Abstract
Anti-transglutaminase antibodies are the diagnostic markers of coeliac disease. A role is suggested for infectious agents in the production of anti-transglutaminase antibodies. The aim was to measure positive anti-transglutaminase antibody levels in children with infectious diseases and to compare immunological and biological characteristics of the anti-transglutaminase antibodies derived from these children with that from coeliac patients. Two hundred and twenty-two children suffering from infectious diseases were enrolled prospectively along with seven biopsy-proven coeliacs. Serum samples were tested for anti-transglutaminase antibodies and anti-endomysium antibodies; positive samples were tested for coeliac-related human leucocyte antigen (HLA)-DQ2/8 and anti-viral antibodies. Purified anti-transglutaminase antibodies from the two study groups were tested for urea-dependent avidity, and their ability to induce cytoskeletal rearrangement and to modulate cell-cycle in Caco-2 cells, using phalloidin staining and bromodeoxyuridine incorporation assays, respectively. Nine of 222 children (4%) tested positive to anti-transglutaminase, one of whom also tested positive for anti-endomysium antibodies. This patient was positive for HLA-DQ2 and was diagnosed as coeliac following intestinal biopsy. Of the eight remaining children, two were positive for HLA-DQ8. Levels of anti-transglutaminase returned to normal in all subjects, despite a gluten-containing diet. Purified anti-transglutaminase of the two study groups induced actin rearrangements and cell-cycle progression. During an infectious disease, anti-transglutaminase antibodies can be produced temporarily and independently of gluten. The infection-triggered anti-transglutaminase antibodies have the same biological properties as that of the coeliacs, with the same in-vivo potential for damage.
Keywords: anti-transglutaminase antibodies, celiac disease, infectious diseases
Introduction
Coeliac disease (CD) is a gluten-dependent autoimmune disorder, developing in genetically susceptible individuals. The principal determinants of genetic susceptibility are the highly variable human leucocyte antigen (HLA) class II DQA and DQB genes, encoding the HLA DQ2 and DQ8 protein molecules, which present gluten peptides to CD4-positive T lymphocytes. However, it is clear that additional factors are critical for the development of CD as up to 30% of people of European ancestry, most of whom eat gluten, express HLA-DQ2/DQ8 class II, but CD develops in only a small proportion of these carriers [1].
Anti-tissue transglutaminase antibodies (anti-tTG) have become the accepted diagnostic indicator of coeliac disease (CD) [2]. The mechanisms underlying the production of anti-tTG are still not understood fully. They might be generated by a mechanism resembling the hapten-carrier model, based on evidence suggesting that gliadin combines somehow with transglutaminase enzymes in the intestinal mucosa to make macromolecular aggregates, which are processed by B cells to synthesize anti-tTG with the help of gliadin-specific CD4+ helper T cells [3]. This would also explain why serum anti-tTG disappear when coeliac patients are put on a gluten-free diet.
Other hypotheses have been put forward: up-regulation of human tTG in inflamed sites may generate new antigenic epitopes by cross-linking or deaminating external or endogenous proteins [4]; again, infectious agents may play a role in the production of autoreactive antibodies against tTG.
With regard to the latter suggestion, it was observed recently that in untreated CD patients a subset of anti-tTG recognized the rotavirus protein VP7, indicating that this viral protein may trigger autoimmunity in individuals predisposed genetically to gluten intolerance [5]. Elsewhere, it was observed that in untreated CD patients, bacteria and fungi, colonizing the small intestine, transformed both the gluten peptides and tTG into a macromolecular aggregate: this promotes the production of anti-tTG, thereby inducing the early phases of gluten-dependent intestinal inflammation [6]. We hypothesized that during an infectious disease there may be an immunological response to tTG by monitoring the serum concentration of anti-tTG in children with infectious diseases. Furthermore, we evaluated the immunological characteristics and biological activities of these particular anti-tTG and compared them with the anti-tTG of untreated coeliacs.
