Abstract
Apolipoprotein E4 (apoE4) is the major genetic risk factor for Alzheimer’s disease (AD) by an as yet to be defined mechanism. Since the structure or biophysical properties of a protein directly determines function, our approach to addressing mechanism is structure:function based. Domain interaction a structural property of apoE4 that distinguishes it from apoE3 is predicted to contribute to the association of apoE4 with AD. We developed a mouse model, the Arg-61 apoE model, which is specific for domain interaction. These mice display synaptic, functional, and cognitive deficits, demonstrating domain interaction is the causative factor. We present evidence that domain interaction results in stressed astrocytes that are dysfunctional and propose that dysfunctional astrocytes are an early player in apoE4-associated AD and that domain interaction is a potential therapeutic target.
INTRODUCTION
Of the three common human apolipoprotein (apo) E isoforms (apoE2, apoE3, and apoE4), apoE4 is the major genetic risk factor for Alzheimer’s disease (AD) [1–3]. All other candidate genes identified in numerous genetic screens of AD populations fall far short of apoE4 for impact on AD pathogenesis. In a typical control population, approximately 20% of the individuals carry at least one APOE4 allele. That percentage would rise to 65% in non-related patients with sporadic AD and to 80% in those with familial AD [4]. This impact is more sobering given the emerging evidence that current therapies, including those targeting Aβ, are relatively ineffective in apoE4 carriers and that there has been a general lack of activity in developing apoE4-targeted therapies. Indeed, apoE4 can act independent of Aβ in AD, and that apoE4 is a viable drug target for treating AD [5].
Several potential mechanisms have been suggested to account for the association of apoE4 with AD for review see [5, 6], but none has been casually linked to disease. Since a basic principle in biology is that the function of a protein or dysfunction in the case of mutations is dictated by its structure and biophysical properties, our approach has been to examine differences in the structures of apoE isoforms to gain insight into both disease mechanisms and, eventually, into designing therapeutic strategies.
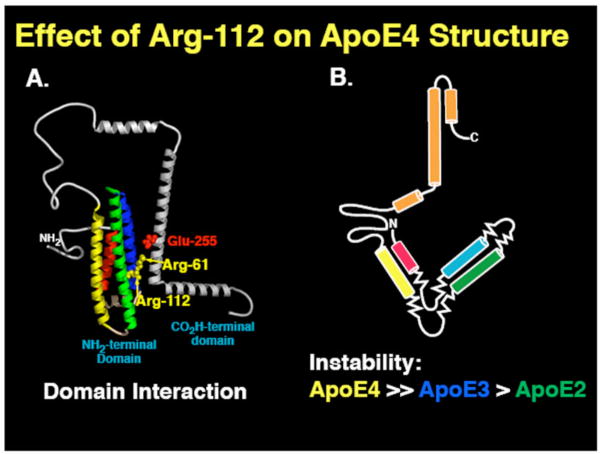
Two key structural differences distinguish apoE4 from apoE2 and apoE3: domain interaction and instability to protein unfolding (Fig. 1) [7, 8]. ApoE4 domain interaction is characterized by interaction of the two apoE structural domains, mediated by a salt bridge between Arg-61 in the N-terminal domain and Glu-255 in the C-terminal domain [7]. This interaction does not occur to the same degree in apoE3 or apoE2 and has been postulated to contribute to neurodegeneration [7]. The other distinguishing apoE4 structural characteristic is its relative instability to protein unfolding compared to the other isoforms (Fig. 1). The apoE4 instability leads to the formation of an ensemble of loosely folded structures referred to as a molten globule state [8]. Interestingly, the order of the isoform instability is identical to the order of isoforms influence on AD.
Fig. 1. The effect of position 112 on the structure of apoE4 and apoE4.
1) ApoE4 and apoE3 differ by a single amino acid at position 112: apoE4 contains arginine and apoE3 cysteine. With the influence of Arg-112 in apoE4, the Arg-61sidechain is positioned to interact with Glu-225. This interaction called domain interaction, results in a compact protein structure. With Cys-112 in apoE3, the Arg-61 side chain is in between two helices where the interaction with Glu-255 is minimized, resulting in a more open structure. 2) ApoE4 unfolds more readily to chemical and thermal denaturation than apoE3 and apoE2 and forms an ensemble of partially unfolded structures referred to as molten globules.
