Abstract
The normal breathing rhythm in mammals is hypothesized to be generated by neurokinin-1 receptor (NK1R)-expressing neurons in the preBötzinger complex (preBötC), a medullary region proposed to contain the kernel of the circuits generating respiration. If this hypothesis is correct, then complete destruction of preBötC NK1R neurons should severely perturb and perhaps even fatally arrest breathing. Here we show that specific and near complete bilateral (but not unilateral) destruction of preBötC NK1R neurons results in both an ataxic breathing pattern with markedly altered blood gases and pH, and pathological responses to challenges such as hyperoxia, hypoxia and anesthesia. Thus, these ~600 neurons seem necessary for the generation of normal breathing in rats.
Breathing is an exceptionally reliable and continuous mammalian behavior that regulates blood gases and pH at rest and in response to diverse challenges such as exercise, sleep or altitude. The rhythm underlying breathing is postulated to depend critically on neurons in the preBötC1,2. Two recent developments allowed us to determine in awake adult rats whether this critical regulatory behavior would be affected by lesions in the preBötC. First, the extent of the preBötC can be anatomically defined by the sub-population of propriobulbar respiratory neurons within the ventrolateral respiratory column expressing NK1R3–5. Second, NK1R neurons can be specifically lesioned by substance P conjugated to saporin (SP-SAP)6, an effect that takes several days, allowing for complete recovery from surgery6.
Results
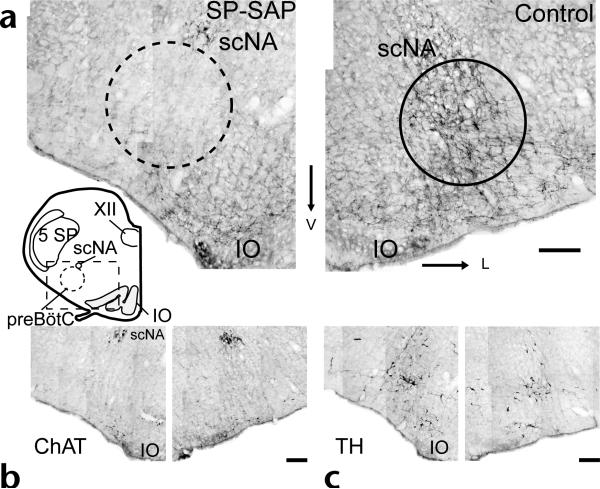
SP-SAP was effective in eliminating preBötC NK1R neurons in adult rats. Injections of 0.1–0.2 pmol of SP-SAP or 0.3 pmol each of unconjugated saporin and SP were made in the pre-BötC. Unilateral SP-SAP injection transiently produced sighs (large inspiratory efforts followed by prolonged expiration), consistent with the effects of injection of SP alone into the pre-BötC in vitro7 and in vivo (P.A.G., D.R.M. and J.L.F., unpublished observations). All rats returned to normal behavior upon recovery from surgery. Two to eighteen days after injection, rats were perfused and their medullas were stained for NK1R immunoreactivity (n = 20). The lesion extent was estimated by counting NK1R immunopositive neuronal soma in the rostral medulla inside a circle of 600-μm diameter approximating the preBötC (ventrolateral to the nucleus ambiguus) and in a rectangle (1600 × 1070 μm) outside this circle (Fig. 1, inset). Counts were estimated from four transverse sections beginning within 100 μm of the rostral border of the lateral reticular nucleus and spanning the preBötC3,4 containing approximately 12–15% of the total preBötC volume. Uninjected controls (n = 4) had 35 ± 5.1 (mean ± s.e.m., per side) NK1R neurons within the preBötC (~600 preBötC NK1R neurons total, which we estimate represent less than 10% of all preBötC neurons) and 82 ± 9 NK1R neurons outside the preBötC. Most of the latter were immediately ventral to the preBötC. Unilaterally SPSAP injected rats (n = 4) had 0 ± 0 NK1R neurons inside and 29 ± 9 NK1R neurons outside the preBötC on the injected side compared to the uninjected side, which had 47 ± 6 inside and 62 ± 6 outside. In contrast, tyrosine hydroxylase (TH)-immunoreactive neurons, which do not express NK1R but which have soma and dendrites within the target site3,4, were largely unaffected (Fig. 1c; unilateral injections, injected side, 111 ± 15; control side; 121 ± 15, n = 4), although some TH neurons at the injection site showed damage. NK1R staining in dorsal brainstem regions such as the solitary tract and motor nucleus of the vagus appeared normal with no signs of toxin-mediated degradation or receptor internalization (n = 20)8, although collateral damage in other regions was possible. In some rats, nucleus ambiguus motoneurons adjacent to the preBötC were mildly damaged (Fig. 1b); however, motoneuronal damage could not account for the changes in breathing frequency or pattern we observed. Bilateral injection of unconjugated saporin and SP had little effect on preBötC NK1R neurons (inside, 30 ± 4; outside, 70 ± 14; per side, n = 2).
