Abstract
Learned associations are hypothesized to develop between drug effects and contextual stimuli during repeated drug administration to produce context-specific sensitization that is expressed only in the drug-associated environment and not in a non-drug paired environment. Neuroadaptations that mediate such context-specific behavior are largely unknown. We investigated context-specific modulation of CREB phosphorylation and four upstream kinases in nucleus accumbens that phosphorylate CREB, including ERK, PKA, CaMKII and IV. Rats received seven once daily injections of cocaine or saline in one of two distinct environments outside their home cages. Seven days later, test injections of cocaine or saline were administered in either the Paired or the Non-paired environment. CREB and ERK phosphorylation were assessed with immunohistochemistry while phosphorylation of the remaining kinases, as well as CREB and ERK, were assessed by Western blotting. Repeated cocaine administration produced context-specific sensitized locomotor responses accompanied by context-specific enhancement of the number of cocaine-induced phosphoCREB and phosphoERK immunoreactive nuclei in a minority of neurons. In contrast, CREB and CaMKIV phosphorylation in nucleus accumbens homogenates were decreased by cocaine test injections. We have recently shown that a small number of cocaine-activated accumbens neurons mediate the learned association between cocaine effects and the drug administration environment to produce context-specific sensitization. The corresponding cocaine and context-specific phosphorylation of ERK and CREB in cocaine-activated accumbens neurons in the present study suggests that this signal transduction pathway is also selectively activated in the same set of accumbens neurons.
Keywords: PKA, CaMK, neuroadaptation, kinase, calcium/calmodulin-dependent kinase
Introduction
Cocaine-induced psychomotor sensitization is the progressive enhancement of stimulant drug effects during repeated administration of cocaine (Post & Rose, 1976; Shuster et al., 1977; Robinson & Becker, 1986; Vanderschuren & Kalivas, 2000). With the appropriate treatment conditions, rats and mice can remain sensitized to cocaine for up to 6 months following repeated cocaine injections (Shuster et al., 1977; Hope et al., 2006). The sensitized response is thought to be mediated by a combination of learned associations and long-lasting pharmacologically-induced alterations (Vanderschuren & Kalivas, 2000; Nestler, 2001; Badiani & Robinson, 2004; Boudreau & Wolf, 2005; Kourrich et al., 2007). Although many pharmacologically-induced alterations have been identified, little is known about the molecular and cellular mechanisms underlying the learned component.
The environment where drug is administered modulates the development of psychomotor sensitization as well as subsequent sensitized responsivity to psychostimulants (Post et al., 1981; Vezina et al., 1989; Post et al., 1992). One well-studied factor is relative novelty of the drug administration environment. Repeated administration of cocaine or amphetamine outside the animal’s home cage induces greater sensitization of psychomotor responses than repeated drug administration in their home cage (Badiani et al., 1995; Robinson et al., 1998; Badiani & Robinson, 2004). A second distinct factor involves the specific sets of stimuli found in different administration environments outside the home cage. Learned associations are hypothesized to develop between drug effects and contextual stimuli during repeated drug administration to produce a form of sensitization called context-specific sensitization that is expressed only in the drug-associated environment and not in a non-drug paired environment (Anagnostaras & Robinson, 1996; Carey & Gui, 1998; Duvauchelle et al., 2000b; Duvauchelle et al., 2000a; Vezina & Leyton, 2009).
Repeated cocaine administration in a novel environment produces context-specific enhancement of cocaine-induced Fos, a commonly used protein marker of neural activity, in nucleus accumbens neurons that mediate context-specific locomotor sensitization to cocaine (Crombag et al., 2002; Todtenkopf et al., 2002; Hope et al., 2006; Mattson et al., 2008; Koya et al., 2009). Enhanced Fos responses have also been observed following repeated amphetamine treatment in the rat’s home cages (Nordquist et al., 2008). From this we hypothesized that upstream signal transduction pathways that activate c-fos transcription should also be sensitized in a context-specific manner.
CREB (cyclic AMP-regulated element-binding protein) is one of the most important transcription factors capable of activating c-fos transcription. CREB is activated via serine-133 phosphorylation by a number of different kinases, including extracellular signal-regulated kinase (ERK or MAP kinase) (Xing et al., 1996; Sgambato et al., 1998; Thomas & Huganir, 2004), cAMP-dependent protein kinase (PKA) (Montminy & Bilezikjian, 1987), and calcium/calmodulin-dependent kinases (CaMKs) (Sheng et al., 1991; Enslen et al., 1994; Matthews et al., 1994; Braun & Schulman, 1995; Tokumitsu & Soderling, 1996; Heist et al., 1998; Tokumitsu et al., 2004). Similar to Fos expression, cocaine-induced CREB and ERK activation is enhanced in nucleus accumbens following sensitization to cocaine in a novel environment (Valjent et al., 2000; Mattson et al., 2005; Brenhouse et al., 2007). In the present study, we assessed whether this enhanced activation is also context-specific for cocaine-induced CREB phosphorylation and the upstream activators ERK, PKA, CaMK II and IV in rat nucleus accumbens following sensitization to cocaine in two distinct novel environments.
Materials and methods
Subjects
Male Sprague-Dawley rats (Charles River, Raleigh, NC, USA) weighing 250–320 g were housed individually in standard plastic cages in a temperature- and humidity-controlled room. They were maintained on a 12:12 h reverse light:dark cycle (lights on at 19 h), and allowed free access to food and water. Rats were acclimatized to these housing conditions for at least 7 days prior to drug treatments. Experimental procedures were approved by the NIDA Animal Care and Use Committee.
Apparatus and environmental contexts of drug treatment
Rats were injected in two distinct environments outside the home cage that had different tactile, visual and auditory cues. Environment A was a 43 × 43 cm square Plexiglas chamber with a smooth floor; each chamber was located inside a light-and sound-attenuating box in a dark quiet room. Environment B was a round Plexiglas chamber (38 cm diameter) with woodchip bedding (Sani-chips; Harlan Teklad, Madison, WI, USA); the doors of the sound-attenuating box were open in a light room with moderate-volume music by Rammstein. Each plexiglas chamber was in an activity monitor with 16 photocell pairs on each axis (Med Associates, St Albans, VT, USA). Distance traveled was calculated from beam breaks using Activity Monitor v.5.9 software (Med Associates).
General procedure for context-specific sensitization to cocaine
During repeated administration, cocaine (15 mg/kg) or saline (1 ml/kg) was injected intraperitoneally (i.p.) into rats once daily for seven days. Rats were immediately placed into either Environment A or B for 60 min and then returned to their home cages after each session. After the last session, rats were kept in their home cages for seven days. On test day, all rats were given i.p. injections of cocaine or saline and placed into Environment A. Thus rats that received repeated injections in Environment A were tested in the same environment (Paired groups) while rats that received repeated injections in Environment B were tested in a different environment (Non-paired groups). This procedure was used for Experiments 1–4.
