Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of disease ranging from hepatocellular steatosis through steatohepatitis to fibrosis and irreversible cirrhosis. The prevalence of NAFLD has risen rapidly in parallel with the dramatic rise in obesity and diabetes,1,2 and is rapidly becoming the most common cause of liver disease in Western countries.3 Indeed, NAFLD is now recognized to be the aetiology in many cases previously labelled as cryptogenic cirrhosis.4
In Western populations, estimates of NAFLD prevalence vary between 20 and 30%,5,6 rising up to 90% in morbidly obese individuals.7 The more severe, and clinically significant form of NAFLD, non-alcoholic steatohepatitis (NASH) is less common, affecting an estimated 2–3% of the general population,8 and up to 37% of the morbidly obese.7 Of particular concern, and with significant implications for future disease burden, is the increasing prevalence of NAFLD in children and young adults. Studies have reported a 3% prevalence of NAFLD in the general paediatric population, rising to 53% in obese children.9,10 NAFLD has a strong association with type 2 diabetes, with steatosis present in 70% of type 2 diabetics screened with ultrasound,11 and thus it is now recognized to represent the hepatic manifestation of the metabolic syndrome.
NAFLD occurs in all ethnic groups although it appears to have a lower prevalence in African-Americans compared with Hispanic and European Americans. This difference remains even after controlling for obesity and insulin resistance (IR)5,12 and may be related to ethnic differences in lipid homeostasis.5
There are no laboratory, imaging or histological findings which can accurately distinguish between NAFLD and alcohol-induced steatosis or steatohepatitis, and the diagnosis can therefore only be made in the absence of a history of significant alcohol intake. Other specific causes of steatosis need to be considered and include metabolic disorders e.g. lipodystrophy and abetalipoproteinaemia, nutritional causes such as rapid weight loss, jejuno-ileal bypass and total parenteral nutrition, and drug-induced. Commonly implicated agents include glucocorticoids, methotrexate, amiodarone, synthetic oestrogens, tamoxifen, diltiazem and highly active anti-retroviral drugs.13–15 Steatosis also commonly occurs in association with hepatitis C, particularly genotype 3, and has an increased prevalence in women with polycystic ovary syndrome, when it is usually associated with IR.16
In the great majority of patients NAFLD develops in association with features of IR and the metabolic syndrome. The metabolic syndrome comprises a cluster of clinical and biochemical features, namely IR, glucose intolerance or diabetes, central obesity, hypertension and dyslipidaemia and is associated with significant cardiovascular morbidity and mortality.17–19
Whilst simple steatosis in the absence of significant fibrosis is considered to be a relatively benign condition,20 the presence of fibrosis predicts both disease progression and liver-related complications over a subsequent 10-year period.21 Decreased survival in this sub-group is due to predominantly cardiovascular causes, although there is a significant increase in liver-related deaths.21 NASH also carries an increased risk of hepatocellular carcinoma (HCC)21 and thus the observation of increased incidence of HCC in type 2 diabetics22 is likely to be due to their high prevalence of NASH.21 In a recent US study, NASH was found to account for at least 13% of overall cases of HCC.23
There are as yet few proven therapies available for patients with NASH, and current strategies are directed towards improving aspects of the metabolic syndrome. Ultimately when such measures fail, liver transplantation remains the only option for patients with end-stage cirrhosis.
Although the pathogenesis of NAFLD/NASH is not yet fully understood, much progress has been made in recent years in elucidating the mechanisms of progression from steatosis to more advanced liver inflammation and fibrosis. In this review, we discuss the current understanding of NAFLD pathogenesis, and anticipate that such knowledge will eventually translate into the development of novel treatment strategies for this increasingly important disease.
NAFLD pathogenesis
The ‘2-hit hypothesis’
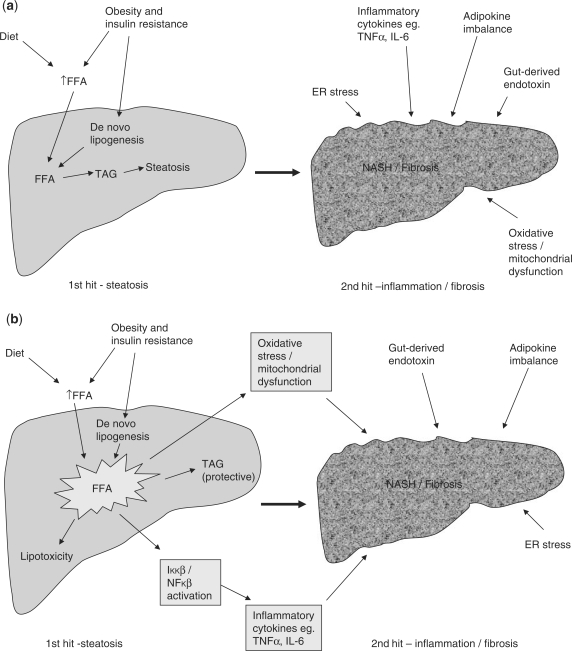
