Abstract
Prostate cancer immunotherapy clinical trials have been performed, but often in immunocompromised patients with limited clinical success. The study aim was to determine whether the stage of prostate cancer development at which immunization occurs affects vaccine efficacy, and if so which tumor-associated immunosuppressive mechanisms may be involved at later stages. Therapeutic vaccination of TRAMP mice with only precancerous PIN lesions confered superior protection than immunization after development of invasive carcinoma. The presence of Treg, upregulation of tumor indoleamine-2,3-dioxygenase and TGFβ and an immunosuppressive intratumoral cytokine milieu were identified in more advanced prostate cancer. These results indicate that prostate cancer immunotherapy trials will be more successful if conducted in patients with less advanced disease.
Keywords: Prostate cancer, immunotherapy, regulatory T cells
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignancy in the United States apart from skin cancers, and is the second-leading cause of cancer-related death in American men [1]. There are several therapeutic options available for prostate cancer that is diagnosed early such as prostatectomy, cryotherapy and radiotherapy, but these can have serious side effects including incontinence and impotence [2–4].
There have been myriad attempts to use immunotherapeutic approaches to treat prostate cancer. The overall aim of prostate cancer immunotherapy is to induce a specific immune response against one or more prostate tumor-associated antigens (TAA) in an effort to precisely target and eradicate cancer cells expressing those antigens. Several prostate cancer TAA, including Prostate Specific Antigen (PSA) [5], Six-Transmembrane Epithelial Antigen of the Prostate (STEAP) [6], Prostate Stem Cell Antigen (PSCA) [7], Prostate Specific Membrane Antigen (PSMA) [8] and Prostatic Acid Phosphatase (PAP) [9] have been identified. Though all have been used in clinical trials of prostate cancer immunotherapies, none have been approved by the FDA for use to treat patients [10]. The overwhelming majority of these trials have been conducted in patients with advanced disease. From an ethical standpoint these patients form an ideal group in which to test experimental therapies because they have failed all other therapeutic options and usually have a life expectancy of less than one year. However, these patients are frequently severely immunocompromised due to their advanced cancer and are thus very poor candidates for immunotherapy trials. While generally unsuccessful, there is evidence that the vaccines investigated thus far can elicit protective, specific immune responses against prostate TAAs, but only in the minority of men who are still immunocompetent. This led us to hypothesize that the failure of therapeutic prostate cancer vaccines to date is not necessarily due to a lack of efficacy on the part of those vaccines per se, but instead represents either a general inability of the patients’ immune systems to respond effectively, or an active inhibition of anti-tumor immunity within the microenvironments of tumors in these patients.
In recent years it has become apparent that there are multiple immunosuppressive mechanisms that can be subverted by tumors in order to blunt patient immune responses mounted against them. The tumor immunology field is currently focused on the activities and functional significance of tumor-associated suppressive immune cells such as regulatory T cells (Treg) [11] and myeloid-derived suppressor cells (MDSC) [12]. In addition, suppression of T cell activity can be mediated by tryptophan depletion due to increased expression of indoleamine-2,3-dioxygenase within the tumor [13], or by increased arginine metabolism due to upregulation of arginase and/or inducible nitrous oxide synthase (iNOS) expression within the tumor [14]. Finally, increased expression of suppressive cytokines such as interleukin(IL)-10 or tumor growth factor β (TGFβ) can result in an immunosuppressive tumor microenvironment [15, 16]. It is possible that these tumor-associated immunosuppressive mechanisms become dominant at later stages of prostate cancer, resulting in the relatively poor efficacy of therapeutic vaccines that are administered at those stages. Therefore, we hypothesized that the efficacy of therapeutic vaccines will be improved if they are administered at the earliest stages of disease, thereby circumventing these problems [10].
Prostate cancer is routinely screened for and is frequently diagnosed early in the course of the disease when the patient has only small, non-invasive tumors or even precancerous prostatic intraepithelial neoplastic (PIN) lesions. In these cases, the (pre)cancerous prostate lesions generally cause symptoms in the patients that are less severe than the serious side-effects of the standard prostate cancer treatments. Therefore, the standard of care in these patients is a period of active surveillance that can last for years before the prostate tumor begins to pose a more serious risk to the health of the patient. Prostate cancer immunotherapy may induce a specific immune response that can potentially mediate long-term protection against tumor outgrowth. This may be achieved with significantly fewer side-effects than can occur with conventional treatments for early prostate cancer. The combination of early detection methods, availability of vaccines and a patient population that has received no other clinical interventions means that prostate cancer represents an ideal proving ground for the application of therapeutic cancer vaccines at early stages of disease.
In our previous studies, we have investigated the efficacy of therapeutic cancer vaccines in the TRAMP (transgenic adenocarcinoma mouse prostate) model of spontaneous prostate cancer. Prostate cancer in TRAMP mice closely mimics the course of the human disease, from the development of precancerous PIN lesions to invasive prostate adenocarcinoma or neuroendocrine tumors and then to metastatic disease [17]. Therefore this model is an ideal system in which to investigate the effects of therapeutic vaccination at different stages of prostate cancer progression. We have made extensive use of a heterologous prime-boost vaccination strategy in which mice are immunized with DNA encoding a particular TAA and boosted using Venezuelan Equine Encephalitis virus replicon particles encoding the same antigen. The prostate TAAs STEAP and PSCA are highly upregulated by prostate cancer cells in humans and mice [18, 19]. Our previous studies indicated that vaccination against these antigens using our protocol elicits strong protective immune responses in TRAMP mice and in C57BL/6 mice that have been challenged with TRAMP-C2 prostate cancer cells [6, 20]. In this study, we explored whether superior efficacy of therapeutic vaccination in TRAMP mice can be elicited if it is administered to animals that have only developed PIN lesions compared to when vaccination of these mice occurs after invasive carcinomas or neuroendocrine tumors have developed, and if so what immunosuppressive mechanisms may be involved in hampering therapeutic vaccination effectiveness at the later stages of disease.
MATERIALS AND METHOD
Mice
C57BL/6 and C3H mice were obtained from Taconic farms (Germantown, NY). TRAMP mice [17] on the C57BL6 background were bred at the University of Southern California. Research was conducted in compliance with the institutional animal use guidelines. TRAMP mice were categorized into groups based on their ages: Young, ≤8 weeks old; Middle-aged, 16–20 weeks old and Old, ≥24 weeks old. These ages reflect different stages of prostate cancer development in the TRAMP model, being prostate intrapepithelial neoplasia (PIN), development of prostate adenocarcinoma or neuroendocrine tumors and presence of high-grade or metastatic tumors, respectively.