Experiment 1: Multiple dose test for sensitized locomotor response
We used a 2 × 2 × 4 factorial design (n=8 per group) that included the factors: repeated administration environment (Environment A, B), repeated administration drug (saline, cocaine) and the within-subject factor, cocaine dose on test day. Rats were kept in their home cages for seven days following repeated administration of cocaine or saline in Environment A or B. On test day, all rats were injected first with saline and placed in Environment A where distance traveled was monitored for 60 min. Rats were then injected each subsequent hour with increasing doses of cocaine (5, 10, 20 mg/kg) and distance traveled was assessed for 60 min after each injection.
Experiment 2: Immunohistochemistry analysis of phosphoCREB and phosphoERK
We used a 2 × 2 × 2 design (n=6–8 per group) that included the factors: repeated administration environment (Environment A, B), repeated administration drug (saline, cocaine) and test injection (saline, cocaine). Rats were kept in their home cages for seven days following repeated administration of cocaine or saline in Environment A or B. On test day, rats were habituated to Environment A for 30 min prior to their test injections. Based on results from Experiment 1, we chose the 20 mg/kg dose of cocaine, along with saline vehicle, for test injections.
Twenty minutes after test injections, rats were deeply anesthetized with isoflurane (approximately 80 sec) and perfused transcardially with 300 ml of 4% paraformaldehyde in 0.1 M sodium phosphate, pH 7.4. Brains were removed and post-fixed for 2 hr before transfer to 20% sucrose in 0.1 M sodium phosphate, pH 7.4, for 48 hr at 4°C. Brains were then frozen in powdered dry ice and stored at −30° C. Coronal sections (30 μM) were cut in a cryostat, collected in cryoprotectant (20% glycerol and 2% DMSO in 0.1 M sodium phosphate, pH 7.4), and stored at −80° C until further processing.
Sections were rinsed 3 × 10 minutes in phosphate-buffered saline (PBS) and placed in blocking buffer (3% normal goat serum and 0.25% Triton X-100 in PBS) for 1 hr at 22°C. Sections were then incubated overnight at 4°C in 1:500 dilution of anti-phosphoCREB antibody (Cat# 9198, Cell Signaling Technology, Boston, MA, USA) or anti-phosphoERK antibody (Cat# 9101, Cell Signaling Technology) in blocking buffer. Sections were washed in PBS and incubated at 22°C with 1:100 dilution of biotinylated goat anti-rabbit IgG secondary antibody (BA-1000; Vector Laboratories, Burlingame, CA, USA) in 1% normal goat serum and 0.25% Triton X-100 in PBS. Sections were washed in PBS and processed with ABC Elite kits (Vector Laboratories) using a 1:80 dilution of each A and B reagent. Sections were washed again in PBS and developed in a solution containing 0.035% diaminobenzidine and 0.04% hydrogen peroxide in 0.1 M sodium phosphate buffer, pH 7.4.
Bright-field images of phosphoCREB and phosphoERK immunoreactivity in nucleus accumbens (1.8 mm anterior to Bregma; (Paxinos & Watson, 1998)) were captured using a CCD camera (Coolsnap Photometrics, Roper Scientific Inc., Trenton, NJ) attached to a Zeiss Axioskop 2 microscope (see Figure 2a). Labeled nuclei (see Figure 2b,c) in two hemispheres per rat were counted using IPLab software (Scanalytics, Inc., Fairfax, VA).
Figure 2.
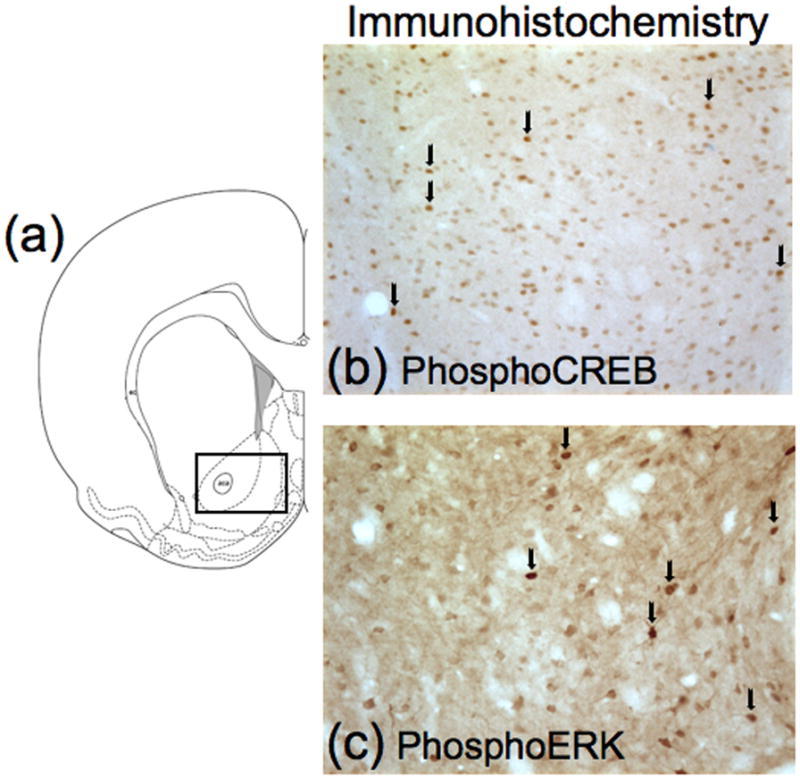
Schematic representation of brain regions and immunohistochemistry images. (a) Schematic of coronal section approximately 1.8 mm anterior to Bregma employed in the immunohistochemistry detection. Drawings of coronal sections and coordinates were obtained from the atlas of Paxinos & Watson (1998). The rectangle represents the area used to protein quantification. (b) Example of phosphoCREB-immunoreactive nuclei in nucleus accumbens. (c) Example of phosphoERK-immunoreactive cells in nucleus accumbens. Arrows indicate immunoreactive cells.
Experiment 3: Western blotting analysis of phosphoCREB and upstream kinases
This experiment used the same treatment procedure used in Experiment 2. We used a 2 × 2 × 2 design (n = 8 per group) that included the factors: repeated administration environment (Environment A, B), repeated administration drug (saline, cocaine) and test injection (saline, cocaine). This time however, rats were rapidly decapitated 20 min after test injections with 20 mg/kg of cocaine or saline. Brains were extracted and frozen in isopentane (−50°C) within 30–45 s of decapitation and stored at −80°C until dissection.
One-millimeter coronal slices of brain (rostral face approximately 2.0 mm from Bregma based on the atlas of Paxinos & Watson (1998)) were cut in a cryostat at −20°C. Tissue punches (blunt 14-gauge needle) were obtained from nucleus accumbens (see Figure 4a) and then sonicated in 1% sodium dodecyl sulfate (SDS). Tissue was kept frozen at all times until sonication in SDS. Protein concentrations of the samples were determined using the bicinchoninic acid assay (Pierce Chemical Company; Rockford, IL, USA). Sample protein concentrations were equalized by diluting with 1% SDS. Samples were then subjected to SDS-polyacrylamide gel electrophoresis for 3 h at 200 V. Proteins were transferred electrophoretically to Immobilon-FL PVDF membranes (Millipore, Billerica, MA, USA) at 0.3 A for 3.5h.