Initial theories for the pathogenesis of NASH were based on a ‘2-hit hypothesis’ (Figure 1a). The ‘first hit’, hepatic triglyceride accumulation, or steatosis, increases susceptibility of the liver to injury mediated by ‘second hits’, such as inflammatory cytokines/adipokines, mitochondrial dysfunction and oxidative stress, which in turn lead to steatohepatitis and/or fibrosis.24,25 However, there is increasing recognition of the role that free fatty acids (FFA) play in directly promoting liver injury, which has led to modification of this theory (Figure 1b). In obesity and IR there is an increased influx of FFA to the liver. These FFA either undergo β-oxidation or are esterified with glycerol to form triglycerides, leading to hepatic fat accumulation. There is now substantial evidence that FFA can directly cause toxicity by increasing oxidative stress and by activation of inflammatory pathways,26 therefore hepatic triglyceride accumulation may be a protective mechanism by preventing the toxic effects of unesterified FFA.27 Additionally, a further component, or ‘third-hit’ has been added to reflect inadequate hepatocyte proliferation (Figure 1c).28 In the healthy liver, cell death stimulates replication of mature hepatocytes which replace the dead cells and reconstitute normal tissue function.28 However oxidative stress, a central feature of NAFLD pathogenesis, inhibits the replication of mature hepatocytes which results in expansion of the hepatic progenitor cell (oval cell) population.29 These cells can differentiate into hepatocyte-like cells, and both oval cell and intermediate hepatocyte-like cell numbers are strongly correlated with fibrosis stage, suggesting that cumulative hepatocyte loss promotes both accumulation of progenitor cells and their differentiation towards hepatocytes.29 Activation of these cells has also been implicated in hepatocellular carcinogenesis.29 In chronic liver injury, the development of fibrosis/cirrhosis is dependent on the efficacy of hepatocyte regeneration, and therefore cell death with impaired proliferation of hepatocyte progenitors represents the proposed ‘third hit’ in NAFLD pathogenesis.28
Figure 1.
(a) The traditional 2-hit hypothesis: steatosis represents the ‘first hit’, which then sensitises the liver to injury mediated by ‘second hits’, such as inflammatory cytokines, adipokines, oxidative stress and mitochondrial dysfunction, leading to steatohepatitis and fibrosis. The presence of high levels of oxidative stress reduces the ability of mature hepatocytes to proliferate, resulting in reduced endogenous liver repair. (b) Modified 2-hit hypothesis: the accumulation of FFA alone has been suggested to be sufficient to induce liver damage, without recourse for a second hit. Indeed, rather than being harmful, triglyceride accumulation in the form of steatosis may actually be protective by preventing FFA-induced inflammation and oxidative stress. (c) The 3-hit hypothesis: oxidative stress reduces the ability of mature hepatocytes to proliferate, resulting in the recruitment of other pathways of liver regeneration, such as HPCs. These cells have the capability of differentiating into both cholangiocytes and hepatocytes and contributing to liver repair. It has been suggested that an inability to mount such a ductular response, as is seen in patients transplanted for NASH who have denervated livers, may be responsible for a more progressive pattern of liver damage. Thus, impaired proliferation of hepatocyte progenitors represents the proposed ‘third hit’ in NAFLD pathogenesis.28
Lipid accumulation/steatosis
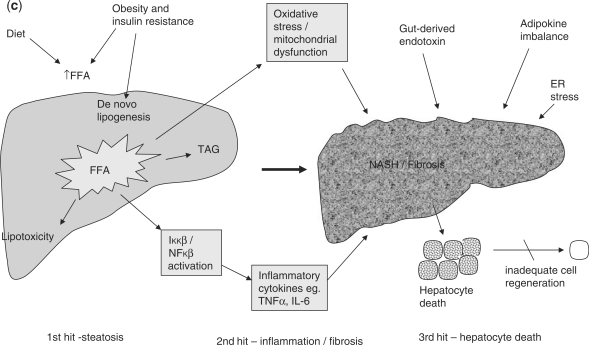
NAFLD is characterized by the accumulation of triglycerides, which are formed from the esterification of FFA and glycerol within the hepatocyte. FFAs arise in the liver from three distinct sources; lipolysis (the hydrolysis of FFA and glycerol from triglyceride) within adipose tissue, dietary sources, and de novo lipogenesis (DNL).30 In contrast, FFA may be utilized either through β-oxidation, re-esterification to triglycerides and storage as lipid droplets, or packaged and exported as very low density lipoprotein (VLDL). Hence hepatic fat accumulation can occur as a result of increased fat synthesis, increased fat delivery, decreased fat export, and/or decreased fat oxidation (Figure 2).30
Figure 2.
Mechanisms of hepatic fat accumulation.
