sudden cardiac and cerebral events are of endogenous origin or merely as a result of the changes in physical activity, psychological stress and sleep over the course of the day.
Investigating circadian rhythms in the responses of physiological systems to exercise is more difficult than examining rhythms in resting physiology, and this difficulty raises some important issues about experimental design and analysis. For example, some ‘hybrid’ protocols have been developed which enable physiological responses to be explored alongside some control over prior sleep, physical activity and diet (Kline et al. 2007; Jones et al. 2008b). There are also statistical approaches for controlling for, or ‘purifying’, the masking influences of sleep and activity on circadian rhythms (Waterhouse et al. 2005). The purpose of the present review is to summarize the research evidence from such protocols and analyses for diurnal variation in the exercise-related responses of those circulatory outcomes that are relevant to sudden cardiac and cerebral events. The review is structured so that the hypothesized links between the human body clock and circulatory function are discussed first. Second, the epidemiological data that indicate sudden cardiac and cerebral events show circadian rhythmicity is discussed. Third, the various circulatory variables are examined in terms of whether the responses to exercise are influenced by time of day.
The body clock and circulatory control
The suprachiasmatic nuclei (SCN) of the hypothalamus mediate circadian rhythms in many biological functions. In mammals, the SCN is comprised of two clusters of nerve cells, which are located in the anterior hypothalamus of the brain (Cassone et al. 1988). The results of molecular level studies show that the clock mechanism in the SCN is essentially cyclic interactions between clock genes and clock proteins (Clayton et al. 2001; Richter et al. 2004). Information about the characteristics of the clock resides within the nucleus of the cells within the SCN. Nevertheless, clock genes have been found in mice and humans that are expressed widely throughout the body (Balsalobre 2002). Indeed, the circadian timing system is thought to be comprised of a hierarchy of biological clocks (some in peripheral tissues as discussed in below), which are commanded by the ‘master’ clock in the SCN (Richter et al. 2004). How the SCN communicate with effectors responsible for the overt physiological rhythms is not well understood.
In mammals, oscillations in circadian clock genes have been discovered not only in the SCN (Okamura et al. 2002), but also in the heart and the liver (Storch et al. 2002), and other peripheral tissues (Nonaka et al. 2001). It has been hypothesized that these peripheral circadian oscillators could contribute to circadian rhythms in BP and rhythmic occurrence of some cardiovascular diseases (McNamara et al. 2001). Nevertheless, it is not clear which modulators connect the central oscillations in the SCN with the peripheral ones in the blood vessels. It is also not well understood how the time information generated by the transcription–translation-based core feedback loop is transmitted to clock-controlled genes that represent the output pathway of the clock.
Although circadian expression of clock genes can occur in healthy tissues relevant to the circulation, such as the heart, aorta and kidney (Maemura et al. 2000; Nonaka et al. 2001), no circadian variation in clock gene expression has been reported in cultured cardiac cells in vitro. Cultured vascular smooth muscle cells show circadian variation in clock gene expression, which can be entrained to a 24-h period by angiotensin II and retinoic acid (McNamara et al. 2000; Nonaka et al. 2001). In terms of heart disease, the circadian expression of core clock genes persists normally in the pressure-overloaded heart. Nevertheless, the circadian variation in transcription factors and genes involved in metabolism is blunted in the hypertrophied heart (Takeda and Maemura 2007). Takeda and Maemura (2007) maintained that peripheral circadian clocks in the circulatory system provide an anticipatory function, enabling the heart and the vasculature to prepare for external stimuli, such as the morning surge in BP. This raises the primary question in the present review of whether the responses of BP and other circulatory variables to physical exertion, which is a potent trigger of cardiac and cerebrovascular events, vary with time of day. Before this question is addressed, it is important to cover the ‘chronoepidemiology’ of sudden cardiac and cerebrovascular events, i.e. to describe the 24-h variation in these outcomes.
Chronoepidemiology of cardiac and cerebral events
Circadian variation in cardiac and cerebral events has been discussed previously in several comprehensive systematic reviews and meta-analyses (Elliott 1998; Smolensky et al. 2007), including one manuscript devoted specifically to circadian variation in events with a known exercise cause (Atkinson et al. 2006). There is also a special issue of Biological Rhythm Research devoted to circadian variation in cardiac events (Manfredini and Waterhouse 2007). Nevertheless, few reviewers have considered cardiac and cerebral events together in the same manuscript, nor discussed in particular circadian variation in the exercise-related responses of variables postulated to be involved in the pathology of these events. Therefore, as a prelude to our later discussions on exercise-related pathophysiology, we summarize below the main observations, which are described in detail in the past reviews (Elliott 1998; Smolensky et al. 2007).
Angina pectoris and myocardial ischemia
Both physical exertion and emotional stress can trigger the pain that is characteristic of angina pectoris, which can also be detected by a depressed ST segment with echocardiography. Angina pectoris has been found to occur most frequently during the morning (Behar et al. 1991). A secondary peak in incidence has also been reported during the afternoon or evening. Nevertheless, the peak incidence of Prinzmetal angina occurs during nocturnal sleep (Waters et al. 1984). Prinzmetal angina is different from angina pectoris in that it is detected by an elevated, not depressed, ST segment with echocardiography, which illustrates that the specific type of cardiovascular disease can have a specific circadian timing.
The frequency and duration of myocardial ischemia (for which angina pectoris is a symptom) peak between 06:00 and 12:00 hours (Mulcahy et al. 1988). Parker et al. (1994) reported that this peak circadian time can be influenced to some degree by the specific sleep-wake habits of patients (i.e. waking and becoming active at earlier or later times of the day). Nevertheless, Krantz et al. (1996) examined the circadian variation in myocardial ischemia whilst participants performed “normal” daily activities out-of-hospital. In contrast to the results of Parker et al. (1994), ischemia was found to peak in the morning, even after allowing for inter-individual changes in activity. Such analyses of descriptive data, which include covariate control for the effects of activity, are rare, but would be illuminating in helping to separate the effects of physical exertion per se on these events from the influence of an endogenous mechanism altering the pathology of risk at different times of day.
Acute myocardial infarction and sudden cardiac death
Although clinicians have been aware for many years that acute myocardial infarction (AMI) seems more common at some times of day compared with others, Muller et al. (1985) were the first research group to formally analyze a large number of cases and found that AMI is most common during the first few hours after waking from nocturnal sleep (Fig. 1). Data from large-scale meta-analyses have subsequently confirmed with good statistical precision that there is a prominent morning peak in incidence of AMI (Cohen et al. 1997) and sudden cardiac death (Fig. 2). These outcomes can be two to three times more likely during the first 3 h after waking from nocturnal sleep than at other times of day. When circadian variation in AMI is examined with a large number of data collection periods over 24 h, a secondary peak in incidence occurring during the late afternoon or early evening has also been reported (Levine et al. 1992). It is interesting that patients with obstructive sleep apnea syndrome (OSAS) do not show the typical morning peak in myocardial infarction. Conversely, these events have been reported to be more common at night in these patients (Kuniyoshi et al. 2008). A discussion of the specific pathophysiological reasons for the unusual timing of cardiac events in OSAS is beyond the scope of the present review. Suffice to say that the circadian peaks in several pathophysiological variables considered in the present review, such as arterial BP (Somers et al. 1995), platelet activation (Sanner et al. 2000), whole-blood viscosity and fibrinolytic activity (Rangemark et al. 1995), have been reported to be shifted to the nocturnal hours in OSAS patients. Pathophysiologic explanations unique to OSAS are also likely, e.g. cardiac wall stress caused by obstructed breathing with negative intrathoracic pressures (Floras and Bradley 2007). Given the common (10–15% of the population) nature of this complaint (frequent nocturnal awakenings and large increases in BP during apnea periods), it is surprising that the pathology of this circadian variation in AMI has not been fully elucidated.
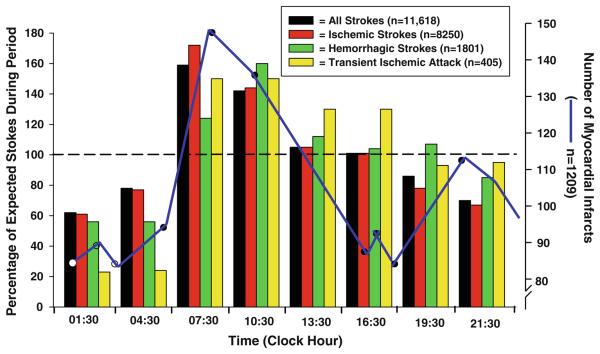
Fig. 1.
