Abstract
Background
Many patients with diabetes fail to achieve American Diabetes Association Guidelines for glycemic, blood pressure and lipid control. Depression is a common comorbidity and may affect disease control through adverse effects on adherence and physician intensification of treatment.
Methods
In a cohort of 4117 patients with diabetes, depression was measured at baseline with the Patient Health Questionnaire-9 (PHQ-9). Patient adherence and physician intensification of treatment were measured in those who had evidence of poor disease control (HbA1c ≥8.0%, LDL ≥130mg/dl, systolic blood pressure ≥140 mm Hg) over this 5-year period. Poor adherence was defined as having medication refill gaps for ≥20% of days covered for medications prescribed for each of these conditions. Treatment intensification was defined as an increased medication dosage in a class, an increase in the number of medication classes, or a switch to a different class within 3 month periods before and after notation of above target levels.
Results
Among patients with diabetes and poor disease control, depression was associated with an increased likelihood of poor adherence to diabetes control medications [OR = 1.98 (95% CI 1.31, 2.98)], antihypertensives [OR = 2.06 (95% CI 1.47, 2.88)] and LDL control medications [OR = 2.43 (95% CI 1.19, 4.97)]. In patients with poor disease control who were adherent to medication or not yet started on a medication, depression was not associated with differences in likelihood of physician intensification of treatment.
Conclusions
In patients with diabetes and poor disease control, depression is an important risk factor for poor patient adherence to medications, but not lack of treatment intensification by physicians.
Keywords: depression, diabetes, adherence, intensification, disease control
INTRODUCTION
American Diabetes Association (ADA) Guidelines have established clear disease control goals for patients with diabetes and their physicians. These goals include keeping HbA1c levels below 7.0%, blood pressure below 130/80 mm Hg and low density cholesterol (LDL) below 100mg/dl (1). Recent studies suggest that only about 10% of patients with diabetes meet all three of these disease control goals concurrently (2). Inadequate disease control in patients with diabetes is linked to adverse macrovascular and microvascular events and mortality (3, 4).
Two major explanations have been proposed for not meeting disease control goals: lack of adherence to self-care regimens such as diet, exercising and taking medications as prescribed (5, 6) and physician's "clinical inertia", a delayed or absent action regarding intensifying treatment regimens in the face of poor control (7, 8). Estimates suggest that as many as 60% to 80% of patients have problems following diet and exercise recommendations and 25% to 50% have problems adhering to medications (5, 6). Poor adherence may be responsible for up to 50% of treatment failures in chronic medical illness and often leads to disease progression, avoidable functional impairment, hospitalizations and mortality (9, 10).
Kerr and colleagues have defined clinical inertia as lack of intensification or change in medication regimens in patients with chronic illnesses even when these patients are clearly above guideline-level disease control targets (11, 12). Clinical inertia has been shown to be associated with poorer disease control, medical complications and costs (13–15). Multiple competing demands such as the acute presenting complaint, multiple chronic illnesses and short, infrequent appointments with lack of proactive follow-up may be all associated with clinical inertia (16). Schmittdeil et al. (17) found that, while a good portion of failure to achieve clinical targets was attributable to poor adherence, lack of treatment intensification played an even larger role. For blood pressure control, clinical uncertainty about differences in blood pressure measurements taken at home and in the clinic and by physicians and nurses have also been associated with clinical inertia (11).
Depression is a common comorbidity in patients with diabetes(18) and has been shown to be associated with poor adherence to medications, diet, exercise, and recommendations for smoking cessation (19). Depression has also been associated with poorer control of cardiovascular risk factors (20, 21). Yet to be explored is whether depression may also promote clinical inertia and failure to intensify treatment. While patients with depression have more scheduled office visits than patients without depression, they also have more missed appointments (22, 23). Patients with depression and comorbid medical illness often present with somatic complaints (24) and impairments in functioning (25) which may present competing demands that divert focus from achieving disease control (26). We are not aware of any studies that have measured the effect of comorbid major depression in patients with diabetes on patient adherence to medication and physician intensification of treatment among patients with poor disease control.
Although ADA guidelines have recommended treating patients with diabetes to keep HbA1c <7.0%, LDL <100 mg/dl and blood pressure <130/80 mm Hg,(1) higher disease control thresholds were chosen for this study to unequivocally identify patients requiring clinical intervention. This paper will prospectively examine the association of depression in patients with diabetes who develop evidence of poor disease control as characterized by HbA1c levels ≥8.0%, systolic blood pressure (SBP) ≥140 mmHg and LDL ≥130 mg/dl on the following:
adherence to medications used for these three clinical conditions; and
physician intensification of treatment for patients who were adherent to medication or not yet on a disease control medication.
We hypothesize that patients with comorbid depression and diabetes and poor disease control will have poorer adherence to disease control medication and less likelihood of physician intensification of treatment.
METHODS
Study Population
Group Health (GH) is a mixed-model prepaid health plan serving over 500,000 members in Washington State. Most GH members receive medical services within the integrated group practice which includes 30 primary care clinics in Western Washington. The GH enrollment is demographically similar to the area population. All study procedures were approved by institutional review boards at GH and the University of Washington.
Study Cohort Selection
The GH diabetes registry includes all GH members meeting any of the following eligibility criteria in the preceding 12 months: filled prescriptions for insulin or an oral hypoglycemic agent, two fasting plasma glucose levels ≥126 mg/dl, two random plasma glucose levels ≥ 200mg/dl, two outpatient diagnoses of diabetes, or any inpatient diagnosis of diabetes (27). The cohort for this longitudinal study, the Pathways Epidemiologic Follow-up Study (28), was sampled in 2001 and 2002 with approximately 700 questionnaires mailed per month to adults in the GH diabetes registry. The 5-year follow-up period was completed between 2005 and 2007. All patients with diabetes were identified at nine primary care clinics in the Seattle/Puget Sound region that were selected based on the socioeconomic, racial and ethnic diversity and number of patients with diabetes that these clinics serve.
