Abstract
Due to recent progress in analytical techniques, metallomics are evolving from detecting distinct trace metals in a defined state to monitoring the dynamic changes in the abundance and location of trace metals in vitro and in vivo. Vascular metallomics is an emerging field that studies the role of trace metals in vasculature. This review will introduce common metallomics techniques including atomic absorption spectrometry, inductively coupled plasma-atomic emission spectrometry, inductively coupled plasma-mass spectrometry and X-ray fluorescence spectrometry with a summary table to compare these techniques. Moreover, we will summarize recent research findings that have applied these techniques to human population studies in cardiovascular diseases, with a particular emphasis on the role of copper in these diseases. In order to address the issue of interdisciplinary studies between metallomics and vascular biology, we will review the progress of efforts to understand the role of copper in neovascularization. This recent progress in the metallomics field may be a powerful tool to elucidating the signaling pathways and specific biological functions of these trace metals. Finally, we summarize the evidence to support the notion that copper is a dynamic signaling molecule. As a future direction, vascular metallomics studies may lead to the identification of targets for diagnosis and therapy in cardiovascular disease.
Keywords: vascular diseases
Introduction
The emerging field of metallomics aims to study the entirety of trace metals in a given material. Basic information about the concepts and recent advances in metallomics is available from several comprehensive reviews [1–3]. With regards to recent progress in this field, the International Symposium on Metallomics was established to bring together scientists from the biological, chemical, environmental, clinical and measurement sciences to create a greater understanding of the role of metals and metal compounds in these systems. In addition, a new international journal named Metallomics has recently been launched, and helps link the many research fields related to biometals.
Because trace metals such as zinc, iron and copper play important roles in cellular and molecular processes in biology [4, 5], the major goal of metallomics in biology and medicine is to facilitate the dissection of the specific biological functions associated with these trace elements. Cardiovascular disease is the leading cause of death in Western countries. Growing evidence shows that trace metals, such as iron [6–8], copper [7] and zinc [9], appear to play an important role in the development of the disease. However, the elucidation of metals signaling pathways and their specific biological functions are still challenges and are driving forces to further extend the domain of metallomics. Thus, a new concept, vascular metallomics, is proposed here to bridge the gap between vascular biology and metallomics. The emerging field of vascular metallomics aims to address the role of trace metals in the vasculature.
Among these trace metals, copper is a redox-active metal and is an essential element that is required for normal cellular function. During the past decade, copper trafficking theory has been established and validated in several laboratories. This theory convincingly defines the regulation of uptake, distribution, sequestration and export of copper. Intracellular copper availability is extraordinarily restricted, as the intracellular milieu has a great capacity to chelate copper [10]. In the cytoplasm copper is distributed between a number of proteins, known as copper chaperones. Copper chaperones compete with chelators and directly insert the copper cofactor into target cuproenzymes, such as cytochrome c oxidase, copper/zinc superoxide dismutase, and ATP7A, thus converting these cuproenzymes from an inactive to an active state [11]. ATP7A has attracted significant attention since its characterization as a copper P-type ATPase and copper egress pump, and the discovery that ATP7A mutation leads to human Menkes disease. We reported that ATP7A is highly expressed in human vasculature [12–14]. These findings will also be discussed in this review.
Common metallomic technologies
The field of metallomics is comprised by a group of powerful quantitative technologies that can be used to determine the availability of metals in the complex biological environment. These technologies include atomic absorption spectrometry (AAS), x-ray fluorescence spectrometry (XRF), inductively coupled plasma-atomic emission spectrometry (ICP-AES) and inductively coupled plasma-mass spectrometry (ICP-MS). A summary of the advantages and disadvantages of each of these techniques is provided in Table 1.
Table 1.
Comparison of common metallomics techniques
| AAS | ICP | XRF | TXRF | |||
|---|---|---|---|---|---|---|
| FAAS | GFAAS | ICP-AES | ICP-MS | |||
| Sample form | solution | solution, slurry and solid | Solution1 | solution1 | solid | liquid |
| Minimum detectable concentration | ppm | ppb | ppb | ppt | ppm | ppb |
| Detection capacities | one metal at a time | one metal at a time | more than one metal at a time | more than one metal at a time | more than one metal at a time | more than one metal at a time |
| Isotope analysis | no | no | no | yes | - | - |
| Metal image | - | - | - | yes | yes | yes |
Note: The ICP-AES and ICP-MS methods are also suitable for analysis of solid materials applying electrothermal vaporisation or laser ablation as sample introduction techniques.
Atomic absorption spectrometry requires the analyte of interest to be in gas phase atoms, and then measures the light that is absorbed by these atoms. There are two main types of atomic absorption instruments: flame-AAS (FAAS) and graphite furnace-AAS (GFAAS). The process of drying, pyrolysis and atomization occur at the same time for FAAS, while those same processes can be separated in time when using GFAAS. This property allows GFAAS to achieve lower detection levels. In addition, FAAS is only capable of analyzing solutions while GFAAS is capable of analyzing solutions, slurries and solids.