Illustration of the 24 h pattern in different types of stroke as well as myocardial infarction. The values represent the incidence of each event per 3 h time interval as a percentage of the 24 h mean number (equal to 100%) for each type of stroke and for transient ischemic attack. These data clearly show that the occurrence of stroke and transient ischemic attack is greatest in the morning at 07:30 and 10:30 hours (modified from Elliott 1998). The blue overlay represents the 24 h pattern in myocardial infarction, which also peaks in the early morning, although there is a secondary peak occurring around 21:00 hours (modified from Smolensky 1983)
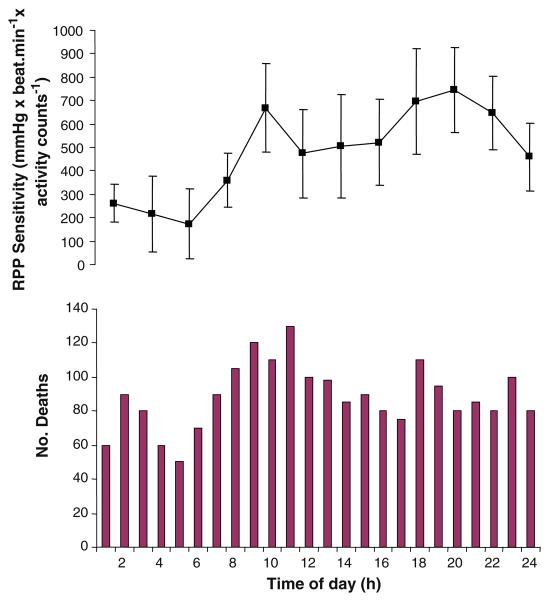
Fig. 2.
Parallelism between 24-h variations in sudden cardiac death (bottom) and the sensitivity of rate pressure product to everyday physical activities (top). Sudden cardiac death data redrawn from Muller (1999). The BP and heart rate data used to calculate rate pressure product were collected by Atkinson et al. (2009) with an ambulatory monitor on 440 patients attending a hypertension clinic. Activity was measured with a wrist accelerometer by Atkinson et al. (2009). A highly significant effect of time of day was found on RPP (P < 0.0005)
Atkinson et al. (2006) considered the question of whether cardiac events, occurring specifically during supervised exercise programmes, are more common at specific times of day. In one study that was discussed, Murray et al. (1993) randomly assigned patients with established heart disease to either a morning (07:30 hours) or afternoon (15:00 hours) supervised exercise training group. The incidence of cardiac events was very low, irrespective of when the patients exercised (2.4–3.0 events per 100,000 patient hours of exercise). The relative risk of a cardiac event was 1.27 in the morning compared with the afternoon, although this estimate was not very precise (95% confidence interval 0.25–6.55). Murray et al. (1993) argued that, even if the true population relative risk was as high as the upper confidence limit of 6.55, the absolute risk would still not be clinically significant. Similarly, Franklin et al. (1998) found that a rehabilitation program involving submaximal exercise gave rise to only three morning and two afternoon non-fatal complications over a 16-year period involving 3,335 patients. Again, this low number of clinical events is indicative of a low absolute risk during supervised training programmes, irrespective of time of day. Nevertheless, freely chosen physical exertion performed out-of-hospital by individuals already at risk might be less safe than during a closely controlled and clinically monitored bout of exercise in a clinic, and therefore more prone to changes in behavior (e.g. physical exertion) with time of day. Unfortunately, there are sparse chronoepidemiological data specifically on cardiac outcomes with a known out-of-hospital cause (Atkinson et al. 2006). Albert et al. (2000) found that relative risks of sudden cardiac death during vigorous compared to light physical activity were quite stable (16.6–19.0) between the hours of 06:00 and midnight, although a much lower relative risk (4.6) was found between midnight and 06:00 hours.
In summary, AMI and sudden cardiac death are rare in closely supervised clinic-based exercise programs, irrespective of time of exercise. There are insufficient data to arrive at any firm conclusions regarding out-of-clinic cardiac events. This lack of sufficient and specific epidemiological data makes the question of whether a person with known risk factors for heart disease should take recreational physical activity in the morning or afternoon unanswered. In the absence of such data, it is pertinent to examine whether known pathophysiological correlates of a sudden cardiac event are most pronounced in the morning hours.
Cerebrovascular disease
Stroke is the culmination of a heterogeneous group of cerebrovascular diseases that is manifested as ischemia or hemorrhage of one or more blood vessels in the brain (Edvinsson and Krause 2002; Hachinski and Norris 1985). Such strokes can result from occlusion of a major cerebral artery (ischemic stroke) or rupture of intracerebral arteries (hemorrhage). Although ischemic hemorrhagic strokes are different entities and involve different underlying pathophysiological mechanisms, there are considerable overlaps between them. For instance, ischemia can lead to hemorrhage by rupture of ischemic vessels or extravasation of blood from leaky vessels. Conversely, hemorrhage can lead to ischemia by compressing the surrounding areas and reducing local blood flow. Acute cerebrovascular events are typically characterized by the sudden onset of several characteristic signs that may or may not be preceded by premonitory symptoms and that evolve and reach maximal intensity within 24 h (Edvinsson and Krause 2002; Hachinski and Norris 1985). Both focal neurological symptoms— including cognitive impairment, muscle weakness, loss of coordination, numbness of the extremities, cranial nerve palsies—and global symptoms—such as headache, nuchal (nape of neck) rigidity, BP and vital signs, and altered mental status (syncope, seizures, and coma)—may be exhibited.
The incidence of stroke exhibits a similar 24-h pattern as for the above acute cardiovascular events (Manfredini et al. 2005; Fig. 1). The 24-h pattern in stroke-onset is independent of stroke subtype, patient demographics, clinical features, and presence or absence of predisposing risk factors (Casetta et al. 2002; Guy and Bornstein 2000). Syncope is another common cerebrovascular event, accounting for 1–3% of all visits to the emergency room (Van Lieshout et al. 2003). The typical symptoms of pre-syncopal symptoms of wooziness, light headedness, and visual dimming are fundamentally caused by insufficient oxygen supply to the brain which, if sustained, results in syncope. The prevalence of syncope in the general population is significant (~5%) and recent reports indicate that the incidence of syncope is nearly twice as common in people under 40 years than in individuals over 60 years (Romme et al. 2008). Epidemiological data indicate that there is a circadian pattern in the frequency of vasovagal episodes, with a peak in the morning (Convertino and Adams 1991; Mineda et al. 2000; van Dijk et al. 2007). Although the mechanisms underlying this increased likelihood of syncope in the early morning have not been examined, recognition of the daily distribution of syncope is useful for patient education and therapeutic strategy. Importantly, this impairment to maintain adequate cerebral perfusion in the early morning may not only underscore the greater occurrence of syncope, but may also be an additional contributor to the greater occurrence of ischemic stroke at this time of day (Fig. 3). Indeed, a classical quote is that “the only difference between syncope and sudden death is that in one you wake up” (Engel 1978).
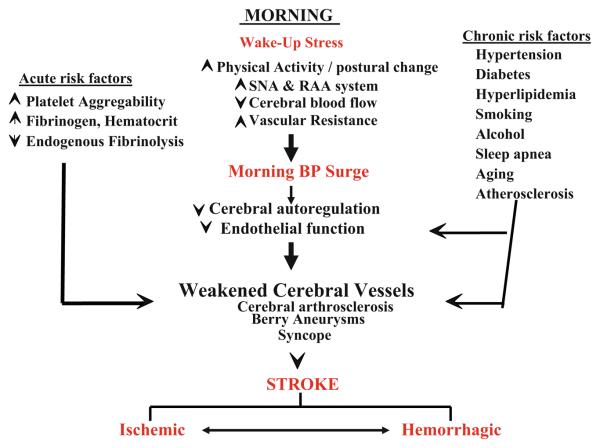
Fig. 3.
Schematic to illustrate some of the underlying pathophysiological mechanisms and related environmental ‘triggers’ responsible for circadian variation of onset of ischemic and hemorrhagic strokes. The key changes in the early morning due to ‘wakeup’ stress and related BP surge; the outlined acute and chronic risk factors may further compromise cerebral vascular health, potentially exacerbating the possibility of stroke. Although ischemic hemorrhagic strokes are different entities and involve different underlying pathophysiological mechanisms, there are considerable overlaps between them. For instance, ischemia can lead to hemorrhage by rupture of ischemic vessels or extravasation of blood from leaky vessels. Conversely, hemorrhage can lead to ischemia by compressing the surrounding areas and reducing local blood flow
Circadian variation in circulatory factors at rest and in response to exercise
It has been proposed that atherosclerotic plaque rupture, and consequent thrombosis, is the mechanism that mediates peak occurrence of cardiovascular events in the morning. Mechanisms surrounding the morning peak in stroke may be more complicated, depending on the type of cerebrovascular event. Although the precise mechanisms are unknown, possible triggers for plaque rupture in the morning could be related to circadian variation in variables such as BP, heart rate, vascular structure and function or the autonomic nervous system via sympathetic outflow. Therefore, it is important to examine how each of these factors is affected by time of day in the resting state or under ‘free-living’ conditions. Nevertheless, such information tells one little about whether the response of a physiological variable to a given stimulus (e.g. exercise) varies with time of day. The mechanisms mediating circadian variation in circulatory control may be due to endogenous circadian rhythms as discussed above. However, everyday life for most individuals consists of intermittent bouts of physical activity which vary in intensity and duration, and such activity can be triggers for sudden cardiac and cerebral events (Albert et al. 2000). Therefore, it is important to isolate the effects of exercise per se in the studies on circadian variation in order to understand the circadian changes in circulatory control. For example, in the morning, circadian variation in circulatory control could be due to the arousal effects of waking from sleep or haemodynamic adjustments after arising from bed and adopting upright posture. Therefore, quantifying the endogeneity of circadian variation in the response of haemodynamic variables is necessary.