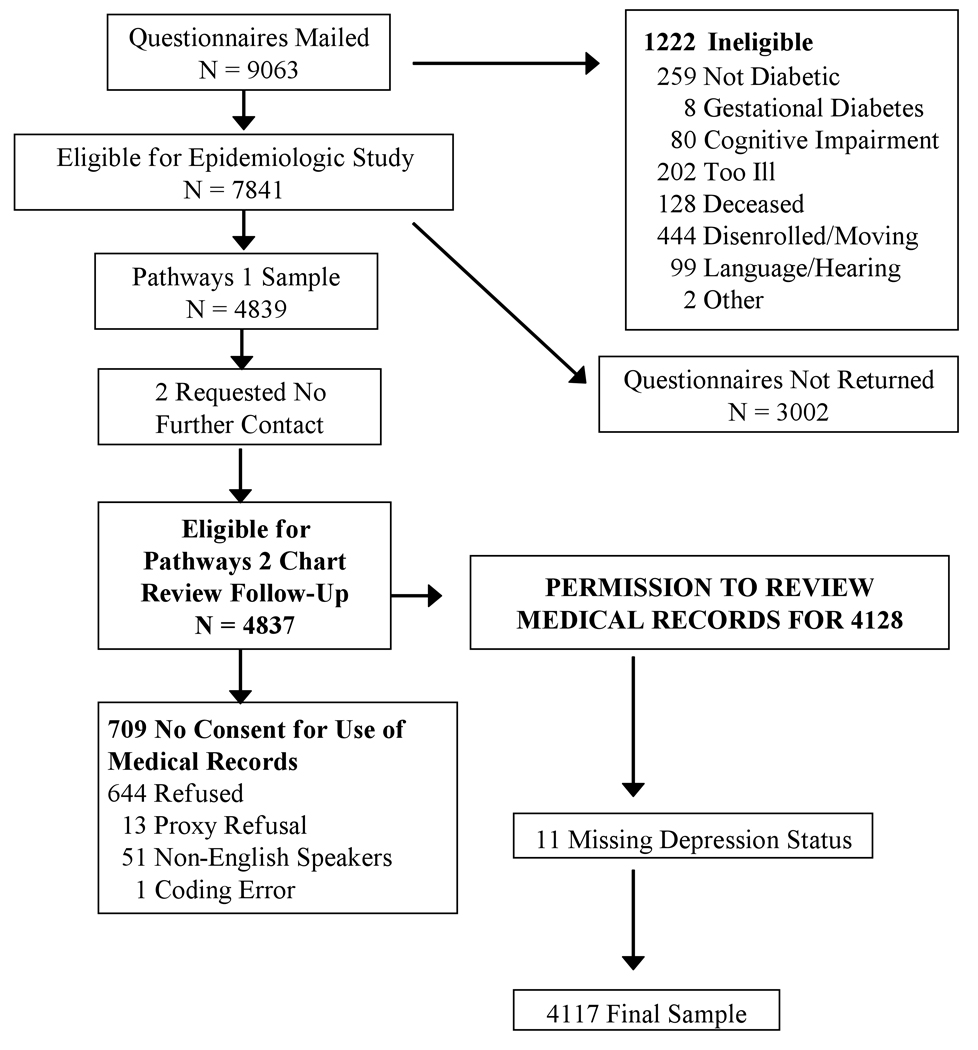
As shown in Figure 1, baseline questionnaire surveys were mailed to 9063 potentially eligible patients, but 1222 were later found to be ineligible due to reasons such as death, disenrollment, erroneous diagnosis of diabetes, or cognitive impairment. Among the 7841 eligible patients, 4839 subjects (61.7%) patients returned the baseline questionnaire. Two patients requested no further contact, 709 did not provide consent for medical record review, and 11 did not have a baseline depression score, leaving a final sample of 4117 for this analysis.
Figure 1.
The baseline questionnaire included questions on age, sex, years of education, employment, race/ethnicity, marital status, height and weight. Questions about clinical status and health habits included: age of onset and duration of diabetes, type of treatment at onset of disease and smoking. Patients were classified as having type 1 diabetes if onset was prior to 30 years of age, insulin was the first treatment prescribed, and they currently receive insulin.
The Patient Health Questionnaire-9 (PHQ-9) was used to screen for depression in the baseline survey. The PHQ-9 diagnosis of probable major depression has been found to have adequate sensitivity (73%) and high specificity (98%) to a diagnosis of major depression based on structured psychiatric interviews (29, 30). A diagnosis of probable major depression on the PHQ-9 requires having five or more symptoms for more than half the days, including either depressed mood or anhedonia (29, 30).
Computerized pharmacy records were used to compute a chronic disease score (Rx Risk), a measure of medical comorbidity based on prescription drug use in the previous 12 months (31). Rx Risk has been shown to predict subsequent hospitalization and mortality rates over the next one-year period (31). A measure using automated data was used to code for 7 types of diabetes complications present at baseline, including retinopathy, neuropathy, nephropathy, cerebrovascular, cardiovascular, peripheral vascular and metabolic (32). This diabetes complication measure has been shown to predict mortality and hospitalization rates over the next one year period (32).
Disease Control
At baseline and during the 5-year follow-up, blood pressure measurements were abstracted from the ambulatory medical records and HbA1c and LDL levels were obtained from automated lab data. The baseline measurements of SBP, HbA1c, and LDL were the levels closest in time before the return date of the screening questionnaire.
