Abstract
Background
The Xanthomonadaceae family contains two xylem-limited plant pathogenic bacterial species, Xanthomonas albilineans and Xylella fastidiosa. X. fastidiosa was the first completely sequenced plant pathogen. It is insect-vectored, has a reduced genome and does not possess hrp genes which encode a Type III secretion system found in most plant pathogenic bacteria. X. fastidiosa was excluded from the Xanthomonas group based on phylogenetic analyses with rRNA sequences.
Results
The complete genome of X. albilineans was sequenced and annotated. X. albilineans, which is not known to be insect-vectored, also has a reduced genome and does not possess hrp genes. Phylogenetic analysis using X. albilineans genomic sequences showed that X. fastidiosa belongs to the Xanthomonas group. Order of divergence of the Xanthomonadaceae revealed that X. albilineans and X. fastidiosa experienced a convergent reductive genome evolution during their descent from the progenitor of the Xanthomonas genus. Reductive genome evolutions of the two xylem-limited Xanthomonadaceae were compared in light of their genome characteristics and those of obligate animal symbionts and pathogens.
Conclusion
The two xylem-limited Xanthomonadaceae, during their descent from a common ancestral parent, experienced a convergent reductive genome evolution. Adaptation to the nutrient-poor xylem elements and to the cloistered environmental niche of xylem vessels probably favoured this convergent evolution. However, genome characteristics of X. albilineans differ from those of X. fastidiosa and obligate animal symbionts and pathogens, indicating that a distinctive process was responsible for the reductive genome evolution in this pathogen. The possible role in genome reduction of the unique toxin albicidin, produced by X. albilineans, is discussed.
Background
The Xanthomonadaceae are a family of Gram negative bacteria belonging to the order Xanthomonadales in the gamma subdivision of the Proteobacteria [1]. Members of this family are typically characterized as environmental organisms and occupy diverse ecological niches, such as soil and water, as well as plant tissues. Many Xanthomonadaceae, especially species from the genera Xanthomonas and Xylella, cause plant diseases and only one, Stenotrophomonas maltophilia, is known to be an opportunistic human pathogen.
Complete genome sequences of several Xanthomonas species and Xylella fastidiosa strains have been determined, making those bacteria attractive models for study of plant-pathogen interactions [2]. X. fastidiosa was the first completely sequenced plant pathogen. Sequence analysis showed that this xylem-limited bacterium, which is insect-vectored to a variety of diverse hosts, had a reduced genome and did not possess hrp genes, which encode a Type III secretion system (T3SS) found in most Gram negative plant pathogenic bacteria [3]. Phylogenetic analysis with rRNA sequences showed that the two major genera of Xanthomonadaceae, Xanthomonas and Stenotrophomonas, form a coherent group excluding X. fastidiosa [4-6]. These characteristics suggested the hypothesis that this species evolved from an ancestor shared with Xanthomonas and Stenotrophomonas by genome reduction during adaptation to life within its hosts [2].
Xanthomonas albilineans is a systemic, xylem-limited pathogen that causes leaf scald, one of the major diseases of sugarcane (interspecific hybrids of Saccharum spp.) [7]. Leaf scald symptoms vary from a single, white, narrow, sharply defined stripe to complete wilting and necrosis of infected leaves, leading to plant death. X. albilineans produces the toxin albicidin that has phytotoxic and antibiotic properties [8]. Albicidin is a potent DNA gyrase inhibitor that targets the chloroplastic DNA gyrase A, inhibits chloroplast DNA replication and blocks chloroplast differentiation, resulting in the white foliar stripe symptoms [8,9]. All attempts to identify hrp genes in X. albilineans failed so far [10,11]. A phylogenetic study with the housekeeping genes ihfA and efp, which did not include S. maltophilia sequences, suggested that X. albilineans was an evolutionary intermediate between several Xanthomonas species and X. fastidiosa [11].
Unlike other xylem-invading xanthomonads that interact with living plant tissues, such as X. campestris pv. campestris or X. oryzae pv. oryzae, X. fastidiosa and X. albilineans appear to be strictly xylem-limited, living only in dead xylem cells or tracheary elements. In order to better understand the evolution of these two xylem-limited Xanthomonadaceae, we sequenced the genome of X. albilineans strain GPE PC73 from Guadeloupe [11]. This sequence was compared to complete genome sequences of other closely related members of the Xanthomonadaceae. This comparative analysis revealed that X. albilineans and X. fastidiosa experienced a convergent reductive genome evolution from a common ancestral parent of the Xanthomonas genus.
Results
General genomic features of X. albilineans
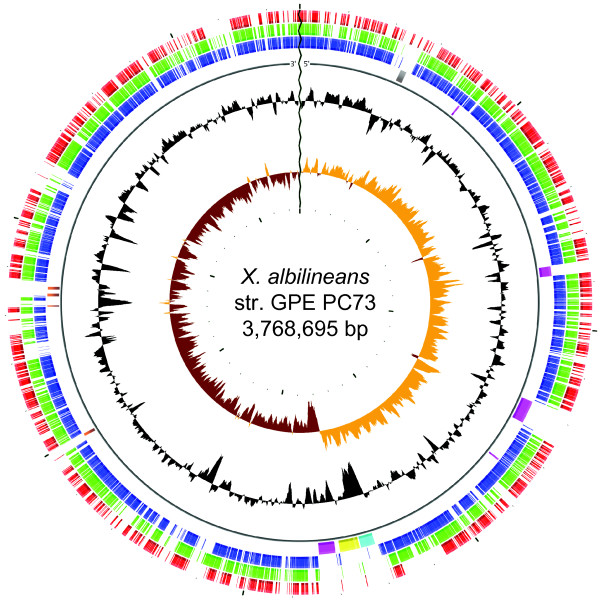
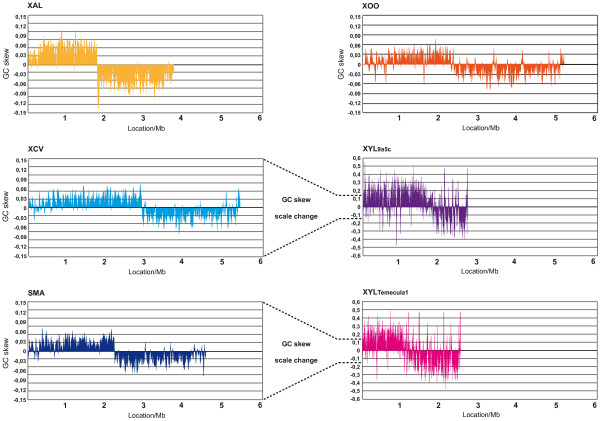
The genome of X. albilineans strain GPE PC73 consists of one circular chromosome of 3,768,695 bp and three extrachromosomal plasmids of 32, 27 and 25 Kbp, respectively. The chromosome exhibits a GC skew pattern typical of prokaryotic genomes that have two major shifts, one near the origin and one near the terminus of replication, with dnaA assigned as base pair 1 of the chromosome (Figure 1). The GC skew pattern of X. albilineans contains a lower number of diagram distortions and has much lower amplitude than the GC skew pattern of X. fastidiosa (Figure 2). However, the amplitude of the GC skew pattern of X. albilineans is significantly higher than the one of other xanthomonads and S. maltophilia (Figure 2). Of the 3115 putative protein-coding sequences (CDSs) manually annotated on the chromosome of X. albilineans strain GPE PC73, 2014 (64%) were assigned putative functions based on homology to other known proteins and domain analyses.
Figure 1.
Circular representation of the X. albilineans chromosome (strain GPE PC73). The scale is shown in megabases around the periphery. Moving inward, the first three circles show CDSs conserved in X. fastidiosa strain 9a5c (in red), S. maltophilia strain R551-3 (in green) and X. axonopodis pv. vesicatoria strain 85-10 (in blue), respectively (forward and reverse-strand conserved CDSs are shown in the same circle). In the next circle, the NRPS gene clusters (except the albicidin biosynthesis gene cluster) are shown in pink, the albicidin biosynthesis gene cluster is shown in yellow, the T3SS SPI-1 gene cluster is shown in blue, genes encoding proteins that contain repeated Rhs elements are shown in brown, and a large phage-related sequence is shown in grey. The black circle shows the G+C content using a 100 base window. The brown and orange circle shows the GC skew (G-C)/(G+C) using a 100 base window.
Figure 2.
Linear representation of the GC skew (G-C)/(G+C) of six strains of Xanthomonadaceae using a 1000 base window. XCV = X. axonopodis pv. vesicatoria strain 85-10; XAL = X. albilineans strain GPE PC73; XOO = X. oryzae pv. oryzae strain MAFF 311018; SMA = S. maltophilia strain R551-3; XYL9a5c = X. fastidiosa strain 9a5c; XYLTemecula1 = X. fastidiosa strain Temecula1. A GC skew pattern very similar to that of X. axonopodis pv. vesicatoria strain 85-10 was observed for X. campestris pv. campestris strain ATCC 33913 and X. axonopodis pv. citri strain 306 (data not shown). A GC skew pattern very similar to that of S. maltophilia strain R551-3 was observed for S. maltophilia strain K279a (data not shown).