Fig. 1.
Local injection of SP-SAP selectively ablates preBötC NK1R neurons. Inset, transverse section of adult rat brainstem showing relative location of preBötC (circle) ventrolateral to scNA. Rectangle (excluding circle) indicates area outside preBötC analyzed for neuron counts (see text). Comparison of NK1R (a), choline acetyltransferase (ChAT) (b) and tyrosine hydroxylase (TH) (c) immunohistochemistry in unilaterally injected rat. scNA, semicompact division of nucleus ambiguus; IO, inferior olive; 5SP, trigeminal nucleus; XII, hypoglossal nucleus. Scale bars, 200 μm.
Bilateral but not unilateral SP-SAP lesions profoundly affected breathing. We measured respiratory period and inspiratory amplitude from unrestrained, awake adult rats. Bilaterally SP-SAP injected rats with near-complete bilateral destruction of preBötC NK1R neurons (see below) exhibited a transformation in breathing pattern 4–5 days after injection, going from a eupneic to a severely ataxic pattern in room air (preBötC–; n = 10; Fig. 2). The changes in breathing pattern were strongly correlated to the loss of preBötC NK1R neurons. PreBötC– rats that were perfused 10–18 days after injection (sufficient time for clearance of dead NK1R neurons) had markedly fewer NK1R neurons within (4 ± 1; range, 0–7; p < 0.001, total, both sides) and somewhat fewer NK1R neurons outside (70 ± 10; range, 66–96; p < 0.05, total) the preBötC, per side (n = 6). Bilaterally injected rats that maintained normal breathing patterns and that were perfused 10–12 days after injection had 30 ± 9 NK1R neurons inside (range, 15–52; p < 0.05) and 105 ± 13 NK1R neurons outside (range, 81–131; p > 0.05, n = 4) per side, with a maximum estimated lesion sparing more than 20% of preBötC NK1R neurons.
Fig. 2.
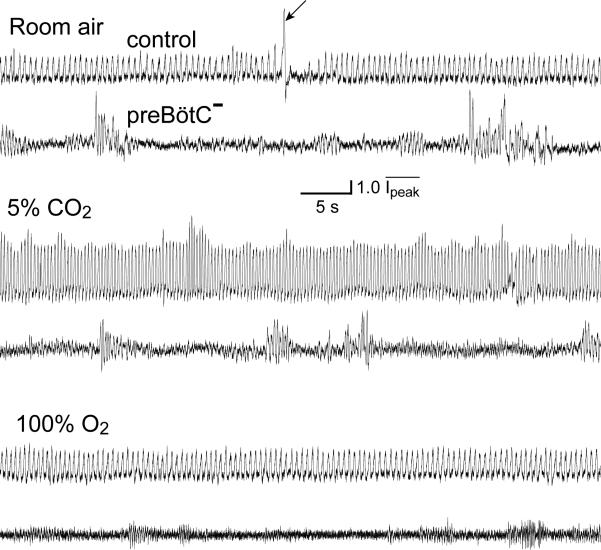
PreBötC– rats breathe with an ataxic pattern. Spontaneous breathing from control (top of each pair) and preBötC– (bottom of each pair) rats. Plethysmographic traces breathing room air (top), 5% CO2/95% O2 (middle) and 100% O2 (bottom). Upward deflection represents inspiration. Control rats breathing room air spontaneously generate an occasional sigh (arrow) followed by an extended expiratory pause; this pattern seems absent in preBötC– rats. (Movies of a preBötC– rat at 2 and 8 days after injection are available on the supplementary information page of Nature Neuroscience online.)