The first date during follow-up between 2001 and 2007 with a disease control measure above target level was identified. Based on medical record review of primary care clinic blood pressure measurements, the blood pressure closest to each incremental one-year follow-up date was recorded. Most guidelines suggest at least two blood pressure readings of >130/80 mm Hg should be required prior to initiating or intensifying treatment (1), but we had access to only one measure of blood pressure per year and considered patients to be eligible for treatment intensification after only one SBP ≥ 140 mm Hg. However, of the 2804 patients with one SBP ≥140, 1904 (68%) had at least one other subsequent reading of ≥140 and another 355 (12.7%) had a subsequent reading of ≥130 in the subsequent 5-year time period (i.e. over 80% remained above ADA guideline recommended systolic blood pressure).
Adherence to Medications
Adherence to medication was based on pharmacy utilization data from GH automated records using the validated continuous, multiple interval measure of gaps in therapy method (CMG) (33, 34). This measure has been validated against objective measures such as serum and urine drug levels, physiologic medication effects such as blood pressure decreases, and increased medical costs (33, 35). Briefly, the CMG was defined as the number of days the patient did not have medication available divided by the number of days the patient should have been on medication. We calculated the CMG for hyperlipidemia, hypertension and hyperglycemia medications filled at least twice in the 12 months before the identified date of an above target level. CMG was first classified for each medication class. Individual class adherence was then combined into a single measure for all medications prescribed for a single condition, weighting the estimate for each medication class by the number of days from the first to the first prescription filled in the 12-month period. Medications that were only filled once were not included in the analysis because CMG cannot be calculated from single fills. We defined poor adherence for each condition as a weighted non-adherence measure of ≥20% across all medications prescribed for the condition (33, 34). Prior studies have shown that when cumulative days of refill gaps exceed 20%, there are associated clinically significant adverse effects (13, 35).
Treatment Intensification
Using methods described by Schmittdiel et al., treatment intensification was assessed for each of the three conditions using GH prescription databases during the three months before and three months after first measurement of above target levels (17). Intensification was defined by any one of the following three treatment changes: 1) an increase in the number of medication classes; 2) an increase in the daily dosage of at least one ongoing medication class; or 3) a switch to a medication in a different medication class (17). Five medication classes were considered for hyperlipidemia (statins, fibrates, bile acid resins, ezetimibe and niacin), five for hypertension (ACE inhibitors/angiotensin receptor blockers, diuretics, beta adrenergic blockers, calcium channel blockers, and other); and four for diabetes control (metformin, sulfonylurea, thiazolidinediones and insulin) (17). Combination pills (such as prinizide) were included in both classes. Daily dosages were classified as low (near initial starting dosage recommendations), medium (maintenance range) and high (above maintenance range) based on package insert recommendations and inspection of actual dosage distributions prescribed in the population (17). The treatment intensification analyses focused on patients with no evidence of poor adherence because it is most appropriate for physicians to recommend changes in intensity of treatment in patients who are already adherent to prescribed medications (17).
Patients who were taking insulin were excluded because medication adherence and treatment intensity cannot be accurately assessed with prescription refill data. However the addition of insulin in a patient with poor glycemic control who was adhering to an oral hypoglycemic was considered evidence of treatment intensification.
Statistical Analyses
We tested for differences in clinical and sociodemographic characteristics comparing participants with and without comorbid major depression using chi-square analyses for the categorical variables and independent group t tests (using 2-tailed statistical significance) for continuous variables. We tested for differences in time to an above-target measure, comparing participants with and without major depression using t tests.
Levels of medication adherence (poor versus adequate adherence) for those who were taking disease control medications (at least two prescription filled) were compared among those with and without comorbid major depression using both unadjusted and adjusted odds ratios generated by logistic regression analyses. Clinical and sociodemographic variables used in multivariate models were chosen apriori based on prior epidemiologic research describing relationships between depression and diabetes.(18, 19, 21) The same analytic strategy was used to examine the relationship between major depression and treatment intensification.
We ran several sensitivity analyses. In the first sensitivity analysis, we examined the relationship between depression and treatment intensification separately for those who were not yet prescribed a medication and those adhering to medication. Second, because physicians may not be aware of poor adherence as a reason for poor disease control, we examined whether depression was associated with lack of intensification of treatment in patients with poor control in all groups combined, i.e. those with poor adherence, those with good adherence, and patients not yet on medication. Third, we repeated the treatment intensification analyses after excluding patients on maximum therapy for hypertension or hyperlipidemia. As described in a previous study of therapy intensification (17), maximal medical therapy was defined as four classes of antihypertensive medications for treatment of hypertension and treatment with ezetimibe at any dosage or simvastatin 80mg, lovastatin 80mg, atorvastatin 80mg and pravastatin 80mg for hyperlipidemia. In the final sensitivity analysis, we extended the window after identification of poor disease control to 6 months from 3 months, since it may take time for the physician to recognize the lack of depression control.
All analyses were performed using SPSS 15.0 and STATA 10.0.
