Abstract
The mammalian CNS is usually not capable of regeneration. However, conditioning dorsal root ganglion neurons by first lesioning their peripheral axons allows for regeneration of their central axons later on within the spinal cord. New work shows that, even if the sequence of lesioning is reversed, regeneration through the CNS lesion can rapidly occur under certain conditions.
Regeneration in the adult central nervous system (CNS) of mammals is unsuccessful as a result of the decreased intrinsic regenerative capacity of affected neurons, myelin-associated inhibitory factors and components of the glial scar [1]. A substantial amount of research suggests that increasing the growth potential of damaged sensory neurons enables them to overcome inhibition in the injured peripheral nervous system (PNS) and CNS. A classic strategy to augment the intrinsic machinery for regeneration in neurons is to lesion their axon then wait for a while, and lesion the axon again. This first, so-called ‘conditioning lesion’ induces a renewed growth state in an adult PNS axon that normally regenerates. Subsequently, the effects of the initial lesion are tested by making a second lesion (the ‘test lesion’) more proximally. If the second lesion is delayed for several days and kept distal to the cell body, this results in an acceleration of the rate of secondary axonal regeneration [2] (Figure 1). Though this paradigm has been investigated for the past 40 years, new work by Frank Bradke and colleagues [3] in this issue of Current Biology sheds new light on the basic mechanisms that underlie this phenomenon.
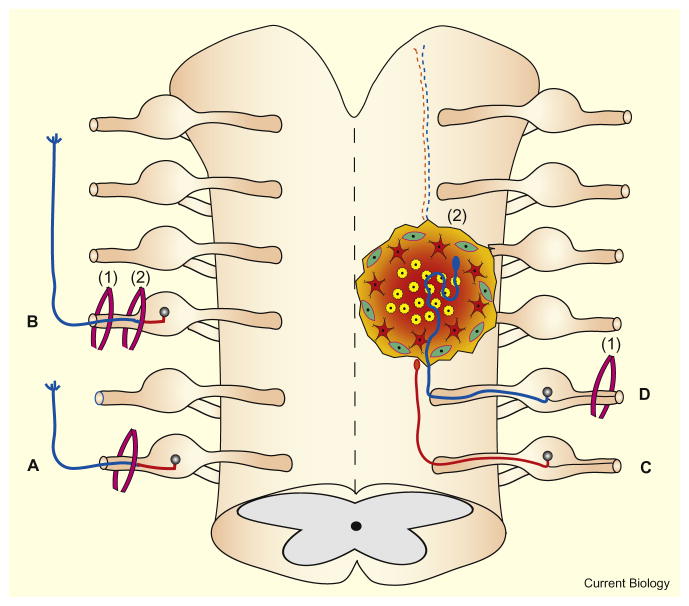
Figure 1. Conditioning lesions and regeneration in the CNS and PNS.
The left side of the figure depicts effects in the PNS and the right side depicts effects in the CNS. (A) After only a single PNS lesion, the rate of regeneration (blue) of severed axons is comparatively low. (B) After a conditioning lesion (1) in the PNS, the regeneration in the distal direction (blue) after a more centrally located test lesion (2) is significantly higher. (C) Regeneration in the CNS is minimal without conditioning as severed axons cannot cross scars. (D) A peripheral conditioning lesion (1) stimulates axonal regeneration within a lesion in the CNS (2).
The conditioning effect has been documented in both sensory and motor neurons in the mammalian PNS but also retinal ganglion cells within the optic nerve in species, such as goldfish, that can regenerate certain types of CNS axons [4]. The dorsal root ganglion (DRG) contains a population of unusual, dendrite-less neurons that maintain two distinct axons, one on either side of the cell body. The centrally directed axon still resides in an environment rich in Schwann cells and extends all the way to the dorsal root entry zone. Importantly, for some as yet unknown reason, a prior lesion of the dorsal root axon in the PNS between the cell body and the spinal cord (as well as its extension into the CNS dorsal columns) neither produces a detectable cell body response or changes in protein synthesis, nor does it appear to alter the regeneration rate of the central or peripheral axons [5]. Regeneration in the root remains sluggish and halts abruptly at the PNS–CNS interface [6]. Regeneration across the dorsal root entry zone or completely through a lesion in the CNS is essentially non-existent [7]. In 1999, Simona Neumann in the lab of Clifford Woolf [8] addressed a fundamental question: Can conditioning overcome potently inhibitory substrates such as those encountered by regenerating axons within the CNS? Indeed, they showed that a peripheral conditioning lesion could lead to enhanced regrowth of sensory fibers in the vicinity of a surgical lesion within the dorsal columns (Figure 1). While such central regeneration was relatively meager (on the order of a few millimeters), the result has stimulated renewed excitement about understanding the conditioning phenomenon.