Figure 4.
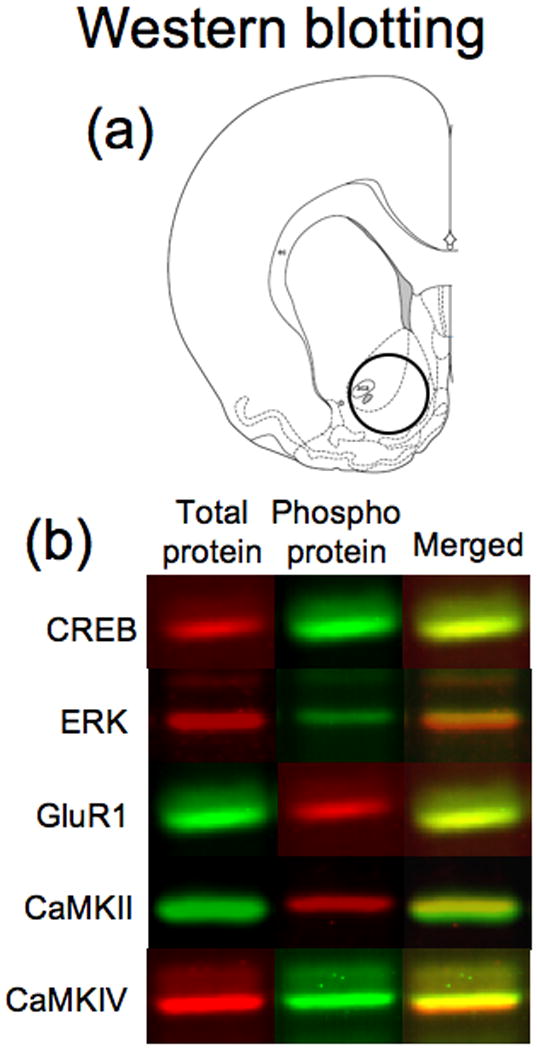
Schematic representation of brain regions and fluorescent images of proteins analyzed by Western Blotting. Schematic representation (a) of rostral face (2.0 mm anterior to Bregma) of 1mm slice employed in the assays. The circle represents the area taken for tissue punches. Drawings of coronal sections and coordinates were obtained from the atlas of Paxinos & Watson (1998). (b) Example of fluorescent bands from Western blots of each analyzed protein.
Membranes were incubated for 1h in blocking buffer (Li-Cor Biosciences, Lincoln, NE, USA) and then incubated overnight at 4°C in fresh Li-Cor blocking buffer containing two primary antibodies for each signaling protein: one antibody specifically labeled the phosphorylated form of the protein while the second antibody labeled total levels of the same protein. Each pair of antibodies were chosen so that one was a rabbit antibody while the other was a mouse antibody. CREB phosphorylation levels were assessed using antibodies against total CREB (1:500; Cat# 9197; Cell Signaling Technology) and phosphoCREB (1:1000; Cat# 05-807; Millipore). ERK activity levels were assessed using antibodies against total ERK (1:2000; Cat# 9102; Cell Signaling Technology) and phosphoERK (1:1000; Cat# 7383; Santa Cruz Biotechnology, Santa Cruz, CA, USA). CaMKII activity levels were assessed by measuring phosphorylation at Thr286, which is autophosphorylated when the enzyme is activated (Lisman et al., 2002; Wayman et al., 2008). We used antibodies against total CaMKII (1:2000; Cat# 05-532; Millipore) and phosphoCaMKII (1:500; Cat# 06-881; Millipore). CaMKIV activity levels were assessed by measuring phosphorylation of Thr196, which is phosphorylated by CaMK kinase to activate the enzyme (Tokumitsu et al., 2004; Wayman et al., 2008). We used antibodies against total CaMKIV (1:300; Cell Signaling Technology) and phosphoCaMKIV (1:10; see Tokumitsu et al. (2004)). Endogenous PKA activity levels were assessed by measuring PKA-specific phosphorylation of Ser845 in the GluR1 subunit of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor (Roche et al., 1996; Chao et al., 2002; Mattson et al., 2005). We used antibodies against total GluR1 (1:200; Cat# 13152; Santa Cruz Biotechnology) and phosphoGluR1 (1:500; Cat# AB5849; Millipore). After incubation with primary antibodies, blots were washed and incubated 1h with anti-mouse and anti-rabbit secondary antibodies labeled with IRDyes 680 and 800 (Li-Cor Biosciences). Fluorescence from both fluorophores (see Figure 4b) was assessed simultaneously using an Odyssey IR fluorescence scanner (Li-Cor Biosciences) and bands were quantified using Odyssey 2.0 software.
Experiment 4: Locomotor activity and western blotting following 10 mg/kg cocaine
This experiment was designed to replicate the results of the previous experiment with a lower dose of cocaine on test day, as well as to evaluate locomotor activation in the same animals. We used a 2 × 2 × 2 design (n =11–12 per group) that included the factors: repeated administration environment (Environment A, B), repeated administration drug (saline, cocaine) and test injection (saline, cocaine). On test day, rats were habituated to Environment A for 30 min prior to their test injections with 10 mg/kg of cocaine or saline. Distance traveled by the rats was assessed for 20 min before we rapidly decapitated the rats and removed their brains for Western blotting analysis similar to experiment 3.
Statistical analysis
Data from experiments 1 to 4 were analyzed with Statistica program using three-way analyses of variance (ANOVA) considering the factorial design in the experiments described above. Distance traveled during the repeated treatment of rats from all experiments was analyzed with two-way ANOVA for each administration environment. Factors considered were repeated administration drug and administration day. Newman-Keuls post-hoc test or Contrast Analysis was used to make specific group comparisons when appropriate. Effects were considered significant when p < 0.05.
Results
Development of cocaine-induced locomotor sensitization
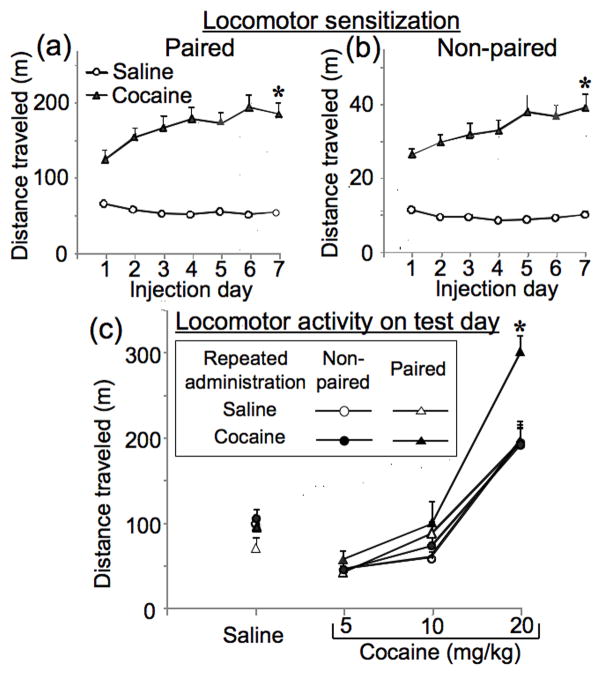
We analyzed distance travelled during the seven-day pretreatment of rats from all experiments (n = 48 per group) to verify development of sensitization in each environment. For rats injected repeatedly in Environment A (Figure 1a), two-way ANOVA indicated a significant interaction between factors (F6,534=10.57, p<0.001). Contrast analysis indicated that cocaine-induced locomotor activity was greater on day 7 than on day 1 following repeated administration of cocaine, but not saline (p<0.001). For rats injected repeatedly in Environment B (Figure 1b), two-way ANOVA indicated a significant interaction between factors (F6,474=2.89, p<0.001). Contrast analysis indicated that cocaine-induced locomotor activity was greater on day 7 than on day 1 following repeated administration of cocaine, but not saline (p<0.001). Thus, sensitization developed during repeated cocaine administration in both environments.