The general features of the X. albilineans chromosome were compared to those of the chromosomes of the following eight Xanthomonadaceae strains: X. oryzae pv. oryzae strain MAFF 311018 (isolated from rice; [12]), X. campestris pv. campestris strain ATCC 33913 (isolated from cabbage; [13]), X. axonopodis pv. vesicatoria strain 85-10 (isolated from pepper; [14]), X. axonopodis pv. citri strain 306 (isolated from citrus; [13]), S. maltophilia strain K279a (isolated from the blood of an infected patient; [15]), S. maltophilia strain R551-3 (isolated from poplar; [16]), X. fastidiosa strain 9a5c (isolated from citrus; [3]) and X. fastidiosa strain Temecula1 (isolated from grapevine; [17]). The chromosome of X. albilineans is 1.4 Mb smaller than the chromosomes of X. axonopodis pv. vesicatoria strain 85-10 and X. axonopodis pv. citri strain 306, but it is 1.2 Mb bigger than the chromosome of X. fastidiosa strain Temecula1 (Table 1). The G+C content of the chromosome of X. albilineans averages 63%. This value is similar to those of other Xanthomonas strains, but it is 12% higher than the G+C content of the chromosome of X. fastidiosa strain Temecula1. The chromosome of X. albilineans shows an average coding density of 84%, which is also similar to other xanthomonads.
Table 1.
General features of nine Xanthomonadaceae chromosomes
| Features |
X. oryzae pv. oryzae strain MAFF 311018 |
X. campestris pv. campestris strain ATCC 33913 |
X. axonopodis pv. vesicatoria strain 85-10 |
X. axonopodis pv. citri strain 306 |
S. maltophilia strain K279a |
S. maltophilia strain R551-3 |
X. fastidiosa strain 9a5c |
X. fastidiosa strain Temecula1 |
X. albilineans strain GPE PC73 |
|---|---|---|---|---|---|---|---|---|---|
| Size (bp) | 4,940,217 | 5,076,187 | 5,178,466 | 5,175,554 | 4,851,126 | 4,573,969 | 2,679,306 | 2,519,802 | 3,768,695 |
| G+C content (%) | 63 | 65 | 65 | 64 | 66 | 66 | 52 | 51 | 63 |
| Coding density (%) | 83 | 84 | 87 | 84 | 88 | 89 | 83 | 79 | 84 |
| Protein-coding sequences (CDS) | 4372 | 4181 | 4487 | 4312 | 4386 | 4039 | 2766 | 2123 | 3115 |
| Average length of all CDS (bp) | 948 | 1027 | 1005 | 1032 | 980 | 1010 | 805 | 964 | 1059 |
| Average length of thea core genome CDS (bp) | 1055 | 1058 | 1060 | 1056 | 1051 | 1048 | 1048 | 1044 | 1050 |
| CDS < 300 bp | 346 | 318 | 428 | 299 | 294 | 261 | 738 | 194 | 283 |
| rRNA operons | 2 | 2 | 2 | 2 | 4 | 4 | 2 | 2 | 2 |
| tRNA | 53 | 54 | 54 | 54 | 74 | 71 | 49 | 49 | 51 |
acore genome CDS correspond to the 1256 orthologs shared by the nine Xanthomonadaceae
Comparative genomic analyses
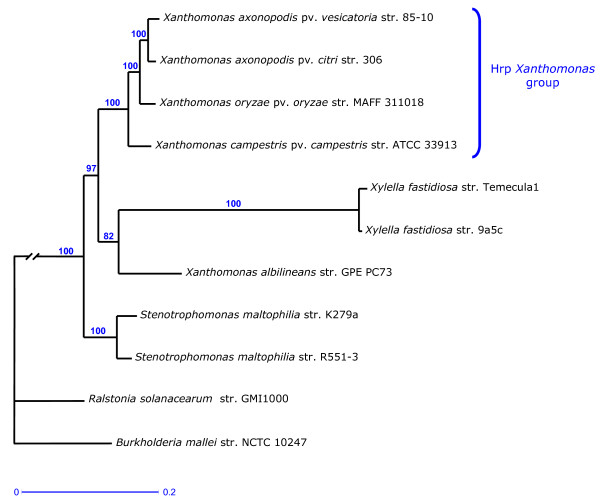
In order to assess phylogenetic relationships among the nine Xanthomonadaceae mentioned above (Table 1), we performed multilocus sequence analysis (MLSA). The phylogenetic tree obtained with the concatenated data set of seven housekeeping genes showed that X. albilineans belongs to the same clade as X. fastidiosa (Figure 3). The clade containing X. albilineans and X. fastidiosa is clearly distinct from the clade containing the four xanthomonads of the Hrp Xanthomonas group (X. campestris pv. campestris, X. axonopodis pv. vesicatoria, X. axonopodis pv. citri and X. oryzae pv. oryzae). On the basis of this phylogenetic tree, we conclude that X. albilineans and X. fastidiosa derived from the progenitor of the Xanthomonas genus (Figure 3).
Figure 3.
Tree of the concatenated nucleotide sequences of seven housekeeping genes (gyrB, atpD, dnaK, efp, groEL, glnA and recA) using the maximum likelihood method and GTR as substitution model. The tree was constructed with Burkholderia pseudomallei strain NCTC 10247 as outgroup. The total length of the concatenated nucleotide sequences was between 10417-10686 bp. Bootstrap percentages retrieved in 500 replications are shown at the nodes. The scale bar (0.2) indicates the number of nucleotide substitutions per site. The long branch separating the Xanthomonadaceae from the two other distant taxa (B. pseudomallei strain NCTC 10247 and Ralstonia solanacearum strain GMI1000) has been shortened.
In order to identify orthologs shared by the nine Xanthomonadaceae strains (Table 1), we performed OrthoMCL comparative analyses [18]. The X. albilineans CDSs shared with X. fastidiosa strain 9a5c, S. maltophilia strain K279a or X. axonopodis pv. vesicatoria strain 85-10 are represented on the circular chromosome (Figure 1). Interestingly, the chromosome of X. albilineans harbours several large regions that do not contain any genes present in the other eight Xanthomonadaceae strains. These regions correspond either to phage related sequences or to large genes encoding proteins that contain repeated Rhs elements that are known to be frequently transferred by horizontal genetic transfer [19]. They also contain several gene clusters specific to X. albilineans: (i) the albicidin biosynthesis gene cluster XALB1 which was previously sequenced from X. albilineans strain Xa23R1 [20] and which contains three nonribosomal peptide synthetases (NRPSs) genes; (ii) three additional NRPS gene clusters to which cannot be ascribed a precise function as they have not been previously identified and no strictly orthologous genes were found in other bacteria; and (iii) a gene cluster encoding a T3SS of the SPI-1 (Salmonella Pathogenicity Island -1) family that is mainly found in free-living animal pathogens [21]. The OrthoMCL analyses identified a total of 522 CDSs of the genome of X. albilineans strain GPE PC73 that are not shared with any of the other eight Xanthomonadaceae complete genome sequences compared in this study. They also identified 1256 CDSs shared by the nine Xanthomonadaceae, which represent a Xanthomonadaceae core gene set which was likely inherited from a common ancestor.
