Abstract
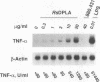
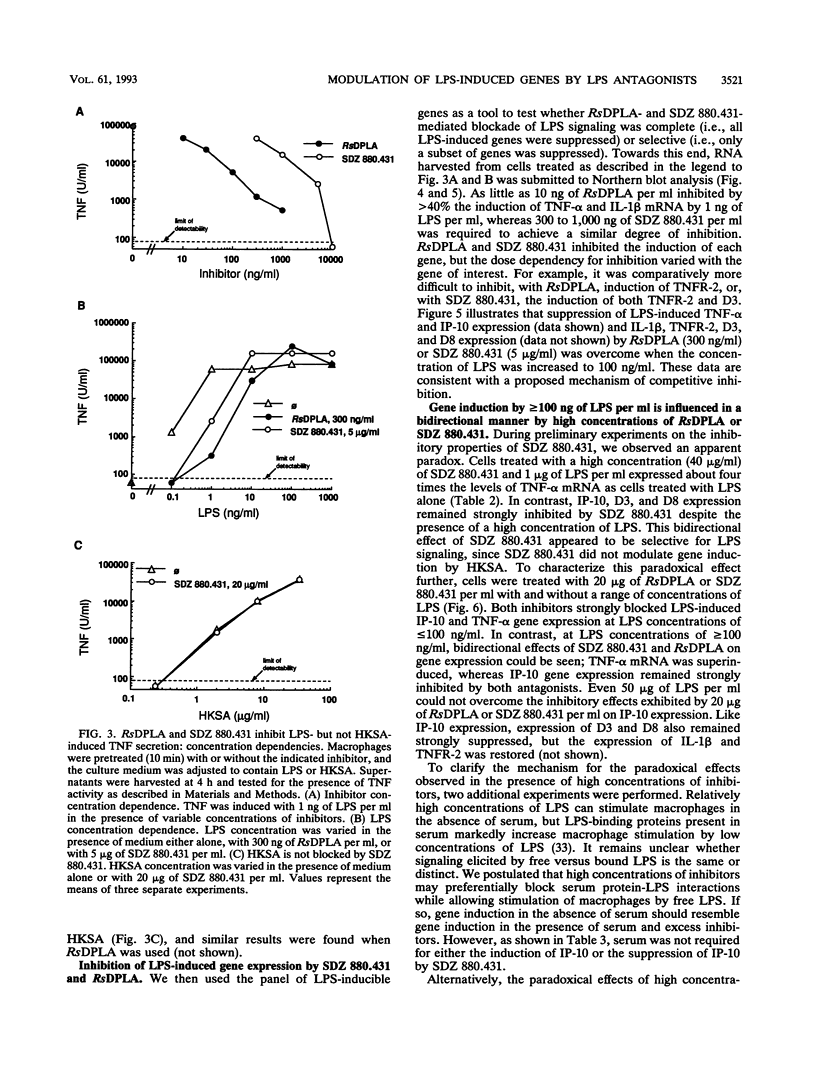
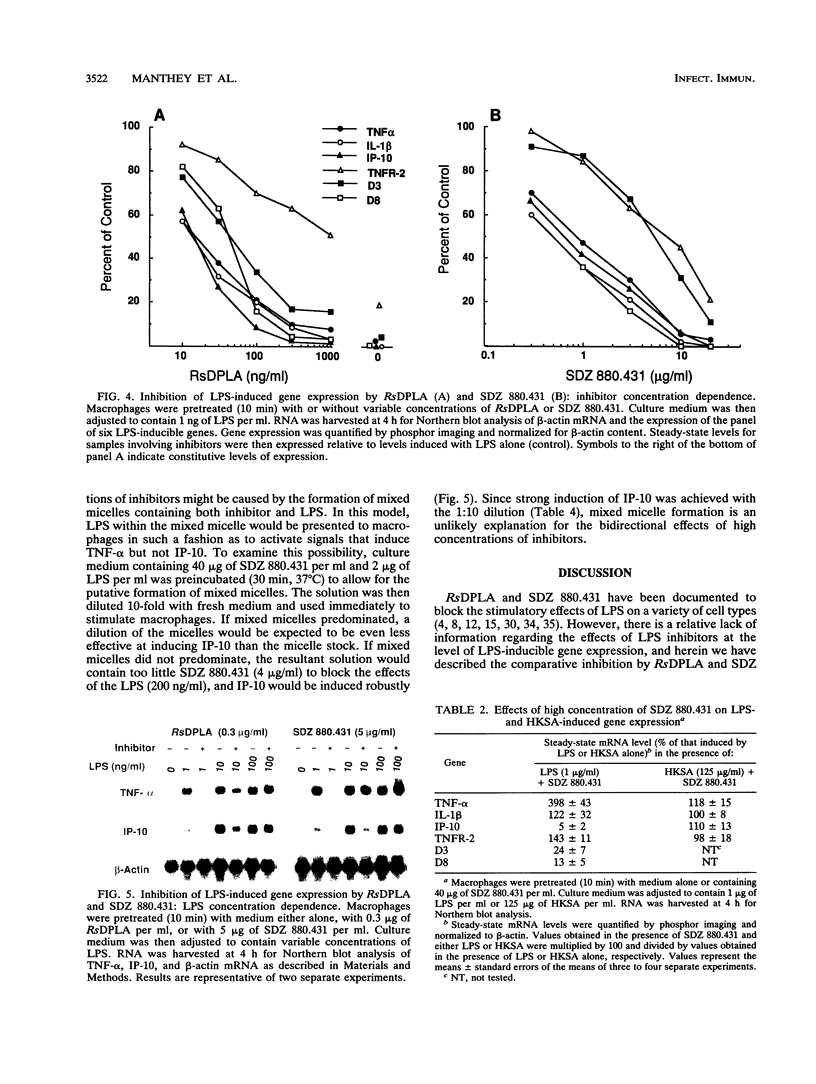
Rhodobacter sphaeroides lipid A (RsDPLA) and SDZ 880.431 (3-aza-lipid X-4-phosphate) are prototypic lipopolysaccharide (LPS) antagonists. Herein, we examined the ability of these structures to regulate murine macrophage tumor necrosis factor (TNF) secretion and LPS-inducible gene expression (tumor necrosis factor alpha [TNF-alpha], interleukin-1 beta [IL-1 beta], IP-10, type 2 TNF receptor [TNFR-2], D3, and D8 genes). We report that RsDPLA alone (> 1 microgram/ml) induced low levels of TNF-alpha secretion and a selective pattern of gene expression in peritoneal exudate macrophages; SDZ 880.431 alone was completely inactive. When LPS was present at a low concentration (1 ng/ml), RsDPLA and SDZ 880.431 blocked TNF secretion and gene induction in a concentration-dependent fashion. In general, gene induction was measurably reduced by 10 to 30 ng of RsDPLA per ml or 300 ng of SDZ 880.431 per ml, but inhibition could be uniformly overridden by increasing the concentration of LPS. Although induction of all six genes by LPS was suppressed by either inhibitor, effective inhibitor concentrations depended on the gene of interest. Induction of TNFR-2 by LPS was relatively resistant to inhibition by RsDPLA, and induction of TNFR-2 and D3 was relatively resistant to inhibition by SDZ 880.431. When LPS was present at > or = 100 ng/ml, correspondingly high concentrations (> or = 20 micrograms/ml) of either inhibitor influenced gene expression in a bidirectional manner. Under these conditions, LPS-induced expression of IP-10, D3, and D8 was suppressed regardless of the LPS concentration used (concentrations tested up to 50 micrograms/ml), while expression of TNF-alpha mRNA was enhanced about fourfold. In toto, RsDPLA and SDZ 880.431, when present at low concentrations, act in a manner consistent with competitive inhibition of LPS, while at higher concentrations, these structures inhibit certain LPS responses noncompetitively and synergize with LPS for other responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. J., Hraba T., Taylor C. E., Myers K. R., Takayama K., Qureshi N., Stuetz P., Kusumoto S., Hasegawa A. Structural features that influence the ability of lipid A and its analogs to abolish expression of suppressor T cell activity. Infect Immun. 1992 Jul;60(7):2694–2701. doi: 10.1128/iai.60.7.2694-2701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S., Tebo J. M., Hamilton T. A. IL-4 suppresses cytokine gene expression induced by IFN-gamma and/or IL-2 in murine peritoneal macrophages. J Immunol. 1992 Mar 15;148(6):1725–1730. [PubMed] [Google Scholar]