Figure 1.
Locomotor sensitization during repeated treatment and in the test day. Distance travelled of Paired group rats (a) was assessed in the square locomotor boxes (Environment A), while locomotor activity of Non-paired group rats (b) was assessed in the round chamber (Environment B) following each daily injection of saline or 15 mg/kg of cocaine. Activity on test day (c) was evaluated in the square locomotor boxes, seven days after repeated administration of saline or cocaine. Rats were injected at one-hour intervals with saline, then 5, 10 and 20 mg/kg of cocaine. Data represent mean ± SEM (N = 8 rats per group) of locomotor activity during 60 min following each injection for Paired or Non-paired groups of rats. # p < 0.001: locomotor activity on day 7 compared to day 1. * p < 0.01: compared to rats repeated injected with saline and tested with the same drug dose.
Experiment 1
Seven days following the last repeated injections, test injections of saline, 5, 10, or 20 mg/kg of cocaine were administered to each rat within a single session to assess expression of sensitization (Figure 1c). Three-way ANOVA (repeated administration drug, test dose, administration environment) indicated an interaction among these three factors (F3,84=4.02, p<0.01). When data were analyzed by two-way ANOVA using the main factors of repeated administration drug and time (each 60-min block corresponded to a separate dose of cocaine), repeated administration drug interacted significantly with time, and thus test dose, but only when injected in the Paired environment (F23,42=5.86, p<0.01), and not in the Non-paired environment. Contrast analysis indicated that when rats were injected in the Paired environment, repeated cocaine administration produced significantly enhanced locomotor responses on test day for the 20 mg/kg cocaine dose (p<0.01), but not for the 5 or 10 mg/kg doses. Thus, our treatment procedure produced a context-specific sensitized locomotor response with only the 20 mg/kg test dose of cocaine.
Experiment 2
PhosphoCREB immunoreactivity
We assessed the number of phosphoCREB-immunoreactive nuclei in nucleus accumbens of rats following the above treatment procedure but using only the 20 mg/kg cocaine dose along with saline vehicle for test injections (images in Figure 2b, graphs in Figure 3a,b). Many nuclei had lower basal levels of immunoreactivity. We selected a higher threshold for counting nuclei with increased levels of phosphoCREB immunoreactivity. Three-way ANOVA (repeated administration drug, test drug, administration environment) indicated an interaction among these three factors (F1,50=31.75, p<0.001). Newman-Keuls post hoc tests indicated that, in general, cocaine test injections increased the number of phosphoCREB-immunoreactive nuclei in rats following either repeated administration of cocaine or saline for rats injected in the Paired environment (p<0.01) or in the Non-Paired environment (p<0.001). Most importantly, repeated cocaine administration increased the number of phosphoCREB-immunoreactive nuclei induced by cocaine injections in the Paired environment (p<0.001); repeated cocaine administration did not increase the number of phosphoCREB-immunoreactive nuclei induced by cocaine injections in the Non-paired environment. Thus, our treatment procedure produced a context-specific enhancement of the number of phosphoCREB-immunoreactive nuclei following cocaine test injections.
Figure 3.
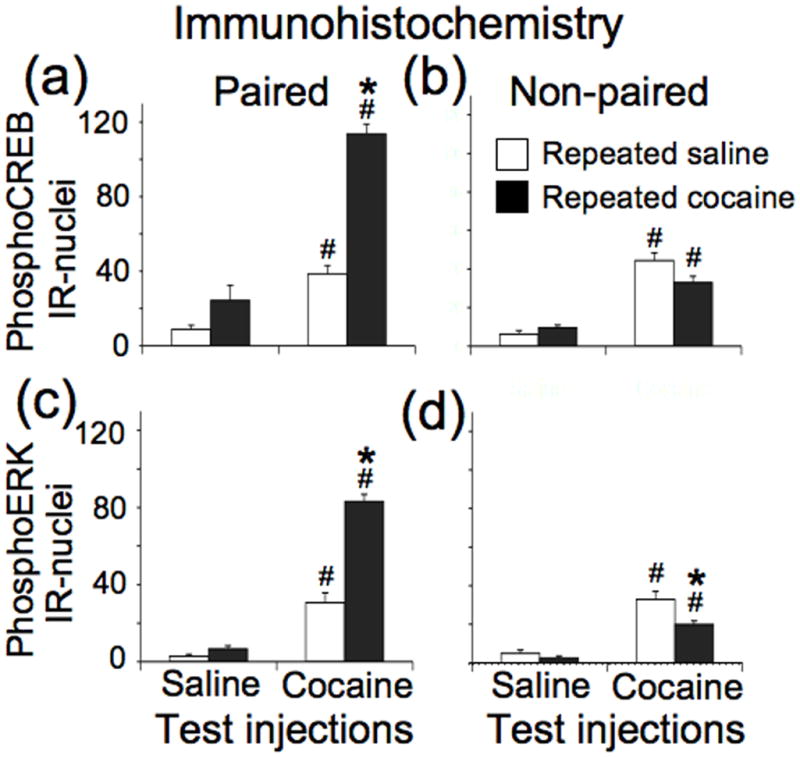
Quantification of immunohistochemical results for CREB and ERK phosphorylation in nucleus accumbens. Data represent mean ± SEM (N = 6–8 rats per group) of phosphoCREB (a, b) and phosphoERK (c, d) immunoreactive nuclei from rats injected on test day in a Paired or Non-paired environment. # p < 0.01: compared to rats that received saline test injections following repeated administration of either saline or cocaine. * p < 0.05: compared to rats that received cocaine test injections following repeated saline administration.