Evidence of convergent genome reductive evolution of X. albilineans and X. fastidiosa
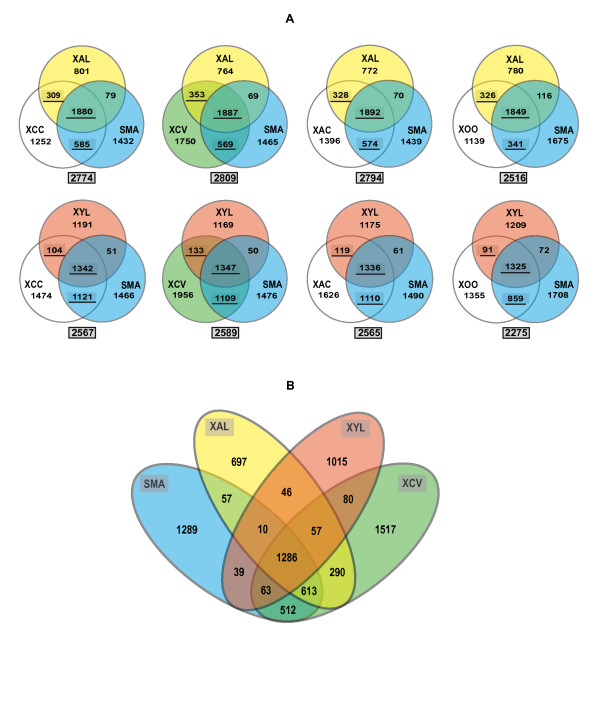
The phylogenetic tree presented in Figure 3 suggests that X. albilineans, X. fastidiosa and the Hrp Xanthomonas group derived from a common ancestor shared with Stenotrophomonas. The chromosome sizes of X. albilineans and X. fastidiosa are smaller than those of any other xanthomonad (Table 1), suggesting that both species evolved from the progenitor of xanthomonads by genome reduction. To examine how the chromosomes of X. albilineans and X. fastidiosa have evolved to result in these different sizes, we determined the order of divergence of X. albilineans, X. fastidiosa, the four xanthomonads of the Hrp Xanthomonas group and S. maltophilia (Figure 3). Orthologous genes shared by S. maltophilia and a species of the Hrp Xanthomonas group, but missing in X. albilineans or X. fastidiosa, may be assumed to have been lost by X. albilineans or X. fastidiosa, respectively. Inversely, genes of a species of the Hrp Xanthomonas group that are shared with S. maltophilia, X. albilineans or X. fastidiosa may be assumed to have been inherited from the progenitor of the Xanthomonas genus. On the basis of OrthoMCL analysis, the numbers of genes lost by X. albilineans strain GPE PC73 and X. fastidiosa strain 9a5c are at least 585 and 1121, respectively (numbers obtained by comparison with X. campestris pv. campestris; 585 genes lost by X. albilineans = the number of CDSs conserved in both X. campestris pv. campestris and S. maltophilia and missing in X. albilineans; 1121 genes lost by X. fastidiosa = the number of CDSs conserved in both X. campestris pv. campestris and S. maltophilia and missing in X. fastidiosa; Figure 4A). The number of ancestral genes inherited from the progenitor of the Xanthomonas genus is higher in X. axonopodis pv. vesicatoria than in any other species of the Hrp Xanthomonas group (2809 ancestral genes present in X. axonopodis pv. vesicatoria = the number of CDSs of X. axonopodis pv. vesicatoria conserved in X. albilineans or S. maltophilia; Figure 4A). Interestingly, X. oryzae pv. oryzae strain MAFF 311018 contains the smallest number of genes lost by X. albilineans or X. fastidiosa, indicating that a significant number of genes lost by X. albilineans or X. fastidiosa were also lost by the xylem-invading pathogen X. oryzae pv. oryzae (Figure 4A). On the basis of OrthoMCL analysis including both X. albilineans strain GPE PC73 and X. fastidiosa strain 9a5c, 512 ancestral genes were lost by both X. albilineans and X. fastidiosa (512 = number of orthologs shared only by X. axonopodis pv. vesicatoria strain 85-10 and S. maltophilia strain R551-3, Figure 4B), 960 ancestral genes were lost only by X. fastidiosa (960 = 613 + 290 + 57 = number of CDSs of X. albilineans strain GPE PC73 conserved in X. axonopodis pv. vesicatoria strain 85-10 or S. maltophilia strain R551-3 and missing in X. fastidiosa strain 9a5c, Figure 4B), and 182 ancestral genes were lost only by X. albilineans (182 = 63 + 39 + 80 = number of CDSs of X. fastidiosa strain 9a5c conserved in X. axonopodis pv. vesicatoria strain 85-10 or S. maltophilia strain R551-3 and missing in X. albilineans strain GPE PC73, Figure 4B).
Figure 4.
Venn diagrams showing the number of orthologous CDSs as determined by OrthoMCL analyses among strains of Xanthomonadaceae. (A) Venn diagrams showing the number of orthologous CDSs among (i) X. albilineans strain GPE PC73 (XAL), S. maltophilia strain R551-3 (SMA) and one of the four following Hrp Xanthomonas strains: X. campestris pv. campestris strain ATCC 33913 (XCC), X. axonopodis pv. vesicatoria strain 85-10 (XCV), X. axonopodis pv. citri strain 306 (XAC) or X. oryzae pv. oryzae strain MAFF 311018 (XOO), and (ii) X. fastidiosa strain 9a5c (XYL), S. maltophilia strain R551-3 (SMA) and one of the four following Hrp Xanthomonas strains: X. campestris pv. campestris strain ATCC 33913 (XCC), X. axonopodis pv. vesicatoria strain 85-10 (XCV), X. axonopodis pv. citri strain 306 (XAC) or X. oryzae pv. oryzae strain MAFF 311018 (XOO). The number of predicted ancestral CDSs of respectively XCC, XCV, XAC and XOO (CDSs conserved in SMA, XAL or XYL) are underlined and the total number of these predicted ancestral CDSs is indicated below each Venn diagram. (B) Venn diagram showing the number of orthologous CDSs among (i) X. albilineans strain GPE PC73 (XAL), S. maltophilia strain R551-3 (SMA), X. axonopodis pv. vesicatoria strain 85-10 (XCV) and X. fastidiosa strain 9a5c (XYL). Numbers do not include paralogous CDSs.
Comparison of the reductive evolutions of X. albilineans and X. fastidiosa
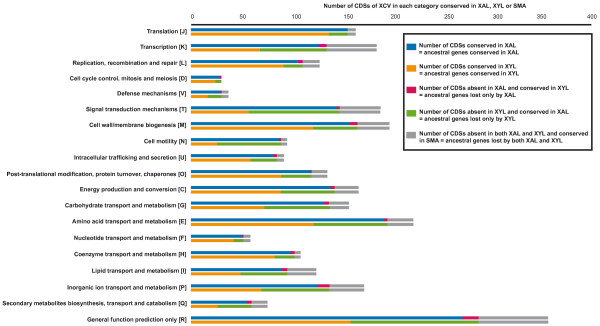
Further comparative analyses were performed to compare genome erosion in X. albilineans and X. fastidiosa. For these analyses, we selected the genome of X. axonopodis pv. vesicatoria, which contains the highest number of genes inherited from the progenitor of the Xanthomonas genus (Figure 4A). OrthoMCL analysis identified 3004 CDSs of X. axonopodis pv. vesicatoria that do not include any transposase genes and that are shared either by X. albilineans strain GPE PC73, X. fastidiosa strain 9a5c or one of the two S. maltophilia strains K279a and R551-3. For each of the 3004 CDSs, we looked for the best BLAST hit within a database that included: (i) all annotated CDSs from the genome sequence of X. albilineans strain GPE PC73 and (ii) all available sequences from public databases excluding all sequences from the xanthomonads. On the basis of these analyses and among the 3004 CDSs, we selected the genes for which the best BLAST hit belonged to X. albilineans, X. fastidiosa or S. maltophilia. We made the hypothesis that these genes were inherited by X. axonopodis pv. vesicatoria from the ancestor of the xanthomonads. These best BLAST hit analyses confirmed that 2864 of these 3004 CDSs have the same ancestor as genes present either in X. albilineans, X. fastidiosa or S. maltophilia and were therefore inherited by X. axonopodis pv. vesicatoria from the progenitor of the Xanthomonas genus. The elimination of paralogs present in at least two copies in X. axonopodis pv. vesicatoria generated a list of 2816 CDSs representing one copy of each gene inherited by X. axonopodis pv. vesicatoria from the progenitor of the Xanthomonas genus. These 2816 CDSs are listed and individually analysed in additional file 1. Among them, 1334 CDSs are shared by both X. fastidiosa and X. albilineans (these represent the ancestral genes conserved by X. fastidiosa and X. albilineans), 480 CDSs are shared only with one of the two S. maltophilia strains (they represent the ancestral genes lost by both X. fastidiosa and X. albilineans), 112 CDSs are shared with X. fastidiosa but not with X. albilineans (they represent the genes lost only by X. albilineans) and 890 CDSs are shared with X. albilineans but not with X. fastidiosa (they represent the genes lost only by X. fastidiosa). The distribution in functional COG categories of these 2816 CDSs is illustrated in Figure 5.
Figure 5.
Distribution in each functional COG category of the putative ancestral genes of X. axonopodis pv.vesicatoria that were conserved or lost by X. albilineans or X. fastidiosa. These putative ancestral genes correspond to the 2816 CDSs of the X. axonopodis pv.vesicatoria strain 85-10 chromosome shared with S. maltophilia strain R551-3, S. maltophilia strain K279a, X. albilineans strain GPE PC73 or X. fastidiosa strain 9a5c. They are listed and individually analysed in Additional file 1. XCV = X. axonopodis pv.vesicatoria strain 85-10; XAL = X. albilineans strain GPE PC73; SMA = S. maltophilia strain R551-3 or S. maltophilia strain K279a; XYL = X. fastidiosa strain 9a5c.
Analyses of the arrangement of these 2816 ancestral genes on the chromosome of X. axonopodis pv. vesicatoria strain 85-10 led to the identification of DNA regions constituting contiguous ancestral genes that are missing in X. albilineans or X. fastidiosa (Additional file 1). During the speciation of X. fastidiosa or X. albilineans, the loss of these DNA regions was due either to a single event of deletion or to the cumulative effect of multiple events (pseudogenization and short deletions). For example, the loss in X. fastidiosa of the large DNA region constituted by ancestral genes from XCV1928 to XCV2044 seems to be due to a single event of deletion since all these ancestral genes are missing in X. fastidiosa (Additional file 1). This large DNA region lost by X. fastidiosa encodes all flagellar proteins and several chemotaxis proteins. The sum of the length of the ancestral genes present in this DNA region is 106,626 bp, strongly suggesting that X. fastidiosa experienced a single deletion of a DNA fragment of a larger size. The other DNA regions constituting contiguous ancestral genes that are missing in X. albilineans or X. fastidiosa are shorter and, for this reason, their loss may result either from a single event of deletion or from multiple mutational events. Analysis of the arrangement on the chromosome of X. axonopodis pv. vesicatoria strain 85-10 of ancestral genes absent in X. albilineans or X. fastidiosa did not allow us to determine if the genes absent in both xylem-limited Xanthomonadaceae were lost by their common ancestor or were lost independently after their divergence.