Immunization
Male TRAMP mice (8 or 16 weeks weeks old) were anesthetized ip with 2.4 mg ketamine (Phoenix Pharmaceutical Inc, St Joseph, MO) and 480μg xylazine (Phoenix). DNA-gold particles were delivered to a shaved area on the abdomen using a helium-driven gene gun (BioRad) with a discharge pressure of 400 psi. Each mouse received 2 μg of either murine PSCA or murine STEAP cDNA vaccine. Fifteen days after gene gun vaccination mice were subcutaneously boosted 1 cm from the tail base with 106 infectious units (IU) of mPSCA-VRP and mSTEAP, respectively. TRAMP mice received an additional dose of either 106 IU mPSCA-VRP or mSTEAP-VRP, as appropriate, at day 60. As control groups, TRAMP mice were vaccinated with empty pcDNA3 plasmid and boosted with 106 IU GFP-VRP. Survival was followed until the defined endpoint which was the development of a palpable tumor, in accordance with our IACUC guidelines.
Isolation of tumor infiltrating lymphocytes (TIL) and flow cytometric analysis
TIL were isolated from individual prostate tumors as previously described [21]. TIL were analyzed by flow cytometry and the total number of cells per gram of tumor calculated as previously described [6].
Measurement of suppression of T cell proliferation by regulatory T cells
A single cell suspension of lymphocytes isolated from the tumor-draining lymph nodes of TRAMP mice were purified into CD4+CD25− (responder) and CD4+CD25+ (suppressor) populations by magnetic separation using a CD4+CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions, except that only half the recommended amount of anti-CD25-PE was used. 5×104 CD4+CD25+ T cells were cocultured for 72 hours with 5×104 allogenic T cell-depleted splenocytes from C3H mice in complete RPMI supplemented with 1 ug/ml activating anti-mouse CD3 antibody, either alone or with autologous CD4+CD25− responder T cells in 1:2, 1:4, 1:8 and 1:16 ratios. As positive controls for maximal proliferation, 5×104 CD4+CD25− responder T cells were cocultured with 5×104 allogenic T cell-depleted splenocytes from C3H mice in complete RPMI supplemented with 1 ug/ml activating anti-mouse CD3 antibody. 3H-thymidine (1 μg/well) was added in the last 8 hours of culture. Responder cell proliferation was measured by 3H-thymidine incorporation using a TopCount NXT microplate scintillation counter (Perkin Elmer, Shelton, CT). The relative proliferation index of responder cells for each mouse at each Treg:Tresponder ratio was calculated by dividing the mean Tresponder proliferation at each ratio by the maximal proliferation (Tresponders cultured in the absence of Treg) of Tresponders in that animal.
Quantification of intratumoral cytokine levels
Prostate tumors were harvested from TRAMP mice, weighed and homogenized at 4°C in 10 μl/mg sterile PBS containing 1x Halt Protease Inhibitor Cocktail (Pierce, Rockford, IL) using a PolyTron PT2100 homogenizer (Kinematica AG, Switzerland). Cytokine levels were quantified with a custom 32-plex Milliplex MAP mouse cytokine immunoassay (Millipore, Billerica MA) using the Bio-Plex multiplex system (Bio-Rad, Hercules, CA) following the manufacturer’s instructions.
Quantitative Real-Time Polymerase Chain Reaction
Prostate tumors were harvested from TRAMP mice, weighed and immediately fixed in RNAlater. After 24 hours, fixed tumors were homogenized at 4°C in 10 μl/mg buffer RLT with β-mercaptoethanol using a PolyTron PT2100 homogenizer (Kinematica AG, Switzerland). Total RNA was isolated using a QIAGEN RNeasy kit according to the manufacturer’s instructions. DNase-treated RNA was reverse transcribed with oligo (dT) and SuperScript III (Invitrogen Life Technologies). Quantitative PCR was performed using Universal PCR Master Mix containing SYBR, following the Applied Biosystems protocol. Primers for GAPDH, TGFβ, indoleamine-2,3-dioxygenase and Foxp3 were obtained (USC DNA Core Facility, Los Angeles, CA). Quantitative PCRs were performed using a PRISM 7700 instrument from Applied Biosystems. The relative level of mRNA expression for each gene in each tumor was first normalized to the expression of GAPDH RNA in that tumor. The mean relative expression of each mRNA in the tumors of young mice was arbitrarily set as 1.
Statistical analysis
Quantitative real-time PCR, flow cytometry, suppression of T cell proliferation and multiplex immunoassay were analyzed by a two tailed, paired Student’s t test. Survival rates were analyzed by the log rank test for survival. Delta survival was calculated by subtracting the cumulative percentage survival of the relevant negative control mouse group from that of the age-matched vaccinated mouse group at each time point at which at least one animal died in either group. Changes in delta survival over time were analyzed by linear regression. Slopes of linear regressions were compared by F-test.
RESULTS
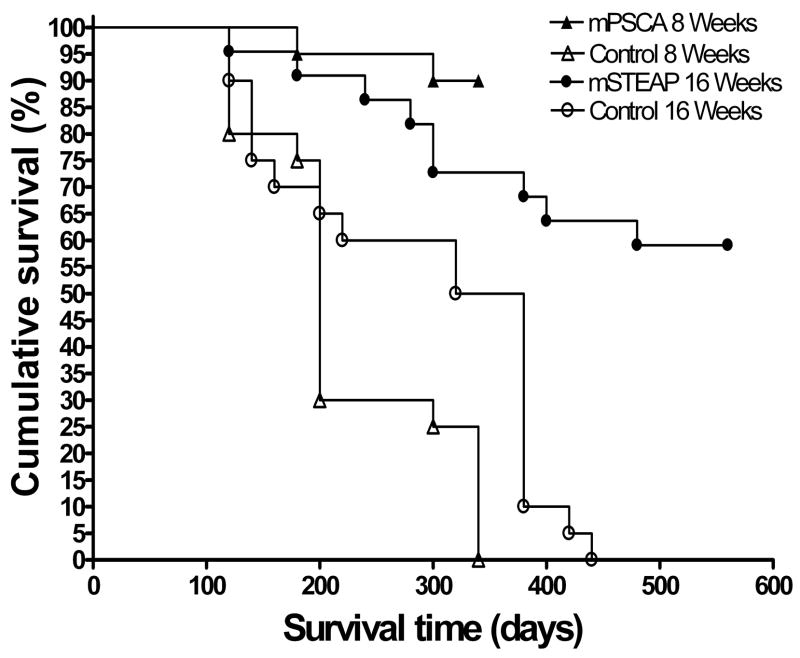
To evaluate whether the stage of prostate cancer progression at which therapeutic vaccination is applied affects the efficacy of the vaccine, we compared the long-term survival rates of TRAMP mice that were vaccinated at two distinct stages of prostate cancer carcinogenesis. TRAMP mice were vaccinated at 8 weeks of age, at which point they have developed precancerous prostate intraepithelial neoplastic (PIN) lesions, or at 16 weeks of age when prostate neuroendocrine carcinomas or adenocarcinomas have developed. The groups of mice were vaccinated using an identical heterologous DNA prime/VRP boost scheme, though the antigens targeted were different (mPSCA at 8 weeks and mSTEAP at 16 weeks). However, we have previously demonstrated that vaccination against both mPSCA [20] and mSTEAP [6] using this immunization scheme induce strong and comparable immune responses in mice. Survival in mice vaccinated at 8 weeks and at 16 weeks was statistically significantly improved compared to age matched controls (p < 0.0001 and p = 0.0001, respectively), indicating that vaccination at both time points yielded excellent protection from prostate cancer development (Figure 1a). In the case of TRAMP mice vaccinated at 8 weeks, there was a dramatic difference in tumor burden between mPSCA-vaccinated mice and negative controls euthanized at 340 days (Figure 1b).