The respiratory period of uninjected (control) rats was 0.53 ± 0.001 s (n = 10) and normalized peak inspiratory amplitude (Ipeak) was 1.00 ± 0.006 (n = 3), with occasional sniffing (period, 0.18 ± 0.001 s) and sighing (Figs. 2 and 3). Ataxia in preBötC– rats was characterized by shortened respiratory periods (0.29 ± 0.01 s, n = 8, p < 0.0001) and an irregular sequence of inspiratory efforts of near normal amplitude interspersed with (prolonged) periods of apnea or very low amplitude inspiration (Ipeak = 0.85 ± 0.004; n = 3, p < 0.001, Figs. 2 and 3). One additional rat (>90% bilateral destruction of preBötC NK1R neurons perfused at day 18) with an ataxic breathing pattern with noticeable apneas did not show periods of low amplitude inspiration. Arterial blood gases and pH values of preBötC– rats four days after injection, when their breathing patterns still seemed normal, suggested a slight hyperventilation giving rise to reduced CO2 with consequent alkalosis and elevated O2 (pH 7.44 ± 0.01; PCO2, 31.5 ± 1.0 mm Hg; PO2, 97.25 ± 2.7 mm Hg; n = 4) compared to control rats (pH 7.40 ± 0.01; PCO2, 36.5 ± 1.9 mm Hg; PO2, 92.5 ± 5.5 mm Hg; n = 2; T = 37.4 ± 0.1°C; n = 17). Five days after injection, however, preBötC– rats had a profile typical of respiratory depression giving rise to CO2 retention and consequent acidosis (pH 7.26 ± 0.07; PCO2, 56.4 ± 3.7 mm Hg; PO2, 76.5 ± 5.5 mm Hg; n = 2; T = 36.8 ± 0.1°C; n = 14).
Fig 3.
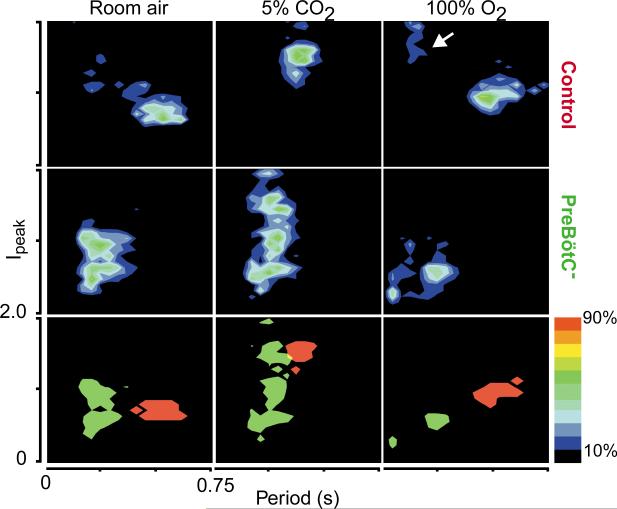
PreBötC rats (3–5 days after injection) and control rats breathe differently. Each of 9 graphs represents grouped data (n = 3, 10 consecutive minutes) of instantaneous respiratory period (0–0.75 s) versus Ipeak (0.0–2.0) for 4800–7000 breaths, plotted as two-dimensional density for control (top), preBötC– (middle) and combined control (red)/preBötC– (green; bottom). Individual breaths (and period) were converted into a two-dimensional histogram (bin width, 0.02 s and 0.02) and plotted as density maps. Top and middle, colors represent the relative number of breaths in a given bin as percentages of breaths in the maximum bin, as indicated in legend at right; densities less than 10% of maximum are black. Bottom, flattened comparison between control (red) and preBötC– (green) rats of all bins with densities ≥25% of maximum. Arrow points to sniffing behavior. Data was obtained from rats breathing room air (left), 5% CO2/95% O2 (middle) and 100% O2 (right).