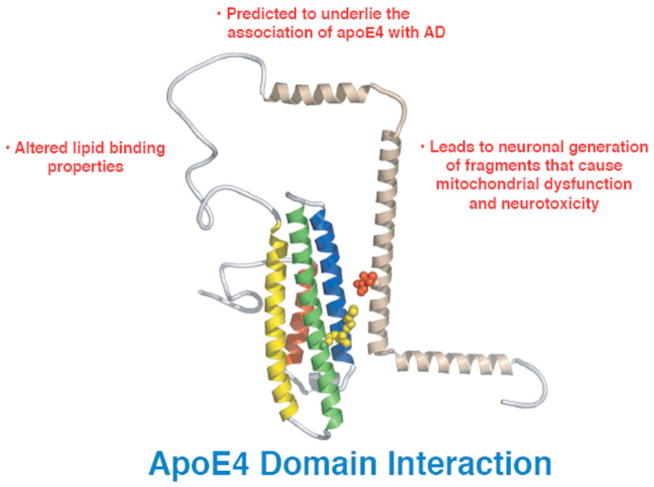
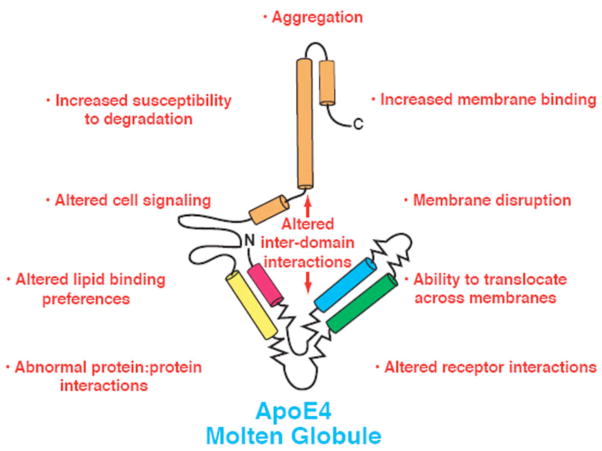
These apoE4 structural features could each independently contribute to AD in multiple ways (Figs. 2 and 3). So what are the relative contributions of each to neurodegeneration and AD and how can we approach this problem? The question cannot be answered with current transgenic and knock in mouse models expressing human apoE isoforms that display the two distinct features simultaneously.
Fig. 2.
Potential mechanisms by which domain interaction could contribute to neurodegeneration and AD.
Fig. 3.
Potential mechanisms by which molten globule formation could contribute to neurodegeneration and AD.
Fortunately, there is a solution. Wild-type (WT) mouse apoE does not display apoE4 domain interaction or its instability. Although mouse apoE contains the equivalent of Arg-112 and Glu-255, which are both required for domain interaction, it lacks the critical Arg-61 equivalent. Introduction of an arginine codon into the mouse Apoe gene by gene targeting generates a specific model for domain interaction, referred to Arg-61 mouse apoE [9]. Arg-61 apoE has domain interaction but does not form a molten globule. Generation of a molten globule mouse model is currently under way [10].
The Arg-61 mice yielded intriguing results. Brain levels of apoE were 40% lower in Arg-61 apoE mice than WT mice [11]. Astrocytes, the prime source of apoE synthesis in uninjured brains, secreted less Arg-61 apoE than WT mice, despite identical mRNA levels [11]. Thus, decreased secretion by astrocytes accounted for the lower brain levels. The significance of this point will be considered later. The Arg-61 mice displayed both pre- and post-synaptic deficits: they had lower immunoreactive levels of synaptophysin and neuroligin than WT mice [12]. Exposure to novel environment exploration revealed a functional deficit in synaptic activity as measured by lower expression of Fos and Arc after stimulation [12]. Finally, the Arg-61 apoE mice displayed a cognitive deficit in the Morris water maize [12]. These features are characteristic of AD. These results demonstrate that introduction of domain interaction into a mouse model results in synaptic, functional, and cognitive deficits. Since the Arg-61 apoE mice contained no mutant forms of amyloid precursor protein and there were no signs of Aβ peptide involvement in this phenotype, we conclude that Arg-61 apoE can cause AD-like effects in an Aβ-independent pathway, as reported [5, 6].
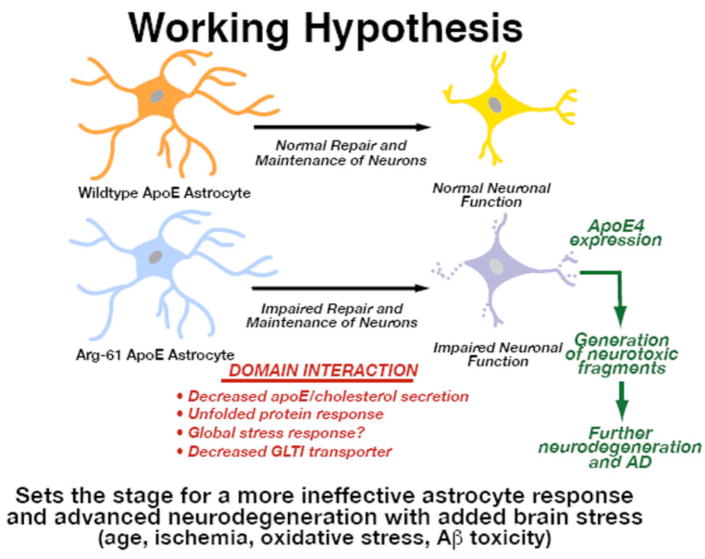
As noted above, Arg-61 astrocytes secrete lower levels of apoE, and Arg-61 apoE does not accumulate in astrocytes. These observations suggest that apoE molecules with domain interaction are recognized as an abnormally folded protein and targeted for degradation. The findings also raise the intriguing possibility that domain interaction elicits an endoplasmic stress (ER) response. ER stress and the unfolded protein response (UPR) are implicated in a number of neurodegenerative diseases [13]. Under ER stress, normal cell function is compromised (14,15). Thus, chronic stress could render a cell permanently dysfunctional. When the three UPR pathways (XBP-1, IRE, and OASIS, an ATF6 family member specific for astrocytes) were examined in Arg-61 astrocytes, all three were activated, in addition to several downstream target genes [11]. Thus, domain interaction results in activation of UPR. In agreement with a dysfunction in the Arg-61 astrocytes, we demonstrated in vivo that levels of the astrocyte-specific glutamate transporter, GLT1, were lower than in WT mice [12]. Ineffective clearance of glutamate could result in excitotoxicity and contribute to the neurodegeneration in this model. Because astrocytes are important for supporting and maintaining neurons, other critical pathways are likely affected by the ER stress.