Blood pressure
In most normotensive and uncomplicated essential hypertensive individuals, the circadian variation in resting BP is characterized by a nocturnal dip (which can be 10–20% lower than the daytime mean level) followed by a morning ‘surge’ (Kawano et al. 1994; Millar-Craig et al. 1978). The 24-h BP pattern can be altered in certain clinical conditions, e.g. patients with secondary hypertension, such as in diabetes and in both renal and congestive heart disease (Hermida et al. 2007; Middeke et al. 1991; Portaluppi et al. 1991). Nevertheless, the variation is robust enough for the morning surge in BP to have been suggested as a reason for the morning peak in sudden cardiovascular events (Kuwajima et al. 1995; Muller et al. 1989). It has been hypothesized that the morning surge in BP may disrupt vulnerable plaques causing rupture and thrombosis leading to a cardiovascular or cerebrovascular incident (Johnstone et al. 1996; Millar-Craig et al. 1978). This hypothesis is supported by the results of a study by Muller et al. (1987), who found that those elderly hypertensive patients who experienced larger than average morning surges in BP were also at an increased risk of stroke.
It has been suggested previously that the change in physical activity associated with the act of rising from bed and beginning ambulation within the first 2 h after waking is related to the surge in BP in the morning (Leary et al. 2002). In addition, a relationship between physical activity and changes in BP has also been observed at other times of day, e.g. during the daytime and sleep periods (Kario et al. 1999). A relevant question is whether such relations between physical activity and BP are themselves affected by time of day. In order to answer this question, Jones et al. (2006) examined circadian variation in the response of BP to everyday physical activities in both hypertensive and normotensive participants. A regression-based index of ‘BP reactivity’ was employed to describe the rate of change in BP measured 15 min after a change in physical activity for twelve 2-h data bins spaced over a 24 h period. Regression slopes were generally positive, although quite variable between the 440 participants studied. Nevertheless, the results of this study revealed that the response of ambulatory BP to everyday physical activities does vary with time of day. The increase in BP to a unit increase in logged physical activity counts was found to be highest between 08:00 and 10:00 hours. Nevertheless, it should be noted that general physical activity was measured using an accelerometer-based device worn on the wrist. Participants also went about their everyday lives, sleeping and eating when they desired during data collection and no controlled exercise intervention was administered. Rather, variability in the physical activity stimulus was controlled for using a statistical approach akin to analysis of covariance.
Subsequent to the study of Jones et al. (2006), a number of related experiments involving normotensive participants and standardized exercise tests were undertaken (Fig. 4). BP is normally reduced after a bout of exercise, but this phenomenon of ‘post-exercise hypotension’ was found to be absent or even reversed when exercise was taken in the morning (Jones et al. 2008a). This finding was not explained by the amount of sleep prior to morning exercise, since a 4-h sleep period prior to the afternoon test sessions did not alter the fact that greater post-exercise hypotension was observed in the afternoon compared with the morning (Jones et al. 2008b), although prior sleep in the afternoon did seem to lead to a lower post-exercise hypotension compared with a protocol in which participants did not sleep prior to the afternoon test session (Fig. 4). Taken together, these findings suggest that circadian variation is evident in the response of BP to exercise, with higher values of BP being observed during and after a set exercise protocol in the morning compared with other times of day. It is also interesting to note in one study (Jones et al. 2009a) that an intermittent protocol comprising three 10-min bouts of exercise mediated greater post-exercise hypotension than a continuous 30-min protocol, both in the morning and in the afternoon. In another recent study (Fullick et al. 2009), 1 h of moderate intensity exercise was undertaken at 19:00 hours, and the hypotensive effects of this exercise were apparent throughout a subsequent period of night work (21:00–05:00). Taken together, these study results suggest that the BP lowering effects of exercise are quite robust, being evident during everyday activities, even at night, and that the incorporation of intermittent rest periods into an exercise bout may maximize the hypotensive effects of exercise.
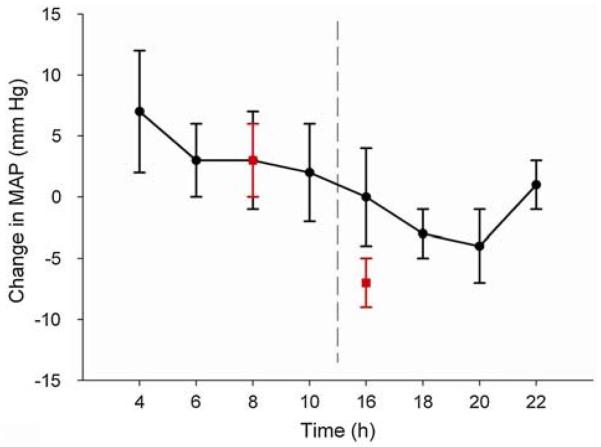
Fig. 4.
Diurnal variation in the post-exercise response of MAP measured during two different experimental protocols. Data shown by the circle symbols were collected during a study (n = 6 participants) in which the amount of sleep prior to the 04:00 and 16:00 test times was controlled at approximately 4 h. Post-exercise hypotension was significantly greater following exercise in the afternoon and evening compared with the early morning, even though the amount of prior sleep was controlled between times of day (Jones et al. 2008b). The square symbols indicate data from another study (n = 12 participants) in which exercise was undertaken at two times of day. Pre-exercise posture and diet were controlled but the amount of prior sleep was not (Jones et al. 2008a). Post-exercise hypotension was, again, more marked in the afternoon. Taken together, these data suggest that the ‘normal’ blood pressure lowering effects of exercise are blunted in the morning compared with the afternoon and early evening and this is not necessarily explained by differences in prior sleep between these times of day
There are two important issues relevant to diurnal variation in the BP responses to exercise that have not yet been addressed. First, it is unclear how long the diurnal variation in BP response persists after a bout of exercise. Second, it is not known whether the diurnal variation in BP response is robust when studied in different populations. Park et al. (2005) reported that afternoon exercise exhibited a greater reduction in systolic BP and this reduction persisted during subsequent nocturnal sleep in so-called “non-dippers” (hypertensive patients who do not normally exhibit a 10% or greater reduction in average night time BP). This finding may be population-specific since a similar study on normotensive participants resulted in no diurnal variation being found in the longer-term ambulatory BP responses to a bout of exercise (Jones et al. 2009c). Besides this factor of disease status being important, it is also possible that age and gender may moderate the diurnal variation in BP response to exercise. Post-exercise hypotension has been found to similar in men and women when the reduction in BP is expressed as a percentage of the initial value (Lynn et al. 2007). Post-exercise hypotension is also apparent in older hypertensive patients (Hagberg et al. 1987), although how time of day interacts with these findings is unknown. It is also unclear whether a period of exercise training elicits different chronic changes in BP depending on whether that training is performed routinely in the morning or evening.
The exact mechanisms governing the acute and longer-term effects of exercise on BP remain to be elucidated. Recently, researchers have focused on the identification of the vasodilatory substance which explains post-exercise hypotension, and Lockwood et al. (2005) provided evidence that this substance is histamine, at least in normotensive people. Interestingly, endogenous concentrations of histamine are known to be lower at night (Tuomisto et al. 2001). Nevertheless, endogenous nitric oxide is another important vasodilatory substance for BP control, which has also been reported to vary with time of day in healthy participants (Bode-Boger et al. 2000). Moreover, melatonin may be an especially interesting candidate for research relevant to diurnal variation in BP response, since this secretary product of the Pineal gland has vasodilatory properties and is also thought to be crucial to the circadian system (Atkinson et al. 2003). Nevertheless, any interactive effects of exercise and time of day on endogenous concentrations of histamine, nitric oxide and melatonin are, at present, unclear (Piepoli et al. 1993; Atkinson et al. 2003). Future researchers should measure these vasodilatory substances alongside the BP measurements following exercise at different circadian times.