In general, AAS is relatively inexpensive, simple to use and has high precision with low interferences. However, AAS can only detect one element at a time and does not allow for isotope analysis. Since the light source is typically a hollow-cathode lamp of the analyte of interest, its major disadvantage is that it is limited to single element detection. In order to improve the detection limits, optimizing techniques such as flow injection [15], preconcentration/matrix separation [16, 17], as well as adding chemical modifiers [18] and increasing the signal to noise ratio may improve the sensitivity of AAS.
Inductively coupled plasma (ICP) is also commonly used for metal determination. This technique can be paired with atomic emission or mass spectrometric detection. ICP-atomic emission spectrometry (ICP-AES) employs an argon plasma to excite atoms and ions of analytes, and then detects the characteristic emission given off by these excited atoms and ions. The emission intensity is proportional to the concentration of the analyte of interest. The fundamental principle of ICP-mass spectrometry (ICP-MS) is similar to that of ICP-AES, in which an argon plasma is used to atomize and ionize the sample, allowing for charged species to enter the mass spectrometer. The mass spectrometer separates the ions based on their mass to charge ratio, which also allows for the specific determination of metals and metal species. Both ICP-AES and ICP-MS are capable of simultaneous detection of more elements, but the minimum detectable concentrations for ICP-MS are lower than those of ICP-AES. ICP-AES can handle a higher dissolved solid sample than ICP-MS, but it does not have the capability for isotope analysis as does ICP-MS. Although ICP requires a higher skill level to run the instrument, it is cost-effective and the detection benefits are well worth the effort.
With regard to the improvement of ICP techniques, a recent metallomics study used size exclusion chromatography in conjunction with ICP-AES to identify metal-containing metalloproteins in whole blood and blood fractions [9]. This study indicates that coupling the separation technique with ICP opens a new door for the identification of metal complexes. In addition, an electrothermal vaporization technique [19] and laser ablation [20] coupled with ICP-MS have been developed to improve the signal to background ratio and sample application, respectively. The latter has been applied to thin slices of brain tissue to determine metal distribution. This technique is now known as elemental imaging [21]. Another hyphenated technique for ICP-MS is the use of high performance liquid chromatography (HPLC) with ICP-MS detection. HPLC is commonly used to separate different metal complexes or metal species in a complex sample matrix for determination using ICP-MS. Rabieh et al. employed the use of ion pair reverse phase HPLC to separate arsenic species in urine before detection with ICP-MS [22].
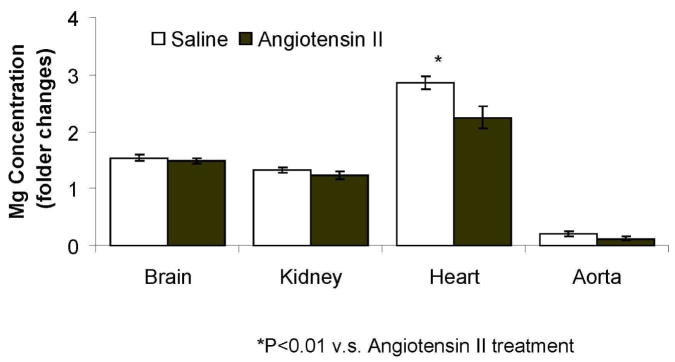
With regard to the application of ICP-MS in vascular metallomics, angiotensin II perfusion in mice has provided a valuable tool to investigate the effect of angiotensin II on the contribution of vascular diseases, particularly hypertension [23, 24]. Recently, we investigated the effect of angiotensin II on magnesium level in brain, heart, aorta and kidney of mice (C57BL/J) using ICP-MS. The experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. After infusion of angiotensin II (1000 ng/kg/min) for 5 days, the level of magnesium was significantly decreased as compared with that infused with saline (Figure 1; n=3 for each group). Although the sample number is limited, the result of this study of mice is consistent with previous findings indicating an association between hypertension and magnesium level [25]. Interestingly, the level of magnesium in aorta was significantly lower than other organs; whereas that in heart is markedly higher than other organs (Figure 1).
Figure 1.
Detection of tissue magnesium level of mice using ICP-MS. ICP-MS used for specific magnesium detection was an Agilent 7500ce by Agilent Technologies (Santa Clara, CA). The instrument was equipped with a microconcentric nebulizer made by Glass Expansion (Pocasset, MA), a Scott double channel spray chamber (2°C), a shielded torch with a sampling depth of 7 mm, nickel sampling and skimmer cones, a CE lens stack, an octopole collision/reaction cell with hydrogen gas (Matheson Gas Products, Parisppany, NJ) pressurization (purity of 99.999%) at a flow rate of 4 ml min−1, a quadrupole mass analyzer with a dwell time of 100 ms per isotope and an electron multiplier for detection. Instrumental parameters were as follows: forward power, 1500 W; plasma gas flow rate, 15.0 L min−1; carrier gas flow rate, 0.99 L min−1; makeup gas flow rate, 0.14 L min−1; monitored isotope, 24Mg. Samples were repeated 3 times and averaged for statistic error. The sample values were normalized to the total protein content.