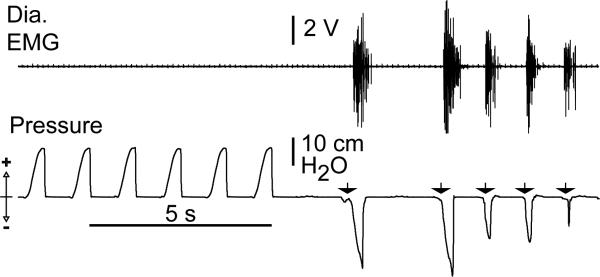
PreBötC– rats had pathological responses to changes in inspired gases. In response to 100% O2, control rats had an increased period (0.6 ± 0.002 s, n = 9, p < 0.001) and an increased Ipeak (1.3 ± 0.008, n = 3, p < 0.0001; Figs. 2 and 3). PreBötC– rats responded with a marked (further) depression of breathing, with a small decrease (~3%) in period (0.28 ± 0.002 s, n = 4, p < 0.05) and a decrease in Ipeak (0.7 ± 0.006, n = 3, p < 0.0001) due primarily to the loss of normal amplitude events (Fig. 3). Two rats developed fatal apneas. The depressive effects of 100% O2 in ataxic animals are in contrast to its normalizing effects in humans with Cheyne–Stokes breathing, which has a vaguely similar respiratory phenotype; this suggests a different etiology for preBötC– rats. In control rats, 5% CO2 and 95% O2 caused a significantly decreased (~25%) period (0.4 ± 0.001 s, p < 0.0001) and increased Ipeak (1.8 ± 0.009, n = 3, p < 0.0001) compared to response when breathing room air (Figs. 2 and 3). PreBötC– rats, in contrast, showed a smaller (~14%) decrease in respiratory period from breathing room air (0.25 ± 0.001 s, n = 5, p < 0.0001) with an increase in Ipeak (1.1 ± 0.007, p < 0.0001) due primarily to the appearance of a number of higher amplitude respiratory events among continued low-amplitude inspiration (Fig. 3). In response to severe hypoxia (4.4% O2/95.6% N2), control and injected rats up to four days after injection showed shortened (~34%) periods (0.35 ± 0.001 s, n = 7, p < 0.0001) and increased amplitude; these rats tolerated this challenge for at least 15 minutes (n = 16; data not shown). PreBötC– rats (n = 8) had a brief increase in frequency and amplitude, followed within 2–7 minutes by apneas of increasing and eventually fatal duration. Only one preBötC– rat recovered spontaneous breathing when quickly returned to room air. In addition, breathing in preBötC– rats was unusually sensitive to depression by anesthesia, similar to humans with Jou-bert's Syndrome9. Thus, in contrast to control rats, no spontaneous respiratory drive could be recorded in preBötC– rats at modest levels of inhaled fluorothane (1–2%; n = 6, Fig. 4). Moreover, premotor circuits driving respiratory motoneurons appeared intact, as lung deflation in anesthetized preBötC– rats induced a robust reflex diaphragmatic output (Fig. 4).
Fig. 4.
Anesthetized (fluorothane, 1–2%) preBötC– rats do not have spontaneous respiratory activity. However, lung deflation induces diaphragmatic activity (Dia. EMG, top). Arrows point to short-lasting (<100 ms) lung deflations that induce bursts of diaphragmatic activity leading to airflow.
Discussion
Although the importance of the preBötC in generating a respiratory-related rhythm in in vitro preparations from neonatal rodents is well established1,2,10, the role of the preBötC in generating the normal rhythm of breathing in intact mammals is controversial1,11. The respiratory pattern of in vitro preparations is somewhat similar in pattern, frequency and chemosensitivity to gasping in anesthetized adult rats7,11 and it has been suggested that hypotheses derived from these preparations have little relevance to normal breathing in intact animals1,11. Thus, we tested the hypothesis that the preBötC is critical for the generation of normal breathing in awake, behaving animals. We found that bilateral injections of SP-SAP that eliminated greater than 80% of preBötC NK1R neurons resulted in a highly irregular breathing pattern in conscious rats. These rats were unable to maintain appropriate blood gases and pH, and had pathological responses to hypoxia or hyper-oxia. However, these lesions did not result in the rapid onset of fatal apnea when these preBötC– rats were conscious. When taken together with previous results1–3,10,12,13, our interpretation is that normal breathing in mammals requires an intact preBötC, with NK1R neurons having a necessary role. In their absence, an ataxic rhythm sufficient to maintain life can still be generated in conscious rats by undetermined structures, which may include non-NK1R expressing neurons in the preBötC or ventrolateral respiratory column14, other respiratory regions13, vestigial brainstem rhythmogenic networks15,16 or even structures normally underlying voluntary or emotional control of respiratory muscles. The extent to which brain regions in addition to the preBötC are also necessary for the normal pattern of breathing is yet to be determined. PreBötC NK1R neurons may contain the kernel for the genetically determined behavior of breathing in mammals, and delineating their properties may provide ways to understand and treat life-threatening and poorly understood central failures of breathing such as central sleep apnea17,18, congenital central hypoventilation syndrome (Ondine's curse)19,20, Joubert's Syndrome21 and Sudden Infant Death Syndrome22.