Figure 1.
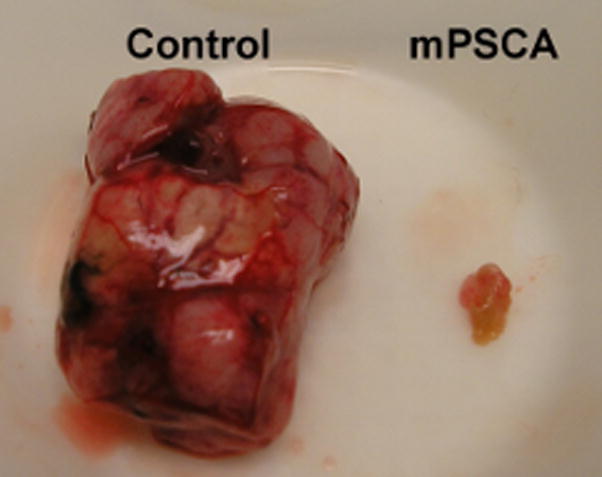
Figure 1a. Therapeutic vaccination against prostate tumor-associated antigens results in long term survival in TRAMP mice. Groups of 20 male 8 week old TRAMP mice were vaccinated by helium-driven gene gun at day 0 with either 2 ug mPSCA-pcDNA or 2 ug empty vector and boosted at days 15 and 60 with 106 IU mPSCA-VRP and 106 IU GFP-VRP, respectively. Groups of 20 male 16 week old mice were vaccinated by helium-driven gene gun with either 2 ug mSTEAP-pcDNA or 2 ug empty vector and boosted at days 15 and 60 with 106 IU mSTEAP-VRP and 106 IU GFP-VRP, respectively.
Figure 1b. Therapeutic vaccination against mPSCA results in reduced prostate tumor burden in TRAMP mice. All surviving mPSCA-vaccinated TRAMP mice and their age-matched controls were euthanized at day 340 and necropsy performed. Representative images of a prostate tumor isolated from a negative control animal (left, “Control”) and that of an mPSCA-vaccinated mouse (right, “mPSCA”) are shown.
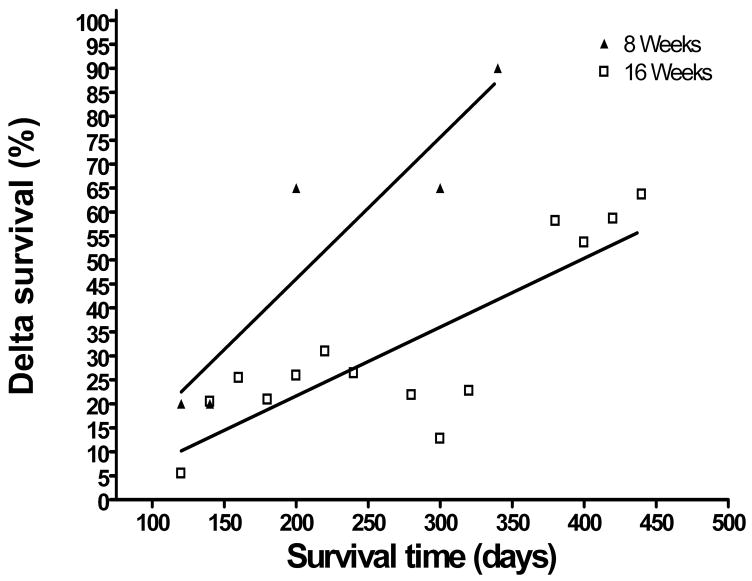
Figure 1c. Therapeutic vaccination against prostate tumor-associated antigens results in superior survival when administered to TRAMP mice with PIN lesions. The difference between the cumulative survival of each group of vaccinated mice and the cumulative survival of their age-matched controls was calculated at each time point at which one or more mice died. This value was termed “delta survival” and was plotted against survival time. Linear regression analysis was performed on each data set, and the slopes of the regression lines compared.
The survival of mice vaccinated at 8 weeks and at 16 weeks could not be directly compared because the mice vaccinated at 16 weeks were castrated prior to immunization. The effect of androgen ablation on long-term TRAMP mouse survival is seen in the statistically significant difference in survival between the non-castrated 8 week negative control group and the castrated 16 week negative control group (p = 0.0074). Both of these groups were immunized with an empty DNA vector and boosted with a GFP-VRP, so the difference in survival between the two groups is due to castration in the 16 week group. We have demonstrated in a previous study that castration only affects the results of prostate cancer immunotherapy when it is carried out three weeks after vaccination [22]. Therefore, the effect of castration on survival in TRAMP mice that were vaccinated at 16 weeks is most likely due to the retardation of androgen-dependent prostate tumor growth rather than any immunological effect. To remove this confounding factor from our analysis, we calculated the difference between the cumulative survival of the vaccinated groups from the cumulative survival of their age- and castration-matched control groups (which we termed “delta survival”) at each time point. Scatter plots of delta survival versus survival time were plotted, and linear regression analysis performed (Figure 1c). There was a very strong correlation between delta survival and survival time in groups of mice vaccinated at 8 weeks (R2 = 0.856) and a weaker correlation at 16 weeks (R2 = 0.695). This indicated that, as expected, the difference in survival probability between vaccinated mice and negative controls steadily increases over time. To determine whether the improvement in survival in mice vaccinated at 8 weeks was better than that of mice vaccinated at 16 weeks, the slopes of the regressions were compared via F-test. The linear regression slope of the 8 week vaccination groups was statistically significantly higher than that of the 16 week groups (F = 5.398, p = 0.0346). This demonstrates that the survival of the group vaccinated at 8 weeks improved statistically significantly more than did the survival of the group vaccinated at 16 weeks when each was compared to their age- and castration-matched control groups.