x-ray fluorescence spectrometry (XRF) utilizes an x-ray beam to excite the electrons of an atom causing the inner shell electrons to be ejected from their shell. Electron excitation leads to vacancies in that shell which are then filled by outer shell electrons. During that process, characteristic x-rays are emitted which generates the measureable fluorescence. Because the x-ray fluorescence spectrum is unique for each element, XRF allows multi-element analysis. In addition, the measurement produces very little damage to the sample. Its minimum detectable concentrations are in the ppm range or lower. Recent developments of high brightness synchrotron sources and advanced x-ray focusing optics had enabled XRF microscopy with spatial resolution of 100–200 nm, allowing elemental imaging with subcellular resolution. In addition, a technique called total reflection x-ray fluorescence spectrometry (TXRF) has been developed in order to reduce the detection limits and to apply the simple internal standardization for quantitative analysis [26]. The TXRF technique was also used for determination of various elements in fractionated part of biological materials [27][28]. TXRF is based on energy dispersive x-ray fluorescence and offers sub-ppm detection levels for a variety of sample types.
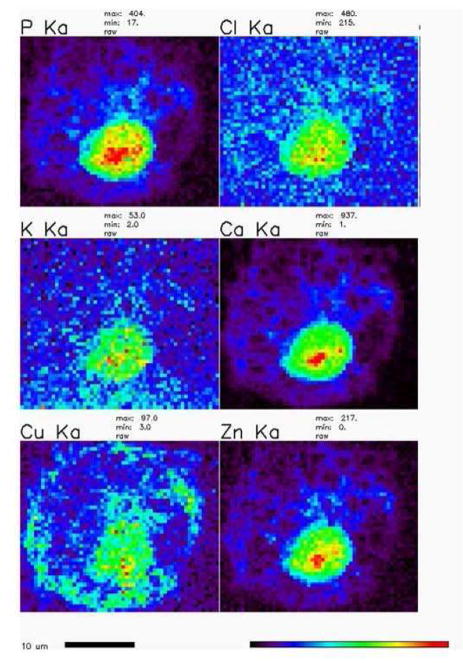
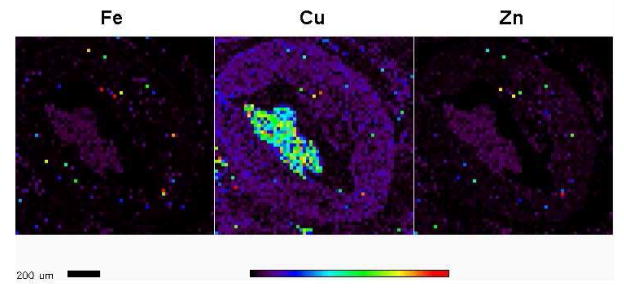
With regard to the application of XRF in vascular metallomics, in our laboratories we use THP-1 cells as a model cell to study the role of ATP7A and copper egress in macrophage. Synchrotron-based XRF microscopy provides the opportunity to detect the spatial copper distribution and concentration in a single cell. Figure 2 illustrates the qualitative spatial distribution and concentration of metal elements including P, Cl, K, Ca, Zn and Cu in THP-1 cells after treatment with copper for a brief period. Moreover, XRF also shows a great potential to investigate the role of metal in vivo. Figure 3 illustrates that XRF can detect small accumulations of metals such as iron, copper and zinc in arterial walls of a murine atherosclerotic model. Note that this experiment was performed using the same cohort of mice of a micro-CT study [29].
Figure 2.
Representative XRF map of qualitative spatial distributions and concentration of metal elements in THP-1 derived macrophages after copper (CuSO4, 200 μM) treatment for 4 hrs. The cells were then imaged with an x-ray microprobe. The conditions of XRF are as follows: 10-keV incident energy of x-rays, a single bounce Si〈111〉 monochromator, 0.3 × 0.2 μm spot size, 0.5 μm scan step, and 1 sec of dwell time.
Figure 3.
Detection of iron, zinc and copper in arterial walls using XRF. XRF was performed using the atherosclerotic aorta of a murine model (ApoE−/− LDLR−/− double knockout mice). Incident photon energy of 13.7 keV was chosen to excite the elements. Single 6-μm thick sections were cut from formalin fixed and paraffin embedded tissue blocks and mounted on silicon nitride membranes (Silson, Northampton, England) with a nominal field-of-view of 4 mm2. An x-ray beam, focused to a spot size of 0.5 μm, was used to raster-scan the 6-μm thick section. Spectral analysis of the excited x-ray fluorescence spectrum at each raster pixel then provided spatial images for each element. Under this condition, copper, iron and zinc were readily detected. Note that the XRF image itself just describes the distribution but not concentration of metal. In a separate format of XRF, element concentrations were determined using calibrated thin-film standards of the elements-of-interest. Among them, iron is the one that is detected at the highest level, followed by zinc and then copper.