PhosphoERK-immunoreactivity
We assessed the number of phosphoERK-immunoreactive nuclei in nucleus accumbens from the same rats used to assess phosphoCREB-immunoreactive nuclei (images in Figure 2c, graphs in Figure 3c,d). Three-way ANOVA indicated an interaction among these three factors (F1,50=31.75, p<0.001). Newman-Keuls post hoc tests indicated that, in general, cocaine test injections increased the number of phosphoERK-immunoreactive nuclei in rats following either repeated administration of cocaine or saline for rats injected in the Paired environment (p<0.01) or in the Non-Paired environment (p<0.001). Most importantly, repeated cocaine administration increased the number of phosphoERK-immunoreactive nuclei induced by cocaine injections in the Paired environment (p<0.001), and decreased the number of phosphoERK-immunoreactive nuclei in the Non-paired environment (p<0.05). Thus, our treatment procedure produced a context-specific enhancement of the number of phosphoERK-immunoreactive nuclei following cocaine test injections.
Experiment 3
We used Western blotting to assess phosphorylated levels and total levels of CREB and upstream kinases capable of phosphorylating CREB from homogenates of nucleus accumbens (see images in Figure 4). Overall, total levels of each protein were not significantly altered by drug administration or environment. Phosphorylation levels are described below as the ratio of phosphorylated protein to total protein levels to account for differences in sample input in each lane.
CREB phosphorylation
Three-way ANOVA (repeated administration drug, test drug, administration environment) of CREB phosphorylation levels indicated only a main effect of test drug injections (F1,56=64.23, p<0.001) (Figure 5a,b). Newman-Keuls post hoc tests indicated that, in general, cocaine test injections decreased CREB phosphorylation levels following either repeated administration of cocaine or saline for rats injected in either the Paired or Non-paired environment (p<0.05). Notably, repeated cocaine administration (compared to repeated saline administration) produced nearly significant higher levels of CREB phosphorylation following cocaine test injections in the Paired (p=0.08), but not in the Non-paired, environment.
Figure 5.
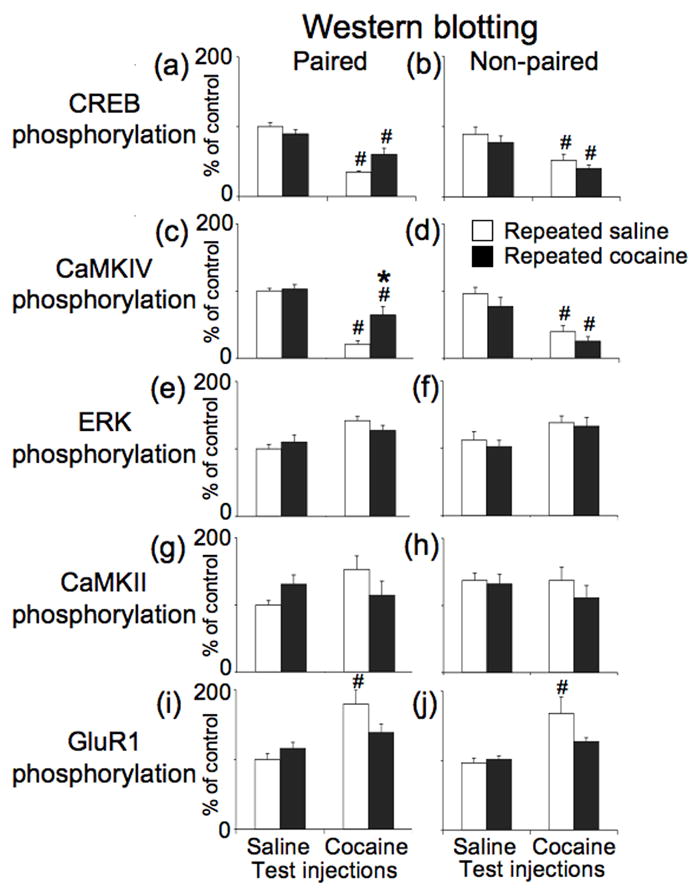
Western blotting analysis of phosphorylation of CREB and upstream kinases in the nucleus accumbens following test injections of saline or 20 mg/kg of cocaine. Data represent mean ± SEM (N = 8 rats per group) phosphorylation levels for CREB (a, b), CaMKIV (c, d), ERK (e, f), CaMKII (g, h) and GluR1 serine-845 (i, j) in rats that received test injections in Paired or Non-paired environments. # p < 0.01: compared to rats that received saline test injections following repeated administration of either saline or cocaine. * p < 0.05: compared to rats that received cocaine test injections following repeated saline administration.
CaMKIV phosphorylation
Three-way ANOVA of CaMKIV phosphorylation levels indicated an interaction for only administration environment and repeated administration drug (F1,56=9.74, p<0.01) (Figure 5c,d). Newman-Keuls post hoc test indicated that, in general, cocaine test injections decreased CaMKIV phosphorylation levels following either repeated administration of cocaine or saline for rats injected in either the Paired or Non-paired environment (p<0.05). Notably, repeated cocaine administration (compared to repeated saline administration) produced significantly higher levels of CaMKIV phosphorylation following cocaine test injections in the Paired (p<0.01), but not in the Non-paired environment. Overall the regulation of CaMKIV phosphorylation was very similar to regulation of CREB phosphorylation in the nucleus accumbens. Although cocaine test injections always decreased phosphorylation levels of CREB and CaMKIV, our repeated drug administration procedure attenuated this acute decrease in a context-specific manner.
ERK phosphorylation
Three-way ANOVA of ERK phosphorylation levels indicated a significant effect for only test injections (F1,55=16.99, p<0.001); cocaine test injections increased ERK phosphorylation levels (Figure 5e,f). However, Newman-Keuls post hoc tests did not indicate significant differences between specific pairs of groups.
CaMKII phosphorylation
Three-way ANOVA of CaMKII phosphorylation levels did not indicate any significant effects (Figure 5g,h).
GluR1 Ser845 phosphorylation
Three-way ANOVA of GluR1 phosphorylation levels indicated significant effects of test injections (F1,56=13.21, p<0.001) and an interaction between repeated administration drug × test drug (F1,56=7.75, p<0.01) (Figure 5i,j). Newman-Keuls post hoc test indicated that cocaine test injections increased GluR1 phosphorylation levels in rats that received repeated saline administration (p<0.05), but not in rats that received repeated cocaine administration in either environment. Thus, repeated cocaine administration attenuates acute cocaine-induced increases in GluR1 phosphorylation levels regardless of the administration environment.
Experiment 4
We also examined locomotor activity and phosphorylation levels using western blotting of nucleus accumbens samples obtained from rats following injections of 10 mg/kg, instead of 20 mg/kg, cocaine on test day (Figure 6). Three-way ANOVA (repeated administration drug, test drug, administration environment) of locomotor activity indicated a significant effect of administration environment (F1,86=5.73, p<0.05), test injections (F1,86=28.76, p<0.001) and an interaction of administration environment with test injections (F1,86=4.48, p<0.05) (Figure 6). Newman-Keuls post hoc test indicated that repeated cocaine administration significantly enhanced cocaine-induced locomotor activity for test injections in the Paired environment (p<0.01), but not in the Non-paired environment. Thus, our treatment procedure produced a context-specific sensitized locomotor response using a 10 mg/kg dose of cocaine injected after only 30 minutes of habituation in the test chamber. This effect was different than our lack of sensitized response in Experiment 1. The longer time for habituation to environmental stimuli in Experiment 1 prior to 10 mg/kg cocaine injections probably contributed to reducing acute locomotor responsivity (Crombag et al., 2001). Also Experiment 4 assessed acute locomotor responses during peak activation by cocaine, which was not diluted by lesser locomotor response assessed during the later non-peak time period 20–60 minutes following drug injections in Experiment 1.