The loss of genes by pseudogenization and short deletions should not affect the position on the chromosome of the genes that precede or follow the lost genes. In order to identify ancestral genes putatively lost by pseudogenization and short deletions, we looked for ancestral lost genes that are present on the chromosome of X. axonopodis pv. vesicatoria between the orthologs of two ancestral genes that are contiguous and conserved in X. fastidiosa or X. albilineans. For example, the rpf (for regulation of pathogenicity factors) gene cluster contains in X. axonopodis pv. vesicatoria two ancestral genes (XCV1913 and XCV1914 which are conserved in S. maltophilia) that are missing in both X. albilineans and X. fastidiosa. The ancestral genes XCV1912 and XCV1915 that precede and follow respectively these two lost genes are orthologs of either Xalc_1342 and Xalc_1343 or XF1110 and XF1111 that are contiguous in X. albilineans and X. fastidiosa, respectively. Using the same strategy we identified 147 and 131 ancestral genes potentially lost by pseudogenization by X. fastidiosa and X. albilineans, respectively (Additional files 2 and 3).
Common genomic features of X. fastidiosa and X. albilineans
The close relationship between X. albilineans and X. fastidiosa is illustrated by the common unique characteristics of their enzymes involved in cellulose degradation. In these two xylem-limited Xanthomonadaceae, endoglucanase EngXCA and 1,4-beta cellobiosidase CbhA possess a cellulose binding domain (CBD) and a long polyserine linker (PSL) at their C termini (Table 2). The endoglucanase EngXCA is conserved in all other Xanthomonas species and also has a CBD, but the linker is much shorter and its serine content is much lower (Table 2). The 1,4-beta cellobiosidase CbhA is conserved in the xylem-invading xanthomonads X. campestris pv. campestris and X. oryzae pv. oryzae but, in these two species, CbhA does not possess any linker nor any CBD (Table 2). The presence of a CBD is known to increase catalytic activity by reducing the "substrate accessibility problem" [22]. The long flexible PSL was proposed to enhance substrate accessibility [23]. The presence of genes encoding enzymes harbouring a PSL and a CBD provides evidence that both X. fastidiosa and X. albilineans are adapted to use plant cell breakdown products as carbon sources.
Table 2.
Comparative analysis of endoglucanase EngXCA and cellobiosidase CbhA encoded by Xanthomonadaceae species
| Enzyme | Xanthomonadaceae species | Accessions | aPSL size | aPSL composition | bCBD |
|---|---|---|---|---|---|
| Endoglucanase EngXCA | X. campestris pv. campestris strain ATCC 33913 | XCC3521 | 29 Aa | 15T, 1G, 1S and 12P | present |
| X. axonopodis pv. vesicatoria strain 85-10 | XCV0670 | 21 Aa | 9P, 9T, 1S, 1A and 1G | present | |
| X. axonopodis pv. citri strain 306 | XAC0612 | 19 Aa | 8P, 8T, 1S, 1A and 1G | present | |
| X. oryzae pv. oryzae strain MAFF 311018 | XOO_3789 | 33 Aa | 15P, 15T, 2S and 1A | present | |
| X. albilineans strain GPE PC73 | XALc_2969 | 96 Aa | 62G, 18S, 15T, 1P | present | |
| X. albilineans strain GPE PC73 | XALc_2967 | 26 Aa | 19G, 2S and 5N | present | |
| X. fastidiosa strain 9a5c | XF0818 | 132 Aa | 93G, 38S and 1T | present | |
| X. fastidiosa strain Temecula1 | PD1851 | 157 Aa | 84G, 43S, 3T, 26A and 1R | present | |
| Cellobiosidase CbhA = GuxA | X. campestris pv. campestris strain ATCC 33913 | XCC3534 | no linker | / | absent |
| X. campestris pv. campestris strain ATCC 33913 | XCC3160 | no linker | / | absent | |
| X. oryzae pv. oryzae strain MAFF 311018 | XOO_3805 | no linker | / | absent | |
| X. albilineans strain GPE PC73 | XALc_0484 | 152 Aa | 99G, 27S and 26T | present | |
| X. fastidiosa strain 9a5c | XF1267 | 146 Aa | 31G, 99S, 4P, 4A, 4F and 4N | present | |
| X. fastidiosa strain Temecula1 | PD0529 | 106 Aa | 15G, 78S, 2P, 5T, 2A, 2F and 2N | present | |
aPSL = Polyserine linker; bCBD = Cellulose binding domain
The OrthoMCL analyses identified 18 orthologs shared only by X. fastidiosa strain 9a5c, X. fastidiosa strain Temecula1 and X. albilineans GPE PC73 (corresponding to the CDSs conserved in these three strains that are missing in all the other six Xanthomonadaceae genome sequences analysed herein). BLAST analyses confirmed that 11 of these 18 CDSs are unique to X. albilineans and X. fastidiosa (Additional file 4). Interestingly, they include the gene metE which encodes the 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase. This enzyme is absolutely required for the biosynthesis of methionine and is therefore present in all Xanthomonadaceae. However, the metE gene present in the two xylem-limited Xanthomonadaceae is closer to the gene metE of Mesorhizobium sp. (amino acid identity = 466/764 = 60%) than the metE present in the other Xanthomonas species and in S. maltophilia (amino acid identity 89/346 = 25%). This strongly suggests that the progenitor of the two xylem-limited Xanthomonadaceae lost the ancestral metE gene (which was conserved in other Xanthomonas species and S. maltophilia) and acquired another metE by horizontal genetic transfer. The 11 genes unique to X. albilineans and X. fastidiosa also include one cysteine protease gene, one ABC transporter gene, one polysaccharide deacetylase gene, one glycosyl transferase gene, one hydrolase gene, one cell filamentation protein gene and four hypothetical protein genes (Additional file 4).
BLAST analyses confirmed that X. albilineans, like X. fastidiosa, lacks the Hrp T3SS that is present in other Xanthomonas species and does not possess any of the known Hrp type III effectors. The Hrp T3SS, which plays a major role in suppressing host plant defense responses in most other pathogenic Xanthomonas strains [24], was therefore probably acquired after the divergence of the Hrp Xanthomonas group and xylem-limited Xanthomonadaceae lineages. No remains of the Hrp gene cluster were found in the complete genome sequence of X. albilineans strain GPE PC73 nor in the available complete genome sequences of X. fastidiosa.
Discussion
In their rather cloistered environmental niche inside xylem vessels, X. albilineans and X. fastidiosa may have largely avoided surveillance by general and specific plant defense systems. Their lack of a T3SS of the Hrp1 or Hrp2 families may be explained by the fact that X. albilineans and X. fastidiosa live and multiply essentially in a dead-cell environment. However, like other bacterial vascular pathogens, they may interact with living xylem parenchyma cells through pit membranes [25]. If they do, they do not use a Hrp TTSS but another system that remains to be identified. The adaptation of X. albilineans and X. fastidiosa to a xylem-limited lifestyle is also illustrated by their enzymes adapted to the use of plant cell breakdown products as carbon sources. The low number of genes unique to both X. albilineans and X. fastidiosa (11, see Additional file 4) may be explained by a very early divergence of the two xylem-limited Xanthomonadaceae lineages, possibly followed by strong selective pressure to adapt to their different biological niches and lifestyles. X. fastidiosa is vector-transmitted by various xylem sap-feeding insects and is able to colonize many plant species (citrus, wine grape, coffee, alfalfa, peach, plum, almond, elm, maple, pear, etc) (reviewed in [26]). On the other hand, X. albilineans is mainly transmitted by mechanical means and is not known to be insect-transmitted, and is able to colonize only sugarcane and few other monocots in the Poaceae family (reviewed in [8]).
The genome of X. albilineans encodes a T3SS that displays similarities with the Burkholderia pseudomallei bsa T3SS which belongs to the injectisome family SPI-1 (Salmonella Pathogenicity Island -1) and which is required for the virulence of this human pathogen. The SPI-1 injectisome family mainly includes T3SSs from human and insect bacterial pathogens or symbionts [21]. Interestingly, the genomes of Erwinia amylovora strain Ea273 and Erwinia tasmaniensis strain Et1/99 both contain two copies of a SPI-1 T3SS [27,28]. The role of these SPI-1 T3SSs in these plant-invading Erwinia spp. remains unknown. E. amylovora is insect-disseminated, although the interactions between this pathogen and its insect hosts remain poorly understood. It was suggested that the presence of a SPI-1 T3SS in Erwinia spp. indicates a common ancestry and close phylogenetic relationship between Erwinia spp. and insect-related enteric bacteria, raising the possibility that an insect host might be serving as a mixing vessel for the exchange of genes between Erwinia strains and other enteric bacteria [27]. Similarly, the presence of a SPI-1 T3SS in the genome of X. albilineans could indicate an insect-associated life style of this plant pathogen.