Methods
Injection of SP-SAP
We used 54 adult Sprague–Dawley male rats. All procedures were approved by the UCLA Animal Care Committee (ARC# 94-159-22). Rats were anesthetized with 100 mg/kg of ketamine (Ketaject, Phoenix Scientific, St. Joseph, Missouri) and 10 mg/kg of Xylazine (AnaSed, Lloyd Laboratories, Shenandoah, Iowa) administered intraperitoneally. Rats were intubated and respiratory flow was recorded (MLT1L flow head from ADInstruments, Grand Junction, Colorado and DRAL501 transducer, Honeywell Data Instruments, Acton, Massachusetts). When necessary, rats were mechanically ventilated. Rats breathed 1–2 volume percent fluorothane (IsoFlo, Abbott Laboratories, North Chicago, Illinois) in 1:1 air:oxygen mixture. Rats were positioned in a stereotaxic frame with bregma 5 mm below lambda. Microinjections (100–150 nl) of SP-SAP (Advanced Targeting Systems, San Diego, California) were made from a glass capillary tube with a 40-μm tip diameter. Coordinates were 0.7 mm rostral, 1.95 mm lateral and 2.6 mm ventral to the obex. When the inferior cerebellar vein was in the way of injection, the micropipette was moved rostrally (50–150 μm) to avoid puncture. A second injection was made just caudal to the vein. Fluorescent microspheres (Molecular Probes, Eugene, Oregon) were added to the solution to allow identification of injection sites. For arterial blood sampling, the femoral artery was cannulated and an artery access port was implanted subcutaneously. Blood oxygen and carbon dioxide levels were assessed with a blood gas analyzer.
Histology
Rats (n = 39, 24 brainstems were of sufficient quality to do cell counts) were transcardially perfused with 4% paraformaldehyde in PBS, cryoprotected in 25% sucrose PBS, embedded in OCT, sectioned at 40 μm on a cryostat and processed free floating. Sections were incubated in primary antibody diluted in sera at 4°C overnight, placed in biotin conjugated species-specific secondary antibody (Vector Laboratories, Burlingame, California), stained using the ABC method (Vector Laboratories) and mounted on gelatin subbed slides. The following antibodies were used: rabbit anti-NK1R (1:20,000, Chemicon, Temucula, California), goat anti-ChAT (1:400, Chemicon) and mouse anti-tyrosine hydroxylase (1:1000, Boehringer Mannheim, Indianapolis, Indiana). Brightfield images were digitally acquired into Photoshop (Adobe Systems, San Jose, California). All images were filtered and adjusted for contrast and light levels in Photoshop for clarity. NK1R somata were visually identified by the presence of at least 2 labeled processes and their location plotted via camera lucida and counted within a 1600 × 1040 μm region bounded laterally by the 5SP and dorsally by the nucleus ambiguus. Neurons within the preBötC were counted by placing a 600-μm circle just ventral to and centered upon the lateral edge of the NA. Cells in sections separated by 200 μm were counted.
Phethysmography
All respiratory recordings of unrestrained rats were obtained using a seven-liter whole-body plethysmograph (Buxco, Sharon, Connecticut) and a pressure transducer (DRAL501, Honeywell Data Instruments). The data was recorded using Axoscope software (Axon Instruments, Union City, California). Respiratory periods were determined using peak detection software written in LabView software (National Instruments, Austin, Texas) or by hand measurement of plethysmographic traces. Peak inspiratory amplitudes were calculated from pressure transducer output (in millivolts) and normalized. For control rats breathing room air and 100% oxygen, mean respiratory period was calculated after removal of periods less than 0.3 s to exclude sniffing. For calculation of mean inspiratory amplitude, values greater than 10 ml were excluded as motion artifacts. All numbers are expressed as mean ± s.e.m. Mean values were calculated from 15-min (period, room air) or 10-min (all else, 5 min after gas exchange) recordings of continuous respiratory behavior. Control means were compared against control room air, and ataxic means were compared against control means for the same gas mixture for statistical analysis (Student's t-test for independent variables) in Origin (Microcal, Northampton, Massachusetts).
Apneic rats
Rats that developed irreversible apnea were anesthetized and mechanically ventilated at a rate adequate to keep end-tidal CO2 at the 5% level. Bipolar wire electrodes were implanted into the costal part of diaphragm (n = 6) and abdominal muscle (n = 2). Needle electrodes were used to monitor ECG. Signals were amplified filtered and integrated. For lung deflation, a constant negative pressure source was used. Intratracheal pressure was monitored.