In order to be therapeutically relevant to regeneration biology in the CNS, the conditioning effect needs to be elicited after and not before a central lesion and it is obviously preferable to avoid sacrifice of peripheral nerves. While the complex sequence of events underlying this phenomenon is still being worked out, the conditioning effect is likely due to the renewed activation or repression of an array of regeneration-associated genes (RAGs) regulated by a variety of transcription factors that, in turn, leads to the synthesis of abundant regeneration-associated proteins. Some cytoskeletal and other proteins made in the cell body are transported down the regenerating axon where they likely contribute to a reservoir of pre-made material that is at the disposal of the growth cone upon re-lesion [9]. However, it is now becoming clear that newly transcribed mRNAs also enter the regenerating axon. Such regeneration-associated mRNAs may be able to be rapidly translated into new growth-inducing proteins near the tip of the re-severed axon [10,11]. Also, it is clear that cAMP [12], as well as inflammation within the ganglion [6], are involved in the conditioning effect since injection of cAMP or inflammatory provoking agents into the DRG can mimic the effects of overt lesioning. Importantly, it has been shown that a regeneration-enhancing effect can be stimulated in adult mammalian retinal ganglion cells by lens injury or an inflammatory agent placed within the vitreous body [13]. These observations provide evidence that certain mammalian neurons whose axons reside solely within the CNS can also be conditioned.
Given the nearly four decades of investigation into the conditioning effect, one would have thought that many if not all of the most fundamental questions about this interesting phenomenon had already been addressed. But now, Frank Bradke and colleagues [3], writing in this issue of Current Biology, have answered two critical, clinically relevant issues: Can DRG neurons become conditioned peripherally after a central lesion? Are there any circumstances where DRG axons injured first in the dorsal columns can be stimulated to later on traverse the lesion upon conditioning? The answer to the first question is surprisingly: ‘yes’. The answer to the second question is also ‘yes’, but depends upon the size of the lesion. It needs to be small enough to avoid scarring, which means really small [3].
First, the authors examined whether centrally injured adult rat DRGs would up-regulate RAGs after a subsequent peripheral lesion. Remarkably, all tested RAGs were upregulated multiple fold in DRG neurons conditioned two, four, or even eight weeks after the CNS lesion. Importantly, a similar upregulation was induced in animals that were either conditioned before CNS injury or underwent peripheral lesion only. As expected, a dorsal column lesion alone did not significantly affect the expression of RAGs compared to unlesioned controls. They also showed that these biochemical indices of conditioning had a biological effect, at least in vitro. Indeed, neurons conditioned after CNS injury grew axons as efficiently as those conditioned before CNS injury both on permissive substrates as well as on inhibitory myelin.
They next asked whether the post-lesioned, conditioned DRG neurons could actually regenerate in vivo across a relatively large lesion. It is possible that the PNS lesion may induce the centrally injured DRG neurons into a growth competent state, but the time delay between lesions leading to inhibitory changes at the injury site may block the execution of their intrinsic potential. Thus, regeneration could never happen across the original large lesion. So how did Bradke and colleagues [3] address this question? They assessed whether centrally injured DRG neurons that were subsequently conditioned two weeks later could regenerate their axon beyond a second, fresh central lesion made after an additional delay of one week and made more proximal toward the cell body. In rats that received a central lesion only, the vast majority of axons retracted from the caudal edge of the lesion and formed dystrophic retraction balls [14]. In contrast, in all rats conditioned after an initial CNS injury, axons regenerated into and a short distance beyond the second, more caudally placed fresh central lesion.