Materials and methods
Patients
The study was designed as a prospective follow-up of children with infectious diseases in order to measure the serum concentration of anti-tTG. The children were recruited on the basis of the following criteria: presence of fever for more than 5 days, with or without gastrointestinal complaints such as diarrhoea, vomiting, abdominal distension; absence of autoimmune diseases; and no relationship with coeliac patients. During a standard admission to the emergency room of the ‘Burlo Garofolo’ Children's Hospital in Trieste, children corresponding to the criteria were enrolled and both serum anti-tTG and anti-endomysium antibodies (AEA) were measured. Those children who tested positive for one or both of these antibodies were then tested for the CD-associated HLA DQ2 and DQ8 molecules, and for serum antibodies to the following pathogens: Epstein–Barr virus (EBV), rotavirus, adenovirus, echovirus and Coxsackievirus. Children testing positive for anti-tTG with or without HLA DQ2/DQ8 molecules were followed for 12 months on a gluten-containing diet and retested for serum CD-related antibodies and anti-viral antibodies. Children testing positive for both anti-tTG and AEA and one of the CD-associated HLA DQ molecules were offered intestinal biopsy for the diagnosis of CD.
As positive controls, age-matched biopsy-proven CD subjects were enrolled to provide serum samples. Serum anti-tTG concentration values of healthy age-matched children (enrolled in a large screening programme of CD during 2000) [7] were used as a negative control group. The anti-tTG were measured using both the same immunoassay and the cut-off values described in the present study.
Before testing for CD-associated antibodies, a detailed explanation of the study was given to the children's parents, and informed consent was obtained. The investigation was approved by the hospital's independent ethics committee (CE/V-71).
Serum antibody tests
Serum anti-tTG antibodies were measured using an immunoenzymatic assay, as described previously [8], characterized by a sensitivity of 98% and a specificity of 95% for both immunoglobulin (Ig)A and IgG assays together. The data were expressed as a percentage of the positive control serum: (optical density of the sample/optical density of the positive control) × 100. AEA were measured using an immunofluorescence assay on human umbilical cord cryosections, as described previously [9], characterized by a sensitivity of 90% and a specificity of 100%. The anti-viral antibodies were measured using both an indirect immunofluorescence assay [EBV viral capsid antigen (VCA) immunofluorescent antibody (IFA) IgG–IgM; Focus Diagnostics, Cypress, CA, USA] and a complement-fixation assay (anti-rotavirus, adenovirus, echovirus and Coxsackievirus antibodies; Virion/Serion, Würzburg, Germany), following the manufacturer's instructions. These assays were performed 1–3 days after clinical observation by two operators (N. D. T., D. M. G) blinded to the patients' clinical and laboratory data.
HLA typing
The known susceptibility alleles for CD, in particular the HLA heterodimer DQ2 DQ8, were determined by polymerase chain reaction (PCR) with allele-specific primers using a Dynal Classic SSP-DQ kit (Dynal Biotech, Hamburg, Germany).
Chromatographic anti-transglutaminase antibody purification
Serum anti-tTG were isolated and purified by an affinity chromatography using a CNBr-activated Sepharose 4B resin (GE Healthcare Bio Sciences, Uppsala, Sweden). Freeze-dried resin powder was resuspended in 1 mM HCl (ice-cold) solution, and the gel obtained was washed immediately with copious amounts of 1 mM HCl solution: 1·5 mg of human recombinant transglutaminase enzyme [10] was diluted into 350 µl of coupling buffer (NaHCO3 0·1 M, pH 8·3 containing 0·5 M NaCl); the protein solution was mixed with the gel suspension and then incubated overnight at 4°C. The gel was transferred to a 1 M ethanolamine pH 8·0 solution to block the remaining active resin groups, and the non-adsorbed proteins were washed away using coupling buffer followed by acetate buffer (0·1 M pH 4·0) containing NaCl 0·5 M. The resin was put into an affinity purification column and washed with 5 ml of phosphate-buffered saline (PBS). The serum, diluted 1 : 100 in PBS/0·05% Tween 20 solution, was pushed through the resin several times for a total of 40 min. After that, the resin was washed with 5 ml of water. Elution was performed using 500 µl of 0·2 M glycine buffer pH 2·2, and the eluate solution was buffered with 75 µl of 1 M Tris-HCL buffer pH 9·1.