In an effort to understand the improvement of survival in mice vaccinated earlier in carcinogenesis, we hypothesized that one or more prostate tumor-mediated immunosuppressive mechanisms become established at later stages of carcinogenesis which are responsible for the reduction in therapeutic vaccine efficacy at later stages of disease. Several immunosuppressive mechanisms have been identified as having a possible role in prostate cancer. Given the significantly worse response to therapeutic vaccination at later stages of prostate cancer progression, we investigated whether any of these mechanisms either become active or are more prevalent at later stages of disease.
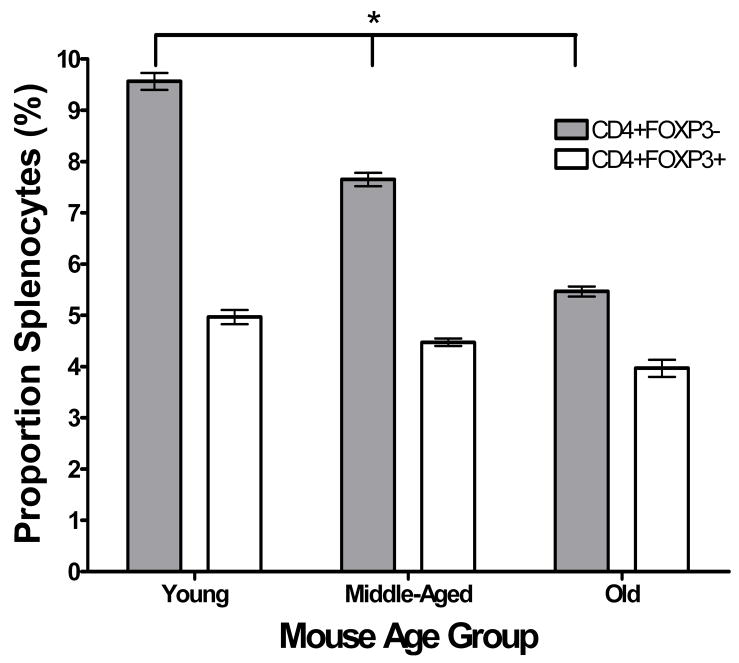
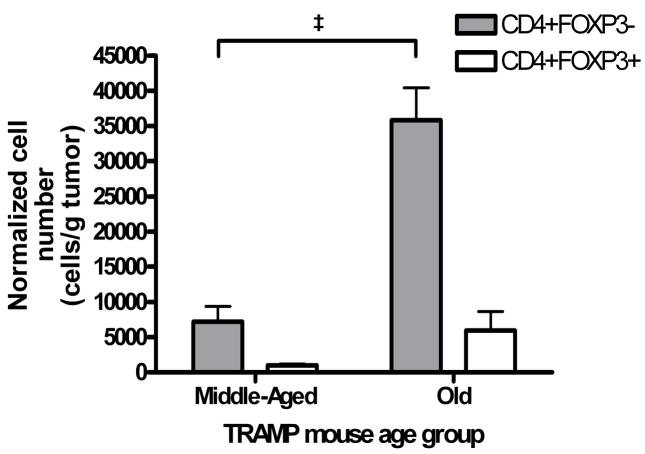
We first assessed whether regulatory T cells (Treg) are more prevalent in either the periphery or prostate tumors of TRAMP mice with more advanced disease. Splenocytes and tumor infiltrating lymphocytes (TIL) were isolated from young (≤ 8 weeks old, n = 3), middle-aged (16–20 weeks old, n = 4) and old (≥ 24 weeks old, n = 3) TRAMP mice and analyzed by flow cytometry. The mean percentage of splenocytes that were CD3+CD4+FOXP3−non-regulatory T cells in young TRAMP mice (9.57 ± 0.28%) was statistically significantly higher than in middle-aged (7.65 ± 0.28%, p < 0.01) and in old (5.47 ± 0.26%, p < 0.01) TRAMP mice (Figure 2a). In addition, the mean percentage of splenic CD3+CD4+FOXP3− T cells was statistically significantly decreased in old compared to middle-aged TRAMP mice (p < 0.01) (Figure 2a). The mean percentage of splenocytes that were CD3+CD4+FOXP3+ Tregs also decreased with age, but not significantly. As a result, the ratio of mean CD3+CD4+FOXP3+ splenocyte percentage to mean CD3+CD4+ splenocyte percentage in TRAMP mice statistically significantly increased with age (p = 0.021, ANOVA), indicating an increased accumulation of Treg in the spleens of TRAMP mice the course of prostate cancer progression. Flow cytometric analysis of tumor infiltrating lymphocytes revealed a statistically significant increase in the mean number of tumor infiltrating CD3+CD4+ FOXP3− cells in old (35844 cells/g tumor) compared to middle-aged (7182 cells/g tumor) TRAMP mice (Figure 2b, p = 0.001). In addition, the mean number of tumor infiltrating CD3+CD4+FOXP3+ Treg cells in old (5962 cells/g tumor) increased compared to middle-aged (1008 cells/g tumor) TRAMP mice (Figure 2b). In contrast to the situation in the periphery, the proportion of tumor infiltrating CD3+CD4+ FOXP3− cells that were CD3+CD4+FOXP3+ Treg cells remained constant (15.6% versus 15.4% in middle-aged and old mice, respectively). However, the absolute number of Treg per gram of tumor increased over the course of disease progression. No data were available from young TRAMP mice because insufficient TIL for flow cytometry could be isolated from the prostates of these animals.
Figure 2.
Figure 2a. Relatively increased numbers of Tregs in periphery of TRAMP mice with increasing age. Splenocytes isolated from groups of young (≤ 8 weeks old, n = 3), middle-aged (16–20 weeks old, n = 4) and old (≥ 24 weeks old, n = 3) TRAMP mice were washed and stained with anti-mouse CD3-FITC and anti-mouse CD4-PE/Cy7. Cells were fixed and permeablised overnight, then stained with anti-mouse FOXP3-PE and analysed by flow cytometry. Events were collected gated on live, CD3+ cells and then further gated on the CD4+FOXP3− fraction and the CD4+FOXP3+ fraction. The * symbol indicates p < 0.05.