RESULTS
Among patients in our sample, those with comorbid depression and diabetes, compared to those with diabetes alone, were significantly more likely to be female, single, younger, smokers and on insulin. They also had greater medical comorbidity, more diabetes complications, higher HbA1c levels at baseline, and a higher BMI (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Depression Groups at Baseline (N = 4117)
| Overall | No Depression | Major Depression |
Test Statistic (X2 = 1 or t(4115) |
|
|---|---|---|---|---|
| N (%) | 4117 | 3622 | 495 | |
| Women | 1981 (48.1) | 1690 (46.7) | 291 (58.8) | 25.66 (p < .001) |
| High school or less | 983 (24.2) | 848 (23.7) | 135 (27.6) | 3.61 (p = .057) |
| Unmarried and not living as married | 1384 (33.9) | 1169 (32.6) | 215 (43.9) | 24.50 (p < .001) |
| Employment full or part time | 1681 (42.2) | 1476 (42.0) | 205 (43.6) | 0.40 |
| Caucasian | 3299 (80.5) | 2910 (80.7) | 389 (78.7) | 0.94 |
| Age in years X̅ (SD) | 63.4 (13.4) | 64.0 (13.3) | 59.4 (13.8) | 7.17 (p < .001) |
| Rx risk score X̅ (SD) | 3133.7 (2441.0) | 3093.0 (2386.3) | 3431.4 (2793.5) | 2.57 (p = .01) |
| Type I diabetes | 184 (4.5) | 165 (4.6) | 19 (3.8) | 0.52 |
| Number of diabetes complications X̅ (SD) | 1.4 (1.3) | 1.4 (1.3) | 1.6 (1.5) | 4.34 (p < .001) |
| Baseline HbA1c X̅ (SD) | 7.8 (1.6) | 7.7 (1.5) | 8.2 (1.7) | 5.08 (p < .001) |
| Baseline Systolic Blood Pressure (N = 3679) X̅ (SD) |
135.7 (20.3) | 135.9 (20.1) | 134.3 (21.4) | 1.58 |
| Baseline LDL (N = 2939) X̅ (SD | 111.2 (34.6) | 111.0 (34.3) | 113.0 (37.1) | 0.99 |
| Duration of diabetes in years X̅ (SD | 9.6 (9.4) | 9.6 (9.6) | 9.6 (8.3) | 0.16 |
| BMI | 31.5 (7.3) | 31.1 (6.8) | 34.8 (9.3) | 8.54 (p < .001) |
| Smoking currently | 347 (8.4) | 276 (7.6) | 71 (14.3) | 25.50 (p < .001) |
| Diabetes Treatment Intensity | ||||
| None or diet | 1040 (25.3) | 953 (26.3) | 87 (17.6) | 43.41 |
| Insulin or insulin + oral hypoglycemic | 1818 (44.2) | 1622 (44.8) | 196 (39.6) | (df = 2) |
| Oral hypoglycemic | 1259 (30.5) | 1047 (28.9) | 212 (42.8) | (p < .001) |
Rx Risk = Pharmacy-based medical comorbidity measure; HbA1c = Hemoglobin A1c; LDL = Low-Density Lipids; BMI = Body Mass Index
Sample sizes may vary with missing data
Of the patients who had poor control on at least one of the three study measures (blood glucose, low density lipids, and blood pressure), the majority had poor control on two (47%) or all three (19%) of the disease control measures. In contrast, of those with poor disease control who were non-adherent on at least one of the medications, only 12.5% were non-adherent on two or more of the three medication classes.
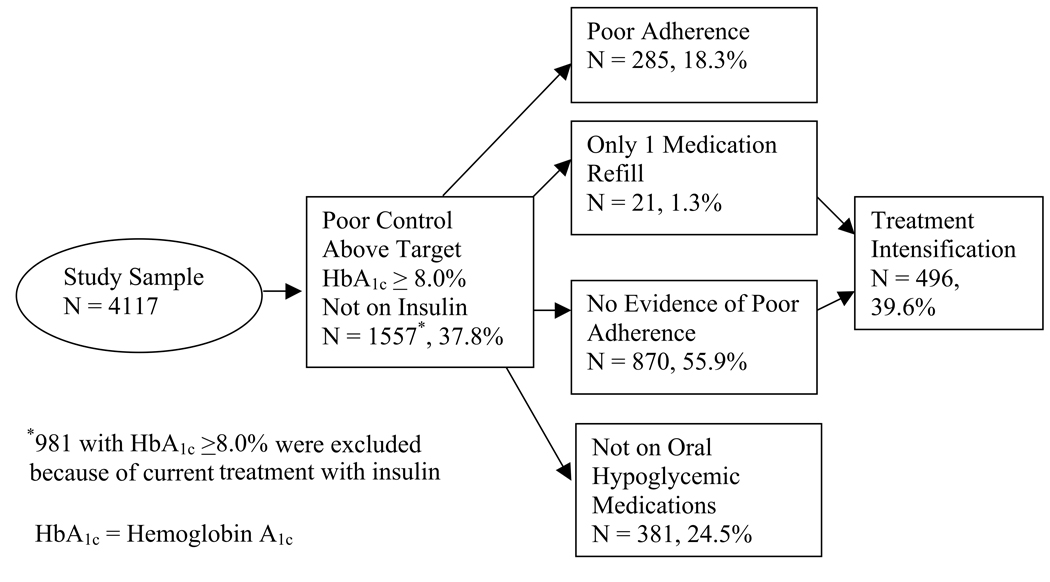
Of the 4117 patients in our analysis, 1557 (37.8%) had evidence of poor glycemic control (HbA1c ≥8.0%) and were not being treated with insulin and were thus eligible for our adherence and treatment intensity analyses (figure 2a). If the 981 patients treated with insulin with HbA1c ≥8.0% were included, a total of 2538 (61.6%) had evidence of poor control. Of the 1557 patients, 870 (55.9%) had evidence of adequate adherence, 285 (18.3%) had evidence of poor adherence (24.7% of those prescribed oral hypoglycemic medications), 381 (24.5%) were not being treated with oral hypoglycemic agents, and 21 (1.3%) had only one fill. A total of 496 (39.6%) of 1251 patients with no evidence of poor adherence to oral hypoglycemic medications or who were not treated with these medications had evidence of treatment intensification.
Figure 2.