Antibody avidity assay
Anti-tTG were captured by human recombinant transglutaminase bound in enzyme-linked immunosorbent assay (ELISA) plate wells that were used for the measurement of anti-tTG. After two washing rounds, low-avidity antibodies were eluted by incubating the wells with 120 µl of PBS solution containing 5 M urea, pH 8·0, for 20 min at room temperature (RT) [11]. The control wells were treated with PBS alone for the determination of reactivity values. After two washes, the remaining anti-tTG were detected by the peroxidase conjugated anti-human IgG and IgA antibodies (Sigma, St Louis, MO, USA). The results were expressed as avidity indices (AI): (optical density of the urea-treated anti-tTG reactivity/optical density of the anti-tTG reactivity without urea) × 100. Results were obtained from three different experiments.
Transglutaminase fragments recognition
The tTG-derived fragments were cloned and expressed as reported [12]. In the present study we used one tTG fragment well recognized by CD patients' sera (fragment 11; amino acids 1–376; 45 kDa) and one not recognized (fragment 8; amino acids 269–687; 60 kDa). The chromatographic purified antibodies of both CD patients and patients with infectious diseases were tested for their ability to recognize full-length transglutaminase and the derived fragments using an ELISA assay. Peroxidase-conjugated anti-human IgA and IgG were used as secondary antibodies in the ELISA assay. The data were expressed as the following ratio: (optical density of the purified antibodies against fragment/optical density of the purified antibodies against full-length transglutaminase) × 100. Results were obtained from three different experiments.
Actin rearrangement assay
Caco-2 cells were cultured as described previously [13]. Cells on glass cover slips were placed for 30 min in serum-free media [supplemented with 0·1% bovine serum albumin (BSA)], then treated for 30 min with the purified anti-tTG or with commercial anti-tTG CUB7402 (NeoMarkers, Fremont, CA, USA) as positive controls or total human immunoglobulins as negative controls. All antibodies were used at a final concentration of 2 µg/ml. Cells were then fixed in 3% paraformaldehyde, permeabilized with 0·2% Triton X-100 and incubated with 0·1 mg/ml fluorescein isothiocyanate (FITC)-labelled phalloidin (Sigma-Aldrich, St Louis, MO, USA) for 45 min. After washing with PBS, stained cells were observed with an Axioskop 40 fluorescent microscope. Images were acquired and processed using the Axiovision software (Carl Zeiss MicroImaging s.p.a., Milano, Italy).
Cell cycle assay
Caco-2 cells were seeded on coverslips and were cultured 24 h later in 0·1% fetal bovine serum medium for 72 h. Cells were then challenged separately for 24 h with purified anti-tTG at 2 and 4 µg/ml, with commercial tTG-antibody CUB7402 at 2 µg/ml and total human Ig at 4 µg/ml. Three hours before paraformaldehyde fixing and Triton X-100 permeabilizing, bromodeoxyuridine (BrdU) (Roche Diagnostic s.p.a., Monza, Italy) was added to the medium (final concentration 100 µM). BrdU incorporation was monitored by treating cells with an anti-BrdU antibody 1 : 100 (Invitrogen) and a mouse secondary tetramethyl rhodamine isothiocyanate (TRITC)-conjugated antibody (Invitrogen) 1 : 100. S-Phase entry was expressed as the ratio: (number of cells incorporating BrdU/total number of cells) × 100. Results were derived from three different experiments.
Statistics
Statistical analysis was performed where appropriate using Student's t-test or Fisher's exact test. Differences were considered to be statistically significant at P < 0·05.
Results
Between October 2007 and June 2008, we enrolled 222 children (104 females, 118 males, median age 6·5 years) who fulfilled the study criteria. One hundred and six children (47·7%) were suffering from sore throat with severe pharyngitis. Half these cases were accompanied with both an enanthem and lymphadenopathy, 42 (19%) from gastrointestinal complaints, 42 (19%) from acute otitis media, 29 (13%) from pneumococcal pneumonia, two (0·9%) from varicella and one (0·4%) from rheumatic fever.
Nine patients (4%, three female, six male, median age 7 years) were tested positive for anti-tTG; two were positive for both IgA and IgG, one for IgA and six for IgG anti-tTG. One patient positive for both IgA and IgG anti-tTG and with rheumatic fever tested positive for AEA while the other eight were AEA-negative. Of the eight subjects testing negative to AEA, two were carriers of HLA DQ8 and the AEA-positive child was tested positive for HLA DQ2. This child was suffering from anaemia (Hb 10 g/100 ml, ferritin 10 ng/ml) and the parents agreed for him to undergo an intestinal biopsy that showed the typical CD intestinal lesions. The patient had been on a gluten-free diet and had been followed for 12 months.