- Golenbock D. T., Hampton R. Y., Qureshi N., Takayama K., Raetz C. R. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem. 1991 Oct 15;266(29):19490–19498. [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Bredon N., Ohmori Y., Tannenbaum C. S. IFN-gamma and IFN-beta independently stimulate the expression of lipopolysaccharide-inducible genes in murine peritoneal macrophages. J Immunol. 1989 Apr 1;142(7):2325–2331. [PubMed] [Google Scholar]
- Henricson B. E., Manthey C. L., Perera P. Y., Hamilton T. A., Vogel S. N. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993 Jun;61(6):2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricson B. E., Perera P. Y., Qureshi N., Takayama K., Vogel S. N. Rhodopseudomonas sphaeroides lipid A derivatives block in vitro induction of tumor necrosis factor and endotoxin tolerance by smooth lipopolysaccharide and monophosphoryl lipid A. Infect Immun. 1992 Oct;60(10):4285–4290. doi: 10.1128/iai.60.10.4285-4290.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D., Gallay P., Barras C., Zaech P., Ulevitch R. J., Tobias P. S., Glauser M. P., Baumgartner J. D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992 Jun 1;148(11):3505–3512. [PubMed] [Google Scholar]
- Kirkland T. N., Qureshi N., Takayama K. Diphosphoryl lipid A derived from lipopolysaccharide (LPS) of Rhodopseudomonas sphaeroides inhibits activation of 70Z/3 cells by LPS. Infect Immun. 1991 Jan;59(1):131–136. doi: 10.1128/iai.59.1.131-136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens R. L., Ulevitch R. J., Munford R. S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992 Aug 1;176(2):485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn W. A., Raetz C. R., Qureshi N., Golenbock D. T. Lipopolysaccharide-induced stimulation of CD11b/CD18 expression on neutrophils. Evidence of specific receptor-based response and inhibition by lipid A-based antagonists. J Immunol. 1991 Nov 1;147(9):3072–3079. [PubMed] [Google Scholar]
- Manthey C. L., Brandes M. E., Perera P. Y., Vogel S. N. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992 Oct 1;149(7):2459–2465. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Narumi S., Finke J. H., Hamilton T. A. Interferon gamma and interleukin 2 synergize to induce selective monokine expression in murine peritoneal macrophages. J Biol Chem. 1990 Apr 25;265(12):7036–7041. [PubMed] [Google Scholar]
- Narumi S., Hamilton T. A. Inducible expression of murine IP-10 mRNA varies with the state of macrophage inflammatory activity. J Immunol. 1991 May 1;146(9):3038–3044. [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T. A. A macrophage LPS-inducible early gene encodes the murine homologue of IP-10. Biochem Biophys Res Commun. 1990 May 16;168(3):1261–1267. doi: 10.1016/0006-291x(90)91164-n. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T. A. Ca2+ and calmodulin selectively regulate lipopolysaccharide-inducible cytokine mRNA expression in murine peritoneal macrophages. J Immunol. 1992 Jan 15;148(2):538–545. [PubMed] [Google Scholar]