The MLSA performed herein resulted in a phylogenetic tree that included X. fastidiosa into the Xanthomonas group. This phylogenetic tree is in accordance with the presence of the unique gum genes in both X. fastidiosa and Xanthomonas species of the Hrp Xanthomonas group. The gum genes, which are involved in the biosynthesis of extracellular polysaccharides and the formation of biofilms, play a key role in pathogenicity of these Xanthomonadaceae. These genes, which were probably acquired by the progenitor of the Xanthomonas genus, were most likely lost by X. albilineans and conserved by X. fastidiosa during their speciation. Our MLSA phylogenetic tree is also in accordance with i/the presence of 11 unique genes, including metE, in X. albilineans and X. fastidiosa, and ii/the alignment of the 5' end of the 16S RNA of Xanthomonadaceae (Additional file 5).
Additionally, based on this MLSA, the same 480 ancestral genes appeared to be lost by both X. albilineans and X. fastidiosa. Interestingly, 209 of the 480 ancestral genes lost by both X. albilineans and X. fastidiosa are also absent in X. oryzae pv. oryzae (a xylem invading pathogen belonging to another phylogenetic clade), indicating that independent but convergent evolution events were involved in genome erosion of X. oryzae pv. oryzae and the xylem-limited Xanthomonadaceae. Some of these genes lost by three xylem-invading pathogens are orthologous of genes with assigned functions and are organized into clusters. The five following ancestral gene clusters were lost by X. albilineans, X. fastidiosa and X. oryzae pv. oryzae: i/ the ancestral genes XCV0258 to XCV265 encoding enzymes involved in the glyoxylate cycle; ii/ the ancestral genes XCV0592 to XCV0602 encoding enzymes involved in malonate metabolism; iii/ the ancestral genes XCV1316 to XCV1334 including one TonB-dependant receptor gene, a two component signal transduction system (TCSTS) and chemotaxis genes, iv/ the ancestral genes XCV2187 to XCV2196 including one TCSTS and a type I secretion system and v/ the ancestral genes XCV2796 to XCV2803 encoding enzymes involved in catabolism of polysaccharides (Additional file 1). These examples support the hypothesis of a link between the convergent erosion of three xylem-invading Xanthomonadaceae and the adaptation to a same restricted environment (the xylem) in which these lost functions are useless. However, only 38 of the 480 ancestral genes lost by both X. albilineans and X. fastidiosa are also absent in another xylem invading pathogen, X. campestris pv. campestris, indicating that adaptation to xylem lifestyle favoured or allowed genome erosion, but did not necessarily induce it. Alternatively, the convergent genome erosion of the two xylem-limited Xanthomonadaceae may be linked to similar insect-associated lifestyles that may have favoured genome erosion because most of the genes required for a plant-associated life style are most likely not required for an insect-associated life style.
Similar striking convergence in fundamental genomic features associated with a restricted lifestyle is very well documented for obligate animal symbionts and pathogens, especially for Buchnera (reviewed in [29]). In these bacteria, gene losses are non-random but can affect all functional categories. The most dramatic losses affect genes that are involved in metabolism but are not required for survival. Another general feature is the loss of most DNA repair systems and transcriptional regulatory mechanisms, indicating that there is reduced need for transcriptional regulation in a stable environment [29]. In X. fastidiosa, and to a lesser extent in X. albilineans, losses also affected genes involved in metabolism and transcriptional regulatory mechanisms (Figure 5). Metabolic capabilities essential for other habitats may have been lost in the genome reduction process coincidently with the adaptation of X. fastidiosa and X. albilineans to the nutrient-poor xylem environment. For X. fastidiosa, genome erosion has been extreme. For example, X. fastidiosa retained only one transcriptional sigma factor gene and one outer membrane efflux protein tolC gene, and it lost all genes involved in synthesis of the flagellar apparatus. This extreme erosion allowed X. fastidiosa to save energy (synthesis and operation of the flagella confer a growth disadvantage of about 2%; [30]).
In obligate animal symbionts and pathogens, the process of genome shrinkage might have taken place in two separate stages [29]. A massive gene loss must have occurred soon after the establishment of the obligate symbiosis, probably by means of large deletions that eliminated a series of contiguous genes. The large DNA region containing the flagellar genes was probably lost by X. fastidiosa during a similar stage. The accumulation of mobile elements, representing a source of chromosomal rearrangements and gene inactivation, seems to have an important role in this first stage. A similar process is likely responsible for the limited genome erosion of X. oryzae pv. oryzae, which possesses a very high number of insertion sequences (IS) covering 20% of the genome [31]. During the second stage of genome reduction in obligate animal symbionts and pathogens, genome shrinkage seems to have mostly occurred through a process of gradual gene loss, scattered along the genome. Such losses seem to follow a pattern that starts with the inactivation of a gene (pseudogenization) by single-nucleotide mutations, and continues with a rapid reduction in length until the original gene is completely eroded [29,32]. A similar process is likely responsible for the genome erosions of X. fastidiosa and X. albilineans (Additional files 2 and 3). Furthermore, the coding density of X. fastidiosa strain Temecula1 is significantly smaller than that of xanthomonads probably because of the degradation of ancestral genes. In X. fastidiosa strain 9a5c, the number of short annotated CDSs is considerably higher than in other Xanthomonadaceae (Table 1), although the functionality of these shortened CDSs, which may result from the degradation of ancestral genes, is questionable.
Obligate animal symbionts and pathogens display rapid evolution and have highly biased nucleotide base compositions with elevated frequencies of adenine and thymine (A+T) (reviewed in [29]). X. fastidiosa also displays rapid evolution (note that the length of the branch separating X. fastidiosa from the ancestor common to X. albilineans and X. fastidiosa is much longer, Figure 3) and has a high A+T content in comparison with other Xanthomonadaceae (Table 1). Furthermore, the GC skew pattern of the chromosome of X. fastidiosa has very high amplitude and contains a high number of diagram distortions (Figure 2). A similar atypical GC skew pattern was observed for the chromosome of a Buchnera aphidicola strain [33]. This latter atypical GC skew coincides with the loss of genes involved in the replication restart process (recA and priA) and may be explained by a higher frequency of cytosine deaminations [34]. The loss of DNA repair genes recX, dinG and dinP may explain, similarly, the very high GC skew of X. fastidiosa. It may also explain the more extensive genome erosion of X. fastidiosa, compared to X. albilineans and X. oryzae pv. oryzae. Alternatively, the most important factor affecting genome erosion of X. fastidiosa may reflect the insect-associated lifestyle specific to this Xanthomonadaceae [26].
The GC skew pattern of the X. albilineans chromosome contains a lower number of distortions and has a significantly higher amplitude than the GC skew pattern of other Xanthomonas species (Figure 2), indicating that no recent events of recombination have occurred in X. albilineans. Furthermore, the synteny between the chromosomes of X. albilineans strain GPE PC73 and X. axonopodis pv. vesicatoria strain 85-10 also indicated that recombination events were limited during the speciation of X. albilineans (Additional file 1). The limited recombination of the chromosome of X. albilineans, its limited erosion, its high G+C content and its low number of IS elements may indicate that a distinctive process was responsible for the reductive genome evolution of this pathogen.
We propose a unique mechanism of genome erosion involving the unique toxin albicidin produced by X. albilineans. Albicidin is a potent DNA gyrase inhibitor with 50% inhibitory concentrations (40 to 50 nM) lower than those of most quinolones [9]. DNA gyrase inhibitors block the religation of cleaved DNA intermediate during gyrase catalysis, resulting in lethal double-stranded DNA breaks [9,35]. In the presence of subinhibitory doses of DNA gyrase inhibitors, the SOS response mediates survival of the bacteria by allowing DNA replication to continue past breaks that would normally block it. In exchange for this survival advantage, there is an increased mutation rate because the polymerases that perform the repair are prone to error [36,37]. Several studies showed that subinhibitory doses of quinolones result in an increased mutation rate in Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Mycobacterium tuberculosis [35,38-40]. X. albilineans has two genes conferring resistance to albicidin: an albicidin efflux pump gene that is present in the albicidin biosynthesis gene cluster XALB1 [20,41] and an albicidin-resistant DNA gyrase A gene elsewhere on the chromosome. This albicidin-resistant DNA gyrase A is unique to X. albilineans [42]. It contains a unique insertion of 43 amino-acids length close to the albicidin binding site. Production of albicidin in ancestral bacteria that possessed both the albicidin biosynthesis gene cluster and a DNA gyrase A sensitive to albicidin may have induced genome erosion. In these ancestral bacteria, most of the albicidin molecules were secreted by the albicidin efflux pump. Occasionally, molecules of albicidin that were not secreted most likely had the same effect as subinhibitory doses of quinolones: the SOS response was induced, thus resulting in DNA repair, recombination and mutagenesis. Successive and cumulative effect of albicidin at each replication cycle eventually resulted in genome erosion. The genome erosion induced by albicidin was likely arrested by evolution of the albicidin-resistant DNA gyrase A.