Rate pressure product
The rate pressure product (RPP) is calculated by multiplying heart rate and systolic BP, and is sometimes referred to as the ‘double product’. Changes in RPP have been found to relate to myocardial oxygen uptake (Nelson et al. 1974; Fletcher et al. 2001), cardiac workload (Campbell and Langston 1995; Campbell et al. 1997) and left ventricular hypertrophy (Azevedo et al. 1993), as well as with cardiovascular mortality, angina pain and AMI (Deedwania and Nelsen 1990). Several decades ago, an RPP threshold of 20,000 beats min−1 mmHg was suggested as being indicative of likely angina pain (Robinson 1967). More recent data indicate that the RPP threshold for silent ischemic events could be as low as 10,000–14,000 beats min−1 mmHg in susceptible patients, such as those with hypertension (Deedwania and Nelsen 1990; Uen et al. 2003). Hermida et al. (2001) described a large amplitude 24-h variation in RPP for individuals living a normal diurnal existence, and it was reported that RPP rose by approximately 2,500 beat min−1 mmHg during the 7 h after waking from nocturnal sleep. The authors maintained that this rise would constitute a clinically significant increase in cardiac workload, which should be controlled with appropriately timed medication. Similar 24-h variations in RPP during everyday living has also been described for patients with liver glycogen storage disease (Young et al. 2002) and patients attending a hypertension clinic (Atkinson et al. 2009).
Evidence is accumulating that the responses of RPP to exercise provide better prognostic value for cardiovascular mortality than resting measurements of RPP, as well as other exercise-related cardiovascular responses (Villella et al. 1999; Prakash et al. 2001; Elhendy et al. 2003; Nieminen et al. 2008). Nieminen et al. (2008) found that the difference between the peak RPP attained during an exercise test and the RPP measured 4 min after the cessation of exercise was the most predictive haemodynamic outcome for sudden cardiac death, cardiovascular mortality and all-cause mortality. Using the similar statistical approach adopted by Jones et al. (2006), Atkinson et al. (2009) examined the response of RPP to everyday physical activities over a 24-h period in normotensive and hypertensive participants. It can be seen in Fig. 2 that this variation in calculated RPP sensitivity to physical activity shows the greatest increase in the morning hours and shows parallelism with the circadian variation in data pertaining to sudden cardiac death. Twenty-four-hour variation in the responses of RPP to exercise has been explored in only one other study. Jones et al. (2008b) found a trend for the post-exercise response of RPP to be one to two times higher when exercise was taken by normotensive participants in the morning compared with other times of day. Although the differences between times of day were not statistically significant with the six participants studied, this trend agrees with the findings reported by Atkinson et al. (2009) and supports data from other studies indicating that RPP is generally more sensitive to exercise. This might explain why it provides better prognostic value for cardiovascular mortality, than BP or heart rate per se (Nieminen et al. 2008), although the prognostic value of RPP sensitivity derived specifically from 24-h ambulatory monitoring remains to be established.
Vascular function
The endothelium is the inner most layer of the artery and is the interface between blood flow and the vessel wall. The endothelium modulates BP and vascular tone, since it produces a number of paracrine substances, including nitric oxide (Green et al. 2008). Nitric oxide diffuses to smooth muscles, where it elicits vasodilation. Nitric oxide is synthesized in endothelial cells from the amino acid L-arginine in a reaction catalyzed by endothelial nitric oxide synthase (Palmer et al. 1988). It is released tonically, under resting conditions, and in response to an increase in flow and associated shear stress through the vessel (Davies and Tripathi 1993). Shear stress is the physiological stimulus for endothelial cell release of vasodilator substances such as NO and prostacyclin and elevated shear rate therefore implies higher levels of vasodilator autacoid production in the vessel wall (Pyke and Tschakovsky 2005).
Circadian variation in vascular tone at rest has been reported previously. Data from a number of studies suggest that forearm vascular resistance, measured at rest, is greater in the morning than the afternoon in normotensive individuals (Casiglia et al. 1998; Panza et al. 1991), but that this pattern is reversed in hypertensive individuals (Casiglia et al. 1998). Potentially, increased vascular tone in the morning could be one mechanism contributing to increased risk of disturbing a vulnerable plaque. However, Veerman et al. (1995) demonstrated that total peripheral resistance dropped sharply after waking in the morning. One reason for these differences could be due to measurement techniques. Veerman et al. (1995) used finger plethysmography and the pulse contour technique compared to forearm blood flow changes using plethysmography. Intriguingly, Jones et al. (2008a) found lower total peripheral resistance in the morning compared with the afternoon using the same method as Veerman et al. (1995) but no diurnal variation in brachial arterial diameter when the same normotensive participants were assessed with high resolution ultrasonography (Jones et al. 2009b). This demonstrates disparity in measurement tools and or physiological differences in the vascular bed under examination.
The ability of the vasculature to dilate has significant implications for protection against diseases including hypertension, stroke and myocardial infarction (Motoyama et al. 1997). Given the central role the endothelium has in the vascular system, including vasomotor control, platelet aggregation, adhesion and development of atherosclerosis (Verma and Anderson 2002), 24-h variation in endothelial function could mediate, at least in part, the elevated cardiovascular risk in the morning. Moreover, endothelial dysfunction is considered to be an early indicator in the atherosclerotic disease process and is predictive of future cardiovascular events (Neunteufl et al. 2000; Perticone et al. 2001; Modena et al. 2002). Endothelial function has been shown to vary with time of day. Shaw et al. (2001) reported greater pharmacologically induced blood flow responses in the evening compared to the morning in resting older individuals. Conversely, other researchers have demonstrated reduced flow-mediated vasodilation in the morning in conduit arteries (Kawano et al. 2002; Otto et al. 2004) and the micro-vessels of the skin (Elherik et al. 2002). Reduced morning endothelial-dependent vasodilation is evident in healthy individuals (Elherik et al. 2002; Otto et al. 2004) and patients with variant angina (Kawano et al. 2002). This reduction has been linked to elevated cardiovascular risk in morning and could potentially be a fundamental factor in circadian variation in cardiac and cerebrovascular events. However, none of the researchers who have investigated endothelial function in the conduit arteries have calculated shear rate, an index of shear stress in order to normalize the findings to the magnitude of the stimulus for flow-mediated dilatation (Pyke and Tschakovsky 2005). Therefore, it is currently unknown if the diurnal variation is due to intrinsic endothelial NO-vasodilator system function or diurnal variation in increased shear stress related to blood flow. It should also be said that several previous studies on diurnal variation in vascular function have not tightly controlled the amount of prior sleep and/or posture. Moreover, some protocols involved the morning and afternoon test sessions being administered in the same 24-h period, which raises the issue of whether serial carryover effects of testing might have influenced the description of diurnal variation.
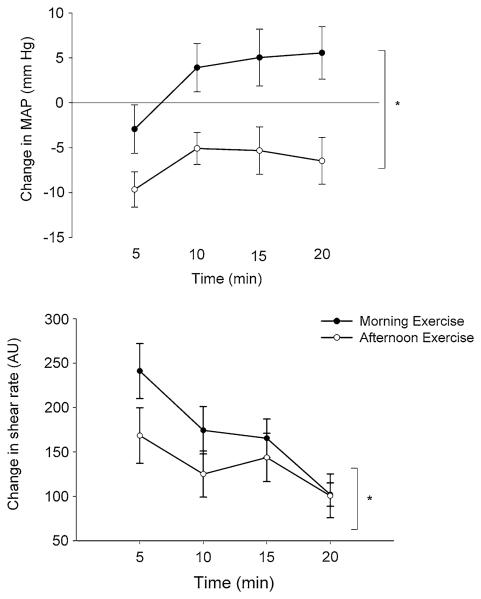
It has been suggested that the differences in BP between times of day could be explained by associated changes in the vasculature (Jones et al. 2008a). In the latter study, the total peripheral resistance of normotensives measured after exercise using finger plethysmography was lower in the morning, suggesting that less vasodilation occurred at this time of day. Nevertheless, post-exercise conduit (brachial and femoral) artery diameter measurements have not been found to be different between the morning and the afternoon and, therefore, these data do not confirm the notion of reduced vasodilation in the morning (Jones et al. 2009b). One important observation in the latter study was the greater post-exercise brachial luminal shear stress in the morning (Fig. 5). This finding suggests that arteries may be exposed to elevated risk of atherothrombotic events in the morning, thus contributing to the elevated cardiovascular risk. The fact that brachial artery diameter did not apparently increase, despite the higher shear stress exposure in the morning, may relate to time of day variation in intrinsic endothelial function. Although, there is evidence of time of day differences in flow-mediated dilation at rest (as described above), information about flow-mediated dilation (normalized for shear rate) measured after exercise and as a function of time of day is currently lacking. An alternative explanation for the lack of difference in diameter found by Jones et al. (2009b) could be due to increased sympathetic nerve activity in the morning, which is considered in the next section.
Fig. 5.