To establish the relative contribution of lipid accumulation in patients with NAFLD, Donnelly et al. used a multiple-stable-isotope method, demonstrating that approximately 60% of liver triglyceride content derived from FFA influx from adipose tissue, 26% from DNL, and 15% from diet.31 This contrasts with healthy individuals in whom DNL contributes <5% of hepatic triglyceride formation.32,33
Triglyceride can also be exported from the liver in VLDL particles, which are formed by the incorporation of triglyceride into apolipoprotein B (apoB) by microsomal transfer protein (MTP).34 Aberrant alterations of MTP/apoB synthesis and secretion have been proposed as potential mechanisms underpinning the pathogenesis of NAFLD leading to a decreased capacity for lipid export.35,36
Insulin resistance
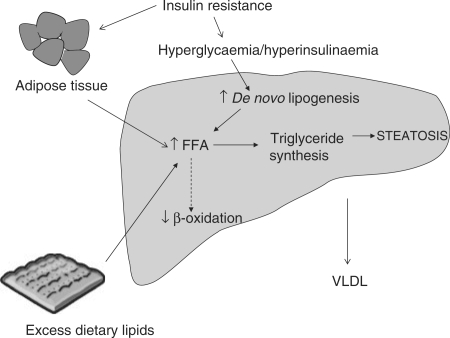
In healthy individuals, binding of insulin to its receptor leads to phosphorylation of several substrates including insulin receptor substrates (IRS)-1, -2, -3 and -4, which propagate the insulin signal.30,37 Insulin stimulation of IRS-1 and -2 leads to activation of intracellular PI3K (phosphoinositide 3-kinase) and AKT/PKB (protein kinase B) pathways, which are intimately involved in mediating the metabolic effects of insulin.30 Ultimately, AKT/PKB activation results in translocation of glucose transporter, GLUT4, containing vesicles to the plasma membrane, thus facilitating glucose uptake. In addition, the expression of key lipogenic genes is increased, with a concomitant decrease in gluconeogenic gene expression via its regulation of forkhead (FOXO) transcription factor activity.
Insulin has a potent action to suppress adipose tissue lipolysis. However, in situations of IR, such as NAFLD, this suppression is impaired resulting in an increased efflux of FFA from adipose tissue.38 The hyperinsulinaemia associated with IR leads to: (i) up-regulation of the transcription factor sterol regulatory element binding protein-1c (SREBP-1c), which is a key transcriptional regulator of genes involved in DNL,15 and (ii) Inhibition of β-oxidation of FFA thus further promoting hepatic lipid accumulation.30
Many of the abnormalities reported in NAFLD interfere with the insulin signalling cascade, and thus contribute to IR. These include FFAs, tumour necrosis factor-alpha (TNF-α), nuclear factor kappa B (NF-κB), ceramide, jun N-terminal kinase 1 (JNK1), SOCS (suppressors of cytokine signalling) and cytochrome CYP2E1.39,40 Increased lipid metabolites such as diacylglycerol (DAG) have been implicated in a protein kinase Cε (PKCε) dependent mechanism, to interfere with insulin signalling through inhibition of insulin receptor activity and modulation of IRS-2 phosphorylation.30 Similar processes occur in skeletal muscle cells, leading to a more generalized state of IR.
Inflammation/steatohepatitis
Inflammatory cytokines and FFA
The presence of steatosis is tightly associated with chronic hepatic inflammation,41 an effect in part mediated by activation of the Iκκ-β/NF-κB signalling pathway. In murine models of high-fat diet (HFD)-induced steatosis, increased NF-κB activity is associated with elevated hepatic expression of inflammatory cytokines such as TNF-α, interleukin-6 (IL-6) and interleukin 1-beta (IL-1β), and activation of Kupffer cells.41 Liver-specific NF-κB inhibition prevents HFD-induced inflammatory gene expression, whereas HFD-induced hyperglycaemia and IR can be reproduced by selective over-expression of constitutively active Iκκ-β in hepatocytes.41 The Iκκ-β/NF-κB pathway in hepatocytes can also be activated directly by FFA, providing a further mechanism by which central obesity with consequent increased hepatic FFA supply can contribute to inflammation (Figure 3).26 Furthermore, the conversion of FFA to hepatic triglyceride may serve as a protective measure to prevent direct hepatic lipotoxicity. This is endorsed by a murine model of NAFLD, where inhibition of DGAT2, the enzyme that catalyzes the final step in triglyceride synthesis, resulted in improvement of hepatic steatosis and IR but exacerbation of injury and fibrosis.27,42
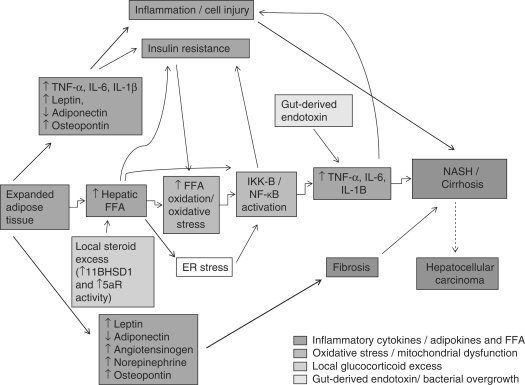
Figure 3.
Proposed pathogenesis of NASH. The likelihood of progression to advanced NASH/cirrhosis results from a complex interplay between genetic predisposition and the mechanisms described earlier.
Both serum and hepatic levels of TNF-α are elevated in patients with NASH,43,44 and levels correlate with histological severity.45 In addition to its proinflammatory effects, TNF-α promotes IR.46 Conversely, inhibition of TNF-α signalling improves IR and histological parameters of NASH.47–49 Similarly, serum IL-6 levels are also elevated in both animal and human models of IR and NAFLD,43,50,51 and levels correlate with increasing liver inflammation and fibrosis.52 The key role of hepatocyte cytokine production in the progression of steatosis to NASH is supported by studies demonstrating that cytokines can replicate all of the histological features associated with NASH, including neutrophil chemotaxis, hepatocyte apoptosis/necrosis, Mallory body formation and stellate cell activation.25 Additionally, data suggests that inflammation and NF-κB activation can promote carcinogenesis,53 and that the chronic inflammatory state associated with hepatic steatosis may also play a key role in HCC development.25
Adipokines
Adipose tissue is not just an inert site of energy storage, but an actively secreting endocrine organ. The functional role of adipocyte-derived cytokines (adipokines), is now increasingly recognized, with leptin and adiponectin amongst the most well described. Leptin is a 16 kDa hormone produced mainly by mature adipocytes whose actions include the regulation of energy intake and expenditure,54 regulation of the immune system,55,56 and promotion of inflammation and fibrogenesis.56,57 Higher leptin levels are observed in obese patients and those with NAFLD,54,58–60 which are commonly regarded as states of leptin resistance.58 It remains plausible that leptin may have a functional role to play in the pathogenesis of NAFLD.