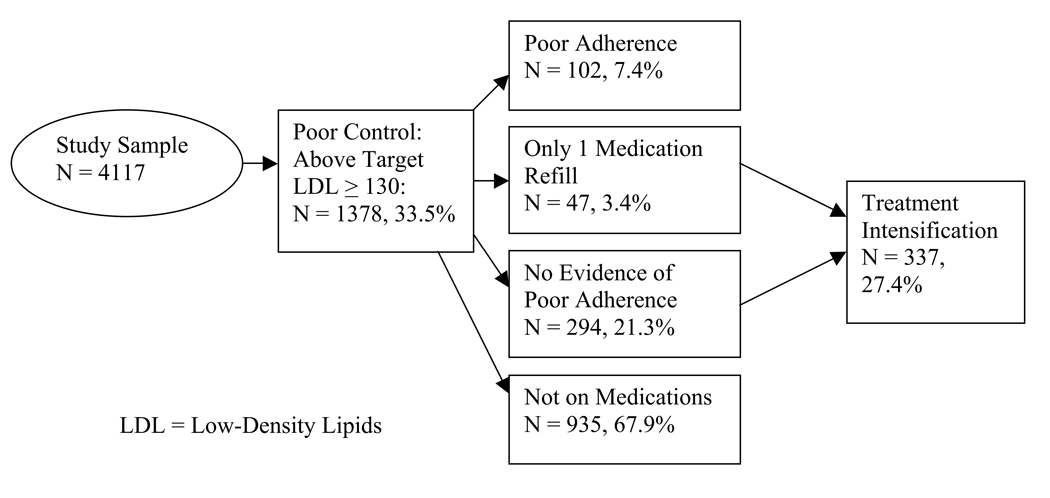
A total of 1378 (33.5%) of the 4117 patients had evidence of one or more LDL levels of ≥130 mg/dl (Figure 2b). Of those in poor control, 102 (7.4%) had evidence of poor adherence (25.8% of those prescribed lipid lowering medication), 294 (21.3%) were adherent, 935 (67.9%) were not being treated with lipid medication, and 47 (3.4%) had only one medication fill. A total of 337 (27.4%) of 1229 patients with no evidence of poor adherence to lipid lowering medications or who were not getting treated with other drugs received treatment intensification.
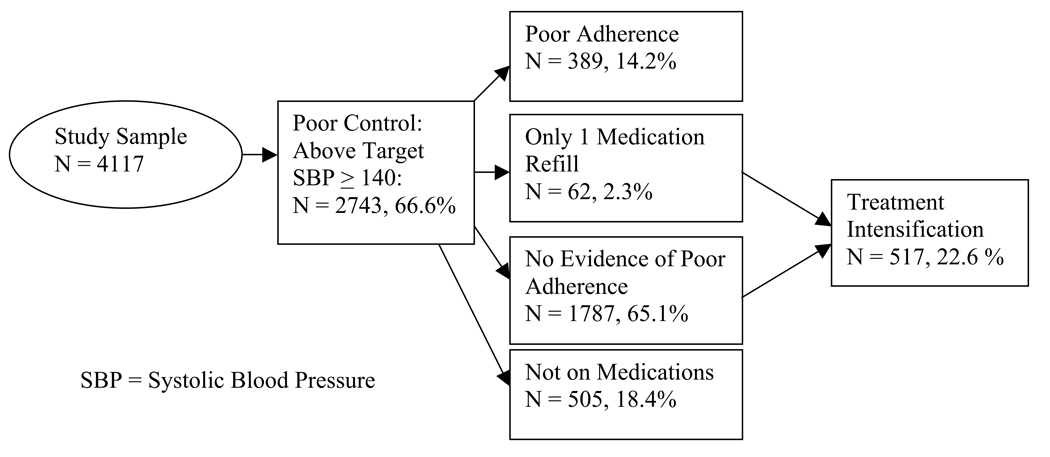
A total of 2743 (66.6%) of 4117 patients had one or more systolic blood pressure readings of ≥140 mm Hg. Of those with poor blood pressure control, 389 (14.2%) had evidence of poor adherence (17.9% of those prescribed antihypertensive medications), 1787 (65.1%) were adherent, 505 (18.4%) were not taking antihypertensive medications, and 62 (2.3%) had only one medication fill. A total of 517 (22.6%) of those 2292 patients showing good adherence or no treatment with antihypertensive agents had evidence of treatment intensification.
In those who crossed the threshold into poor control, patients with comorbid depression and diabetes compared to those with diabetes alone had significantly shorter time to the first HbA1c level ≥8.0% [depressed 0.9 years post baseline entry versus nondepressed 1.1 years post baseline (p < .001)], and to LDL level of ≥130mg/dl [depressed 1.1 years after baseline, non-depressed 1.3 years post baseline (p = .04)], but did not differ in time to the first systolic blood pressure reading of ≥140mm Hg (depressed in 1.3 years post baseline, nondepressed 1.4 years post-study entry).
We examined levels of adherence for each target disease control measure overall and separately by depression status. For hyperglycemia, 285 (24.7%) of the 1155 patients treated with oral hypoglycemics demonstrated poor adherence to glycemic-lowering medication. For hyperlipidemia, 102 (25.8%) of the 396 treated with lipid lowering medications demonstrated poor adherence. For hypertension, 389 (17.9%) of 2176 patients treated with antihypertensive medication had poor adherence. For those above target on any of the three measures, patients with comorbid major depression and diabetes compared to those with diabetes alone were significantly more likely to be non-adherent to all three types of disease control medications (Table 2).
TABLE 2.
Medication Nonadherence in Patients with Diabetes and Poor Glycemic, Blood Pressure and Lipid Control by Depression Status
| % Nonadherent in those with No Depression |
% Nonadherent in those with Major Depression |
Unadjusted OR for Medication Nonadherence for Major Depression |
Adjusteda OR for Medication Nonadherence for Major Depression |
|
|---|---|---|---|---|
| Diabetes Control | 238/1028 (23.2%) | 47/127 (37.0%) |
1.95*** (1.32 – 2.88) |
1.98*** (1.31 – 2.98) |
| Hypertension Control | 322/1944 (16.6%) |
67/232 (28.9%) |
2.04*** (1.50 – 2.78) |
2.06*** (1.47 – 2.88) |
| LDL Control | 85/352 (24.1%) |
17/44 (38.6%) |
1.98* (1.03 – 3.80) |
2.43** (1.19 – 4.97) |
Adjusted for diabetes treatment intensity, gender, age, hemoglobin A1c, Rx risk (pharmacy-based medical comorbidity measure), number of complications, body mass index, current smoking, education level and marital status;
p < .05;
p < .01;
p < .001)
For patients with poor disease control who were adherent to or not yet on medication, treatment intensification only occurred in 39.6% of patients with poor blood sugar control, 27.4% of those with poor LDL control and 22.6% of those with poor blood pressure control. Among these patients, rates of treatment intensification did not differ significantly by depression status (Table 3).