The synaptic, functional, and cognitive deficits in the Arg-61 apoE mouse model clearly result from domain interaction, and domain interaction in astrocytes elicits ER stress and UPR (Fig. 4). Arg-61 apoE or human apoE4 astrocytes are chronically dysfunctional due to apoE4 domain interaction, and their support of neuronal maintenance and repair is not as effective as it is in WT mouse or human apoE3 and apoE2. Under normal conditions and early in life, these astrocytes can cope with minimal effects. However, with age and added stress to the brain (e.g., ischemia, oxidative damage, Aβ toxicity), the response of astrocytes to this stress is inadequate, resulting more extensive neurodegeneration and initiation of disease. Stressed neurons would require additional apoE for repair. However, increased astrocyte expression of apoE4 would further exacerbate the ER stress. In turn, the stressed neurons would turn on their own apoE expression for repair [16]. In the case of apoE4, neuronal expression would result in the generation of C-terminal-truncated fragments that are neurotoxic [17]. Generation of these fragments would lead to more extensive neurodegeneration and AD. Thus, domain interaction in Arg-61 apoE mice or apoE4 carriers sets the stage for advanced neurodegeneration and indicates the astrocytes play a previously unappreciated key role in apoE4-mediated neurodegeneration and AD.
Fig. 4.
Implications of astrocyte ER stress on neurodegeneration and AD.
In addition, our work with the Arg-61 apoE model establishes that domain interaction is a viable therapeutic target. Using a small molecule to bind at the domain interaction interface and thus convert apoE4 to an apoE3-like structure is a potential strategy. In fact, this has already been accomplished as a “proof of principle” [14]. Furthermore, the involvement of ER stress and astrocyte dysfunction in apoE4-mediated disease suggests that strategies to reduce astrocyte stress represent another viable approach. Astrocytes are ideally positioned upstream to impact collectively the neuronal pathways and processes in AD that were discussed at this conference.
References
- 1.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 3.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. J Am Med Assoc. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 5.Mahley RW, Weisgraber KH, Huang Y. Proc Natl Acad Sci USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Weisgraber KH, Mucke L, Mahley RW. J Mol Neurosci. 2004;23:189–204. doi: 10.1385/JMN:23:3:189. [DOI] [PubMed] [Google Scholar]
- 7.Dong L-M, Weisgraber KH. J Biol Chem. 1996;271:19053–19057. doi: 10.1074/jbc.271.32.19053. [DOI] [PubMed] [Google Scholar]
- 8.Morrow JA, Hatters DM, Lu B, Höchtl P, Oberg KA, Rupp B, et al. J Biol Chem. 2002;277:50380–50385. doi: 10.1074/jbc.M204898200. [DOI] [PubMed] [Google Scholar]
- 9.Raffai RL, Dong LM, Tow B, Farese RV, Weisgraber KH. Generation of a mouse model for human apolipoprotein (apo) E4 using gene targeting. Circulation. 2000 [Google Scholar]
- 10.Hatters DM, Peters-Libeu CA, Weisgraber KH. J Biol Chem. 2005;280:26477–26482. doi: 10.1074/jbc.M503910200. [DOI] [PubMed] [Google Scholar]
- 11.Ramaswamy G, Xu Q, Huang Y, Weisgraber KH. J Neurosci. 2005;25:10658–10663. doi: 10.1523/JNEUROSCI.1922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong N, Scearce-Levie K, Ramaswamy G, Weisgraber KH. Alzheimer’s and Dementia. 2008 doi: 10.1016/j.jalz.2008.01.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm D, Wootz H, Korhonen L. Cell Death Differentiation. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 14.Ye S, Huang Y, Müllendorff K, Dong L, Giedt G, Meng EC, et al. Proc Natl Acad Sci USA. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 16.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, et al. Proc Natl Acad Sci USA. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]