Figure 6.
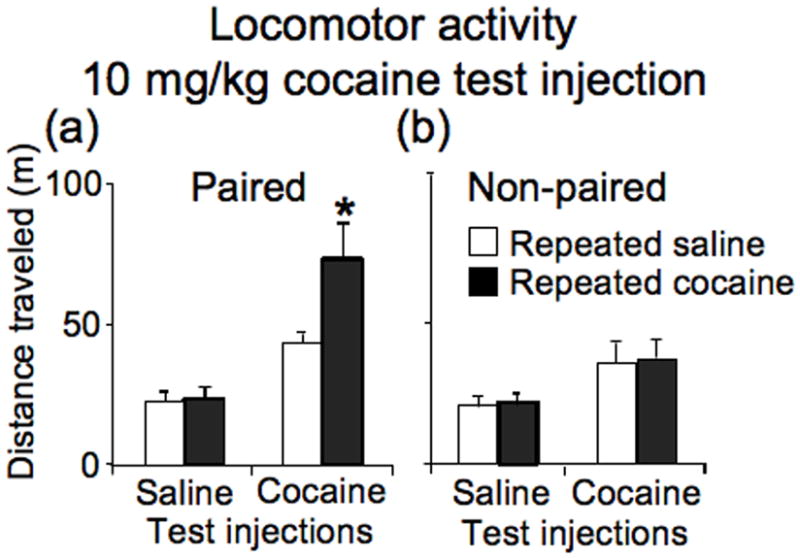
Locomotor activity following test injections of saline or 10 mg/kg of cocaine in the square locomotor boxes, seven days after repeated administration of either saline or cocaine. Data represent mean ± SEM (N = 11–12 rats per group) of locomotor activity during 20 min session for (a) Paired or (b) Non-paired groups of rats. * p < 0.01: compared to rats that received cocaine test injections following repeated saline administration.
Using western blotting, total levels of each protein were again not significantly altered by drug administration or environment. Overall, the data from this experiment indicated smaller alterations in phosphorylation levels compared to those following test injections with 20 mg/kg of cocaine in Experiment 3.
CREB phosphorylation
Three-way ANOVA (repeated administration drug, test drug, administration environment) of CREB phosphorylation levels indicated a main effect of administration environment (F1,88=4.00, p<0.05) and test drug injections (F1,88=17.87, p<0.001) (Figure 7a,b). Newman-Keuls post hoc test indicated that cocaine test injections decreased CREB phosphorylation levels only following repeated cocaine administration in the Paired environment (p<0.05).
Figure 7.

Western blotting analysis of phosphorylation of CREB and upstream kinases in the nucleus accumbens following test injections of saline or 10 mg/kg of cocaine. Data represent mean ± SEM (N = 11–12 rats per group) phosphorylation levels of CREB (a, b), CaMKIV (c, d), ERK (e, f), CaMKII (g, h) and GluR1 serine-845 (i, j) from rats that received test injections in Paired or Non-paired environments. # p < 0.05: compared to rats that received saline test injections following repeated administration of either saline or cocaine.
CaMKIV phosphorylation
Three-way ANOVA of CaMKIV phosphorylation levels indicated a main effect of test injection (F1,88=22.13, p<0.001) and near significant effect of repeated administration environment (F1,88=3.25, p=0.07) (Figure 7c,d). Newman-Keuls post hoc test indicated that cocaine test injections in the Paired environment decreased the CaMKIV phosphorylation only following repeated cocaine administration in the Paired environment (p<0.05).
ERK phosphorylation
Three-way ANOVA of ERK phosphorylation levels indicated a main effect of test injection (F1,88=6.95, p<0.01); in general, cocaine test injections produced a small increase in phosphorylation levels (Figure 7e,f). However, Newman-Keuls post hoc test did not indicate significant differences between specific pairs of groups.
CaMKII phosphorylation
Three-way ANOVA of CaMKII phosphorylation levels indicated a main effect of administration environment (F1,88=4.18, p<0.05) (Figure 7g,h). However, Newman-Keuls post hoc test did not indicate significant differences between specific pairs of groups.
GluR1 Ser845 phosphorylation
Three-way ANOVA of GluR1 phosphorylation levels indicated a main effect of test injection (F1,88=10.74, p<0.01); in general, cocaine test injections produced a small increase in phosphorylation levels (Figure 7i,j). However, Newman-Keuls post hoc test did not indicate significant differences between specific pairs of groups.
Discussion
Repeated cocaine administration to rats in an environment outside their home cage results in context-specific locomotor sensitization accompanied by selective alterations in cocaine-induced CREB and ERK phosphorylation in nucleus accumbens. The phosphorylation data raise two major issues. The immunohistochemical data indicate that repeated cocaine administration produces context-specific enhancement of cocaine-induced CREB and ERK phosphorylation in a minority of nucleus accumbens neurons. Meanwhile we infer from the Western blotting data that cocaine decreases phosphorylation levels in the majority of accumbens neurons when cocaine is administered outside the home cage. Overall, sensitized locomotor responsivity to cocaine appears to be context-specific and corresponds with context-specific enhancement of cocaine-induced CREB and ERK phosphorylation in a minority of accumbens neurons, but not with phosphorylation in homogenates from nucleus accumbens.
Many investigators have shown context-dependent sensitized responses to cocaine and other psychostimulants following repeated drug administration in the animal’s home cage versus a relatively novel environment outside the home cage (reviewed in Badiani & Robinson, 2004). However it is clear from the extensive work of Badiani, Robinson, and others that this novelty factor is distinct from learned associations between drug and specific stimuli sets in the drug administration environment. Thus for studies that compared repeated drug administration in the home cage versus administration outside the home cage, these two factors are intertwined in an undefined manner. We controlled for the novelty factor in the present study by repeatedly administering cocaine in two different novel environments. We used a full factorial version of the experimental design used in the “Third world” experiment of Anagnostaras & Robinson (1996), which indicated context-specific expression of sensitization of amphetamine-induced rotations in rats with unilateral 6-hydroxydopamine lesions. By controlling for novelty, our locomotor data indicate sensitized locomotor responsivity was context-specific, and not just context-dependent. It should be noted that sensitization can be context-independent (Heidbreder et al., 1996; Vanderschuren et al., 2000). This has been shown most often using higher doses of cocaine injections in the home cage. Some of the neuroadaptations mediating context-independent sensitization in these studies may be induced along with the learned associations mediating context-specific sensitization in our treatment regimen.