Change from preceding baseline in mean post-exercise MAP (top) and shear rate (bottom) measured in the morning and afternoon in 12 participants (drawn with data from Jones et al. 2009b). Shear rate measured 5 min after exercise was significantly greater in the morning in accordance with the greater MAP at this time of day. Asterisk refers to the presence of a significant interaction between time of day and post-exercise time
Sympathetic nerve activity
The autonomic nervous system follows a circadian pattern, which is apparent when measuring sympathetic outflow directly or via plasma catecholamines (Linsell et al. 1985). Sympathetic nerve activity is a mechanism that can cause alterations in vascular tone and BP. Elevated sympathetic nerve activity in the morning has been postulated to explain the increased incidence of cardiac events (Muller et al. 1985). Panza et al. (1991) measured forearm blood flow and vascular resistance responses to phentolamine (an alpha-adrenergic-antagonist) and sodium nitroprusside (a direct vasodilator) at three different times of day (07:00, 14:00 and 21:00 hours) in resting individuals. Basal forearm vascular resistance was significantly higher in the morning than in the afternoon and evening. However, following infusion of phentolamine, there was no circadian variation in vascular resistance. These data indicate that the alterations in vascular tone in the morning are due to increased α-sympathetic-mediated vasoconstrictor activity.
Previous research data support the notion of counterbalanced local vasodilator and central sympathetic constrictor control of conduit (large) artery function in vivo (Hijmering et al. 2002). Nevertheless, it is likely that organ and regional differences occur in sympathetic nerve activity. In one study which involved sympathetic nerve activity responses to morning and afternoon exercise, measurements of muscle sympathetic nerve activity (i.e. the skeletal muscle vascular bed) were made in the leg using microneurography whilst performing hand grip exercise (Middlekauff and Sontz 1995). The results of that study suggested that no differences were evident in exercise-mediated muscle sympathetic nerve activity responses between times of day, which indicates that circadian variation is not due to sympathetic outflow. However, all these measurements were made in the supine posture and previous research has shown that plasma noradrenaline levels increase when subjects change from a supine to an upright posture (Dodt et al. 1997; Winther et al. 1992). None of these studies involved careful examination of the post-exercise recovery period and it has been suggested that plasma noradrenaline may be too insensitive a marker of sympathetic nerve activation, since it represents only a small proportion of noradrenaline releases at the sympathetic nerve terminal (Eisenhofer et al. 1986).
Hemostasis
Variables relevant to the hemostatic system show circadian variation when measured under resting conditions, with the changes suggestive of greater occurrence of thromboembolic events in the morning. The vascular endothelium has a direct role in the hemostatic system, producing vasoactive and thromboactive substances to maintain a non-thrombogenic surface. The endothelium therefore effects coagulation, platelet aggregation and fibrinolysis. Circadian variation in resting hemostatic marker profiles have been reported previously, including platelet hyperactivity, hyper-coagulability, hypofibrinolysis, increased blood viscosity and vascular spasm in the morning (Andreotti et al. 1988; Feng et al. 1999; Burke et al. 1999; Willich et al. 1993).
A number of researchers have examined the responses of hemostatic marker profiles in response to exercise. Winther et al. (1992) examined platelet aggregation and fibrinolytic activity between 08:00 and 12:00 hours compared to later in the day. Exercise was systematically introduced toward the end of this period, which included previous interventions with posture. Exercise was found not to increase platelet aggregation to levels beyond that produced by the upright posture. In support of these results, Jimenez et al. (1993) compared fibrinolytic activity prior to and following hand gripping exercise lower fibrinolytic activity in the morning. Szymanski and Pate (1994) examined whether time of day for exercise influenced the responses of fibrinolytic activity and found a greater degree of tissue plasminogen activation in the evening exercise bouts compared to the morning.
Cerebrovascular function
There is an approximate 6-h difference between the timing of circadian rhythms in cerebrovascular function and core body temperature, so that cerebrovascular function continues to decline during the early to midmorning hours, when body temperature is, in fact, increasing. Cerebral vasomotor reactivity to hypocapnia and hypercapnia has been found to be most reduced in the morning (Ainslie et al. 2007; Ameriso et al. 1994; Qureshi et al. 1999). The term cerebrovascular CO2 reactivity reflects an ‘index’ of the ability of the cerebrovascular bed to dilate or constrict in response to changes in arterial PCO2 (Ainslie and Duffin 2009). For more than 20 years, transcranial Doppler has been used extensively to study CBF regulation and cerebrovascular CO2 reactivity in otherwise healthy subjects as well as in patients with various forms of cerebrovascular disease (Ainslie and Duffin 2009). For example, the measurement of cerebrovascular reactivity to CO2 has been applied in clinical practice to evaluate cerebrovascular function [e.g. in patients with carotid artery stenosis (Widder et al. 1994), hypertension (Serrador et al. 2005), stroke (Wijnhoud et al. 2006), heart failure (Xie et al. 2005), and a related impairment has been linked to cerebral ischemic events (Wijnhoud et al. 2006; Ainslie 2009)]. Interestingly, links between systemic endothelial function and cerebrovascular CO2 reactivity have been reported (Ainslie et al. 2007; Hoth et al. 2007; Lavi et al. 2006), indicating a common pathway between these responses.
Ainslie et al. (2007) reported recently that cerebral autoregulation is also reduced in the early morning shortly after waking, when compared to later in the day. Cerebral autoregulation adjusts cerebral arteriolar caliber, or cerebrovascular resistance, to ensure that cerebral blood flow levels are matched to metabolic needs, and it comprises two main components: static and dynamic. Static cerebral autoregulation keeps cerebral blood flow constant, over gradual and progressive changes in cerebral perfusion (Paulson et al. 1990). Dynamic cerebral autoregulation refers to the rapid regulation of cerebral blood flow in response to changes in BP that occur in a few seconds (Zhang et al. 2002). Impairment in both static and dynamic cerebral autoregulation have been extensively linked to the increased occurrence in cerebrovascular events and related mortality (see Panerai 2008 for recent review). The marked BP ‘surge’ during the morning, via rupture of a fragile atherosclerotic plaque and subsequent thrombosis, is a clear factor in the etiology of sudden cardiac events. Moreover, such surges in BP, because of a reduction in cerebral autoregulation, and in combination with acute and chronic risk factors, may also exacerbate the likelihood of hemorrhagic and ischemic strokes (Fig. 3).
It is tempting to suggest that the low CBF, in addition of early morning reductions in cerebrovascular reactivity and autoregulation may, in part, help explain the well established diurnal variation of the onset of cerebrovascular accidents, especially in the presence of acute and chronic risk factors (Wroe et al. 1992). As mentioned above, this early morning occurrence of cerebrovascular events, is in agreement with studies on MI and sudden death (Muller et al. 1985). The increased incidence of these events has been attributed, in part, to the surge of BP (Stergiou et al. 2002; Tsementzis et al. 1985) and platelet aggregability (Feng et al. 1999; Tofler et al. 1987) in the morning upon getting out of bed. Even in the absence of surges in BP, the phase of CBF reaches its lowest values during the hours before 12 p.m. (Conroy et al. 2005). This further suggests that the endogenous rhythm of CBF may be associated with the risk of cerebrovascular accidents in the morning hours even without changes in posture or activity.
Perspectives and recommendations for future research
It is clear that significant diurnal variation exists in several life-threatening cardiac and cerebral events. Such events are, however, generally rare, making precise identification of moderating factors on these outcomes difficult. Chronobiologists are interested in unraveling the causes of such 24-h variation in clinical outcomes. One approach is to examine findings from studies in which 24-h variation in the proposed pathophysiological mechanisms for these events has been examined. A myriad of such variables, including BP, hemostasis and vascular function, vary with time of day and could all contribute to the morning peak in cardiac and cerebral events. Nevertheless, it is relevant to attempt to separate the influences of the stress of physical activity per se from the notion that diurnal variation exists in the stress response to physical activity (and therefore bringing one closer to elucidating whether the endogenous body clock is exerting an influence).
In recent years, most attention has been devoted to diurnal variation in BP responses to exercise. Data from both descriptive studies involving ambulatory BP and experiments involving controlled exercise bouts suggest that the sensitivity of BP is greatest during the morning hours. To date, BP is the only variable which has been examined for diurnal variation in exercise-related responses using a protocol which attempts to separate the influences of time of day per se and sleep (Jones et al. 2009a). This protocol involved a 3.5-h period of sleep being taken before both the morning and afternoon test-times. To encourage participants to sleep in the afternoon, some amount of prior sleep restriction was necessary, although this sleep disruption was less than in some other ‘semi-constant’ routine protocols (Kline et al. 2007). It would be valuable for future researchers to examine the effects of time of day on the exercise-related responses of other circulatory variables with similar protocols.
One important function, which could be investigated using chronobiological protocols, is the baroreflex control of BP. Sleep-related changes in baroreflex sensitivity have been reflected to be more effective in buffering (i.e. increased sensitivity) against increased sympathetic activation associated with transition into REM nearer the end of sleep than compared with early sleep (Legramante et al. 2003). Thus, one possibility is that this increased sensitivity may be still present at the time following waking and would influence heart rate response following nocturnal sleep. It is important to note that REM sleep can occur during the daytime sleep bout. Ideally, therefore, measurement of sleep architecture and baroreflex sensitivity should be measured in future studies. However, no study to date has examined whether diurnal variation exists in baroreceptor resetting. The implications of such changes may be particularly relevant to at risk groups if a known impaired in baroreflex control of BP, especially patients with autonomic failure, OSA or ischemic heart disease.