Figure 2b. Increased infiltration of CD4+ T cells and Treg into the prostate tumor with increasing age. Tumor infiltrating lymphocytes isolated from groups of young ( 8 weeks old, n = 3), middle-aged (16–20 weeks old, n = 4) and old ( 24 weeks old, n = 3) TRAMP mice were washed and stained with anti-mouse CD3-FITC, anti-mouse CD8-PE/Cy5 and anti-mouse CD4-PE/Cy7. Cells were fixed and permeablised overnight, then stained with anti-mouse FOXP3-PE and analysed by flow cytometry. Events were collected gated on live, CD3+CD8− cells and then further gated on the total CD4+FOXP3− fraction and the CD4+FOXP3+ fraction. The‡ symbol indicates p < 0.001.
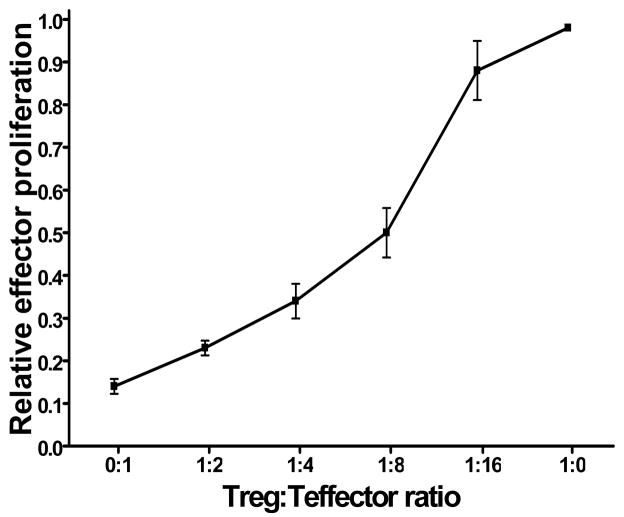
To assess the suppressive capacity of Tregs in early prostate tumor development, lymphocytes were isolated from the prostate tumor draining lymph nodes of young TRAMP mice and divided into CD4+CD25− effector and CD4+CD25+ regulatory T cell populations. The proliferation of the CD4+CD25− effector steadily decreased when cocultured with increasing numbers of CD4+CD25+ regulatory T cells, indicating that this population is functionally suppressive (Figure 3). Taken together, these data indicate an increase in functionally suppressive Treg during prostate cancer progression, which may interfere with the efficacy of therapeutic vaccination.
Figure 3. CD4+CD25+ T cells isolated from prostate tumor draining lymph nodes are functionally suppressive.
T cells from tumor-draining lymph nodes isolated from young (≤ 8 weeks old, n = 3) TRAMP mice were purified into CD4+CD25− (responder) and CD4+CD25+ populations by magnetic separation. 5×104 CD4+CD25+ T cells were cocultured for 72 hours with 5×104 allogenic T cell-depleted splenocytes from C3H mice in complete RPMI supplemented with 1 ug/ml activating anti-mouse CD3 antibody, either alone or with autologous CD4+CD25– responder T cells in 1:2, 1:4, 1:8 and 1:16 ratios. As positive controls for maximal proliferation, 5×104 CD4+CD25− responder T cells were cocultured with 5×104 allogenic T cell-depleted splenocytes from C3H mice in complete RPMI supplemented with 1 ug/ml activating anti-mouse CD3 antibody. 3H-thymidine (1 μg/well) was added in the last 8 hours of culture. Responder cell proliferation was measured by 3H-thymidine incorporation. The relative proliferation index of responder cells for each mouse at each Treg:Tresponder ratio was calculated by dividing the mean Tresponder proliferation at each ratio by the maximal proliferation (Tresponders cultured in the absence of Treg) of Tresponders in that animal.
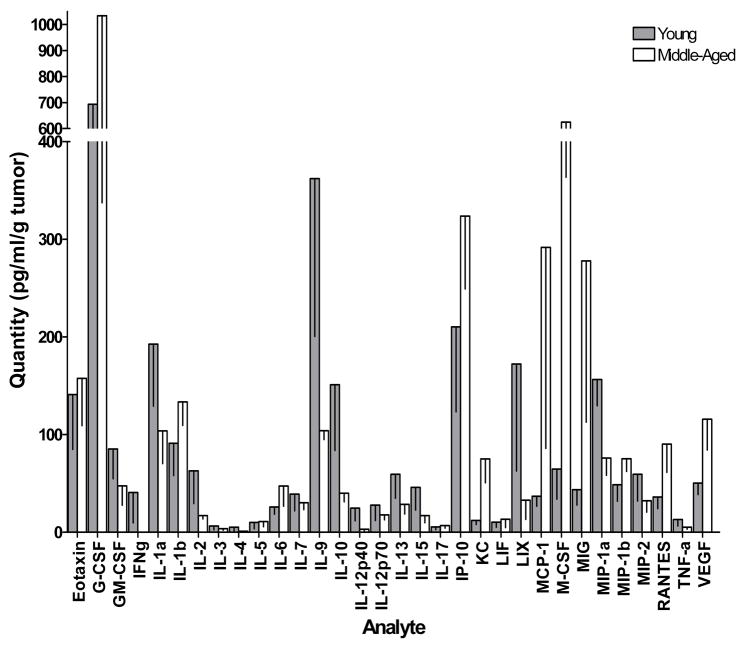
To determine whether the cytokine profile of the tumor microenvironment becomes immunosuppressive or inhibitory over the course of prostate cancer development, expression of a panel of cytokines and chemokines was measured by multiplex immunoassay (Figure 4). The expression of several cytokines and chemokines, including IL-1a, IL-2, IL-4, IL-5, IL-9, IL-10, IL-12(p40 and p70), IL-13, IL-15, IFNγ, G-CSF, GM-CSF, LIX, MIP-1a, MIP-2 and TNFα was reduced in the prostate tumors of middle-aged TRAMP mice compared to the prostates of young TRAMP mice. Conversely, there were increases in the expression of M-CSF, MIG, RANTES and VEGF. In the case of mean MIP-1a expression, the reduction was statistically significant (p = 0.023). In contrast, mean KC and MCP1 expression was statistically significantly increased in middle aged mice compared to young mice (p = 0.027 and p = 0.028, respectively). Overall, there was a trend towards a reduction in both Th1 and Th2 function in middle-aged mice compared to young mice, while expression of angiogenic factors (KC and RANTES) increased with age.
Figure 4. The cytokine/chemokine expression profile of spontaneous TRAMP prostate tumors changes over time.