Conditioned inhibition by the non-drug paired environment has been hypothesized to mask expression of sensitized responses whose underlying mechanisms are inherently non-associative (Stewart & Vezina, 1991; Anagnostaras et al., 2002). In the experiments that form the basis for this hypothesis, the non-drug paired environment used to assess sensitized responsivity on test day has usually been explicitly paired with saline vehicle during the previous repeated administration procedure. Although explicit pairing of saline with the test environment may indeed produce conditioned inhibition of sensitized drug responses, it does not appear to be necessary for context-specific sensitization. Non-paired rats in our study and in the “Third world” experiment of Anagnostaras & Robinson (1996) did not express sensitized responsivity even though they were never exposed to the test environment prior to test day. This prevented any conditioned associations, inhibitory or not, with the test environment. Similar effects have been noted in other sensitization studies that compared repeated drug administration in different novel environments (Vezina & Stewart, 1987; Duvauchelle et al., 2000b; Duvauchelle et al., 2000a; Rademacher et al., 2007; Mattson et al., 2008). Overall these studies suggest that the drug-paired environment can play a facilitatory role in the expression of sensitization (see review of Vezina & Leyton, 2009). It should be noted that we consider the learned association mediating the sensitized response in the presence of drug to be distinct from the learned association mediating ‘conditioned locomotion’ in the absence of drug. ‘Conditioned locomotion’ has been clearly established as distinct from sensitization in the presence of drug (Stewart & Vezina, 1991; Carey & Gui, 1998; Robinson et al., 1998; Michel & Tirelli, 2002; Carey et al., 2005).
Immunohistochemical analyses of CREB and ERK phosphorylation in nucleus accumbens of these rats indicate a correlation between enhanced cocaine-induced phosphorylation and sensitized locomotor responses. Acute cocaine administration on test day increased the number of phosphoCREB-immunoreactive nuclei compared to saline administration, similar to previous studies with cocaine (Mattson et al., 2005; Miller & Marshall, 2005) and amphetamine (Konradi et al., 1994; Simpson et al., 1995; Turgeon et al., 1997; Self et al., 1998). We had previously found that repeated cocaine administration to rats outside their home cage enhances cocaine-induced CREB phosphorylation in nucleus accumbens (Mattson et al., 2005). We now show that this enhancement of cocaine-induced CREB phosphorylation is expressed only in the drug-paired environment, but not in the non-paired environment, and is thus context-specific.
The pattern of cocaine-induced alterations of phosphoERK immunoreactivity by drug and environment was very similar to that for phosphoCREB immunoreactivity. Acute cocaine administration on test day increased the number of phosphoERK-immunoreactive nuclei compared to saline administration, similar to previous studies with cocaine (Valjent et al., 2000; Valjent et al., 2004; Zhang et al., 2004; Mattson et al., 2005; Miller & Marshall, 2005). Previous studies further indicate that repeated cocaine administration to rats outside their home cage enhances cocaine-induced ERK phosphorylation in nucleus accumbens (Valjent et al., 2000; Mattson et al., 2005). We now show that this enhancement of cocaine-induced ERK phosphorylation is expressed only in the drug-paired environment, but not in the non-paired environment, and is thus context-specific. Previous studies indicate that cocaine administration to rats in a novel environment increases CREB phosphorylation via ERK activation (Brami-Cherrier et al., 2002; Mattson et al., 2005; Miller & Marshall, 2005), and not via PKA activation (Mattson et al., 2005). Thus context-specific enhancement of cocaine-induced ERK activation is likely responsible for the corresponding enhancement of cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats.
Context-specific sensitized locomotor responses to cocaine correlate with context-specific enhancement of cocaine-induced Fos expression in approximately 2–3% of sparsely distributed nucleus accumbens neurons when cocaine or amphetamine is repeatedly administered in a novel environment (Crombag et al., 2002; Todtenkopf et al., 2002; Hope et al., 2006; Rademacher et al., 2007; Koya et al., 2009). In the present study, the same repeated cocaine treatment regimen used in Koya et al (2009) produced context-specific enhancement of cocaine-induced phosphoCREB and phosphoERK immunoreactivity in a similarly small proportion of sparsely distributed accumbens neurons. Considering that Fos protein is a marker of neuronal activation and the c-fos gene is activated through ERK and CREB, enhanced cocaine-induced CREB and ERK phosphorylation is likely be induced in the same minority of activated accumbens neurons as Fos (Sgambato et al., 1998; Vanhoutte et al., 1999). From this, we hypothesize that learned associations between drug and specific sets of stimuli in the drug-paired environment produce context-specific enhancement of cocaine-induced activation of the ERK-CREB-Fos signal transduction pathway in a minority of strongly activated neurons in nucleus accumbens that mediate the expression of context-specific sensitization (Koya et al., 2009).
Our Western blotting assays provide a different view where cocaine decreased CREB phosphorylation levels in homogenates of nucleus accumbens. We hypothesize that cocaine increases phosphorylation levels in a small number (approximately 2–3% from Koya et al., 2009) of activated neurons while cocaine decreases phosphorylation levels in the majority of surrounding deactivated neurons. Immunohistochemical labeling of phosphoCREB-immunoreactive nuclei is limited to those neurons that were sufficiently activated to increase phosphorylation levels. Decreased phosphorylation in surrounding neurons would not be detected in these immunohistochemical assays. On the other hand, Western blotting does not distinguish between activated neurons and non-activated neurons. In homogenates used for western blotting experiments, increased CREB phosphorylation levels in a minority of activated neurons are likely diluted by unaltered or even decreased phosphorylation levels in the majority of nonactivated or deactivated neurons. The observation that cocaine decreased phosphorylation levels was somewhat unexpected since other studies observed increased phosphorylation with injections of cocaine and other psychostimulants to rats most often in their home cages. The difference may be due to higher basal levels of phosphorylation in a novel environment than basal levels produced in the home cage (Mattson et al., 2005). Higher basal levels in a novel environment allow observation of drug-induced decreases in phosphorylation levels. In contrast, lower basal phosphorylation levels in the home cage in other studies make it difficult to observe cocaine-induced decreases in phosphorylation levels using Western blotting, but easier to observe cocaine- or amphetamine-induced increases in CREB phosphorylation in the minority of activated neurons (Konradi et al., 1994; Kano et al., 1995).
The pattern of CREB phosphorylation alterations in homogenates corresponded well with the pattern of alterations in CaMKIV phosphorylation, but not with ERK, CaMK II or Ser845 GluR1 phosphorylation. CaMKIV can phosphorylate CREB (Enslen et al., 1994; Matthews et al., 1994; Sun et al., 1996; Tiraboschi et al., 2004). Assuming that these phosphorylation patterns reflect alterations in the majority of accumbens neurons, CaMKIV activity may be an important regulator of CREB phosphorylation in these neurons when cocaine is injected into rats in a novel environment. Test injections with cocaine increased endogenous PKA activity, as indicated by GluR1 serine-845 phosphorylation, similar to our previous study (Mattson et al., 2005). None of the alterations of phosphorylation levels in homogenates were context-specific, which contrasted with context-specific enhanced phosphorylation in the minority of activated neurons observed with immunohistochemistry.