- Ohmori Y., Strassman G., Hamilton T. A. cAMP differentially regulates expression of mRNA encoding IL-1 alpha and IL-1 beta in murine peritoneal macrophages. J Immunol. 1990 Nov 15;145(10):3333–3339. [PubMed] [Google Scholar]
- Pennica D., Hayflick J. S., Bringman T. S., Palladino M. A., Goeddel D. V. Cloning and expression in Escherichia coli of the cDNA for murine tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6060–6064. doi: 10.1073/pnas.82.18.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera P. Y., Manthey C. L., Stütz P. L., Hildebrandt J., Vogel S. N. Induction of early gene expression in murine macrophages by synthetic lipid A analogs with differing endotoxic potentials. Infect Immun. 1993 May;61(5):2015–2023. doi: 10.1128/iai.61.5.2015-2023.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Will J. A., Burhop K. E., Raetz C. R. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986 Jun;52(3):905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Kurtz R. Diphosphoryl lipid A obtained from the nontoxic lipopolysaccharide of Rhodopseudomonas sphaeroides is an endotoxin antagonist in mice. Infect Immun. 1991 Jan;59(1):441–444. doi: 10.1128/iai.59.1.441-444.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Meyer K. C., Kirkland T. N., Bush C. A., Chen L., Wang R., Cotter R. J. Chemical reduction of 3-oxo and unsaturated groups in fatty acids of diphosphoryl lipid A from the lipopolysaccharide of Rhodopseudomonas sphaeroides. Comparison of biological properties before and after reduction. J Biol Chem. 1991 Apr 5;266(10):6532–6538. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum C. S., Koerner T. J., Jansen M. M., Hamilton T. A. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988 May 15;140(10):3640–3645. [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Feist W., Heine H., Kirikae T., Kirikae F., Kusumoto S., Kusama T., Brade H., Schade U., Rietschel E. T. Modulation of endotoxin-induced monokine release in human monocytes by lipid A partial structures that inhibit binding of 125I-lipopolysaccharide. Infect Immun. 1992 Dec;60(12):5145–5152. doi: 10.1128/iai.60.12.5145-5152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dervort A. L., Doerfler M. E., Stuetz P., Danner R. L. Antagonism of lipopolysaccharide-induced priming of human neutrophils by lipid A analogs. J Immunol. 1992 Jul 1;149(1):359–366. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Zhang X., Morrison D. C. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J Immunol. 1993 Feb 1;150(3):1011–1018. [PubMed] [Google Scholar]
- Zuckerman S. H., Qureshi N. In vivo inhibition of lipopolysaccharide-induced lethality and tumor necrosis factor synthesis by Rhodobacter sphaeroides diphosphoryl lipid A is dependent on corticosterone induction. Infect Immun. 1992 Jul;60(7):2581–2587. doi: 10.1128/iai.60.7.2581-2587.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]