Spontaneously arising prostate tumors harvested from groups of young (≤ 8 weeks old, n = 5) and old (≥ 24 weeks old, n = 4) were homogenized at 4°C in 10 μl/mg sterile PBS containing 1x protease inhibitors. Cytokine levels were quantified with a custom 32-plex Milliplex MAP mouse cytokine immunoassay (Millipore, Billerica MA) using the Bio-Plex multiplex system (Bio-Rad, Hercules, CA). The * symbol indicates p < 0.05.
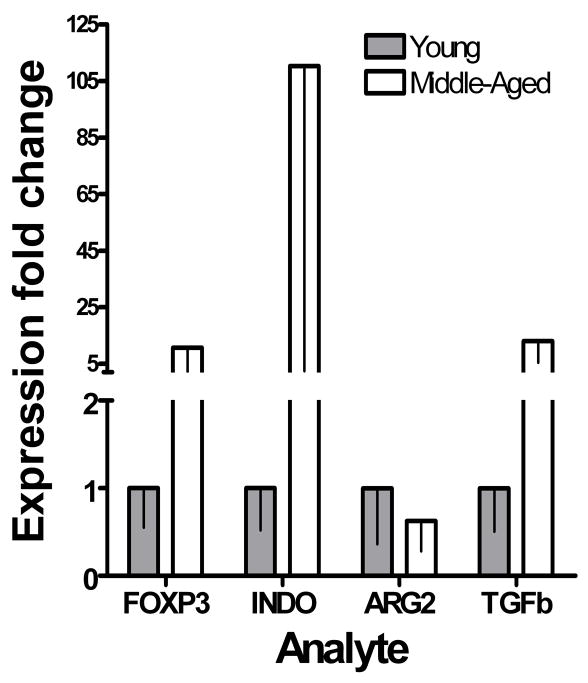
Expression of several immunosuppressive factors, including TGFβ and indoleamine 2,3-dioxygenase has been shown to be increased in multiple tumor types. To assess whether their expression increases with prostate tumor progression, we quantified their expression in the prostate tumors of TRAMP mice by quantitative real-time PCR. Relative expression of both TGFβ and indoleamine 2,3-dioxygenase mRNA normalized to GAPDH were increased in middle-aged mice compared to young mice (Figure 5). The relative expression of FOXP3 mRNA normalized to GAPDH was also increased in the prostates of middle-aged mice compared to young mice, as was expected given the increase in the number of FOXP3-expressing Treg per gram of prostate tumor with increasing age (Figure 2b). Taken together, these data suggest a reduction in pro-inflammatory cytokines combined with an increase in the expression of immunosuppressive factors during disease progression may lead to an inhibition of prostate cancer-specific immunity and reduced efficacy of vaccination in the later stages of prostate cancer. These data support our hypothesis that immunosuppressive mechanisms become active over the course of prostate cancer progression, and that immunization at early stages of disease may yield superior vaccine efficacy by avoiding their effects.
Figure 5. Increased expression of immunosuppressive molecules in prostate cancer.
Spontaneously arising prostate tumors harvested from groups of young (≤ 8 weeks old, n = 3) and old (≥ 24 weeks old, n = 3) mice, fixed in RNAlater and were homogenized at 4°C in 10 μl/mg buffer RLT with β-mercaptoethanol. Total RNA was isolated using a QIAGEN RNeasy kit according to the manufacturer’s instructions. Complementary DNA was generated using this RNA as a template, then quantitative real-time PCR performed.
DISCUSSION
Here we demonstrate that a heterologous DNA prime/VRP boost prostate cancer therapeutic vaccination strategy yields superior long term protection when it is applied at a precancerous stage of disease compared to when it is administered after prostate cancer has developed in the TRAMP mouse model of human prostate cancer. In addition, we have identified several immunosuppressive mechanisms that may be involved in the differential efficacy of a therapeutic vaccine at different stages of prostate carcinogenesis in TRAMP mice.
A wide variety of immunotherapeutic strategies for treating prostate cancer have undergone clinical trials, but none have yet been FDA approved for use in patients. A fundamental problem with these clinical trials is that they have been carried out almost exclusively in terminally ill patients who have failed all other therapeutic options. These patients are frequently immunocompromised, and are therefore poor candidates in whom to test immunotherapies. With this in mind, we have proposed that therapeutic prostate cancer vaccines should be applied in the preventive setting [10]. Prior to seeking approval for clinical trials that involve immunizing early-stage prostate cancer patients, it is vital to demonstrate that application of a therapeutic cancer vaccine at the early stages of disease is likely to yield superior results than when it is administered in the advanced stages of disease. Therefore, we decided to evaluate whether therapeutic vaccination confers superior protection in the TRAMP mouse model of human prostate cancer when it is applied at a precancerous stage of cancer development (PIN lesions at 8 weeks) compared to when the mice have more advanced disease (adenocarcinomas or neuroendocrine tumors at 16 weeks) [23]. To evaluate whether the stage of prostate cancer progression at which therapeutic vaccination is applied affects the efficacy of the vaccine, we compared the long-term survival rates of TRAMP mice that were vaccinated at 16 weeks of age to the survival rates of TRAMP mice that had been vaccinated at 8 weeks of age as part of a previous study [20].
The two long-term survival studies evaluated here were not originally designed to be compared to each other. Most notably, the antigens targeted were different (mPSCA at 8 weeks and mSTEAP at 16 weeks). However, we have previously demonstrated that vaccination elicits strong and comparable immune responses against both mPSCA [20] and mSTEAP [6] using this immunization scheme. Here we show that vaccination of TRAMP mice against either mPSCA and mSTEAP yields very strongly statistically significant improvements in survival compared to age-matched negative controls (Figure 1a). Another difference in the design of the two studies is that the mice vaccinated at 16 weeks were castrated prior to vaccination. In the time since this study was initiated, we have demonstrated that castration only elicits an immunological effect on prostate cancer vaccine efficacy when it is carried out three weeks after vaccination [22]. Therefore, we concluded that the apparent improvement in long-term TRAMP mouse survival due to castration (Figure 1a, compare 8 weeks control curve to 16 weeks control curve) is a direct result of androgen ablation on prostate tumor growth rather than an immunological effect. In order to analyze the effect on survival of vaccination at 8 weeks and at 16 weeks without the confounding factor of castration, the difference between the survival of the vaccinated groups and their age- and castration-matched control groups (which we termed delta survival) was calculated for each time point. Linear regression analysis revealed that the improvement in survival of mice vaccinated at 8 weeks compared to their age-matched controls was statistically significantly better than that of mice vaccinated at 16 weeks compared to their age- and castration-matched controls (Figure 1c). It should also be noted that of the mice vaccinated at 8 weeks with mPSCA, 90% had not yet reached the survival endpoint at 340 days post-vaccination compared to 0% in age-matched controls. Unfortunately, despite not reaching their survival endpoint the mPSCA vaccinated mice were euthanized at 340 days, according to the study design that was then being followed. At the time of their euthanasia, the mPSCA-vaccinated mice were in outstanding condition. They looked outwardly healthy, were eating and behaving exactly as would be expected of one year old wild type C57BL/6 mice and showed no signs of pain or distress. Upon necropsy, the prostates of these animals were outwardly normal. This was in stark contrast with the few animals of the control group that reached the survival endpoint on the same day and were euthanized. These mice were weak, obviously unhealthy and had very large, palpable tumors compared to the mPSCA vaccinated mice. Figure 1b shows the dramatic difference in tumor burden in mPSCA-vaccinated TRAMP mice and controls euthanized at 340 days. Overall, we conclude that therapeutic vaccination against prostate cancer TAAs confers superior survival benefits if it is applied when PIN lesions are present but invasive adenocarcinomas or neuroendocrine tumors have not yet developed.