Acquisition of the albicidin biosynthesis genes by the ancestor of X. albilineans conferred a selective advantage because of the potent antibiotic activity of albicidin. The DNA damage caused by albicidin may rapidly have induced the mutation of DNA gyrase A gene and thus stopped the process of genome erosion, possibly explaining the distinctive genomic characteristics of X. albilineans. Albicidin inhibits the growth of X. axonopodis pv. vesicatoria (data not shown), suggesting that the DNA gyrase A of the ancestral Xanthomonas was sensitive to albicidin. Transfer of the albicidin biosynthesis gene cluster to X. axonopodis pv. vesicatoria led to production of functional albicidin [43], demonstrating that the production of albicidin per se is not lethal for a producer that possesses an albicidin-sensitive DNA gyrase A. No remains of the albicidin biosynthesis genes were found in the complete genome sequences of X. fastidiosa. Therefore, albicidin is most likely not responsible for genome erosion of X. fastidiosa. However, we cannot exclude the hypothesis that albicidin biosynthesis genes were lost during evolution of X. fastidiosa. For example, cluster XALB1 could have been lost concurrently with the flagellar biosynthesis gene cluster because these two gene clusters are close on the chromosome of X. albilineans.
Conclusions
During their descent from a common ancestral parent, the two xylem-limited Xanthomonadaceae experienced a convergent reductive evolution. Adaptation to the nutrient-poor xylem elements and to the cloistered environmental niche of xylem vessels probably favoured this convergent evolution. Alternatively, the most important factor affecting genome erosion of X. fastidiosa and X. albilineans may reflect insect-associated lifestyles specific to these Xanthomonadaceae. X. albilineans and X. fastidiosa evolved differently: genome erosion has occurred to different extents and specific genes have been acquired independently by X. albilineans and X. fastidiosa. For example, X. albilineans has acquired a T3SS of the SPI-1 family that is mainly found in free-living animal pathogens and four NRPS gene clusters that are involved in the biosynthesis of albicidin and probably other unknown small molecules. The toxin albicidin may be responsible for the distinctive genome erosion of X. albilineans. Much progress has been recently made in understanding how X. fastidiosa spreads within the xylem vessels as well as the traits that contribute to its acquisition and transmission by sharpshooter vectors (For review, [26]). A similar in-depth functional analysis will be necessary to identify the genes that are required for X. albilineans to spread and succeed within sugarcane xylem vessels.
Methods
Bacterial strain
X. albilineans strain GPE PC73 was isolated from a diseased stalk of sugarcane cv. H63-1418 in Guadeloupe (France, [11]). Sequenced strain GPE PC73 is referred to as CFBP 7063 in the French Collection of Plant Pathogenic Bacteria ([44]http://www.angers.inra.fr/cfbp/).
Genome sequencing, assembly and finishing
The complete genome sequence of X. albilineans was determined using the whole-genome shotgun method. Three libraries (A, B, and C) were constructed; two of them were obtained after mechanical shearing of genomic DNA and cloning of generated 3 Kbp and 10 Kbp inserts into plasmids pcdna2,1 (Invitrogen) (A) and pCNS (B) (pSU18 derived), respectively. Larger DNA fragments of about 25 Kbp (generated after partial digestion with Sau3A) were introduced into plasmid pBeloBac11 to generate a BAC library (C). Plasmid DNAs were purified and end-sequenced (33792 clones for A, 10752 for B and 4800 for C) by dye-terminator chemistry with ABI3730 sequencers (Applied Biosystems, Foster City, USA) leading to an approximately 17-fold coverage. The Phred/Phrap/Consed software package ([45]http://www.phrap.com) was used for sequence assembly and quality assessment. A total of 2151 additional sequence reactions were necessary for gap closure and sequence polishing that consisted of random sequencing of subclones (for 1625 sequence reactions) supplemented with 145 sequences of PCR-products and 381 sequences of oligonucleotide-targeted regions. Final error estimation rate as computed by phred/phrap/consed was less than 0.04 errors per 10 Kbp. The sequences reported here have been deposited in the EMBL GenBank database, and accession numbers are FP565176, FP340279, FP340278 and FP340277 for the chromosome and for plasmids plasmI, plasmII and plasmIII, respectively.
Gene prediction and annotation
Sequence analysis and annotation were performed using iANT (integrated ANnotation Tool; [46]) as described for R. solanacearum [47]. The probabilistic Markov model for coding regions used by the gene prediction software FrameD [48] was constructed with a set of CDS sequences obtained from the public databank Swiss-Prot as revealed by BLASTX analysis. The alternative matrices were built using genes first identified in ACURs (Alternative Codon Usage Regions) based on homology and taken from the R. solanacearum annotation process [47]. Predicted CDSs were reviewed individually by gene annotators for start codon assignment. The corresponding products were automatically annotated using a protocol based on HAMAP scan [49], InterPro domain annotation and BLASTP analysis. Results were individually expertized to generate the proposed annotations. Proteins were classified according to MultiFun classification [50]. The complete annotated genetic map, search tools (SRS, BLAST), annotation and process classification are available at http://iant.toulouse.inra.fr/X.albilineans[51].
Phylogenetic analysis
A phylogenetic tree was constructed from MLSA, with the maximum likelihood method and GTR as substitution model (with I: 0.01 and G: 0.52). The seven loci chosen, gyrB, groEL, recA, dnaK, efp, atpD and glnA, are typically selected housekeeping genes located at the following positions of the X. albilineans chromosome: 0.004, 0.348, 1.369, 1.983, 2.245, 3.442, and 3.655 Mb from the origin of replication, respectively. The total length of the concatenated group of full length CDSs nucleotide sequences was 10417 bp-10686 bp. The tree obtained with the concatenated data set of the seven housekeeping genes was constructed with B. pseudomallei strain NCTC 10247 as outgroup. Multiple alignments of the nucleotide sequences of the 7 housekeeping genes (gyrB, atpD, dnaK, efp, groEL, glnA, recA) for the 11 taxons were performed using ClustalW (The nucleotide alignment is provided in Additional file 6). The phylogenetic tree was calculated with PHYML ([52,53]; http://atgc.lirmm.fr/phyml/; version 2.4.4).
OrthoMCL analysis
OrthoMCL clustering analyses were performed using the following parameters: P-value Cut-off = 1 × 10-5; Percent Identity Cut-off = 0; Percent Match Cut-off = 80; MCL Inflation = 1.5; Maximum Weight = 316. We modified OrthoMCL analysis by inactivating the filter query sequence during the BLASTP pre-process. All CDSs of X. axonopodis pv. vesicatoria strain 80-15 listed in Additional file 1 were assessed as having a best BLASTP hit within sequences belonging to X. albilineans, X. fastidiosa or S. maltophilia. Best BLASTP hit analyses were performed with database UniProt by excluding all accessions from the xanthomonads using expectation value lower than 1 × 10-5.
Abbreviations
ACURs: alternative codon usage regions; CBD: cellulose binding domain; CDSs: protein-coding sequences; Hrp: hypersensitive response and pathogenicity; IS: insertion sequences; MLSA: multilocus sequence analysing; NRPSs: nonribosomal peptide synthetases; PSL: long polyserine linker; rpf: regulation of pathogenicity factors; SPI-1: Salmonella pathogenicity island -1; T3SS: Type III secretion system; TCSTS: two component signal transduction system.
Authors' contributions
IP and MR contributed to manual annotation of the genome, analysed the data, drafted part of the manuscript and coordinated the project. VB, AC, SM, BS (Segurens) performed sequencing of the genome. SC (Carrere) and JG performed automatic annotation of the genome and OrthoMCL analysis. RK and SC (Cociancich) contributed to manual annotation of the genome and drafted part of the manuscript. CM, VV and MA conceived the study and revised the manuscript. AD, M-A J, EL, SP, BS (Szurek) contributed to manual annotation of the genome and revised the manuscript. PR conceived the study, contributed to manual annotation of the genome and drafted part of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
List and individual analysis of the 2816 ancestral genes identified in the genome of X. axonopodis pv. vesicatoria strain 85-10. List and individual analysis of the 2816 CDSs of X. axonopodis pv. vesicatoria strain 85-10 identified by OrthoMCL and Best hit BLAST analyses as conserved in X. albilineans strain GPE PC73, X. fastidiosa strain 9a5c, S. maltophilia strain R551-3 or S. maltophilia strain K279a.
List of ancestral genes potentially lost by pseudogenization and short deletions in X. fastidiosa. Analysis of lost ancestral genes that are present on the chromosome of X. axonopodis pv. vesicatoria between the orthologs of two ancestral genes that are contiguous and conserved in X. fastidiosa.
List of ancestral genes potentially lost by pseudogenization and short deletions in X. albilineans. Analysis of lost ancestral genes that are present on the chromosome of X. axonopodis pv. vesicatoria between the orthologs of two ancestral genes that are contiguous and conserved in X. albilineans.
List and description of the 11 genes unique to X. albilineans and X. fastidiosa. Summary of BLAST analyses results of the 11 genes unique to X. albilineans and X. fastidiosa.