TABLE 3.
Increase in Intensity of Treatment among Patients with Diabetes and Poor Glycemic, Blood Pressure or Lipid Control Who are Adherent or Not Yet on Medications by Depression Status
| % Intensified in those with No Depression |
% Intensified in those with Major Depression |
Unadjusted OR for Increase in Intensity of Treatment in those with Major Depression |
Adjusteda OR for Increase in Intensity of Treatment in those with Major Depression |
|
|---|---|---|---|---|
| Diabetes Control |
442/1135 (38.9%) |
54/116 (46.6%) |
1.37 (0.93 – 2.00) |
1.18 (0.78 – 1.79) |
| Hypertension Control |
459/2068 (22.2%) |
58/224 (25.9%) |
1.22 (0.89 – 1.68) |
1.26 (0.90 – 1.76) |
| LDL Control | 301/1085 (27.7%) |
36/144 (25.0%) |
0.87 (0.58 – 1.30) |
0.79 (0.52 – 1.20) |
Adjusted for diabetes treatment intensity, gender, age, hemoglobin A1c, Rx risk (pharmacy-based medical comorbidity measure), number of complications, body mass index, current smoking, education level and marital status
p < .05;
p < .01;
p < .001
As a sensitivity analysis we examined the relationship between depression and treatment intensification separately for those who were not being prescribed medication and those who were adhering to medication. The rates of treatment intensification were very similar in these two subgroups and were not associated with depression (data not shown). In a second sensitivity analysis, we repeated the treatment intensification analysis in the combined larger group of patients with poor disease control who were not adhering to medication, those adhering to medication, and those not yet started on medication and found again that treatment intensification was not related to depression for patients with poor lipid control [adjusted OR = 1.00 (95% CI 0.70, 1.44)] or glycemic control [adjusted OR = 0.82 (95% CI 0.55, 1.23)], but was related to a significantly higher rate of treatment intensification in those with poor blood pressure control [adjusted OR = 1.43 (95% CI 1.06, 1.92)].
We also repeated the treatment intensification analyses after excluding those who were on the maximum medical therapy level. This did not apply to the patients with poor glucose control since patients with insulin treatment were not included in this analysis. A total of 44 patients were removed from the LDL analysis and 151 from the hypertension analysis. The rates of treatment intensification did not change, and again were not found to be related to depression (data not shown). Finally, we extended the 3 month period after identification of poor disease control to 6 months and found only a 6 to 8% increase in intensification in any of the three measures with no differences by depression status.
DISCUSSION
In this 5 year prospective study of primary care patients with diabetes, approximately one-third had evidence of hyperlipidemia (LDL ≥130 mg/dl), and about two-thirds had evidence of one or more measures indicating poor glycemic control (HbA1c ≥8.0%) or systolic hypertension (SBP ≥140 mm Hg). Of those treated with medication, poor adherence was observed in approximately 25% of patients with HbA1c ≥8.0%, 18% of those with SBP ≥140mm Hg and 26% of those with LDL ≥130mg/dl. Of those adhering to medication or not yet prescribed medication, evidence of treatment intensification occurred in only 40% of those with HbA1c ≥8.0%, 23% of those with SBP ≥140mm Hg and 27% of those with LDL ≥130mg/dl. These findings of poor adherence in 18% to 26% of patients with poor disease control are consistent with data from a large study of patients with diabetes completed at in another health plan showing 20% to 23% of patients with diabetes had poor adherence to medications across the same three measures of disease control (17). The rates of intensification of treatment found in the current study for those with no evidence of poor adherence (23% to 40%) were slightly lower than those found in the prior study (30 to 47%)(17). Both studies show the lack of therapy intensification among patients with poor clinical control was much more common than poor medication adherence. Such clinical inertia is an appropriate target for evidence-based diabetes care management teams (36).
The patterns were altered when the impact of comorbid depression was assessed. Depression was associated with a 2-fold to almost 2.5-fold greater likelihood of having poor adherence to the three categories of disease control medications among patients with diabetes and poor disease control. Additionally, among patients with diabetes and evidence of poor disease control, depression was associated with modestly shorter time to observation of poor glycemic and LDL lab values, although not systolic blood pressure control. These data are consistent with several other studies showing higher rates of nonadherence to oral hypoglycemic, lipid lowering and blood pressure medications in patients with comorbid depression and diabetes compared to those with diabetes alone (19, 37). In patients with diabetes, depression has also been associated in patients with diabetes with poor adherence to diet, exercise and cessation of smoking which may also worsen disease control and health (19, 37).
On the other hand, depression was not associated with a lack of treatment intensification by primary care physicians among patients with poor disease control who were adherent or not yet on disease control medications. When the patients with poor disease control were combined into the larger group with poor adherence, those with good adherence and those not yet started on medication, depression was shown to be associated with a significantly greater chance of treatment intensification of high blood pressure medication but not lipid lowering or oral hypoglycemic medications. The findings that depression is either not associated with lack of treatment intensification or actually increases treatment intensification in patients with poor blood pressure control may be explained by the well documented higher utilization of primary care visits by patients with comorbid depression and diabetes (38), which may provide more frequent opportunities for treatment intensification. Similarly, prior studies have shown that comorbid depression is associated with decrements in patient self-care activities, but not differences in diabetes care measures ordered by physicians such as HbA1c or LDL tests or retinal examinations (19).