In an effort to explain the difference in survival between TRAMP mice vaccinated at different stages of disease, we investigated whether several different tumor-mediated immunosuppressive mechanisms become present or are more active at later points of prostate cancer development. There are conflicting data regarding the role of CD4+CD25+FOXP3+ regulatory T cells in prostate cancer. It has been concluded that these cells are more prevalent in the blood of prostate cancer patients [24] and more active [25], while a conflicting study asserted that peripheral tolerance in prostate cancer was not mediated by Treg [26]. Consistent with the first two of these studies, our data indicate a relative increase in the numbers of Tregs in periphery of TRAMP mice with increasing age (Figure 2a). The percentage of splenic CD4+FOXP3− T cells decreased with increasing TRAMP mouse age, but the percentage of splenic CD4+FOXP3+ Tregs stays relatively constant, suggesting progressively increasing systemic immunosuppression. We next assessed whether Treg also accumulate within the prostate tumors of TRAMP mice. We found that though the number of Tregs per gram of prostate tumor did indeed increase over time, it was part of a general accumulation of CD4+ T cells within the prostate tumor as it grew (Figure 2b). The constant presence of Treg within the tumor infiltrating lymphocyte population coupled with the fact that the prostate tumors of unvaccinated TRAMP mice will grow rapidly and inevitably kill the animal suggests that despite increased immune infiltration of the prostate, the constant presence of Treg impedes the ability of the attempted immune response to control tumor growth. What is not clear from these data is whether the Treg that infiltrate the tumor do so as part of a general accumulation of lymphocytes within the tumor, whether they are attracted there specifically and independently of the mechanism attracting effector T cells, or whether they are induced in situ from tumor infiltrating effector T cells. Resolving this question will be crucial in the development of mechanisms to prevent Treg infiltration to or induction within the prostate tumor, and is an area of active research for our group.
Though Treg are capable of infiltrating prostate tumors, it was unknown whether they were functionally active. Given the difficulty in isolating sufficient numbers of live Treg from prostate tumors, we investigated the suppressive capacities of CD4+CD25+ Treg isolated from the tumor-draining lymph nodes of TRAMP mice (Figure 3). The data show that there are functionally suppressive Treg in the draining lymph nodes of prostates of young mice.
If the microenvironment within prostate tumors becomes increasingly immunosuppressive as they advance, it is expected that this would be reflected in the intratumoral cytokine milieu. To investigate this, we quantified the expression of a panel of cytokines and chemokines within TRAMP prostate tumors by multiplex immunoassay (Figure 4). Our data indicate a general reduction of Th1 and Th2 type cytokines in more advanced tumors, suggesting that the tumor microenvironment is indeed more immunosuppressive in these tumors. There were decreases in the expression of G-CSF, GM-CSF, IFNγ, IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15 and TNFα in the prostate/prostate tumor tissues of young mice versus those of old mice. Conversely, there were increases in the expression of M-CSF, MIG, RANTES and VEGF. Though these differences were not statistically significant, they were large and indicated a trend towards differential expression of these cytokines and chemokines at different stages of prostate cancer development. Of particular interest was the somewhat counterintuitive reduction in IL-10 expression in more advanced tumors. Though it is generally considered and anti-inflammatory cytokine, several studies have indicated that IL-10 has strong anti-tumor effects [27]. Thus, its downregulation in more advanced tumors is not unexpected. Interestingly, there was a statistically significant decrease in the expression of macrophage inflammatory protein-1α (MIP-1α) but not MIP-1β. A previous study has demonstrated that MIP-1α stimulated the release of IL-1 and TNFα by the peripheral blood monocytes of women with breast cancer but not those of healthy women [28]. In contrast, the same study showed that MIP-1β could stimulate release of these cytokines only healthy women and not in breast cancer patients. These findings are consistent with our own results, and suggest that downregulation of MIP-1α that we observed is a mechanism of prostate tumor immune escape that may be responsible for the decreases in IL-1a and TNFα that occur in more advanced prostate tumors. A statistically significant increase in the expression of monocyte chemoattractant protein 1 (MCP1) was observed in more advanced prostate tumors, which given that this chemokine normally drives Th2 responses was initially puzzling [29]. However, it has been shown that vaccine-induced eradication of r-p185 carcinoma was dramatically increased in MCP1 knockout mice [30]. This result would be consistent with our own, and suggests an important role for this protein in tumor progression. Finally, we observed a statistically significant increase in keratinocyte-derived cytokine (KC, CXCL1). This cytokine is driven by prostaglandin E2 and promotes angiogenesis [31]. The upregulation of KC coupled with that of VEGF may result in an increase in prostate tumor vasculature, which might explain the observed general increase in tumor infiltrating lymphocytes in mice with more advanced disease.
Finally, we sought to determine whether other immunosuppressive mechanisms thought to be involved in tumor immune evasion are upregulated in more advanced prostate tumors. Quantitative real-time PCR data revealed increases in TGFβ and indoleamine-2,3-dioxygenase in the prostate tumors of older TRAMP mice. The increase in TGFβ suggests that the increase in the numbers of regulatory T cells observed within advanced prostate tumors is at least partially due to in situ Treg induction, but this requires further study. The increase in indoleamine-2,3-dioxygenase indicates that advanced prostate tumors may be directly capable of downregulating the activity of tumor infiltrating lymphocytes. In addition, indoleamine-2,3-dioxygenase has a role in the generation of inducible Treg [32, 33] and in preventing their further conversion in to proinflammatory Th17 cells [34]. These data suggest that increased expression of immunosuppressive molecules involved in the induction and maintenance of inducible Treg is an important event in the establishment of an immunosuppressive prostate tumor microenvironment in TRAMP mice. It is not clear whether the tumor cells upregulate expression of these molecules themselves, or whether they are produced by infiltrating immune cells. We are currently conducting studies to establish this. We saw no change in arginase 2 expression in the prostate tumors of older compared to younger mice, suggesting that the loss of vaccine efficacy at this stage of prostate tumor development is not a result of loss of T cell function due to decreased availability of arginine.