From the study results examined in the present review, it is apparent that systemic pressures seem to be less labile to the vasodilating after-effects of physical activity in the morning. It is possible that this reduced vasodilation could be due to reduced sympatholysis and this could be confirmed in an experiment in which exercise responses are examined in the morning and afternoon under conditions of sympathetic blockade. Valuable information could also be obtained if arterial diameter and blood flow is measured in both the upper (inactive) and lower (active) limbs during a period of cycling exercise, since there may be inter-limb differences in vasomotor control in active and inactive vascular beds during exercise.
Finally, from an integrative systems approach, in addition to the traditional mechanisms describing CBF regulation (e.g. autoregulation, partial pressure of arterial carbon dioxide), a variety of other factors—such as cardiac output, the arterial baroreflex, and chemoreflex control—independently, synergistically, and sometimes antagonistically participate in the regulation of CBF. Integrative physiological research exploring these complex interactions—especially from a diurnal variation perspective—is currently lacking. Future studies with particular focus on these integrative physiological mechanisms are clearly warranted in both health and disease states.
Acknowledgments
G. Atkinson leads the project, “Shiftwork and Health: optimal timing of meals and physical activity”, which is funded by the National Prevention Research Initiative (http://www.npri.org.uk) with support from the following organisations: British Heart Foundation; Cancer Research UK; Chief Scientist Office, Scottish Government Health Directorate; Department of Health; Diabetes UK; Economic and Social Research Council; Health and Social Care Research and Development Office for Northern Ireland; Medical Research Council; Welsh Assembly Government; and World Cancer Research Fund.
References
- Ainslie PN. Have a safe night: intimate protection against cerebral hyperperfusion during REM sleep. J Appl Physiol. 2009;106:1031–1033. doi: 10.1152/japplphysiol.00091.2009. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement and interpretation. Am J Physiol. 2009;296:1473–1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Murrell C, Peebles K, et al. Early morning impairment in cerebral autoregulation and cerebrovascular CO2 reactivity in healthy humans: relation to endothelial function. Exp Physiol. 2007;92:769–777. doi: 10.1113/expphysiol.2006.036814. [DOI] [PubMed] [Google Scholar]
- Albert CM, Mittleman MA, Chae C, et al. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- Ameriso SF, Mohler JG, Suarez M, Fisher M. Morning reduction of cerebral vasomotor reactivity. Neurology. 1994;44:1907–1909. doi: 10.1212/wnl.44.10.1907. [DOI] [PubMed] [Google Scholar]
- Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuation in fibrinolytic factors and possible relevance to time of onset of myocardial-infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62:635–637. doi: 10.1016/0002-9149(88)90669-8. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Drust B, Reilly T, Waterhouse J. Relevance of melatonin to sports medicine and science. Sports Med. 2003;33:809–831. doi: 10.2165/00007256-200333110-00003. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Drust B, George K, Reilly T, Waterhouse J. Chronobiological considerations for exercise and heart disease. Sports Med. 2006;36:487–500. doi: 10.2165/00007256-200636060-00003. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Leary A, George KP, Murphy MB, Jones H. 24-Hour variation in the reactivity of rate-pressure-product to everyday physical activity in patients attending a hypertension clinic. Chronobiol Int. 2009;26:958–973. doi: 10.1080/07420520903044455. [DOI] [PubMed] [Google Scholar]
- Azevedo J, Arroja I, Jacques A, et al. Double ambulatory product (blood pressure and heart rate), mild arterial hypertension and left ventricular hypertrophy. Rev Port Cardiol. 1993;12:663–673. [PubMed] [Google Scholar]
- Balsalobre A. Clock genes in mammalian peripheral tissues. Cell Tissue Res. 2002;309:193–199. doi: 10.1007/s00441-002-0585-0. [DOI] [PubMed] [Google Scholar]
- Behar S, Reicher-Reiss H, Goldbourt U, Kaplinsky E. Circadian variation in pain onset in unstable angina pectoris. Am J Cardiol. 1991;67:91–93. doi: 10.1016/0002-9149(91)90107-v. [DOI] [PubMed] [Google Scholar]
- Bode-Boger SM, Boger RH, Kielstein JT, et al. Role of endogenous nitric oxide in circadian blood pressure regulation in healthy humans and in patients with hypertension or atherosclerosis. J Invest Med. 2000;48:125–132. [PubMed] [Google Scholar]
- Burke AP, Farb A, Malcom GT, Liang Y, Smialek JE, Virmani R. Plaque rupture and sudden death related to exertion in men with coronary artery disease. J Am Med Assoc. 1999;281:921–926. doi: 10.1001/jama.281.10.921. [DOI] [PubMed] [Google Scholar]
- Campbell RL, Langston WGA. Comparison of cardiac rate-pressure product and pressure-rate quotient in healthy and medically compromised patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:145–152. doi: 10.1016/s1079-2104(05)80193-3. [DOI] [PubMed] [Google Scholar]
- Campbell RL, Langston WGA, Ross GA. A comparison of cardiac rate-pressure product and pressure-rate quotient with Holter monitoring in patients with hypertension and cardiovascular disease. A follow-up report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:125–128. doi: 10.1016/s1079-2104(97)90056-1. [DOI] [PubMed] [Google Scholar]
- Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59:48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- Casiglia E, Staessen J, Ginocchio G, et al. Characterisation of hypertensive patients according to 24 h peripheral resistance. Jpn Heart J. 1998;39:355–362. doi: 10.1536/ihj.39.355. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Speh JC, Card JP, et al. Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms. 1988;3:71–92. doi: 10.1177/074873048800300106. [DOI] [PubMed] [Google Scholar]
- Clayton JD, Kyriacou CP, Reppert SM. Keeping in time with the human genome. Nature. 2001;409:829–831. doi: 10.1038/35057006. [DOI] [PubMed] [Google Scholar]
- Cohen MC, Rohtla KM, Lavery CM, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Conroy D, Spielman A, Scott R. Daily rhythm of cerebral blood flow velocity. J Circadian Rhythms. 2005;3:3. doi: 10.1186/1740-3391-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convertino VA, Adams WC. Enhanced vagal baroreflex response during 24 h after acute exercise. Am J Physiol. 1991;260:R570–R575. doi: 10.1152/ajpregu.1991.260.3.R570. [DOI] [PubMed] [Google Scholar]
- Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- Deedwania PC, Nelsen J. Pathophysiology of silent myocardial ischemia during daily life. Circulation. 1990;82:1296–1304. doi: 10.1161/01.cir.82.4.1296. [DOI] [PubMed] [Google Scholar]
- Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30:71–76. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology: biological timekeeping. Sinauer Associates; Sunderland: 2004. [Google Scholar]
- Edvinsson L, Krause DN. Cerebral blood flow and metabolism. Williams & Wilkins, Lippincott; Philadelphia: 2002. [Google Scholar]
- Eisenhofer G, Goldstein DS, Kopin IJ. Plasma dihydroxyphenylglycol for estimation of noradrenaline neuronal reuptake in the sympathetic nervous system in vivo. Clin Sci. 1986;76:171–182. doi: 10.1042/cs0760171. [DOI] [PubMed] [Google Scholar]
- Elhendy A, Modesto KM, Mahoney DW, Khandheria BK, Seward JB, Pellikka PA. Prediction of mortality in patients with left ventricular hypertrophy by clinical, exercise stress, and echocardiographic data. J Am Coll Cardiol. 2003;41:129–135. doi: 10.1016/s0735-1097(02)02667-0. [DOI] [PubMed] [Google Scholar]
- Elherik K, Khan F, McLaren M, Kennedy G, Belch JJF. Circadian variation in vascular tone and endothelial cell function in normal males. Clin Sci. 2002;102:547–552. [PubMed] [Google Scholar]
- Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- Engel GL. Psychologic stress, vasodepressor (vasovagal) syncope, and sudden-death. Ann Intern Med. 1978;89:403–412. doi: 10.7326/0003-4819-89-3-403. [DOI] [PubMed] [Google Scholar]
- Feng DL, Murillo J, Jadhav P, et al. Upright posture and maximal exercise increase platelet aggregability and prostacyclin production in healthy male subjects. Br J Sports Med. 1999;33(6):401–404. doi: 10.1136/bjsm.33.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- Floras JS, Bradley TD. Treating obstructive sleep apnea: is there more to the story than 2 millimeters of mercury? Hypertension. 2007;50:289–291. doi: 10.1161/HYPERTENSIONAHA.107.092106. [DOI] [PubMed] [Google Scholar]
- Franklin BA, Bonzheim K, Gordon S, Timmis GC. Safety of medical supervised outpatient cardiac rehabilitation exercise therapy. Chest. 1998;114:902–906. doi: 10.1378/chest.114.3.902. [DOI] [PubMed] [Google Scholar]