Overall, sensitized cocaine-induced locomotor responses were context-specific and corresponded with context-specific enhancement of cocaine-induced CREB and ERK phosphorylation in a minority of accumbens neurons that we have previously shown to mediate context-specific sensitization (Koya et al., 2009). It is likely that this leads to context-specific alterations in gene expression in these neurons. The contrasting effects of cocaine on phosphorylation in accumbens homogenates suggest that CREB and ERK phosphorylation is regulated differently in the majority of surrounding non- or deactivated neurons. Based on our previous study (Koya et al., 2009), activation of these latter neurons do not appear necessary for context-specific sensitization, but could still play a supporting role sensitization. Differential regulation of ERK and CREB in activated versus inactivated neurons is likely to lead to differences in gene expression in these two populations of neurons that could underlie their different roles in context-specific sensitization.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, NIDA. M. T. Marin was supported by a grant from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). C. S. Planeta was supported by grant from FAPESP.
Abbreviations
- ANOVA
analyses of variance
- CaMK
calcium/calmodulin-dependent kinase
- CREB
cyclic AMP-regulated element-binding protein
- ERK
extracellular signal-regulated kinase
- PKA
cAMP-dependent protein kinase
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacology. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Badiani A, Anagnostaras SG, Robinson TE. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 1995;117:443–452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Howe ML, Stellar JR. Differential activation of cAMP response element binding protein in discrete nucleus accumbens subregions during early and late cocaine sensitization. Behav Neurosci. 2007;121:212–217. doi: 10.1037/0735-7044.121.1.212. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Gui J. Cocaine conditioning and cocaine sensitization: what is the relationship? Behav Brain Res. 1998;92:67–76. doi: 10.1016/s0166-4328(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E, Shanahan A. Stimulus gated cocaine sensitization: interoceptive drug cue control of cocaine locomotor sensitization. Pharmacol Biochem Behav. 2005;82:353–360. doi: 10.1016/j.pbb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J Neurochem. 2002;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effects on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24:680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Jedynak JP, Redmond K, Robinson TE, Hope BT. Locomotor sensitization to cocaine is associated with increased Fos expression in the accumbens, but not in the caudate. Behav Brain Res. 2002;136:455–462. doi: 10.1016/s0166-4328(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castaneda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav Neurosci. 2000a;114:1156–1166. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Asami S, Robens J, Kressin K, Castaneda E. Effects of cocaine context on NAcc dopamine and behavioral activity after repeated intravenous cocaine administration. Brain Res. 2000b;862:49–58. doi: 10.1016/s0006-8993(00)02091-6. [DOI] [PubMed] [Google Scholar]
- Enslen H, Sun P, Brickey D, Soderling SH, Klamo E, Soderling TR. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Heist EK, Srinivasan M, Schulman H. Phosphorylation at the nuclear localization signal of Ca2+/calmodulin-dependent protein kinase II blocks its nuclear targeting. J Biol Chem. 1998;273:19763–19771. doi: 10.1074/jbc.273.31.19763. [DOI] [PubMed] [Google Scholar]
- Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ. Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci. 2006;24:867–875. doi: 10.1111/j.1460-9568.2006.04969.x. [DOI] [PubMed] [Google Scholar]
- Kano T, Suzuki Y, Shibuya M, Kiuchi K, Hagiwara M. Cocaine-induced CREB phosphorylation and c-Fos expression are suppressed in Parkinsonism model mice. Neuroreport. 1995;6:2197–2200. doi: 10.1097/00001756-199511000-00023. [DOI] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14:5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Golden SA, Harvey BK, Guez DH, Berkow A, Simmons DE, Bossert JM, Nair SG, Uejima JL, Marin MT, Mitchell TB, Farquhar D, Ghosh S, Mattson BJ, Hope BT. Targeted disruption of sparsely distributed cocaine-activated accumbens neurons prevents context-specific psychomotor sensitization. Nat Neurosci. 2009;12:1069–1073. doi: 10.1038/nn.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Koya E, Simmons DE, Mitchell TB, Berkow A, Crombag HS, Hope BT. Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur J Neurosci. 2008;27:202–212. doi: 10.1111/j.1460-9568.2007.05984.x. [DOI] [PubMed] [Google Scholar]
- Michel A, Tirelli E. Conditioned hyperkinesia induced by cocaine in mice is dose-dependent but not correlated with the unconditioned response or the contextually-sensitized response. Behav Pharmacol. 2002;13:59–71. doi: 10.1097/00008877-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987;328:175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Vanderschuren LJ, Jonker AJ, Bergsma M, De Vries TJ, Pennartz CM, Voorn P. Expression of amphetamine sensitization is associated with recruitment of a reactive neuronal population in the nucleus accumbens core. Psychopharmacology. 2008;198:113–126. doi: 10.1007/s00213-008-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Fontana D, Pert A. Conditioned sensitization to the psychomotor stimulant cocaine. Ann N Y Acad Sci. 1992;654:386–399. doi: 10.1111/j.1749-6632.1992.tb25983.x. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Napier TC, Meredith GE. Context modulates the expression of conditioned motor sensitization, cellular activation and synaptophysin immunoreactivity. Eur J Neurosci. 2007;26:2661–2668. doi: 10.1111/j.1460-9568.2007.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci. 1998;18:8814–8825. doi: 10.1523/JNEUROSCI.18-21-08814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Simpson JN, Wang JQ, McGinty JF. Repeated amphetamine administration induces a prolonged augmentation of phosphorylated cyclase response element-binding protein and Fos-related antigen immunoreactivity in rat striatum. Neuroscience. 1995;69:441–457. doi: 10.1016/0306-4522(95)00274-m. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- Sun P, Lou L, Maurer RA. Regulation of activating transcription factor-1 and the cAMP response element-binding protein by Ca2+/calmodulin-dependent protein kinases type I, II, and IV. J Biol Chem. 1996;271:3066–3073. doi: 10.1074/jbc.271.6.3066. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Mihalakopoulos A, Stellar JR. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–1069. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Soderling TR. Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J Biol Chem. 1996;271:5617–5622. doi: 10.1074/jbc.271.10.5617. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Hatano N, Inuzuka H, Yokokura S, Nozaki N, Kobayashi R. Mechanism of the generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase IV. J Biol Chem. 2004;279:40296–40302. doi: 10.1074/jbc.M406534200. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Schoffelmeer AN, Wardeh G, De Vries TJ. Dissociable effects of the kappa-opioid receptor agonists bremazocine, U69593, and U50488H on locomotor activity and long-term behavioral sensitization induced by amphetamine and cocaine. Psychopharmacology. 2000;150:35–44. doi: 10.1007/s002130000424. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioned locomotion and place preference elicited by tactile cues paired exclusively with morphine in an open field. Psychopharmacology (Berl) 1987;91:375–380. doi: 10.1007/BF00518195. [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56 Suppl. 2009;1:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Giovino AA, Wise RA, Stewart J. Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav. 1989;32:581–584. doi: 10.1016/0091-3057(89)90201-3. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]