Overall, our data indicate that therapeutic vaccination against prostate TAA confers superior protection on TRAMP mice in terms of survival when they are vaccinated at the earliest possible stage of prostate cancer development. In addition, we have identified for future study multiple prostate tumor mediated immunosuppressive mechanisms that may be responsible for the reduction in efficacy of therapeutic prostate cancer immunotherapy at the later stages of disease progression, the neutralization of which would assist in the successful use of therapeutic vaccination at those later stages.
Acknowledgments
This work was supported by DOD grant DAMD 17-02-1-0244, NIH training grant T32 GM 067587 (A.G.), DOD PCRP Prostate Cancer Training Award DAMD PC073417 (A.G.), DOD PRCP Postdoctoral Traineeship Award DAMD PC041078 (M.G.H.) and the Margaret E. Early Medical Research Trust.
S.K. was supported by a post-doctoral fellowship by the ARCS Foundation.
W.M.K. holds the Walter A. Richter Cancer Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002 Jan-Feb;52(1):23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996 Jun;14(6):1756–64. doi: 10.1200/JCO.1996.14.6.1756. [DOI] [PubMed] [Google Scholar]
- 3.Hillman GG, Triest JA, Cher ML, Kocheril SV, Talati BR. Prospects of immunotherapy for the treatment of prostate carcinoma--a review. Cancer Detect Prev. 1999;23(4):333–42. doi: 10.1046/j.1525-1500.1999.99027.x. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ED, Rosenblum M, Ziada AM, Lange PH. Hormone refractory prostate cancer. Urology. 1999 Dec;54(6A Suppl):1–7. doi: 10.1016/s0090-4295(99)00447-1. [DOI] [PubMed] [Google Scholar]
- 5.Noguchi M, Kobayashi K, Suetsugu N, Tomiyasu K, Suekane S, Yamada A, et al. Induction of cellular and humoral immune responses to tumor cells and peptides in HLA-A24 positive hormone-refractory prostate cancer patients by peptide vaccination. Prostate. 2003 Sep 15;57(1):80–92. doi: 10.1002/pros.10276. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Hernandez Mde L, Gray A, Hubby B, Kast WM. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res. 2007 Feb 1;67(3):1344–51. doi: 10.1158/0008-5472.CAN-06-2996. [DOI] [PubMed] [Google Scholar]
- 7.Ross S, Spencer SD, Holcomb I, Tan C, Hongo J, Devaux B, et al. Prostate stem cell antigen as therapy target: tissue expression and in vivo efficacy of an immunoconjugate. Cancer Res. 2002 May 1;62(9):2546–53. [PubMed] [Google Scholar]
- 8.Zhu ZY, Zhong CP, Xu WF, Lin GM, Ye GQ, Ji YY, et al. PSMA mimotope isolated from phage displayed peptide library can induce PSMA specific immune response. Cell Res. 1999 Dec;9(4):271–80. doi: 10.1038/sj.cr.7290026. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Harada M, Yano H, Ogasawara S, Takedatsu H, Arima Y, et al. Prostatic acid phosphatase as a target molecule in specific immunotherapy for patients with nonprostate adenocarcinoma. J Immunother. 2005 Nov-Dec;28(6):535–41. doi: 10.1097/01.cji.0000175490.26937.22. [DOI] [PubMed] [Google Scholar]
- 10.Gray A, Raff AB, Chiriva-Internati M, Chen SY, Kast WM. A paradigm shift in therapeutic vaccination of cancer patients: the need to apply therapeutic vaccination strategies in the preventive setting. Immunological reviews. 2008 Apr;222:316–27. doi: 10.1111/j.1600-065X.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 11.Curiel TJ. Regulatory T cells and treatment of cancer. Current opinion in immunology. 2008 Apr;20(2):241–6. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews. 2009 Mar;9(3):162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunological reviews. 2008 Apr;222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 14.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nature reviews. 2005 Aug;5(8):641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 15.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007 Sep 15;13(18 Pt 1):5262–70. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 16.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunological reviews. 2006 Aug;212:163–9. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 1998 Feb 17;95(4):1735–40. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001 Aug 1;61(15):5857–60. [PubMed] [Google Scholar]
- 20.Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ, Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer research. 2008 Feb 1;68(3):861–9. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Hernandez ML, Hernandez-Pando R, Gariglio P, Berumen J. Interleukin-10 promotes B16-melanoma growth by inhibition of macrophage functions and induction of tumour and vascular cell proliferation. Immunology. 2002 Feb;105(2):231–43. doi: 10.1046/j.1365-2567.2002.01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009 May 1;69(6):571–84. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997 Nov 1;57(21):4687–91. [PubMed] [Google Scholar]
- 24.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006 Nov 15;177(10):7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 25.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008 Feb 15;14(4):1032–40. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 26.Degl’Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, et al. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer research. 2008 Jan 1;68(1):292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 27.Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends in immunology. 2003 Jan;24(1):36–43. doi: 10.1016/s1471-4906(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 28.Nath A, Chattopadhya S, Chattopadhyay U, Sharma NK. Macrophage inflammatory protein (MIP)1alpha and MIP1beta differentially regulate release of inflammatory cytokines and generation of tumoricidal monocytes in malignancy. Cancer Immunol Immunother. 2006 Dec;55(12):1534–41. doi: 10.1007/s00262-006-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsukawa A, Lukacs NW, Standiford TJ, Chensue SW, Kunkel SL. Adenoviral-mediated overexpression of monocyte chemoattractant protein-1 differentially alters the development of Th1 and Th2 type responses in vivo. J Immunol. 2000 Feb 15;164(4):1699–704. doi: 10.4049/jimmunol.164.4.1699. [DOI] [PubMed] [Google Scholar]
- 30.Curcio C, Di Carlo E, Clynes R, Smyth MJ, Boggio K, Quaglino E, et al. Nonredundant roles of antibody, cytokines, and perforin in the eradication of established Her-2/neu carcinomas. The Journal of clinical investigation. 2003 Apr;111(8):1161–70. doi: 10.1172/JCI17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Wang H, Brown J, Daikoku T, Ning W, Shi Q, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. The Journal of experimental medicine. 2006 Apr 17;203(4):941–51. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008 Oct 15;181(8):5396–404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009 May 22; doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009 Jun 11;113(24):6102–11. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]