Among 1276 age-matched healthy children (538 females, 738 males, median age 6·7 years), we identified 11 subjects (0·8%) who tested positive for anti-tTG (four positive for IgA, mean value 30%; five for IgG, mean value 70%; two for both IgA and IgG, mean value 43% and 73%, respectively) but negative for both AEA and CD-associated HLA DQ molecules. Due to the absence of both CD-related HLA and AEA tests, these anti-tTG data were considered as false positive results for the diagnosis of CD. The prevalence of the anti-tTG positive test among the infected children was significantly higher than in healthy children [eight of 222 versus 11 of 1276, P = 0·003, odds ratio (OR) 4·29, 95% confidence interval (CI): 1·4–11·8].
Of the nine children testing positive for anti-tTG, five tested positive for IgM anti-EBV antibodies and two for IgG Coxsackievirus antibodies.
During 12 months of a gluten-containing diet the anti-tTG returned to normal levels in all eight subjects, as did the IgM anti-EBV and the anti-Coxsackievirus antibodies.
After 12 months on a gluten-free diet the coeliac patient's anaemia improved (Hb 13 g/100 ml, 23 ng/ml), his anti-tTG concentration returned to normal levels, and he tested negative for AEA (Table 1).
Table 1.
Clinical and laboratory findings of the subjects with infectious diseases tested positive for anti-tissue transglutaminase antibodies (tTG); normal values of anti-tTG for IgA <16 and for IgG <42.
| Anti-tTG |
AEA |
Clinical findings |
Presence of antiviral antibodies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment |
Follow-up |
Enrolment |
Follow-up |
|||||||||
| Sex | Age (years) | IgA | IgG | IgA | IgG | IgA | IgA | DQ typing HLA | Enrolment | Follow-up | Enrolment | Follow-up |
| F | 4 | 52·4 | 70·4 | 3·8 | 7·4 | − | − | − | Pharyngitis and lymphadenopathy | Improved | IgM anti-EBV | − |
| F | 8 | 145·4 | 125·5 | 7·5 | 5·4 | + | − | DQ2 | Rheumatic fever, anaemia, coeliac disease | Anaemia and rheumatic fever improved | − | − |
| F | 10 | 4·9 | 46·9 | 3·0 | 9·3 | − | − | DQ8 | Pharyngitis and lymphadenopathy | Improved | IgM anti-EBV | − |
| M | 5 | 23·5 | 14·9 | 5·3 | 2·9 | − | − | − | Pharyngitis and lymphadenopathy | Improved | IgM anti-EBV | − |
| M | 5 | 49·0 | 63·3 | 10·4 | 12·6 | − | − | DQ8 | Pharyngitis and lymphadenopathy | Improved | IgG anti-Coxsackie | − |
| M | 6 | 5·0 | 47·2 | 5·0 | 8·4 | − | − | − | Pharyngitis and lymphadenopathy | Improved | IgG anti-Coxsackie | − |
| M | 6 | 3·4 | 87·9 | 4·1 | 17·5 | − | − | − | Pharyngitis and lymphadenopathy | Improved | IgM anti-EBV | − |
| M | 7 | 2·7 | 50·0 | 2·5 | 10·0 | − | − | − | Pharyngitis and lymphadenopathy and varicella | Improved | − | − |
| M | 13 | 1·9 | 46·9 | 2·3 | 12·2 | − | − | − | Pharyngitis and lymphadenopathy | Improved | IgM anti-EBV | − |
Positive values are expressed in bold type. F: female; M: male; Ig: immunoglobulin; HLA: human leucocyte antigen; AEA: anti-endomysium antibodies; EBV: Epstein–Barr virus.
Antibody avidity assay
We obtained highly purified antibodies from five patients with infectious diseases and from seven biopsy-proven CD. The eluted anti-tTG antibodies did not identify cow's milk proteins and gliadin proteins using an ELISA assay (data not shown). The anti-tTG reactivity was measured to normalize the quantity of antibodies to be used in the avidity assay. The purified anti-tTG of five patients with infectious diseases (one tested positive for IgA anti-tTG) and of seven CD patients gave no statistically different results in avidity assays for both IgA and IgG anti-tTG [AI mean value of patients with infectious disease 65·1 ± standard deviation (s.d.) 9·3 versus 54·6 ± s.d. 11·3 of coeliacs: P = 0·12].