In contrast to leptin, secretion and circulating levels of adiponectin are inversely proportional to body fat content,61 and are reduced in patients with NAFLD.44,62 Adiponectin is anti-inflammatory and increases insulin sensitivity,63 and the administration of recombinant adiponectin improves hepatomegaly, as well as the biochemical and histological parameters of NAFLD in a murine model.64,65 Adiponectin antagonises the effects of TNF-α, which itself suppresses adiponectin production.61 The importance of adiponectin in NAFLD is supported by studies showing that serum adiponectin levels can help to distinguish NASH from simple steatosis.44,66,67 Other adipose tissue derived factors found in excess in NAFLD include TNF-α, IL-6, angiotensinogen and resistin, all of which antagonise the lipogenic effects of insulin,28 but their precise role in the pathogenesis of NAFLD remains to be determined (Figure 3).
Oxidative stress and mitochondrial dysfunction
The role of oxidative stress and mitochondrial dysfunction in NASH is well-established, with more advanced disease correlating with greater degrees of oxidative stress.68–71 β-oxidation within the normal liver takes place in the mitochondria, but in the context of NAFLD72 this process can become overwhelmed as a result of increased FFA load, giving rise to reactive oxygen species (ROS).71 ROS induce oxidative stress, with subsequent activation of inflammatory pathways,73 and also mitochondrial damage. Structural mitochondrial abnormalities71 and a reduction in mitochondrial respiratory chain activity have been observed in human studies of NASH.74 Elevated expression and activity of the hepatic microsomal fatty acid oxidizing enzyme cytochrome P450 2E1 (CYP2E1) has been observed in human and animal models of NASH and represents a potent source of ROS.75,76 Importantly, transgenic over-expression of CYP2E1 activity is associated with oxidative stress, IR and hepatic fat accumulation.77
ER stress and bacterial overgrowth
Other mechanisms implicated in NASH pathogenesis include endoplasmic reticulum (ER) stress and gut-derived endotoxinaemia.25 ER stress can be caused by a variety of biological stresses, including hyperinsulinaemia and hyperlipidaemia,25,78 and can result in activation of various pathways leading to IR, inflammation, apoptosis and mitochondrial dysfunction. ER stress is known to be important in alcohol-induced steatohepatitis and further study of its role in NASH is warranted.25
Evidence is also emerging for a role of bacterial overgrowth in the pathogenesis of NASH. Bacterial overgrowth results in production of ethanol79 and release of bacterial lipopolysaccharides, both of which can activate TNF-α production in Kupffer cells and thus induce hepatic inflammation.80 Small intestinal bacterial overgrowth and increased gut permeability have been found more frequently in patients with NASH when compared with controls.81,82 This has led to the suggestion that this may explain the onset of NASH and liver fibrosis as a complication of jejunoileal bypass surgery.83,84 This hypothesis is further supported by evidence that alteration of gut flora with antibiotics85 and probiotics49,86,87 can reduce hepatic inflammation in both animals and humans.
Glucocorticoids
GCs from both exogenous and endogenous sources are a well-recognized cause of NAFLD. Patients with Cushing's syndrome, who have increased circulating GC levels develop a characteristic metabolic phenotype of central obesity, IR and diabetes. Importantly, a significant proportion of these patients will also develop hepatic steatosis.88 The mechanisms by which GCs promote hepatic fat accumulation include inhibition of fatty acid β-oxidation and promotion of hepatocyte DNL. However most patients with NAFLD have normal circulating cortisol levels, suggesting that tissue-specific mechanisms are driving the metabolic dysfunction.
This has led to emerging interest in two enzyme systems which play a key role in local GC metabolism and consequent GC availability to bind and activate the GC receptor. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) converts inactive cortisone to the active GC cortisol,89 and thus increases local GC levels and amplifies GC action. Inhibition of 11βHSD1 has been shown to lower body weight and lipid levels and improve glucose tolerance in animal models,90 and increase hepatic insulin sensitivity in humans.91,92
In parallel, the A-ring reductase enzymes, 5-α- and 5-β reductase, are responsible for the metabolism of cortisol to its inactive tetrahydrometabolites. Increased hepatic 5-α reductase (5αR) activity has been demonstrated in patients with IR89 and NAFLD,93 which may represent a compensatory mechanism to decrease local GC availability in an attempt to prevent development or progression of NAFLD. In animal models, both pharmacological and transgenic inhibition of 5αR activity has been shown to increase susceptibility to development of IR and fatty liver.94 Hence reduction of local hepatic GC exposure by modulation of 11βHSD1 and the A-ring reductases may represent a potential therapeutic intervention for preventing the development and progression of NAFLD.