Copper levels in human cardiovascular disease
Growing evidence of the importance of metals in biological processes makes detection of metals in tissues and cells very critical. Population studies in human disease have applied several different metallomics techniques in order to find answers to problems that plague mankind [30–33]. Table 2 summarizes recent human population studies of cardiovascular diseases that have applied classic metallomics approaches, with a particular emphasis on the role of copper in these diseases. In addition, a recent review by Brewer [7] provides additional prospective in this topic.
Table 2.
Human population studies of cardiovascular diseases with metallomics analytical techniques
| Year | Protocol | Nation | Results | Metallomics analytical techniques | References |
|---|---|---|---|---|---|
| 1981 | In 106 patients undergoing coronary arteriography. The patients were classified into three groups according to severity of CHD as assessed by coronary angiography: those without coronary lesions (n = 31) and those with moderate (n = 34) or severe CHD (n = 41). | Germany | Patients with severe CHD had higher serum copper than those without CHD Metal concentrations in patients with moderate CHD did not differ significantly from control values. | GFAAS | (Manthey, Stoeppler et al. 1981) [34] |
| 1988 | Cardiovascular disease (n = 62) deaths and their matched controls were taken from a cohort of 10,532 persons examined in 1975–1978. | Netherlands | The adjusted risk of death from cancer and cardiovascular disease was about four times higher for subjects in the highest serum copper quintile compared with those with normal levels. | AAS | (Kok, Van Duijn et al. 1988) [30] |
| 1991 | Longitudinal study of a cohort of middle aged men followed up for 24 months. 126 men aged 42, 48, 54, or 60 at examination randomly selected from a population based sample of 2682 men. | Finland | The mean increase in the maximal common carotid intima media thickness after two years was greater in men with high serum copper concentrations. | FAAS | (Salonen, Salonen et al. 1991) [35] |
| 1996 | 230 men dying from cardiovascular diseases and 298 controls matched for age, place of residence, smoking and follow-up time. Mean follow-up time was 10 years. | Finland | High serum copper is associated with increased cardiovascular mortality | (Reunanen, Knekt et al. 1996) [31] | |
| 1997 | 162 rural (86 men and 96 women) and 152 urban (80 men and 72 women) subjects between 26 to 65 years of age. | India | Serum levels of copper were significantly higher in patients with CAD compared to the rest of the subjects. | (Singh, Gupta et al. 1997) [32] | |
| 1998 | Group 1:81 healthy elderly (age >80) Group 2: 62 elderly with chronic degenerative diseases (age>80) Group 3: 81 healthy adults (age 54±1.77) |
Italy | Serum copper was significantly higher in group 2 than in group 1 or 3. | AAS | (Mezzetti, Pierdomenico et al. 1998) [37] |
| 2000 | 151 deaths from coronary heart disease occurred among 4,574 participants aged > or =30 years. | US | At baseline, the age-adjusted serum copper concentration was about 5% higher among participants | AAS | (Ford 2000) [36] |
| 2000 | Sixty-five patients with peripheral vascular disease and 65 sex- and age-matched healthy control subjects | Norway | The patients with suprainguinal types of vascular lesions had significantly higher concentrations of copper in plasma | TXRF | (Mansoor, Bergmark et al. 2000) [38] |
| 2004 | 53 human plaques, Diseased human artery samples from patients undergoing carotid endarterectomy. 14 healthy controls, Healthy artery specimens (aortae, mammary, and radial) from patients undergoing heart bypass and transplantation operations. | Australia | Levels of copper were also detected (7.51 versus 2.01 pmol/mg tissue, lesion versus healthy control, respectively, P<0.05). | ICP-MS | (Stadler, Lindner et al. 2004) [39] |
| 2006 | Data from the Paris Prospective Study 2, a cohort of 4035 men age 30–60 years at baseline. During 18-year follow up, 339 deaths occurred, 176 as a result of cancer and 56 of cardiovascular origin. | France | High serum copper, and concomitance of low serum zinc with high serum copper to an increased mortality risk in middle-aged men. | (Leone, Courbon et al. 2006) [33] |
An imbalance of trace elements and metals appears to be a possible cause of coronary heart disease (CHD). Manthey, et al. investigated the concentrations of several critical metals, including magnesium, chromium, copper, manganese, selenium and zinc in serum from three different groups of patients. Group 1 patients had no signs of CHD, group 2 patients showed signs of moderate CHD and group 3 patients had severe CHD [34]. The authors used a combination of analytical techniques that included FAAS for the detection of magnesium and zinc, GFAAS for the detection of chromium, manganese, copper, cadmium and lead, and neutron activation analysis for the detection of selenium. They established that there is a relationship between magnesium, copper and manganese serum concentrations and there is a significant different between group 1 and group 3 patients. Most of the metals detected were in the ppm to ppb range.