Transglutaminase fragments recognition
Chromatographic purified anti-tTG derived from both patients with infections and with CD were compared for their reactivity against fragments of the tTG protein: fragments 11 and 8. The purified anti-tTG from the children with infectious disease did not produce significant reactivity differences between the two fragments (mean value 80 ± s.d. 20 ratio values of fragment 11 versus 67 ± s.d. 21 of fragment 8, for infected children, P = 0·35). In contrast, the coeliacs' purified anti-tTG showed significant reactivity differences (81 ± s.d. 19 of fragment 11 versus 35 ± s.d. 13 of fragment 8 for coeliacs, P = 0·002) (Fig. 1).
Fig. 1.
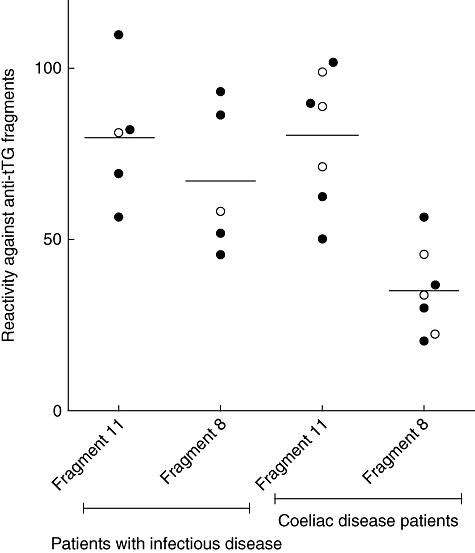
Reactivity of the purified anti-tissue transglutaminase antibodies (tTG) against the transglutaminase-derived fragments in the two study groups. The purified anti-tTG from the children with infectious disease did not produce significantly reactive differences between the two fragments (P = 0·35), while the coeliacs' purified anti-tTG showed significantly reactive differences (P = 0·002). The open and filled circles represent the samples tested for immunoglobulin (Ig)A and for IgG, respectively.
Actin rearrangement assay
By challenging Caco-2 cells with purified anti-tTG from both coeliacs and patients with infections and with anti-tTG CUB7402, actin rearrangements and large membrane ruffling formations were found in comparison to untreated cells and those treated with total human Ig, which maintained a regular geometrical shape (Fig. 2).
Fig. 2.
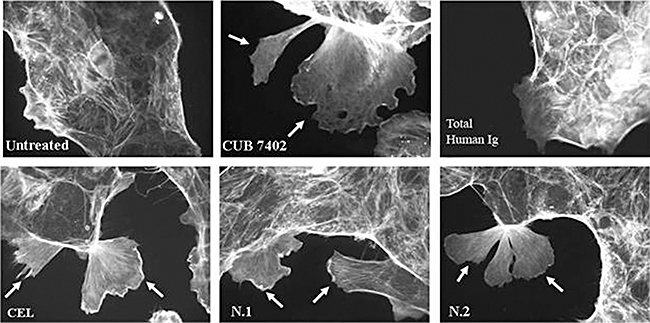
Actin rearrangement of Caco-2 cells after incubation with 2 µg of purified anti-tissue transglutaminase antibodies (tTG) from one coeliac disease (CD) patient (CEL) and from two patients with infectious diseases (N.1 and N.2), and from anti-tTG clone CUB7402, are also shown. No actin rearrangement was seen in the cells treated with total human immunoglobulin and in the untreated cells. Arrows indicate the membrane ruffles.
Cell cycle assay
We induced Caco-2 cells into S-phase, measured as a percentage of cells that incorporated BrdU, in a significant and well-reproducible manner. Clone CUB7402 induced Caco-2 cells into S-phase and the higher BrdU incorporation was at 2 µg/ml (Fig. 3). By treating cells with purified anti-tTG from CD patients and patients with infectious diseases we observed an increase in the number of proliferating cells, as determined by BrdU incorporation [mean values of BrdU incorporation: infected patients 45 ± s.d. 2·3; coeliacs 51 ± s.d. 1·7; all values are significantly different (P < 0·05) from the BrdU incorporation values obtained with total human immunoglobulins, 37·6 ± s.d. 1·5] (Fig. 3).