These results suggest that innovations are needed to alert physicians and health care terms to poor disease control, medication nonadherence, and failure to initiate or intensify treatment for those with poor disease control. Electronic medical records could easily be used for this purpose. However, one study found that simply alerting physicians to poor adherence did not successfully address this problem (39). Most reviews of evidence-based interventions to improve patient level outcomes have suggested that multi-faceted approaches using health care teams with allied health professionals are necessary to increase frequency of contacts, monitor disease outcomes and adherence, and facilitate return appointments to physicians for patients with persistently poor disease control (36, 40). Specialty input to enhance decision support for primary care providers is also key (36, 40).
Integrating screening and treatment of depression may enhance disease management of patients with diabetes, particularly if it can improve adherence. Major depression occurs in up to 18% of patients with type 2 diabetes (18, 41). In addition to being a substantial health burden that negatively affects quality of life in patients with diabetes, depression adversely affects adherence to self-care regimens (19) and is associated with higher HbA1c levels (18), increased macrovascular and microvascular complications (42, 43) and mortality (42, 44, 45). Recent studies have shown that primary care-based collaborative care approaches to screening and treatment of depression in patients with diabetes were associated with improved quality of depression care, improved depressive and functional outcomes (46, 47) and a high probability of decreased total medical costs compared to usual primary care (48, 49). These collaborative care interventions utilize nurses (supervised by a psychiatrist and primary care physician) to enhance patient education and provide more frequent follow-up to monitor adherence to medication, side-effects, depression symptom outcomes and facilitate return appointments to primary care for those with persistent symptoms (46, 47).
Some study limitations should be mentioned. The study sample was an insured population from one region of the United States, thus limiting generalizability. The approximately 40% nonresponse rate also limits generalizability to the larger population sampled. Group Health has introduced several quality of care initiatives for patients with diabetes (27), and, thus, estimates of poor adherence and failure to intensify treatment may be an underestimate compared to other health care systems. Pharmacy utilization data from which we derived our measure of medication adherence (CMG) may overestimate the actual degree of pill taking (all dispensed pills are not necessarily consumed). Because we defined poor adherence as a weighted nonadherence measure of ≥20% across all medications in a class and did not have a daily adherence measure, we may have missed more minor variations in adherence associated with depression. It is also possible that some patients received medications from non-Group Health pharmacies, in which case their medication use would not be captured by the analyses reported here. However, prior research in the GH population suggests that it is uncommon for patients to fill prescription at non-GH pharmacies (50). Lack of intensification may not always be an accurate measure of clinician inertia because there may be clinically appropriate reasons not to intensify regimens such as having severe comorbid medical conditions or patient refusal to add new medications to an already complex regimen (51, 52). Finally, patients with poor glucose control who were being treated with insulin were not included because neither CMG or treatment intensification can be estimated in these patients using automated data.
CONCLUSIONS
We found lower than expected rates of treatment intensification in primary care patients with diabetes and poor glycemic, lipid and systolic blood pressure control. Clinical inertia was a much more dominant problem than poor adherence when the population was viewed as a whole. The patterns differed among patients with diabetes and poor disease control with and without comorbid depression. Depressed patients were significantly more likely to have poor adherence to oral hypoglycemics, lipid lowering, and antihypertensive medications, but they did not have lower rates of treatment intensification.
Acknowledgements
This research was supported by NIMH R01 MH073686 (Von Korff, principal investigator) and NIMH K-24 MH069741 (Katon, principal investigator)
List of Abbreviations
- ADA
American Diabetes Association
- GH
Group Health
- LDL
low density lipoprotein
- HbA1c
hemoglobin A1c
- CMG
continuous multiple interval measure of gaps
- SBP
systolic blood pressure
REFERENCES
- 1.American Diabetes Association. Clinical Practice Recommendations. Diabetes Care Suppl. 2007;1:S1–S103. [Google Scholar]
- 2.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- 6.Sacket D, Snow J. The Magnitude of adherence and non-adherence. In: Haynes RB, Taylor DW, Sacket D, editors. Adherence in Health Care. Baltimore, MD: Johns Hopkins University Press; 1979. pp. 11–22. [Google Scholar]
- 7.Berlowitz DR, Ash AS, Glickman M, Friedman RH, Pogach LM, Nelson AL, Wong AT. Developing a quality measure for clinical inertia in diabetes care. Health Serv Res. 2005;40:1836–1853. doi: 10.1111/j.1475-6773.2005.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodondi N, Peng T, Karter AJ, Bauer DC, Vittinghoff E, Tang S, Pettitt D, Kerr EA, Selby JV. Therapy modifications in response to poorly controlled hypertension, dyslipidemia, and diabetes mellitus. Ann Intern Med. 2006;144:475–484. doi: 10.7326/0003-4819-144-7-200604040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson J. Noncompliance may cause half of antihypertensive drug "failures.". JAMA. 1999;282:313–314. doi: 10.1001/jama.282.4.313. [DOI] [PubMed] [Google Scholar]
- 10.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 11.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 12.Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Ann Intern Med. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- 13.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 14.Ziemer DC, Miller CD, Rhee MK, Doyle JP, Watkins C, Jr, Cook CB, Gallina DL, El-Kebbi IM, Barnes CS, Dunbar VG, Branch WT, Jr, Phillips LS. Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ. 2005;31:564–571. doi: 10.1177/0145721705279050. [DOI] [PubMed] [Google Scholar]