Comparison of the 5' end of 16S RNA of eight Xanthomonadaceae. Alignment of the 5' end of 16S RNA of the following strains: XAC = Xanthomonas axonopodis pv. citri str. 306, XOO = Xanthomonas oryzae pv. oryzae str. MAFF 311018, XCV = Xanthomonas axonopodis pv. vesicatoria str. 85-10, XCC = Xanthomonas campestris pv. campestris str. ATCC 33913, SMA = Stenotrophomonas maltophilia str. R551-3, XAL = Xanthomonas albilineans str. GPE PC73, XYL_9a5c = Xylella fastidiosa str. 9a5c and XYL_Tem = Xylella fastidiosa str. Temecula1. The yellow-highlighted region is specific to X. albilineans and X. fastidiosa.
Alignment obtained with ClustalW of the concatenated sequences of the housekeeping genes gyrB, atpD, dnaK, efp, groEL, glnA, and recA of nine Xanthomonadaceae. Alignment obtained with ClustalW of the concatenated sequences of the housekeeping genes gyrB, atpD, dnaK, efp, groEL, glnA, and recA of X. albiline (X. albilineans str. GPE PC73), StenoK279a (S. maltophilia str. K279a), StenoR551 (S. maltophilia str. R551-3), Vesicatori (X. axonopodis pv. vesicatoria str. 85-10), Citri (X. axonopodis pv. citri str. 306), Oryzae (X. oryzae pv. oryzae str. MAFF 311018), Campestris (X. campestris pv. campestris str. ATCC 33913), Xyl9a5C (X. fastidiosa str. 9a5c), XylTemecul (X. fastidiosa str. Temecula1), Burkholder (B. pseudomallei str. NCTC 10247) and Ralstonia (R. solanacearum str. GMI1000). This alignment was not modified manually.
Contributor Information
Isabelle Pieretti, Email: isabelle.pieretti@cirad.fr.
Monique Royer, Email: monique.royer@cirad.fr.
Valérie Barbe, Email: vbarbe@genoscope.cns.fr.
Sébastien Carrere, Email: Sebastien.Carrere@toulouse.inra.fr.
Ralf Koebnik, Email: Ralf.Koebnik@mpl.ird.fr.
Stéphane Cociancich, Email: stephane.cociancich@cirad.fr.
Arnaud Couloux, Email: acouloux@genoscope.cns.fr.
Armelle Darrasse, Email: Armelle.Darrasse@angers.inra.fr.
Jérôme Gouzy, Email: gouzy@toulouse.inra.fr.
Marie-Agnès Jacques, Email: Marie-Agnes.Jacques@angers.inra.fr.
Emmanuelle Lauber, Email: Emmanuelle.Lauber@toulouse.inra.fr.
Charles Manceau, Email: charles.manceau@angers.inra.fr.
Sophie Mangenot, Email: mangenot@genoscope.cns.fr.
Stéphane Poussier, Email: Stephane.Poussier@agrocampus-ouest.fr.
Béatrice Segurens, Email: segurens@genoscope.cns.fr.
Boris Szurek, Email: boris.szurek@mpl.ird.fr.
Valérie Verdier, Email: Valerie.Verdier@ird.fr.
Matthieu Arlat, Email: Matthieu.Arlat@toulouse.inra.fr.
Philippe Rott, Email: philippe.rott@cirad.fr.
Acknowledgements
We thank Mélanie Marguerettaz and Florence Barthod for their help with the preparation of the figures, and Caitilyn Allen, Dean W. Gabriel, and Philippe Roumagnac for critical reviewing of the manuscript.
References
- Saddler GS, Bradbury JF. In: Bergey's Manual of Systematic Bacteriology. Brenner DJ, Krieg NR, Staley JT, Garrity GM, editor. New York: Springer; 2005. The Proteobacteria; p. 63. full_text. [Google Scholar]
- Meyer DF, Bogdanove AJ. In: Plant Pathogenic Bacteria: Genomics and Molecular Biology. Jackson RW, editor. Norfolk: Caister Academic Press; 2009. Genomics-driven advances in Xanthomonas biology; pp. 147–161. [Google Scholar]
- Simpson AJG, Reinach FC, Arruda P, Abreu FA, Acencio M, Alvarenga R, Alves LMC, Araya JE, Bala GS, Baptista CS. The genome sequence of the plant pathogen Xylella fastidiosa. Nature. 2000;406:151–157. doi: 10.1038/35018003. [DOI] [PubMed] [Google Scholar]
- Nesme X, Vaneechoutte M, Orso S, Hoste B, Swings J. Diversity and genetic relatedness within genera Xanthomonas and Stenotrophomonas using restriction endonuclease site differences of PCR-amplified 16S rRNA gene. Syst Appl Microbiol. 1995;18:127–135. [Google Scholar]
- Lu H, Patil P, Van Sluys MA, White FF, Ryan RP, Dow JM, Rabinowicz P, Salzberg SL, Leach JE, Sonti R. Acquisition and evolution of plant pathogenesis-associated gene clusters and candidate determinants of tissue-specificity in Xanthomonas. PLoS ONE. 2008;3:e3828. doi: 10.1371/journal.pone.0003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AM, de Lima WC, Moreira LM, de Oliveira MC, Souza RC, Civerolo E, de Vasconcelos ATR, Sluys MV. In: Plant Pathogenic Bacteria: Genomics and Molecular Biology. Jackson RW, editor. Norfolk: Caister Academic Press; 2009. Common genes and genomic breaks: a detailed case study of the Xylella fastidiosa genome backbone and evolutionary insights; pp. 113–133. [Google Scholar]
- Rott P, Davis M. In: A guide to sugarcane diseases. Rott P, Bailey R, Comstock J, Croft B, Saumtally A, editor. Montpellier: CIRAD-ISSCT; 2000. Leaf scald; p. 339. [Google Scholar]
- Birch RG. Xanthomonas albilineans and the antipathogenesis approach to disease control. Mol Plant Pathol. 2001;2:1–11. doi: 10.1046/j.1364-3703.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Hashimi SM, Wall MK, Smith AB, Maxwell A, Birch RG. The phytotoxin albicidin is a novel inhibitor of DNA gyrase. Antimicrob Agents Chemother. 2007;51:181–187. doi: 10.1128/AAC.00918-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite RP Jr, Minsavage GV, Bonas U, Stall RE. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1994;60:1068–1077. doi: 10.1128/aem.60.4.1068-1077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoiseau P, Daugrois J-H, Pieretti I, Cociancich S, Royer M, Rott P. High variation in pathogenicity of genetically closely related strains of Xanthomonas albilineans, the sugarcane leaf scald pathogen, in Guadeloupe. Phytopathol. 2006;96:1081–1091. doi: 10.1094/PHYTO-96-1081. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Inoue Y, Takeya M, Sasaki A, Kaku H. Genome sequence of Xanthomonas oryzae pv. oryzae suggests contribution of large numbers of effector genes and insertion sequences to its race diversity. Jpn Agric Res. 2005;39:275–287. [Google Scholar]
- da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, Monteiro-Vitorell CB, Van Sluys MA, Almeida NF, Alves LM. Comparison of the genomes of two Xanthomonas pathogens with differing host. Nature. 2002;417:459–463. doi: 10.1038/417459a. [DOI] [PubMed] [Google Scholar]
- Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Buttner D, Caldana C, Gaigalat L, Goesmann A, Kay S. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol. 2005;187:7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman L, Gould V, Dow JM, Vernikos G, Okazaki A, Sebaihia M, Saunders D, Arrowsmith C, Carver T, Peters N. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008;9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, Lelie D Van Der. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol. 2009;75:748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sluys MA, De Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, Camargo LEA, Da Silva ACR, Moon DH, Takita MA, Lemos EGM. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol. 2003;185:1018–1026. doi: 10.1128/JB.185.3.1018-1026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stoeckert CJ Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-D, Zhao S, Hill CW. Rhs elements comprise three subfamilies which diverged prior to acquisition by Escherichia coli. J Bacteriol. 1998;180:4102–4110. doi: 10.1128/jb.180.16.4102-4110.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer M, Costet L, Vivien E, Bes M, Cousin A, Damais A, Pieretti I, Savin A, Megessier S, Viard M. Albicidin pathotoxin produced by Xanthomonas albilineans is encoded by three Large PKS and NRPS genes present in a gene cluster also containing several putative modifying, regulatory, and resistance genes. Mol Plant Microbe Interact. 2004;17:414–427. doi: 10.1094/MPMI.2004.17.4.414. [DOI] [PubMed] [Google Scholar]
- Cornelis GR. The type III secretion injectisome. Nat Rev Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem. 2004;382:769–781. doi: 10.1042/BJ20040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MB, Ekborg N, Taylor L, Hutcheson SW, Weiner RM. Identification and analysis of polyserine linker domains in prokaryotic proteins with emphasis on the marine bacterium Microbulbifer degradans. Protein Sci. 2004;13:1422–1425. doi: 10.1110/ps.03511604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S, Bonas U. How Xanthomonas type III effectors manipulate the host plant. Curr Opinion Microbiol. 2009;12:37–43. doi: 10.1016/j.mib.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Hilaire E, Young SA, Willard LH, McGee JD, Sweat T, Chittoor JM, Guikema JA, Leach JE. Vascular defense responses in rice: Peroxidase accumulation in xylem parenchyma cells and xylem wall thickening. Mol Plant Microbe Interact. 2001;14:1411–1419. doi: 10.1094/MPMI.2001.14.12.1411. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Almeida RPP, Lindow S. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu Rev Phytopathol. 2008;46:243–271. doi: 10.1146/annurev.phyto.45.062806.094342. [DOI] [PubMed] [Google Scholar]