- Fullick SJ, Morris C, Jones H, Atkinson G. Prior exercise lowers blood pressure during simulated night-work with different meal schedules. Am J Hypertens. 2009;22:835–841. doi: 10.1038/ajh.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol. 2008;105:766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy AY, Bornstein NM. Are there any unique epidemiological and vascular risk factors for ischaemic strokes that occur in the morning hours? Eur J Neurol. 2000;7:179–181. doi: 10.1046/j.1468-1331.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Norris JW. The acute stroke. FA Davis Co; Philadelphia: 1985. [Google Scholar]
- Hagberg JM, Montain SJ, Martin WH., 3rd Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1987;63(1):270–276. doi: 10.1152/jappl.1987.63.1.270. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Fernandez JR, Ayala DE, Mojon A, Alonso I, Smolensky M. Circadian rhythm of double (rate-pressure) product in healthy normotensive young subjects. Chronobiol Int. 2001;18:475–489. doi: 10.1081/cbi-100103970. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Ayala DE, Smolensky MH. Chronotherapy in hypertensive patients: administration-time dependent effects of treatment on blood pressure regulation. Expert Rev Cardiovasc Ther. 2007;5:463–475. doi: 10.1586/14779072.5.3.463. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ESG, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated dilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Hoth KF, Tate DF, Poppas A, Forman DE, Gunstad J, Moser DJ, Paul RH, Jefferson AL, Haley AP, Cohen RA. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–312. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A, Tofler GH, Chen X, Stubbs ME, Solomon HS, Muller JE. Effects of nadolol on hemodynamic and hemostatic responses to potential mental and physical triggers of myocardial infarction in subjects with mild hypertension. Am J Cardiol. 1993;72:47–52. doi: 10.1016/0002-9149(93)90217-z. [DOI] [PubMed] [Google Scholar]
- Johnstone MT, Mittleman M, Tofler G, Muller JE. The patho-physiology of the onset of morning cardiovascular events. Am J Hypertens. 1996;9:22S–28S. doi: 10.1016/0895-7061(95)00403-3. [DOI] [PubMed] [Google Scholar]
- Jones H, Atkinson G, Leary AC, George K, Murphy M, Waterhouse J. Reactivity of ambulatory blood pressure to physical activity varies with time of day. Hypertension. 2006;47:778–784. doi: 10.1161/01.HYP.0000206421.09642.b5. [DOI] [PubMed] [Google Scholar]
- Jones H, George K, Edwards B, Atkinson G. Effects of time of day on post-exercise blood pressure: circadian or sleep-related influences? Chronobiol Int. 2008a;25:987–998. doi: 10.1080/07420520802548044. [DOI] [PubMed] [Google Scholar]
- Jones H, Pritchard C, George K, Edwards B, Atkinson G. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 2008b;104:481–489. doi: 10.1007/s00421-008-0797-4. [DOI] [PubMed] [Google Scholar]
- Jones H, George K, Atkinson G. Timing of exercise within the waking period does not alter blood pressure during subsequent nocturnal sleep in normotensive individuals. J Exerc Sci Fit. 2009 under review. [Google Scholar]
- Jones H, Taylor CE, Lewis NCS, et al. Post-exercise blood pressure reduction is greater following intermittent than continuous exercise and is influenced less by diurnal variation. Chronobiol Int. 2009b;26:293–306. doi: 10.1080/07420520902739717. [DOI] [PubMed] [Google Scholar]
- Jones H, Green DJ, George K, Black MA, Atkinson G. Evidence for elevated vascular shear stress following morning exercise. Med Sci Sport Exerc. 2009b doi: 10.1249/MSS.0b013e318195109c. in press. [DOI] [PubMed] [Google Scholar]
- Kario K, Schwartz JE, Pickering TG. Ambulatory physical activity as a determinant of diurnal blood pressure variation. Hypertension. 1999;34:685–691. doi: 10.1161/01.hyp.34.4.685. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Tochikubo O, Minamisawa K, Miyajima E, Ishii M. Circadian variation of haemodynamics in patients with essential hypertension: comparison between early morning and evening. J Hypertens. 1994;12:1405–1412. [PubMed] [Google Scholar]
- Kawano H, Motoyama T, Yasue H, et al. Endothelial function fluctuates with diurnal variation in the frequency of ischemic episodes in patients with variant angina. J Am Coll Cardiol. 2002;40:266–270. doi: 10.1016/s0735-1097(02)01956-3. [DOI] [PubMed] [Google Scholar]
- Kline CE, Durstine JL, Davis JM, et al. Circadian variation in swim performance. J Appl Physiol. 2007;102:641–649. doi: 10.1152/japplphysiol.00910.2006. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Kop WJ, Gabbay FH. Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation. 1996;93:1364–1371. doi: 10.1161/01.cir.93.7.1364. [DOI] [PubMed] [Google Scholar]
- Kuniyoshi FHS, Garcia-Touchard A, Gami AS, et al. Day–night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima I, Mitani K, Miyao M, Suzuki Y, Kuramoto K, Ozawa T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: relation to arising time. Am J Hypertens. 1995;8:29–33. doi: 10.1016/0895-7061(94)00154-4. [DOI] [PubMed] [Google Scholar]
- Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- Leary AC, Struthers AD, Donnan PT, MacDonald MT, Murphy MB. The morning surge in blood pressure and heart rate is dependent on levels of physical activity after waking. J Hypertens. 2002;20:865–870. doi: 10.1097/00004872-200205000-00020. [DOI] [PubMed] [Google Scholar]
- Legramante JM, Marciani MG, Placidi F, Aquilani S, Romigi A, Tombini M, Massaro M, Galante A, Iellamo F. Sleep-related changes in baroreflex sensitivity and cardiovascular autonomic modulation. J Hypertens. 2003;21:1555–1561. doi: 10.1097/00004872-200308000-00021. [DOI] [PubMed] [Google Scholar]
- Levine RL, Pepe PE, Fromm RE, Jr, Curka PA, Clark PA. Prospective evidence of a circadian rhythm for out-of-hospital cardiac arrests. JAMA. 1992;267:2935–2937. [PubMed] [Google Scholar]
- Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrin Metab. 1985;60:1210–1215. doi: 10.1210/jcem-60-6-1210. [DOI] [PubMed] [Google Scholar]
- Lockwood JM, Wilkins BW, Halliwill JR. H-1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol. 2005;563:633–642. doi: 10.1113/jphysiol.2004.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn BM, McCord JL, Halliwill JR. Effects of the menstrual cycle and sex on postexercise hemodynamics. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1260–R1270. doi: 10.1152/ajpregu.00589.2006. [DOI] [PubMed] [Google Scholar]
- Maemura K, de la Monte SM, Chin MT, et al. CLIF, a novel cycle-like factor, regulates the circadian oscillation of plasminogen activator inhibitor-1 gene expression. J Biol Chem. 2000;275(47):36847–36851. doi: 10.1074/jbc.C000629200. [DOI] [PubMed] [Google Scholar]
- Manfredini R, Waterhouse JM. Preface. Biol Rhythm Res. 2007;38:141. [Google Scholar]
- Manfredini R, Boari B, Smolensky MH, et al. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22(3):417–453. doi: 10.1081/CBI-200062927. [DOI] [PubMed] [Google Scholar]
- McNamara P, Seo SP, Rudic RD, Sehgal A, Charkravarti D, Fitzgerald GA. Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to rest a peripheral clock. Cell. 2001;105:877–889. doi: 10.1016/s0092-8674(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Middeke M, Kluglich M, Holzgreve H. Circadian blood pressure rhythm in primary and secondary hypertension. Chronobiol Int. 1991;8:451–459. doi: 10.3109/07420529109059181. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Sontz EM. Morning sympathetic nerve activity is not increased in humans: implications for mechanism underlying the circadian pattern of cardiac risk. Circulation. 1995;91:2549–2555. doi: 10.1161/01.cir.91.10.2549. [DOI] [PubMed] [Google Scholar]
- Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet. 1978;1:795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- Mineda Y, Sumiyoshi M, Tokano T, et al. Circadian variation of vasovagal syncope. J Cardiovasc Eleciroptiysiol. 2000;11:1078–1080. doi: 10.1111/j.1540-8167.2000.tb01751.x. [DOI] [PubMed] [Google Scholar]
- Minors D, Waterhouse J. The use of constant routines in unmasking the endogenous component of human circadian rhythms. Chronobiol Int. 1984;3:205–216. doi: 10.3109/07420528409063897. [DOI] [PubMed] [Google Scholar]
- Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive post-menopausal women. JACC. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Motoyama T, Kawano H, Kugiyama K, et al. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol. 1997;273(4 Pt 2):H1644–H1650. doi: 10.1152/ajpheart.1997.273.4.H1644. [DOI] [PubMed] [Google Scholar]