- 15.Oliveria SA, Lapuerta P, McCarthy BD, L'Italien GJ, Berlowitz DR, Asch SM. Physician-related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–420. doi: 10.1001/archinte.162.4.413. [DOI] [PubMed] [Google Scholar]
- 16.Parchman ML, Pugh JA, Romero RL, Bowers KW. Competing demands or clinical inertia: the case of elevated glycosylated hemoglobin. Ann Fam Med. 2007;5:196–201. doi: 10.1370/afm.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, Selby JV. Why don't diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23:588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katon W, von Korff M, Ciechanowski P, Russo J, Lin E, Simon G, Ludman E, Walker E, Bush T, Young B. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 19.Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, Ciechanowski P, Ludman EJ, Bush T, Young B. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Swain BE, Gerzoff RB, Karter AJ, Waitzfelder BE, Brown AF, Ackermann RT, Duru OK, Ferrara A, Herman W, Marrero DG, Caputo D, Narayan KM. Understanding the gap between good processes of diabetes care and poor intermediate outcomes: Translating Research into Action for Diabetes (TRIAD) Med Care. 2007;45:1144–1153. doi: 10.1097/MLR.0b013e3181468e79. [DOI] [PubMed] [Google Scholar]
- 21.Katon WJ, Lin EH, Russo J, Von Korff M, Ciechanowski P, Simon G, Ludman E, Bush T, Young B. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004;19:1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciechanowski P, Russo J, Katon W, Simon G, Ludman E, Von Korff M, Young B, Lin E. Where is the patient? The association of psychosocial factors and missed primary care appointments in patients with diabetes. Gen Hosp Psychiatry. 2006;28:9–17. doi: 10.1016/j.genhosppsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Ferrara A, Liu JY, Selby JV. Missed appointments and poor glycemic control: an opportunity to identify high-risk diabetic patients. Med Care. 2004;42:110–115. doi: 10.1097/01.mlr.0000109023.64650.73. [DOI] [PubMed] [Google Scholar]
- 24.Ludman EJ, Katon W, Russo J, Von Korff M, Simon G, Ciechanowski P, Lin E, Bush T, Walker E, Young B. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26:430–436. doi: 10.1016/j.genhosppsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Von Korff M, Katon W, Lin EH, Simon G, Ludman E, Oliver M, Ciechanowski P, Rutter C, Bush T. Potentially modifiable factors associated with disability among people with diabetes. Psychosomatic Medicine. 2005;67:233–240. doi: 10.1097/01.psy.0000155662.82621.50. [DOI] [PubMed] [Google Scholar]
- 26.Nutting PA, Rost K, Smith J, Werner JJ, Elliot C. Competing demands from physical problems: effect on initiating and completing depression care over 6 months. Arch Fam Med. 2000;9:1059–1064. doi: 10.1001/archfami.9.10.1059. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch DK, Price MJ, Hindmarsh M, Wagner EH. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998;1:12–22. [PubMed] [Google Scholar]
- 28.Lin E, Heckbert S, Rutter C, Katon W. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. doi: 10.1370/afm.998. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O'Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, Katon WJ. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records. Description and validation. Med Care. 1988;26:814–823. doi: 10.1097/00005650-198808000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 35.Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004;27:2800–2805. doi: 10.2337/diacare.27.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24:1821–1833. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JS, Safren SA, Delahanty LM, Cagliero E, Wexler DJ, Meigs JB, Grant RW. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabet Med. 2008;25:1102–1107. doi: 10.1111/j.1464-5491.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon G, Katon W, Lin E, Ludman E, Von Korff M, Ciechanowski P, Young B. Diabetes complications and depression as predictors of health care costs. Gen Hosp Psychiatry. 2005;27:344–351. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Bambauer KZ, Adams AS, Zhang F, Minkoff N, Grande A, Weisblatt R, Soumerai SB, Ross-Degnan D. Physician alerts to increase antidepressant adherence: fax or fiction? Arch Intern Med. 2006;166:498–504. doi: 10.1001/archinte.166.5.498. [DOI] [PubMed] [Google Scholar]
- 40.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–618. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 41.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 42.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 43.Lin E, Rutter C, Katon W, Heckbert S, Ciechanowski P, Oliver M, Ludman E, Young B, McCulloch D, Von Korff M. Depression and adverse outcomes of diabetes. In Review. [Google Scholar]
- 44.Katon WJ, Rutter C, Simon G, Lin EH, Ludman E, Ciechanowski P, Kinder L, Young B, Von Korff M. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 45.Katon W, Fan MY, Unutzer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med. 2008;23:1571–1575. doi: 10.1007/s11606-008-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 47.Williams JW, Jr, Katon W, Lin EH, Noel PH, Worchel J, Cornell J, Harpole L, Fultz BA, Hunkeler E, Mika VS, Unutzer J. The effectiveness of depression care management on diabetes-related outcomes in older patients. Ann Intern Med. 2004;140:1015–1024. doi: 10.7326/0003-4819-140-12-200406150-00012. [DOI] [PubMed] [Google Scholar]
- 48.Simon GE, Katon WJ, Lin EH, Rutter C, Manning WG, Von Korff M, Ciechanowski P, Ludman EJ, Young BA. Cost-effectiveness of systematic depression treatment among people with diabetes mellitus. Arch Gen Psychiatry. 2007;64:65–72. doi: 10.1001/archpsyc.64.1.65. [DOI] [PubMed] [Google Scholar]
- 49.Katon W, Unutzer J, Fan MY, Williams JW, Jr, Schoenbaum M, Lin EH, Hunkeler EM. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care. 2006;29:265–270. doi: 10.2337/diacare.29.02.06.dc05-1572. [DOI] [PubMed] [Google Scholar]
- 50.Saunders K, Davis R, Stergachis A. Group Health Cooperative. In: Strom B, editor. Pharmacoepidemiology. Fourth Edition. West Sussex, England: John Wiley and Sons; 2005. pp. 223–239. [Google Scholar]
- 51.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 52.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]