How do conditioned DRG axons manage to cross an old or new lesion? The use of transgenic mice that express green fluorescent protein (GFP) in a few neurons [15] allowed for an unprecedented level of analysis of regenerating axons in vivo. Also, the ability to repeatedly image the same axon tip over time guarantees that any axons found within or beyond the lesion are truly regenerated fibers and not simply axons that had been spared by the initial injury. Normally injured axons within the CNS rapidly withdraw from the site of axotomy during a period of hours to weeks. Such retraction or ‘dieback’ of the proximal axon was first described by Ramon y Cajal [7]. Since these original observations, there have been differing reports as to the nature of axonal retraction, its cause, extent, and timing, as well as discussion of whether it is a passive or active process. While the protracted period of long-distance dieback is now thought to be due to direct interactions between dystrophic axons and activated macrophages [16], it has been suggested that the early phase of proximal retraction is similar to Wallerian degeneration of the distal axon [17]. Interestingly, Bradke and colleagues [3] found that the acute attempts at regeneration of conditioned neurons in the CNS mirror somewhat those that have been reported in the periphery. During the first 5–7 hours post-injury the axons do not undergo dieback. Instead small sprouts emerge, not from the nodes of Ranvier as in the periphery, but rather directly from the cut tips of many axons. Similarly, neurons conditioned after an initial central lesion also showed the rapid production of small sprouts at the fresh lesion. After time, the sprouts elongated further and, although some grew haphazardly, others penetrated and even grew a bit beyond the fresh injury site. In contrast, unconditioned neurons did not form sprouts but, rather, their severed axons were tipped with club shaped endings that retracted from the lesion. These results show that conditioning does at least two things. It not only enables primary sensory neurons to rapidly grow through a fresh CNS lesion but it gives them a head start on the typical unconditioned axon that tends to retract backwards. Importantly, the events described in this paper may help to pinpoint certain proteins that might be critical for these most essential features of the conditioning effect.
Finally, the authors address perhaps the most interesting question concerning why normal or even post-CNS lesioned conditioned axons cannot regenerate through the primary CNS lesion. Is it possible that the lesion becomes inhospitable for normal as well as conditioned axons over some period of time? Alternatively, axons may regenerate through the second central lesion because this injury simply removes the degenerating tip of the axon enabling growth from a freshly cut axon stump. To distinguish between these extrinsic and intrinsic possibilities the authors did something rather challenging and heroic. They used a two-photon laser to lesion central sensory axons without creating traumatic tissue or scarring and then conditioned and imaged them after such minimal CNS injury. Would the axons now regenerate? After transection of single GFP-labeled sensory axons in the spinal cord and after conditioning, the regenerative axonal sprouts, which took a few days to get going, grew in different directions, but by about 6 days, the sprouting became robust and many axons grew right through the tiny central lesion. For the first time it has been shown unequivocally that axon regeneration can occur rather quickly through a primary central lesion upon subsequent conditioning. However, this can only occur when the axotomizing lesion is small enough not to evoke scarring. The result gives strong support to the notion that the lesion environment is a most crucial determinant in axon regeneration failure [1].
This new work [3] is deserving of an accolade not only for the new information that it has provided, but also for the many new questions that it has raised. The authors mention that in the setting of such small lesions, even unconditioned axons showed continuous modest growth. Therefore, if given enough time, might unconditioned, normal axons regenerate at least into or even past the minimal lesion? What are the major obstacles that appear after larger lesions? Scar associated extracellular matrix molecules, such as proteoglycans, macrophage attack of the dystrophic axon as well as the release of exuberant myelin inhibitory factors are obvious candidates, but are there others? Why, in the end, do even conditioned axons regenerate such relatively short distances once they have supposedly passed the lesion site? Given that microtransplanted DRGs can regenerate axons long distances within normal or lesioned white matter, why don’t conditioned DRGs keep going once beyond the glial scar [1]? Now that we have learned that conditioning can happen in a more clinically relevant setting, optimizing this effect in many types of injured neurons will surely become a focus in CNS regeneration biology.
References
- 1.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 2.McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- 3.Ylera B, Ertürk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F. Chronically injured adult sensory neurons acquire axon growth competence following a lesion of their peripheral axons. Curr Biol. 2009;19:930–936. doi: 10.1016/j.cub.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 4.McQuarrie IG, Grafstein B. Effect of a conditioning lesion on optic nerve regeneration in goldfish. Brain Res. 1981;216:253–264. doi: 10.1016/0006-8993(81)90128-1. [DOI] [PubMed] [Google Scholar]
- 5.Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal ganglion cells accelerates regeneration of only their peripheral axons. J Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramon y Cajal S. Degeneration and Regeneration in the Nervous System. London: Oxford University Press; 1928. [Google Scholar]
- 8.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 9.Tashiro T, Komiya Y. Changes in organization and axonal transport of cytoskeletal proteins during regeneration. J Neurochem. 2006;56:1557–1563. doi: 10.1111/j.1471-4159.1991.tb02051.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanz S, Fainzilber M. Retrograde signaling in injured nerve - The axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- 11.Willis DE, Twiss JL. The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 13.Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey A, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci. 2004;24:6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 16.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28:9330–9341. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]