Fibrosis
Fibrosis, and its more advanced form cirrhosis, represents the final common pathway of almost all chronic liver diseases including NASH. Advanced fibrosis results in liver failure and portal hypertension with its associated complications of ascites and life-threatening variceal bleeding, as well as an increased risk of HCC. The pathogenesis of fibrosis is not within the remit of this review and is well covered elsewhere.95
There are certain aspects of liver fibrosis and repair which are relatively specific to NASH and are worth considering here. In most conditions of liver injury repair arises by replication of mature hepatocytes,96 however the presence of ongoing injurious factors such as NASH or viral infection, is associated with high levels of oxidative stress which reduce the ability of these mature hepatocytes to proliferate. In this situation, other pathways of liver regeneration, hepatic progenitor cells (HPCs), are recruited. HPCs are transit-amplifying cells which reside in the Canal of Hering, and which on proliferation form a complex of small ductules and cholangiocytes known as a ductular reaction. This term was first used to identify the expanded population of epithelial cells at the interface of the biliary tree and the hepatocytes, and refers to proliferation of pre-existing ductules, progenitor cell activation and appearance of intermediate hepatocytes.97 Subsequently these cells have the capability of differentiating into both cholangiocytes and hepatocytes and contributing to liver repair. An inability to mount such a ductular response, as is seen in patients transplanted for NASH who have denervated livers, may be responsible for a more progressive pattern of liver damage.
Controversy continues to exist in the literature about the interplay of the ductular reaction and fibrosis in NAFLD. Initial work demonstrated a close association between the expansion of HPCs and the ductular reaction in liver biopsy specimens of NASH. The extent of ductular reactions in turn strongly correlated with the degree of fibrosis, suggesting that HPC expansion/ductular reaction may be responsible for stimulating a progressive periportal fibrosis.98 Possible mechanisms for this include the secretion of profibrogenic cytokines (TGF-β, IL6, IL8 and MCP1) by the ductular reaction,99 as well as direct epithelial mesenchymal transition of the cholangiocytes to myofibroblasts.100 This hypothesis is attractive in that it provides a rationale for the generation of portal fibrosis in NAFLD which is a key feature of progressive disease. More recent work in the CDE model of murine NAFLD has suggested that liver fibrosis precedes the proliferation of HPCs, suggesting that fibrosis does not occur purely as a result of HPC expansion.101 The timing of the presence of the differing histological findings is less clear on further evaluation,102 raising the possibility of an altogether more complex interaction between fibrosis and HPC expansion, in which both cellular processes can stimulate each other respectively. Indeed, the creation of a progenitor cell niche, with deposition of matrix, may act not only as a migratory pathway for HPCs to migrate along from the portal tract into the parenchyma, but also provide trophic factors for the survival of these cells.103
Genetic predisposition
Although hepatic steatosis is common in patients with obesity and IR only a minority progress to NASH and cirrhosis, suggesting an important interplay between genetic predisposition and environmental factors.72 Polymorphisms in genes related to lipid metabolism, IR, oxidative stress, cytokines/adipokines and fibrogenesis may all increase susceptibility to NASH development.104 Several studies have identified single nucleotide polymorphisms (SNPs) which influence fibrosis development in other liver diseases, particularly chronic hepatitis C.105–107 Studies in NASH have so far demonstrated polymorphisms in the angiotensinogen and TGF-β1 genes to be associated with advanced hepatic fibrosis in obese patients.108 In addition, SNPs in the angiotensin II type 1 receptor are associated with an increased risk of NAFLD and NAFLD-related fibrosis.109 Further studies are needed to identify more candidate genes which will undoubtedly be informative not only as to the pathogenesis and prognosis of the disease, but also may represent novel treatment targets.
Current and emerging therapies
Current therapies
Despite an increasing understanding of the mechanisms of NAFLD pathogenesis, there are few effective therapies available. Current treatments are primarily directed towards improving the metabolic parameters which contribute to disease pathogenesis, such as weight loss and exercise, reducing IR and improving diabetic control.
In addition to lifestyle changes, current therapies utilized for patients with NAFLD include insulin sensitizers, e.g. metformin and the thiazolidinediones, weight loss drugs, e.g. orlistat and sibutramine, and consideration of bariatric surgery for morbidly obese patients. Liver transplantation remains the only curative treatment option for end-stage cirrhosis.
The urgent need for specific treatments for NAFLD has rendered this a critical area of research, with particular focus on developing treatments which can reverse or prevent the more advanced and clinically relevant stages of NASH. The presence of fibrosis predicts likelihood of liver related complications and therefore therapies which can prevent or reverse fibrosis are important goals.
New and emerging therapies
Therapies currently undergoing evaluation in NASH include antioxidants, such as vitamins C, E110,111 and betaine,112 iron depletion,113 ursodeoxycholic acid,114,115 statins116–118 and pentoxifylline.47,119 Whilst none of these treatments has yet shown convincing evidence of benefit, further trials are ongoing.