Copper and selenium concentrations in human serum was also studied by Salonen and colleagues [35]. Using FAAS for the detection of copper and GFAAS for the detection of selenium, they reported concentrations in the ppb range. In this study, they examined the relationship between serum copper and selenium concentrations, and low density lipoprotein cholesterol concentration and intima-media thickness. They determined that if there was a high copper concentration in the serum, then the other factors examined in this study would cause a variation in the intima-media thickness. Salonen and coworkers reported values as low as 78 ppb and 712 ppb for selenium and copper, respectively.
Since copper plays such an active role in oxidation/reduction reactions within the body, the levels of serum copper from patients who have died from CHD were studied by Ford [36]. FAAS was used to measure the amount of copper in the sera of patients that participated in the Second National Health and Nutrition Examination Survey. Copper concentrations were reported as low as 370 ppb for this study. It is also demonstrated that there is a strong correlation between serum copper concentration and coronary heart disease mortality.
The copper/zinc ratio in the body has also been studied as a potential cause of oxidative stress that characterize age-related degenerative diseases. Mezzetti, et al. investigated the copper/zinc ratio in three different groups of patients [37]. Group 1 consisted of patients that were healthy and above the age of eighty, while group 2 consisted of patients that had an age related degenerative disease such as a stroke, Parkinson’s or Alzheimer’s and above the age of 80. Group 3 patients were in the range of 20–70 years of age and healthy. Copper and zinc concentrations in serum were analyzed using FAAS and the values reported were as low as 1.2 ppm. It was concluded that the copper/zinc ratio does play an active role in oxidative stress modulation.
All of these studies employed a type of AAS in order to analyze trace metals, such as copper, selenium and zinc. The concentrations of the metals in serum were mainly in the high ppb to low ppm range. Since most of the studies discussed in this section investigated more than one trace metal, and AAS can only be used for single metal analysis, there should have been at least two sets of each sample to enable the measurement of both metals. Therefore, XRF and ICP, which both allow for multi-element detection, appear to be better options for these studies if the equipment is available.
XRF offers multi-element detection capabilities as well as the ability to handle various sample matrices. Mansoor and coworkers used total x-ray fluorescence spectrometry (TXRF) in order to investigate the correlation between copper and other nutritional elements and plasma total homocysteine levels in patients with peripheral vascular disease (PVD) [38]. By measuring the intensity of characteristic fluorescence x-rays of copper, they were able to obtain a concentration of 20.3 μg/g copper in plasma. These results indicate a strong correlation between copper concentration and plasma total homocysteine levels in PVD patients.
Compared to XRF, ICP-MS can offer faster analysis times with lower limits of detection. Stadler, et al. investigated the presence of iron and copper in atherosclerotic plaques through the use of ICP-MS. In this study, diseased human artery samples and healthy artery samples were obtained from patients undergoing surgery. The tissue samples were digested in acid and analyzed with ICP-MS to determine the total metal concentration in three different sample types. The values obtained were in the nmol/mg range. Using ICP-MS, the authors were able to detect and quantify a very low level of iron and copper in three different tissue samples. They could positively conclude that there is a connection between iron and copper concentration and heart disease [39]. Not only did ICP-MS offer low detection limits, the technique also allows for the simultaneous detection of iron and copper, so the limited patient samples acquired.
Copper in angiogenesis
As previously mentioned, the major goal of metallomics is to dissect the specific biological effects of these trace elements. While studies have found that the total copper content in the samples from the patients with heart disease appears to be increased, the elucidation of copper-dependent mechanism remains a challenge and this challenge extends the domain of metallomics. Indeed, classic total element determination has evolved to include a combination of metal analytical techniques with various molecular approaches. For example, the concept of metallomics has been introduced in the field of epigenetics to address the increasing evidence that trace metals may be involved in DNA methylation, modification of histone proteins and RNA interference (see review by Wrobel, et al. [3]). For additional information about the potential role of copper in cardiovascular disease in animal models, it has been summarized elegantly in a recent review by Saari [40].