- Triplett LR, Zhao Y, Sundin GW. Genetic differences between blight-causing Erwinia species with differing host specificities, identified by suppression subtractive hybridization. Appl Environ Microbiol. 2006;72:7359–7364. doi: 10.1128/AEM.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube M, Migdoll A, Müller I, Kuhl H, Beck A, Reinhardt R, Geider K. The genome of Erwinia tasmaniensis strain Et1/99, a non-pathogenic bacterium in the genus Erwinia. Environ Microbiol. 2008;10:2211–2222. doi: 10.1111/j.1462-2920.2008.01639.x. [DOI] [PubMed] [Google Scholar]
- Moya A, Pereto J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- Macnab RM. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt FC, Curtis R, Ingraham JL, Lin ECC, Magasanik B, Resnikoff WS, Riley M, Schaechter M, Umbarger HE, editor. Washington DC: ASM Press; 1996. Flagella and motility; pp. 123–145. [Google Scholar]
- Salzberg S, Sommer D, Schatz M, Phillippy A, Rabinowicz P, Tsuge S, Furutani A, Ochiai H, Delcher A, Kelley D. Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics. 2008;9:204. doi: 10.1186/1471-2164-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Mclaughlin HJ, Sorek R. The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science. 2009;323:379–382. doi: 10.1126/science.1167140. [DOI] [PubMed] [Google Scholar]
- Van Ham RCHJ, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Fernández JM, Jiménez L, Postigo M, Silva FJ. Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci USA. 2003;100:581–586. doi: 10.1073/pnas.0235981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L, Andersson SGE. Strong asymmetric mutation bias in endosymbiont genomes coincide with loss of genes for replication restart pathways. Mol Biol Evol. 2006;23:1031–1039. doi: 10.1093/molbev/msj107. [DOI] [PubMed] [Google Scholar]
- Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi V, Susan JA. DNA repair in Mycobacterium tuberculosis. What have we learnt from the genome sequence? Mol Microbiol. 1998;29:1331–1339. doi: 10.1046/j.1365-2958.1998.01038.x. [DOI] [PubMed] [Google Scholar]
- Boshoff HI, Reed MB, Barry CE, Mizrahi V. DnaE2 polymerase contributes to in vivo survival and the emergence of drug resistance in Mycobacterium tuberculosis. Cell. 2003;113:183–193. doi: 10.1016/S0092-8674(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Phillips I, Culebras E, Moreno F, Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987;20:631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- Fung-Tomc J, Kolek B, Bonner DP. Ciprofloxacin-induced, low-level resistance to structurally unrelated antibiotics in Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1289–1296. doi: 10.1128/aac.37.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'sullivan DM, Hinds J, Butcher PD, Gillespie SH, Mchugh TD. Mycobacterium tuberculosis DNA repair in response to subinhibitory concentrations of ciprofloxacin. J Antimicrob Chemother. 2008;62:1199–1202. doi: 10.1093/jac/dkn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock JM, Huang G, Hashimi SM, Zhang L, Birch RG. A DHA14 drug efflux gene from Xanthomonas albilineans confers high-level albicidin antibiotic resistance in Escherichia coli. J Appl Microbiol. 2006;101:151–160. doi: 10.1111/j.1365-2672.2006.02899.x. [DOI] [PubMed] [Google Scholar]
- Hashimi SM, Huang G, Maxwell A, Birch RG. DNA gyrase from the albicidin producer Xanthomonas albilineans has multiple-antibiotic-resistance and unusual enzymatic properties. Antimicrob Agents Chemother. 2008;52:1382–1390. doi: 10.1128/AAC.01551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien E, Pitorre D, Cociancich S, Pieretti I, Gabriel DW, Rott PC, Royer M. Heterologous Production of albicidin: a promising approach to overproducing and characterizing this potent inhibitor of DNA gyrase. Antimicrob Agents Chemother. 2007;51:1549–1552. doi: 10.1128/AAC.01450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French Collection of Plant Pathogenic Bacteria. http://www.angers.inra.fr/cfbp/
- Phred/Phrap/Consed software package. http://www.phrap.com
- Thébaut P, Servant F, Schiex T, Kahn D, Gouzy J. JOBIM Conf Proc. ENSA, LIRM. Montpellier; 2000. pp. 361–365. [Google Scholar]
- Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, Arlat M, Billault A, Brottier P, Camus JC, Cattolico L. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature. 2002;415:497–502. doi: 10.1038/415497a. [DOI] [PubMed] [Google Scholar]
- Schiex T, Gouzy J, Moisan A, De Oliveira Y. FrameD: a flexible program for quality check and gene prediction in prokaryotic genomes and noisy matured eukaryotic sequences. Nucl Acids Res. 2003;31:3738–3741. doi: 10.1093/nar/gkg610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattiker A, Michoud K, Rivoire C, Auchincloss AH, Coudert E, Lima T, Kersey P, Pagni M, Sigrist CJA, Lachaize C. Automated annotation of microbial proteomes in SWISS-PROT. Comput Biol Chem. 2003;27:49–58. doi: 10.1016/S1476-9271(02)00094-4. [DOI] [PubMed] [Google Scholar]
- Serres MH, Riley M. MultiFun, a multifunctional classification scheme for Escherichia coli K-12 gene products. Microb Comp Genomics. 2000;5:205–222. doi: 10.1089/omi.1.2000.5.205. [DOI] [PubMed] [Google Scholar]
- Complete data regarding annotation of the genome of the X. albilineans strain GPE PC73. http://iant.toulouse.inra.fr/X.albilineans
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- PHYML. http://atgc.lirmm.fr/phyml/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List and individual analysis of the 2816 ancestral genes identified in the genome of X. axonopodis pv. vesicatoria strain 85-10. List and individual analysis of the 2816 CDSs of X. axonopodis pv. vesicatoria strain 85-10 identified by OrthoMCL and Best hit BLAST analyses as conserved in X. albilineans strain GPE PC73, X. fastidiosa strain 9a5c, S. maltophilia strain R551-3 or S. maltophilia strain K279a.
List of ancestral genes potentially lost by pseudogenization and short deletions in X. fastidiosa. Analysis of lost ancestral genes that are present on the chromosome of X. axonopodis pv. vesicatoria between the orthologs of two ancestral genes that are contiguous and conserved in X. fastidiosa.
List of ancestral genes potentially lost by pseudogenization and short deletions in X. albilineans. Analysis of lost ancestral genes that are present on the chromosome of X. axonopodis pv. vesicatoria between the orthologs of two ancestral genes that are contiguous and conserved in X. albilineans.
List and description of the 11 genes unique to X. albilineans and X. fastidiosa. Summary of BLAST analyses results of the 11 genes unique to X. albilineans and X. fastidiosa.
Comparison of the 5' end of 16S RNA of eight Xanthomonadaceae. Alignment of the 5' end of 16S RNA of the following strains: XAC = Xanthomonas axonopodis pv. citri str. 306, XOO = Xanthomonas oryzae pv. oryzae str. MAFF 311018, XCV = Xanthomonas axonopodis pv. vesicatoria str. 85-10, XCC = Xanthomonas campestris pv. campestris str. ATCC 33913, SMA = Stenotrophomonas maltophilia str. R551-3, XAL = Xanthomonas albilineans str. GPE PC73, XYL_9a5c = Xylella fastidiosa str. 9a5c and XYL_Tem = Xylella fastidiosa str. Temecula1. The yellow-highlighted region is specific to X. albilineans and X. fastidiosa.
Alignment obtained with ClustalW of the concatenated sequences of the housekeeping genes gyrB, atpD, dnaK, efp, groEL, glnA, and recA of nine Xanthomonadaceae. Alignment obtained with ClustalW of the concatenated sequences of the housekeeping genes gyrB, atpD, dnaK, efp, groEL, glnA, and recA of X. albiline (X. albilineans str. GPE PC73), StenoK279a (S. maltophilia str. K279a), StenoR551 (S. maltophilia str. R551-3), Vesicatori (X. axonopodis pv. vesicatoria str. 85-10), Citri (X. axonopodis pv. citri str. 306), Oryzae (X. oryzae pv. oryzae str. MAFF 311018), Campestris (X. campestris pv. campestris str. ATCC 33913), Xyl9a5C (X. fastidiosa str. 9a5c), XylTemecul (X. fastidiosa str. Temecula1), Burkholder (B. pseudomallei str. NCTC 10247) and Ralstonia (R. solanacearum str. GMI1000). This alignment was not modified manually.