Glucagon-like peptide-1 (GLP-1)-based therapy may represent a novel therapeutic option for slowing the progression of NAFLD. In diabetic patients GLP-1 analogues, such as Exenatide, have been shown to increase insulin secretion, suppress glucagon secretion, slow gastric emptying and increase satiety in association with modest weight loss,120 and in animal models GLP-1 agonists reduced IR, markers of oxidative stress and hepatic steatosis.121
Antifibrotic therapies
Hepatic fibrosis is the product of hepatic myofibroblasts which predominantly arise from activation of hepatic stellate cells (HSCs), that reside in the space of Disse.122 HSCs express the nuclear peroxisome proliferator activated receptor gamma (PPARγ). Studies have demonstrated that PPARγ ligation by PPARγ agonists, such as the thiazolidinediones (Rosiglitazone and Pioglitazone), leads to reduced HSC activation. Encouraging results with these agents have been demonstrated in patients with NAFLD with improvements in both liver biochemistry and histology.123–125 However side-effects including congestive cardiac failure,126 osteoporosis127 and weight gain125 are of concern. Benefits also appear to be reversed on discontinuation of therapy.128
Angiotensin has been shown to promote myofibroblast survival and liver fibrosis,129 and thus the beneficial effects of ACE inhibitors and angiotensin receptor blockers (ARBs) are likely to include antifibrotic properties. Several clinical studies of antioxidants such as vitamin E, which have been shown to suppress fibrosis in vitro, have so far failed to demonstrate that dietary supplementation of vitamin E improves histological fibrosis in humans.110,111 A large number of cytokines are intimately involved in the proliferative, contractile and fibrogenic activities of HSCs, antagonism of which represents another potential target for antifibrotic therapies.130 Potential candidates include platelet-derived growth factor (PDGF), transforming growth factor beta-1 (TGFβ-1),130 connective tissue growth factor (CTGF),131 endothelin-1 (ET-1), thrombin, vascular endothelial growth factor (VEGF), fibroblast growth factor and insulin-like growth factor, which also exert their effects through tyrosine-kinase receptors.130 Consistent with this, studies in animal models of liver fibrosis have demonstrated antifibrotic effects using tyrosine kinase inhibitors such as imatinib132,133 which is currently licensed for use in chronic myeloid leukaemia and gastrointestinal stromal tumours.
Other potential targets alluded to earlier include inhibiting ER stress,134 and modulation of the gut liver axis using pre- and probiotics.49,85–87,135
Conclusions
NAFLD now represents one of the commonest causes of liver disease in the Western world, and the rising levels of obesity, diabetes and metabolic syndrome will ensure that it remains a major cause of morbidity and mortality. Although simple steatosis carries a relatively benign prognosis, a significant proportion of patients will progress to NASH and later cirrhosis with risk of HCC.
The traditional ‘2-hit’ hypothesis of NAFLD pathogenesis has been modified several times; in most patients however NAFLD does appear to begin with lipid accumulation, or steatosis, which is in turn driven by obesity and IR. Progression to steatohepatitis and fibrosis depends on additional factors such as FFAs, inflammatory cytokines and adipokines, oxidative stress and mitochondrial dysfunction in a complex interplay with genetic predisposition.
Current treatment strategies for NASH focus on improving components of the metabolic syndrome, such as obesity and IR, with no liver-specific agents yet available. However, modulation of any of the multiple mechanisms involved in NASH pathogenesis could provide useful targets to prevent the development of fibrosis and its associated complications. This knowledge, and the significant advances that continue to be made in our understanding of the pathogenesis of NASH, are required to inform the development of novel therapeutic strategies for this increasingly important condition.
Funding
JK Dowman is a Wellcome Trust Clinical Research Fellow in Gastroenterology, and is therefore receiving funding from the Wellcome Trust.
Conflict of interest: None declared.
References
- 1.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–58. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 2.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–17. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl. 1):S104–12. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–4. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 5.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 6.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 7.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–6. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 9.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–9. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 10.Franzese A, Vajro P, Argenziano A, Puzziello A, Iannucci MP, Saviano MC, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–32. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–8. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 12.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 14.Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond) 2008;115:141–50. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of fatty liver. Endocr Rev. 2008;29:939–60. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 16.Setji TL, Holland ND, Sanders LL, Pereira KC, Diehl AM, Brown AJ. Nonalcoholic steatohepatitis and nonalcoholic Fatty liver disease in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1741–7. doi: 10.1210/jc.2005-2774. [DOI] [PubMed] [Google Scholar]
- 17.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 18.Olufadi R, Byrne CD. Clinical and laboratory diagnosis of the metabolic syndrome. J Clin Pathol. 2008;61:697–706. doi: 10.1136/jcp.2007.048363. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds K, He J. Epidemiology of the metabolic syndrome. Am J Med Sci. 2005;330:273–9. doi: 10.1097/00000441-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Day CP. Natural history of NAFLD: remarkably benign in the absence of cirrhosis. Gastroenterology. 2005;129:375–8. doi: 10.1053/j.gastro.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 22.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–54. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 24.Day CP, James OF. Steatohepatitis: a tale of two ‘hits’? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 25.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–10. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 28.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–9. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 29.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829–38. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hudgins LC, Hellerstein MK, Seidman CE, Neese RA, Tremaroli JD, Hirsch J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J Lipid Res. 2000;41:595–604. [PubMed] [Google Scholar]