- Mulcahy D, Keegan J, Crean P. Silent myocardial ischaemia in chronic stable angina: a study of its frequency and characteristics in 150 patients. Br Heart J. 1988;60:417–423. doi: 10.1136/hrt.60.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- Murray PM, Herrington DM, Pettus CW, Miller HS, Cantwell JD, Little WC. Should patients with heart disease exercise in the morning or afternoon? Arch Intern Med. 1993;153:833–836. [PubMed] [Google Scholar]
- Nelson RR, Gobel FL, Jorgensen CR, Wang K, Wang Y, Taylor HL. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation. 1974;50:1179–1189. doi: 10.1161/01.cir.50.6.1179. [DOI] [PubMed] [Google Scholar]
- Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. doi: 10.1016/s0002-9149(00)00857-2. [DOI] [PubMed] [Google Scholar]
- Nieminen T, Leino J, Maanoja J, et al. The prognostic value of haemodynamic parameters in the recovery phase of an exercise test. The Finnish cardiovascular study. J Hum Hypertens. 2008;22:537–543. doi: 10.1038/jhh.2008.38. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation. 2001;104:1746–1748. doi: 10.1161/hc4001.098048. [DOI] [PubMed] [Google Scholar]
- Okamura H, Yamaguchi S, Yagita K. Molecular machinery of the circadian clock in mammals. Cell Tissue Res. 2002;309:47–56. doi: 10.1007/s00441-002-0572-5. [DOI] [PubMed] [Google Scholar]
- Otto ME, Svatikova A, de Mattos Barretto RB, et al. Early morning attenuation of endothelial function in healthy humans. Circulation. 2004;109:2507–2510. doi: 10.1161/01.CIR.0000128207.26863.C4. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to α-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- Park S, Jastremski CA, Wallace JP. Time of day for exercise on blood pressure reduction in dipping and nondipping hypertension. J Hypertens. 2005;19:597–605. doi: 10.1038/sj.jhh.1001901. [DOI] [PubMed] [Google Scholar]
- Parker J, Testa M, Jimenez A. Morning increase in ambulatory ischemia in patients with stable coronary artery disease: importance of physical activity and increased cardiac demand. Circulation. 1994;89:604. doi: 10.1161/01.cir.89.2.604. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Perticone F, Ceravolo R, Puji A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Piepoli M, Coats A, Adamopoulos S, et al. Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J Appl Physiol. 1993;75:1807–1814. doi: 10.1152/jappl.1993.75.4.1807. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Montanari L, Massari M, Di Chiara V, Capanna M. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. Am J Hypertens. 1991;4:20–26. doi: 10.1093/ajh/4.1.20. [DOI] [PubMed] [Google Scholar]
- Prakash M, Myers J, Froelicher VF, et al. Diagnostic exercise tests on 4000 consecutive men. Am Heart J. 2001;142:127–135. doi: 10.1067/mhj.2001.115795. [DOI] [PubMed] [Google Scholar]
- Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, Winter C, Bliwise DL. Sleep fragmentation and morning cerebrovasomotor reactivity to hypercapnia. Am J Respir Crit Care Med. 1999;160:1244–1247. doi: 10.1164/ajrccm.160.4.9810111. [DOI] [PubMed] [Google Scholar]
- Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–194. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- Richter HG, Torres-Farfán C, Rojas-Garcia PP, Campino C, Torrealba F, Serón-Ferré M. The circadian timing system: making sense of day/night gene expression. Biol Res. 2004;37:11–28. doi: 10.4067/s0716-97602004000100003. [DOI] [PubMed] [Google Scholar]
- Robinson BF. Relationship of heart rate and systolic blood pressure to the onset of pain and angina pectoris. Circulation. 1967;25:1073–1083. doi: 10.1161/01.cir.35.6.1073. [DOI] [PubMed] [Google Scholar]
- Romme JJ, van Dijk N, Boer KR, et al. Influence of age and gender on the occurrence and presentation of reflex syncope. Clin Auton Res. 2008;18:127–133. doi: 10.1007/s10286-008-0465-0. [DOI] [PubMed] [Google Scholar]
- Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:648–652. doi: 10.1034/j.1399-3003.2000.16d14.x. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005;98:151–159. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- Shaw JA, Chin-Dusting JP, Kingwell BA, Dart AM. Diurnal variation in endothelium-dependent vasodilatation is not apparent in coronary artery disease. Circulation. 2001;103:806–812. doi: 10.1161/01.cir.103.6.806. [DOI] [PubMed] [Google Scholar]
- Smolensky MH. Aspects of human chronopathology. In: Reinberg A, Smolensky MH, editors. Biological rhythms and medicine. Cellular, metabolic, physiologic, and pharmacologic aspects. Springer; Heidelberg: 1983. pp. 131–209. [Google Scholar]
- Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007;8:668–680. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou G, Vemmos K, Pliarchopoulou K, Synetos A, Roussias L, Mountokalakis T. Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke. 2002;33:1480–1486. doi: 10.1161/01.str.0000016971.48972.14. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Szymanski LM, Pate RR. Effects of exercise intensity, duration and time of day on fibrinolytic-activity in physically active men. Med Sci Sports Exerc. 1994;26:1102–1108. [PubMed] [Google Scholar]
- Takeda N, Maemura K. Chronobiology of acute myocardial infarction molecular biology. Biol Rhythm Res. 2007;38:233–245. [Google Scholar]
- Tofler G, Brezinski D, Schafer A, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316:1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- Tsementzis SA, Gill JS, Hitchcock ER, Gil SK, Beevers DG. Diurnal variation of activity during the onset of stroke. Neurosurgery. 1985;17:901–904. doi: 10.1227/00006123-198512000-00005. [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Lozeva V, Valjakka A, Lecklin A. Modifying effects of histamine on circadian rhythms and neuronal excitability. Behav Brain Res. 2001;124:129–135. doi: 10.1016/s0166-4328(01)00222-4. [DOI] [PubMed] [Google Scholar]
- Uen S, Baulmann J, Dusing R, Glanzer K, Vetter H, Mengden T. ST-segment depression in hypertensive patients is linked to elevations in blood pressure, pulse pressure and double product by 24-h Cardiotens monitoring. J Hypertens. 2003;21:977–983. doi: 10.1097/00004872-200305000-00023. [DOI] [PubMed] [Google Scholar]
- van Dijk N, Boer MC, De Santo T, et al. Daily, weekly, monthly, and seasonal patterns in the occurence of vasovagal syncope in an older population. Eurospace. 2007;9:823–828. doi: 10.1093/europace/eum104. [DOI] [PubMed] [Google Scholar]
- Van Lieshout JJ, Wieling W, Karemaker JM, Secher NH. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol. 2003;94:833–848. doi: 10.1152/japplphysiol.00260.2002. [DOI] [PubMed] [Google Scholar]
- Veerman DP, Imholz BP, Wieling W, Wesseling KH, van Montfrans GA. Circadian profile of systemic hemodynamics. Hypertension. 1995;26:55–59. doi: 10.1161/01.hyp.26.1.55. [DOI] [PubMed] [Google Scholar]
- Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- Villella M, Villella A, Barlera S, Franzosi MG, Maggioni AP. Prognostic significance of double product and inadequate double product response to maximal symptom limited exercise stress testing after myocardial infarction in 6296 patients treated with thrombolytic agents. Am Heart J. 1999;137:443–452. doi: 10.1016/s0002-8703(99)70490-4. [DOI] [PubMed] [Google Scholar]
- Waterhouse J, Kao S, Weinert D, et al. Measuring phase shifts in humans following a simulated time-zone transition: agreement between constant routine and purification methods. Chronobiol Int. 2005;22:829–858. doi: 10.1080/07420520500263375. [DOI] [PubMed] [Google Scholar]
- Waters DD, Miller D, Bouchard A, Bosch X, Theroux P. Circa-dian variation in variant angina. Am J Cardiol. 1984;54:61–64. doi: 10.1016/0002-9149(84)90304-7. [DOI] [PubMed] [Google Scholar]
- Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke. 1994;25:1963–1967. doi: 10.1161/01.str.25.10.1963. [DOI] [PubMed] [Google Scholar]
- Wijnhoud AD, Koudstaal PJ, Dippel DW. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J Clin Ultrasound. 2006;34:70–76. doi: 10.1002/jcu.20193. [DOI] [PubMed] [Google Scholar]
- Willich SN, Maclure M, Mittleman M. Sudden cardiac death. Support for a role of triggering in causation. Circulation. 1993;87:1442–1450. doi: 10.1161/01.cir.87.5.1442. [DOI] [PubMed] [Google Scholar]
- Winther K, Hillegass W, Tofler GH, et al. Effects on platelet aggregation and fibrinolytic activity during upright posture and exercise in healthy men. Am J Cardiol. 1992;70:1051–1055. doi: 10.1016/0002-9149(92)90359-7. [DOI] [PubMed] [Google Scholar]
- Wroe SJ, Sandercock P, Bamford J, Dennis M, Slattery J, Warlow C. Diurnal variation in incidence of stroke: Oxfordshire community stroke project. Br Med J. 1992;304:155–157. doi: 10.1136/bmj.304.6820.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Khayat R, et al. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2005;172:371–378. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol. 2002;34(2):223–231. doi: 10.1006/jmcc.2001.1504. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral auto-regulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]