Neovascularization refers to the formation of new blood vessels. This important biological process is known to be sensitive to copper levels [41, 42]. Lowering copper levels appears to be a potentially effective antiangiogenic approach to cancer treatment (see Review [43]). Recent developments in x-ray fluorescence microscopy (XFM) analysis provide an exciting resource for highly sensitive visualization and quantitation of metals in biological samples (see Review by Fahrni [44]). Using this technique, Finney, et al. [45] recently observed that cellular copper traffics from intracellular compartments to the tips of nascent endothelial cell filopodia and extracellular space in response to the angiogenic stimulus, VEGF. This finding indicates that copper egress is induced by VEGF, and this dynamic developmental process is required for endothelial tube formation. Since we previously identified that ATP7A, a key regulator of copper egress, is abundant in vascular endothelial cells [12], it is reasonable to speculate that VEGF induced copper egress from endothelial cells is mediated by ATP7A. Moreover, VEGFR1 is a member of the VEGFR family, and it binds VEGF, placental growth factor, and VEGF-B. In adults, VEGFR1 is expressed not only in endothelial cells but also in macrophages, where it promotes macrophage recruitment, inflammatory diseases, cancer metastasis, and atherosclerosis via its kinase activity. Particularly, the recruitment of monocytes/macrophages by VEGF, through VEGFR1, is an early and essential step in an immune amplification cascade that leads to inflammatory response of angiogenesis. [46, 47] Activation of VEGFR1 results in epithelial to mesenchymal transition, which is linked to increased invasion and migration of tumor cells [48–50]. We have reported [14] that ATP7A expression was increased in a human monocytic cell line after exposure to PMA, a potent promoter of neovascularization and cancer [51]. Our data also indicated that VEGFR1 expression is regulated by ATP7A mediated copper egress. This finding further supports a role of copper egress in neovascularization. As future directions, it would be interesting to examine the participation of copper trafficking pathway other than ATP7A in neovascularization. To gain more information about copper trafficking pathway, recent reviews by Prohaska [52] and Lutsenko, et al [53] are very helpful.
Copper as a dynamic signaling molecule
Accumulating evidence indicates that copper is a signaling molecule [54] that can alter cellular functions through at least four different mechanisms. First, copper facilitates the generation of free radicals, which, in turn, catalyze the oxidation of biomolecules such as lipids, proteins, and nucleic acids (reviewed by Kehrer et al [55]. Copper overload has long been hypothesized to result from the redox cycling Haber-Weiss reaction in which cuprous ions react with H2O2 to form reactive oxygen species. However, this effect is believed to be minimal under a pathophysiological condition [56]. The intracellular environment has a great capacity to chelate copper, so free copper is extraordinarily restricted in cells [10]. Thus, as a future direction for metallomics, it is important to finely tune the pathological concentrations of copper that are associated with its detrimental effects. Second, copper may regulate gene expression through binding to copper-responsive regulatory elements of gene promoters. For example, overexpression of ATP7A has been shown to significantly reduce β-amyloid precursor protein (APP) levels and down-regulate APP mRNA expression, presumably through this mechanism [57]. Sen, et al. reported that copper regulates VEGF expression and neovascularization related to wound healing [58]. Moreover, our data showed that inhibition of ATP7A expression resulted in a marked decrease in VEGFR1 expression in macrophages [14]. Third, copper can regulate protein-protein interactions. In a previous in vitro study, we reported that copper increased the interaction between ATP7A and ecSOD, and that a copper chelator (bathocuproine disulfonate) attenuated this interaction [12]. Finally, copper directly binds to and regulates proteins containing cysteine, histidine or glutamic acid residues, including enzymes involved in antioxidant defense (SOD1 and ecSOD), cellular respiration (cytochrome c oxidase), catecholamine synthesis (dopamine-β-hydroxylase), pigmentation (tyrosinase) and integration of the vascular wall (lysyl oxidase). Note that the antioxidative effect of copper is, at least in part, related to its role in the maintenance of the full activity of several antioxidants such as SOD1 and ecSOD. The basic information about the detailed mechanisms by which copper is inserted into SOD1 and ecSOD is available from a recent review by Cullota [59]. As another function direction, it is also important to define the physiological range of tissue copper that is associated with the beneficial effects of copper using metallomics-based approaches.
Acknowledgments
This work was supported by an AHA National Scientist Development Grant (0835268N) and a NIH grant (HL65342). Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Abbreviations
- AAS
atomic absorption spectrometry
- CHD
coronary heart disease
- FAAS
flame-AAS
- GFAAS
graphite furnace-AAS
- HPLC
high performance liquid chromatography
- ICP
inductively coupled plasma
- ICP-AES
ICP-atomic emission spectrometry
- ICP-MS
ICP-mass spectrometry
- ppb
parts per billion (such as μg/L)
- ppm
parts per million (such as mg/L)
- PVD
peripheral vascular disease