- 33.Parks EJ. Dietary carbohydrate's effects on lipogenesis and the relationship of lipogenesis to blood insulin and glucose concentrations. Br J Nutr. 2002;87(Suppl. 2):S247–53. doi: 10.1079/BJNBJN/2002544. [DOI] [PubMed] [Google Scholar]
- 34.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 36.Namikawa C, Shu-Ping Z, Vyselaar JR, Nozaki Y, Nemoto Y, Ono M, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781–6. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–8. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 38.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 40.McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40(3 Suppl. 1):S17–29. doi: 10.1097/01.mcg.0000168645.86658.22. [DOI] [PubMed] [Google Scholar]
- 41.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;3; 282:22678–88. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 43.Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–74. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 45.Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, et al. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–63. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 46.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 47.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–8. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 48.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–24. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 50.Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–27. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- 51.Tarantino G, Conca P, Pasanisi F, Ariello M, Mastrolia M, Arena A, et al. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol. 2009;21:504–11. doi: 10.1097/MEG.0b013e3283229b40. [DOI] [PubMed] [Google Scholar]
- 52.van der PD, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48:449–57. doi: 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 53.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 54.Mantzoros CS. The role of leptin in human obesity and disease: a review of current evidence. Ann Intern Med. 1999;130:671–80. doi: 10.7326/0003-4819-130-8-199904200-00014. [DOI] [PubMed] [Google Scholar]
- 55.Lord G. Role of leptin in immunology. Nutr Rev. 2002;60(10 Pt 2):S35–8. doi: 10.1301/002966402320634913. [DOI] [PubMed] [Google Scholar]
- 56.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 57.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang XD, Fan Y, Zhang H, Wang P, Yuan JP, Li MJ, et al. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol. 2008;14:2888–93. doi: 10.3748/wjg.14.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uygun A, Kadayifci A, Yesilova Z, Erdil A, Yaman H, Saka M, et al. Serum leptin levels in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2000;95:3584–9. doi: 10.1111/j.1572-0241.2000.03297.x. [DOI] [PubMed] [Google Scholar]
- 60.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403–9. doi: 10.1053/jhep.2002.34738. [DOI] [PubMed] [Google Scholar]
- 61.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin–a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 62.Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 63.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 64.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, et al. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–73. doi: 10.1002/hep.22365. [DOI] [PubMed] [Google Scholar]
- 66.Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–21. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lemoine M, Ratziu V, Kim M, Maachi M, Wendum D, Paye F, et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431–8. doi: 10.1111/j.1478-3231.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 68.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 69.Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, et al. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005;100:850–5. doi: 10.1111/j.1572-0241.2005.41500.x. [DOI] [PubMed] [Google Scholar]
- 70.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 71.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 72.Petta S, Muratore C, Craxi A. Non-alcoholic fatty liver disease pathogenesis: The present and the future. Dig Liver Dis. 2009;41:615–25. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–78. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- 74.Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- 75.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–53. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 76.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–33. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 77.Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:973–83. doi: 10.1097/MEG.0b013e328328f461. [DOI] [PubMed] [Google Scholar]
- 78.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–8. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology JID - 0374630. 2000;119:1340–7. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 80.Solga SF, Diehl AM. Non-alcoholic fatty liver disease: lumen-liver interactions and possible role for probiotics. J Hepatol. 2003;38:681–7. doi: 10.1016/s0168-8278(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 81.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 83.Drenick EJ, Fisler J, Johnson D. Hepatic steatosis after intestinal bypass–prevention and reversal by metronidazole, irrespective of protein-calorie malnutrition. Gastroenterology. 1982;82:535–48. [PubMed] [Google Scholar]
- 84.Hocking MP, Davis GL, Franzini DA, Woodward ER. Long-term consequences after jejunoileal bypass for morbid obesity. Dig Dis Sci. 1998;43:2493–9. doi: 10.1023/a:1026698602714. [DOI] [PubMed] [Google Scholar]
- 85.Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513–9. doi: 10.1016/0016-5085(91)90224-9. [DOI] [PubMed] [Google Scholar]
- 86.Loguercio C, Federico A, Tuccillo C, Terracciano F, D'Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL #3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 87.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, et al. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905–11. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 88.Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, et al. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149:543–8. doi: 10.1530/eje.0.1490543. [DOI] [PubMed] [Google Scholar]
- 89.Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008;57:2652–60. doi: 10.2337/db08-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermanowski-Vosatka A, Balkovec JM, Cheng K, Chen HY, Hernandez M, Koo GC, et al. 11beta-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med. 2005;202:517–27. doi: 10.1084/jem.20050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andrews RC, Rooyackers O, Walker BR. Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:285–91. doi: 10.1210/jc.2002-021194. [DOI] [PubMed] [Google Scholar]