Fig. 3.
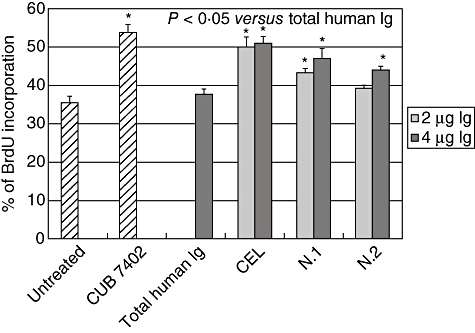
Induction of S-phase entry in Caco-2 cells by different amounts of total purified anti-tissue transglutaminase antibodies (tTG) from one coeliac disease (CD) patient (CEL) and two patients with infectious diseases (N.1 and N.2). Total human immunoglobulins were used as negative control. The effects of 2 µg/ml CUB7402 are also shown. Data are reported as mean values ± standard deviation from three separate experiments. *P < 0·05 versus total human Ig.
Discussion
This is the first study where anti-tTG have been shown to be produced temporarily during an infectious disease, independently of coeliac disease. Acute viral infections in children and adults have long been suggested to induce an autoimmune response, including generation of autoantibodies in which their titres are low and the autoimmune course transient [14].
In our study, more than half the children who tested positive to anti-tTG temporarily presented clinical and serological evidence of EBV infection. This supports the hypothesis that EBV contributes to antibody-mediated autoimmunity. Recent studies suggest that EBV latent membrane proteins might contribute directly to the development of autoimmunity [15] by inducing the expansion and survival of autoreactive B cells with the production of autoantibodies. This accounts for the transient appearance of autoantibodies during mononucleosis. In our cases the anti-tTG disappeared, together with the anti-EBV antibodies, along with the children's clinical improvement.
These findings indicate that anti-tTG should no longer be seen as a province of CD, but might represent an immunological phenomenon depending on yet-to-be identified triggers (overexpression of the autoantigen, viral infection). Thus, autoantigenic challenges caused by infectious agents and/or inflammatory reactions during the lifetime could be responsible for the age-dependent increase of anti-tTG in the general population, as we observed among eight different healthy age groups [8].
Our data are in line with recent data regarding serum anti-tTG concentrations in patients with acute coronary syndromes, in which antibody concentrations were correlated significantly with severity of cardiac event and with serum values of the biochemical markers of myocardial necrosis [16]. Furthermore, anti-tTG concentrations decreased to the baseline after 150 days of cardiological events, confirming an association with the cardiac event and pointing away from an underlying genetic predisposition to gluten intolerance. None of the anti-tTG-positive patients tested positive to AEA assay. Taken together, these observations reinforce the idea that there are differences between coeliac and non-coeliac patients with regard to the immunological machinery involved in generating anti-tTG.
The humoral autoimmune response in CD involves one main conformational immunodominant epitope located in the core of tTG spanning from the N-terminus to the catalytic region, proved by reactivity to the tTG-11 fragment. However, significantly lower reactivity was registered to the tTG-8 fragment mapped outside that region [12]. In this study, the purified anti-tTG from children with infections did not recognize the AEA antigen and showed an unselective recognition pattern of the two tTG fragments. These findings suggest that the anti-tTG of the infected children are characterized by a wider polyclonal reactivity which ensures stronger binding to the antigen molecule, as demonstrated by the avidity assay. Nevertheless, the infected children's anti-tTG had similar features to the CD patients' anti-tTG, as they modulated the cytoskeletal rearrangement of Caco-2 cells in vitro and induced their entry in S-phase. These findings are highly topical, given the lively ongoing debate regarding the role of anti-tTG in the pathogenesis of CD in promoting the small-bowel mucosal remodelling seen in untreated coeliacs [17].
These antibodies might have a modulatory effect in remodelling inflamed tissues in non-coeliac patients, at least during the time of autoantibody production. Our hypothesis is supported by the evidence that in an animal model artificially induced anti-tTG lead to organ damage during their production [18,19].