- TXRF
total reflection x-ray fluorescence spectrometry
- XRF
x-ray fluorescence spectrometry
References
- 1.Szpunar J. Metallomics: a new frontier in analytical chemistry. Anal Bioanal Chem. 2004;378(1):54–6. doi: 10.1007/s00216-003-2333-z. [DOI] [PubMed] [Google Scholar]
- 2.Shi W, Chance MR. Metallomics and metalloproteomics. Cell Mol Life Sci. 2008;65(19):3040–8. doi: 10.1007/s00018-008-8189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrobel K, Wrobel K, Caruso JA. Epigenetics: an important challenge for ICP-MS in metallomics studies. Anal Bioanal Chem. 2009;393(2):481–6. doi: 10.1007/s00216-008-2472-3. [DOI] [PubMed] [Google Scholar]
- 4.Levenson CW. Trace metal regulation of neuronal apoptosis: from genes to behavior. Physiol Behav. 2005;86(3):399–406. doi: 10.1016/j.physbeh.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Mertz W. The essential trace elements. Science. 1981;213(4514):1332–8. doi: 10.1126/science.7022654. [DOI] [PubMed] [Google Scholar]
- 6.Alpert PT. New and emerging theories of cardiovascular disease: infection and elevated iron. Biol Res Nurs. 2004;6(1):3–10. doi: 10.1177/1099800404264777. [DOI] [PubMed] [Google Scholar]
- 7.Brewer GJ. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med (Maywood) 2007;232(2):323–35. [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 9.Beattie JH, I, Kwun S. Is zinc deficiency a risk factor for atherosclerosis? Br J Nutr. 2004;91(2):177–81. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 10.Rae TD, et al. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–8. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 11.Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134(5):1003–6. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- 12.Qin Z, et al. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. Faseb J. 2006;20(2):334–6. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- 13.Qin Z, et al. Role of Menkes ATPase in angiotensin II-induced hypertension: a key modulator for extracellular superoxide dismutase function. Hypertension. 2008;52(5):945–51. doi: 10.1161/HYPERTENSIONAHA.108.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afton S, et al. Copper egress is induced by PMA in human THP-1 cells: a potential regulator of VEGFR1. Biometals. 2009 doi: 10.1007/s10534-009-9210-y. In press. [DOI] [PubMed] [Google Scholar]
- 15.Hill Steve JSC, Dawson John B, Evans E Hywel, Fisher Andrew, Price W John, Smith Clare MM, Sutton Karen L, Tyson Julian F. Advances in atomic emission absorption and fluorescence spectrometry, and related techniques. Journal of Analytical Atomic Spectrometry. 2000;15:763–805. [Google Scholar]
- 16.Yebra-Biurrun MC, RMC-R Fast ultrasound-assisted extraction of copper, iron, manganese and zinc from human hair samples prior to flow injection flame atomic absorption spectrometric detection. Anal Bioanal Chem. 2007;388:711–716. doi: 10.1007/s00216-007-1264-5. [DOI] [PubMed] [Google Scholar]
- 17.Renmin Gong DZ, Zhong Keding, Feng Min, Liu Xingyan. Determination of trace copper in water samples by flame atomic absorption spectrometry after preconcentration on a phosphoric acid functionalized cotton chelator. Journal of the Serbian Chemical Society. 2008;73(2):249–258. [Google Scholar]
- 18.Feyime Sahin MV, Ataman O Yavuz. Effect of nitric acid for equal stabiliation and sensitivity of different selenium species in electrothermal atomic absorption spectrometry. Analytica Chemica Acta. 2005;547:126–131. [Google Scholar]
- 19.Yen-Jia Tseng Y-DT, Jiang Shiuh-Jen. Electrothermal vaporization dynmanic reaction cell inductively coupled plasma mass spectrometry for the determination of Fe, Co, Ni, Cu, and Zn in biological samples. Anal Bioanal Chem. 2007;387:2849–2855. doi: 10.1007/s00216-007-1143-0. [DOI] [PubMed] [Google Scholar]
- 20.Becker JS, Depboylu AMC, Dobrowolska J, Zoriy MV. Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (Slugs-Genus Arion) measured by laser ablation inductively coupled plasma mass spectrometry. Analytical Chemistry. 2007;79:6074–6080. doi: 10.1021/ac0700528. [DOI] [PubMed] [Google Scholar]
- 21.Becker J Sabine, Susanne Becker MZJ, Dobrowolska Justina, Matusch Andreas. Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) in elemental imaging of biological tissues and in proteomics. Journal of Analytical Atomic Spectrometry. 2007;22:736–744. [Google Scholar]
- 22.Sasan Rabieh AVHaJM. Determination of arsenic species in human urine using high performance liquid chromatography (HPLC) coupled with inductively copuled plasma mass spectrometry (ICP-MS) Journal of Analytical Atomic Spectrometry. 2008;23:544–549. [Google Scholar]
- 23.Ruiz-Ortega M, et al. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001;38(6):1382–7. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 24.Qin Z. Newly developed angiotensin II-infused experimental models in vascular biology. Regul Pept. 2008;150(1–3):1–6. doi: 10.1016/j.regpep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Gums JG. Magnesium in cardiovascular and other disorders. Am J Health Syst Pharm. 2004;61(15):1569–76. doi: 10.1093/ajhp/61.15.1569. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda Y, Horiuchi T. Optical flats for use in x-ray spectrochemical microanalysis. Rev Sci Instrum. 1971;42(7):169–70. doi: 10.1063/1.1685282. [DOI] [PubMed] [Google Scholar]
- 27.Mukhtar S, SJH Suitability of total reflection x-ray fluorescence spectrometry for elemental speciation studies. Journal of Analytical Atomic Spectrometry. 1991;6:339–341. [Google Scholar]