- 92.Walker BR, Connacher AA, Lindsay RM, Webb DJ, Edwards CR. Carbenoxolone increases hepatic insulin sensitivity in man: a novel role for 11-oxosteroid reductase in enhancing glucocorticoid receptor activation. J Clin Endocrinol Metab. 1995;80:3155–9. doi: 10.1210/jcem.80.11.7593419. [DOI] [PubMed] [Google Scholar]
- 93.Konopelska S, Kienitz T, Hughes B, Pirlich M, Bauditz J, Lochs H, et al. Hepatic 11beta-HSD1 mRNA expression in fatty liver and nonalcoholic steatohepatitis. Clin Endocrinol (Oxf) 2009;70:554–60. doi: 10.1111/j.1365-2265.2008.03358.x. [DOI] [PubMed] [Google Scholar]
- 94.Livingstone D, Walker B, Andrews R. Susceptibility to hyperinsulinaemia and fatty liver with loss of 5alpha-reductase 1 occurs in rats and mice and is not androgen dependent. Endocrine Abstracts 19. 136. 2009. [Google Scholar]
- 95.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–48. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–50. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- 97.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–45. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 98.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052–64. doi: 10.1111/j.1478-3231.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 100.Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168:1500–12. doi: 10.2353/ajpath.2006.050747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van Hul NK, Abarca-Quinones J, Sempoux C, Horsmans Y, Leclercq IA. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–35. doi: 10.1002/hep.22820. [DOI] [PubMed] [Google Scholar]
- 102.Clouston AD, Jonsson JR, Powell EE. Hepatic progenitor cell-mediated regeneration and fibrosis: chicken or egg? Hepatology. 2009;49:1424–6. doi: 10.1002/hep.22893. [DOI] [PubMed] [Google Scholar]
- 103.Pinzani M. Liver fibrosis. Springer Semin Immunopathol. 1999;21:475–90. doi: 10.1007/s002810000037. [DOI] [PubMed] [Google Scholar]
- 104.Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27:44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- 105.Sermasathanasawadi R, Kato N, Muroyama R, Dharel N, Shao RX, Chang JH, et al. Association of interferon regulatory factor-7 gene polymorphism with liver cirrhosis in chronic hepatitis C patients. Liver Int. 2008;28:798–806. doi: 10.1111/j.1478-3231.2008.01725.x. [DOI] [PubMed] [Google Scholar]
- 106.Okamoto K, Mimura K, Murawaki Y, Yuasa I. Association of functional gene polymorphisms of matrix metalloproteinase (MMP)-1, MMP-3 and MMP-9 with the progression of chronic liver disease. J Gastroenterol Hepatol. 2005;20:1102–8. doi: 10.1111/j.1440-1746.2005.03860.x. [DOI] [PubMed] [Google Scholar]
- 107.Guo J, Loke J, Zheng F, Hong F, Yea S, Fukata M, et al. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–8. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dixon JB, Bhathal PS, Jonsson JR, Dixon AF, Powell EE, O'Brien PE. Pro-fibrotic polymorphisms predictive of advanced liver fibrosis in the severely obese. J Hepatol. 2003;39:967–71. doi: 10.1016/s0168-8278(03)00459-8. [DOI] [PubMed] [Google Scholar]
- 109.Yoneda M, Hotta K, Nozaki Y, Endo H, Uchiyama T, Mawatari H, et al. Association between angiotensin II type 1 receptor polymorphisms and the occurrence of nonalcoholic fatty liver disease. Liver Int. 2009;29:1078–85. doi: 10.1111/j.1478-3231.2009.01988.x. [DOI] [PubMed] [Google Scholar]
- 110.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–9. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 111.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 112.Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. Am J Gastroenterol. 2001;96:2711–7. doi: 10.1111/j.1572-0241.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- 113.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–8. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- 114.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–8. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 115.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 116.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57:1711–8. doi: 10.1016/j.metabol.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 117.Gomez-Dominguez E, Gisbert JP, Moreno-Monteagudo JA, Garcia-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–7. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 118.Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A Randomized Placebo-controlled Trial. J Clin Gastroenterol. 2009;43:990–4. doi: 10.1097/MCG.0b013e31819c392e. [DOI] [PubMed] [Google Scholar]
- 119.Satapathy SK, Garg S, Chauhan R, Sakhuja P, Malhotra V, Sharma BC, et al. Beneficial effects of tumor necrosis factor-alpha inhibition by pentoxifylline on clinical, biochemical, and metabolic parameters of patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1946–52. doi: 10.1111/j.1572-0241.2004.40220.x. [DOI] [PubMed] [Google Scholar]
- 120.Iltz JL, Baker DE, Setter SM, Keith CR. Exenatide: an incretin mimetic for the treatment of type 2 diabetes mellitus. Clin Ther. 2006;28:652–65. doi: 10.1016/j.clinthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 121.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henderson NC, Iredale JP. Liver fibrosis: cellular mechanisms of progression and resolution. Clin Sci (Lond) 2007;112:265–80. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 123.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 124.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 125.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 126.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 127.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–10. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 128.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424–9. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 129.Oakley F, Teoh V, Ching AS, Bataller R, Colmenero J, Jonsson JR, et al. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology. 2009;136:2334–44. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 130.Albanis E, Friedman SL. Antifibrotic agents for liver disease. Am J Transplant. 2006;6:12–9. doi: 10.1111/j.1600-6143.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 131.Friedman SL, Rockey DC, Bissell DM. Hepatic fibrosis 2006: report of the Third AASLD Single Topic Conference. Hepatology. 2007;45:242–9. doi: 10.1002/hep.21459. [DOI] [PubMed] [Google Scholar]
- 132.Yoshiji H, Noguchi R, Kuriyama S, Ikenaka Y, Yoshii J, Yanase K, et al. Imatinib mesylate (STI-571) attenuates liver fibrosis development in rats. Am J Physiol Gastrointest Liver Physiol. 2005;288:G907–13. doi: 10.1152/ajpgi.00420.2004. [DOI] [PubMed] [Google Scholar]
- 133.Gonzalo T, Beljaars L, van de BM, Temming K, van Loenen AM, Reker-Smit C, et al. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321:856–65. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- 134.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, et al. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]