Note : Supplementary movies of a preBötC– rat at two and eight days after injection are available on the Nature Neuroscience web site (http://neuroscience.nature.com/web_specials).
Acknowledgements
We thank M. Sofroniew, N. Brecha, T. Otis, R. Fregosi and L. Kruger for assistance and G. Li for histological work. A. Monnier participated in early experiments. Funding was provided by a Ford Foundation Pre-Doctoral Fellowship for Minorities and the Porter Physiology Development Program of the American Physiological Society to P.A.G., and by the National Institutes of Health (HL40959). W.A.J. is on leave of absence from the Medical Research Centre, Polish Academy of Sciences, Warsaw, Poland.
Footnotes
The first two authors contributed equally to this work
References
- 1.Rekling JC, Feldman JL. PreBötzinger Complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 2.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger Complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger Complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Stornetta RL, Rosin DL, Guyenet PG. Neurokinin-1 receptor-immunoreactive neurons of the ventral respiratory group in the rat. J. Comp. Neurol. 2001;434:128–146. doi: 10.1002/cne.1169. [DOI] [PubMed] [Google Scholar]
- 5.Guyenet PG, Wang H. Pre-Bötzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J. Neurophysiol. 2001;86:438–446. doi: 10.1152/jn.2001.86.1.438. [DOI] [PubMed] [Google Scholar]
- 6.Mantyh PW, et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 7.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 8.Mantyh PW, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 9.Habre W, Sims C, D'Souza M. Anaesthetic management of children with Joubert syndrome. Paediatr. Anaesth. 1997;7:251–253. doi: 10.1046/j.1460-9592.1997.d01-65.x. [DOI] [PubMed] [Google Scholar]
- 10.Ballayni K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog. Neurobiol. 1999;59:583–684. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Remmers JE. Central neural control of breathing. In: Altose M, Kawakami Y, editors. Lung Biology in Health and Disease: Control of Breathing in Health and Disease. M. Dekker; New York: 1999. pp. 1–41. [Google Scholar]
- 12.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin. Exp. Pharmacol. Physiol. 2000;27:126–131. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- 13.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 14.Speck DF, Feldman JL. Effects of microstimulation and microlesion in the ventral and dorsal respiratory groups in medulla of cat. J. Neurosci. 1982;2:744–757. doi: 10.1523/JNEUROSCI.02-06-00744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champagnat J, Fortin G. Primordial respiratory-like rhythm generation in the vertebrate embryo. Trends Neurosci. 1997;20:119–124. doi: 10.1016/s0166-2236(96)10078-3. [DOI] [PubMed] [Google Scholar]
- 16.Gdovin MJ, Torgerson CS, Remmers JE. The fictively breathing tadpole brainstem preparation as a model for the development of respiratory pattern generation and central chemoreception. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 1999;124:275–286. doi: 10.1016/s1095-6433(99)00116-6. [DOI] [PubMed] [Google Scholar]
- 17.Guilleminault C, Robinson A. Central sleep apnea. Neurol. Clin. 1996;14:611–628. doi: 10.1016/s0733-8619(05)70276-0. [DOI] [PubMed] [Google Scholar]
- 18.Thalhofer S, Dorow P. Central sleep apnea. Respiration. 1997;64:2–9. doi: 10.1159/000196635. [DOI] [PubMed] [Google Scholar]
- 19.Gozal D. Congenital central hypoventilation syndrome: an update. Pediatr. Pulmonol. 1998;26:273–282. doi: 10.1002/(sici)1099-0496(199810)26:4<273::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Nattie E, Bartlett D, Jr., Rozycki A. Central alveolar hypoventilation in a child: an evaluation using a whole body plethysmograph. Amer. Rev. Resp. Dis. 1975;112:259–265. doi: 10.1164/arrd.1975.112.2.259. [DOI] [PubMed] [Google Scholar]
- 21.Maria BL, Boltshauser E, Palmer SC, Tran TX. Clinical features and revised diagnostic criteria in Joubert syndrome. J. Child Neurol. 1999;14:583–590. doi: 10.1177/088307389901400906. [DOI] [PubMed] [Google Scholar]
- 22.Guilleminault C, Robinson A. Developmental aspects of sleep and breathing. Curr. Opin. Pulm. Med. 1996;2:492–499. [PubMed] [Google Scholar]