- 28.Klaus Guenther AvB, Strompen Christoph. Element determination by total-reflection x-ray fluorescence spectrometry at intial step of element speciation in biological matrices. Analytica Chemica Acta. 1995;309:327–332. [Google Scholar]
- 29.Langheinrich AC, et al. Quantitative X-ray imaging of intraplaque hemorrhage in aortas of apoE(−/−)/LDL(−/−) double knockout mice. Invest Radiol. 2007;42(5):263–73. doi: 10.1097/01.rli.0000258085.87952.ea. [DOI] [PubMed] [Google Scholar]
- 30.Kok FJ, et al. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol. 1988;128(2):352–9. doi: 10.1093/oxfordjournals.aje.a114975. [DOI] [PubMed] [Google Scholar]
- 31.Reunanen A, et al. Serum calcium, magnesium, copper and zinc and risk of cardiovascular death. Eur J Clin Nutr. 1996;50(7):431–7. [PubMed] [Google Scholar]
- 32.Singh RB, et al. Epidemiologic study of trace elements and magnesium on risk of coronary artery disease in rural and urban Indian populations. J Am Coll Nutr. 1997;16(1):62–7. doi: 10.1080/07315724.1997.10718650. [DOI] [PubMed] [Google Scholar]
- 33.Leone N, et al. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17(3):308–14. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 34.Manthey J, et al. Magnesium and trace metals: risk factors for coronary heart disease? Association between blood levels and angiographic findings. Circulation. 1981;64(4):722–9. doi: 10.1161/01.cir.64.4.722. [DOI] [PubMed] [Google Scholar]
- 35.Salonen JT, et al. Interactions of serum copper, selenium, and low density lipoprotein cholesterol in atherogenesis. Bmj. 1991;302(6779):756–60. doi: 10.1136/bmj.302.6779.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford ES. Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol. 2000;151(12):1182–8. doi: 10.1093/oxfordjournals.aje.a010168. [DOI] [PubMed] [Google Scholar]
- 37.Mezzetti A, et al. Copper/zinc ratio and systemic oxidant load: effect of aging and aging-related degenerative diseases. Free Radic Biol Med. 1998;25(6):676–81. doi: 10.1016/s0891-5849(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 38.Mansoor MA, et al. Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clin Chem. 2000;46(3):385–91. [PubMed] [Google Scholar]
- 39.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24(5):949–54. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 40.Saari JT. Copper deficiency and cardiovascular disease: role of peroxidation, glycation, and nitration. Can J Physiol Pharmacol. 2000;78(10):848–55. doi: 10.1139/cjpp-78-10-848. [DOI] [PubMed] [Google Scholar]
- 41.Ziche M, Jones J, Gullino PM. Role of prostaglandin E1 and copper in angiogenesis. J Natl Cancer Inst. 1982;69(2):475–82. [PubMed] [Google Scholar]
- 42.Raju KS, et al. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982;69(5):1183–8. [PubMed] [Google Scholar]
- 43.Brewer GJ. Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Discov Today. 2005;10(16):1103–9. doi: 10.1016/S1359-6446(05)03541-5. [DOI] [PubMed] [Google Scholar]
- 44.Fahrni CJ. Biological applications of X-ray fluorescence microscopy: exploring the subcellular topography and speciation of transition metals. Curr Opin Chem Biol. 2007;11(2):121–7. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Finney L, et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci U S A. 2007;104(7):2247–52. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cursiefen C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami M, et al. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28(4):658–64. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 48.Wey JS, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104(2):427–38. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 49.Fan F, et al. Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene. 2005;24(16):2647–53. doi: 10.1038/sj.onc.1208246. [DOI] [PubMed] [Google Scholar]
- 50.Yang AD, et al. Vascular endothelial growth factor receptor-1 activation mediates epithelial to mesenchymal transition in human pancreatic carcinoma cells. Cancer Res. 2006;66(1):46–51. doi: 10.1158/0008-5472.CAN-05-3086. [DOI] [PubMed] [Google Scholar]
- 51.Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42(2):469–77. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- 52.Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88(3):826S–9S. doi: 10.1093/ajcn/88.3.826S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutsenko S, et al. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87(3):1011–46. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 54.Mathie A, et al. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol Ther. 2006;111(3):567–83. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Armendariz AD, et al. Gene expression profiling in chronic copper overload reveals upregulation of Prnp and App. Physiol Genomics. 2004;20(1):45–54. doi: 10.1152/physiolgenomics.00196.2003. [DOI] [PubMed] [Google Scholar]
- 56.Leeuwenburgh C, et al. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272(6):3520–6. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 57.Bellingham SA, et al. Copper depletion down-regulates expression of the Alzheimer’s disease amyloid-beta precursor protein gene. J Biol Chem. 2004;279(19):20378–86. doi: 10.1074/jbc.M400805200. [DOI] [PubMed] [Google Scholar]
- 58.Sen CK, et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am J Physiol Heart Circ Physiol. 2002;282(5):H1821–7. doi: 10.1152/ajpheart.01015.2001. [DOI] [PubMed] [Google Scholar]
- 59.Culotta VC, Yang M, O’Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763(7):747–58. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]