In conclusion, a low prevalence of high levels of tTG can be found in patients suffering from infectious diseases. The infection-derived anti-tTG exert the same biological properties as CD-derived antibodies. From a clinical viewpoint, AEA or the tTG fragment-based ELISA assay should be used to validate the anti-tTG-positive response in subjects with infectious diseases before considering a diagnosis of CD.
Acknowledgments
This work was supported by IRCCS Burlo Garofolo RC 28/07, 20/05.
Disclosure
The authors have declared that there is no conflict of interest.
References
- 1.Green P, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Hill I. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology. 2005;128:S25–S32. doi: 10.1053/j.gastro.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Sollid L, Molberg O, McAdam S, et al. Autoantibodies in celiac disease: tissue anti-transglutaminase – guilt by association? Gut. 1997;41:851–2. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccocioppo R, Di Sabatino A, Ara C, et al. Gliadin and tissue transglutaminase complexes in normal and coeliac duodenal mucosa. Clin Exp Immunol. 2003;134:516–245. doi: 10.1111/j.1365-2249.2003.02326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanoni G, Navone R, Lunardi C, et al. In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. Plos Med. 2006;3:1637–53. doi: 10.1371/journal.pmed.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skovbjerg H, Noren O, Anthonsen D, et al. Gliadin is a good substrate of several transglutaminases: possible implication in the pathogenesis of celiac disease. Scand J Gastroenterol. 2002;37:812–7. [PubMed] [Google Scholar]
- 7.Tommasini A, Not T, Kiren V, et al. Mass screening for celiac disease using antihuman transglutaminase antibody assay. Arch Dis Child. 2004;89:512–5. doi: 10.1136/adc.2003.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldas V, Not T, Tommasini A, et al. Anti-transglutaminase antibodies and age. Clin Chem. 2004;50:1856–60. doi: 10.1373/clinchem.2004.036012. [DOI] [PubMed] [Google Scholar]
- 9.Not T, Città A, Lucchesi A, et al. Anti-endomysium antibody on human umbilical cord vein tissue: an inexpensive and sensitive diagnostic tool for the screening of coeliac disease. Eur J Pediatr. 1997;156:616–8. doi: 10.1007/s004310050676. [DOI] [PubMed] [Google Scholar]
- 10.Sblattero D, Berti I, Trevisiol C, et al. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000;95:1253–7. doi: 10.1111/j.1572-0241.2000.02018.x. [DOI] [PubMed] [Google Scholar]
- 11.Westerlund A, Ankelo M, Simell S, et al. Affinity maturation of immunoglobulin A anti-tissue transglutaminase autoantibodies during development of coeliac disease. Clin Exp Immunol. 2007;148:230–40. doi: 10.1111/j.1365-2249.2007.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sblattero D, Florian F, Azzoni E, et al. The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur J Biochem. 2002;269:5175–81. doi: 10.1046/j.1432-1033.2002.03215.x. [DOI] [PubMed] [Google Scholar]
- 13.Barone M, Caputo I, Ribecco M, et al. Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology. 2007;132:1245–53. doi: 10.1053/j.gastro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007;19:636–43. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 15.Swanson-Mungerson M, Longnecker R. Epstein–Barr virus latent membrane protein 2A and autoimmunity. Trends Immunol. 2007;28:213–18. doi: 10.1016/j.it.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Di Tola M, Barillà F, Trappolini M, et al. Anti tissue transglutaminase antibodies in acute coronary syndrome: an alert signal of myocardial tissue lesion? J Intern Med. 2008;263:43–51. doi: 10.1111/j.1365-2796.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- 17.Korponay-Szabo I, Halttunen T, Szalai Z, et al. In vivo targeting of intestinal and extraintestinal trasglutaminase 2 by celiac autoantibodies. Gut. 2004;53:641–8. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Niro R, Sblattero D, Florian F, et al. Anti-idiotypic response in mice expressing human autoantibodies. Mol Immunol. 2008;45:1782–91. doi: 10.1016/j.molimm.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Freitag T, Schulze-Koops H, Niedobitek G, et al. The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev. 2004;3:13–20. doi: 10.1016/S1568-9972(03)00054-5. [DOI] [PubMed] [Google Scholar]


