Abstract
Recently, several proteins have been identified that are related in their sequence to the p53 tumor-suppressor protein. One of these proteins, which is termed p73, exhibits sequence homology to the p53 transcriptional activation, DNA binding, and oligomerization domains. The adenovirus E1B 55-kDa protein, the adenovirus E4orf6 protein, and SV40 T antigen each can bind to p53 and inhibit p53 function. Here we demonstrate that the adenovirus E4orf6 protein, but not the E1B 55-kDa protein or T antigen, interacts with p73. The E4orf6 protein inhibits p73-mediated transcriptional activation and cell killing in a manner similar to its effect on p53. Thus, only a subset of viral oncoproteins that antagonize p53 function also interacts with the related p73 protein.
The p53 tumor-suppressor protein binds to DNA and modulates transcription (1). It is a key regulator of the cell cycle and apoptosis, and loss of p53 function can promote unregulated cell proliferation. As a consequence, the p53 gene is commonly mutated in human cancers. Oncoproteins encoded by DNA tumor viruses inhibit the function of p53 (2). The adenovirus E1B 55-kDa protein and SV40 T antigen bind to p53 and sequester it in an inactive complex. The human papillomavirus E6 protein interacts with p53 and promotes its ubiquitin-dependent degradation. Recently, we determined that a second adenovirus protein, the E4orf6 protein, also binds to p53 (3). In contrast to the adenovirus E1B 55-kDa protein, which binds to the amino-terminal transcriptional activation domain of p53 (4–6), E4orf6 binds within its carboxyl-terminal region (3). Nevertheless, like the E1B protein, E4orf6 inhibits the ability of p53 to activate transcription. Consistent with its ability to bind to p53 and antagonize its function, E4orf6 cooperates with the adenovirus E1A protein to transform rat cells, and it enhances the tumorigenic potential of cells expressing both E1A and E1B oncoproteins (7–9). E4orf6 shuttles between the nucleus and cytoplasm in infected cells (10, 11). Shuttling is almost certainly essential for the mRNA transport function of E4orf6 during adenovirus infection, but it is not yet clear whether shuttling is important for its oncogenic activity.
Recently, several proteins with sequence homology to p53 have been described (12–16). The p73 member of this protein family (12, 13) exhibits striking sequence similarity to the DNA-binding, transactivation, and oligomerization domains of p53. Like p53, p73 can bind to DNA and activate transcription. When it is overexpressed, p73 can block cell proliferation and induce apoptosis in a manner analogous to p53. Here we demonstrate that the adenovirus E4orf6 protein can bind to p73 and inhibit its function. The E4orf6 protein might contribute to adenovirus-mediated oncogenesis by antagonizing p73 as well as p53 function.
MATERIALS AND METHODS
Plasmids and Cells.
The mammalian expression vectors pcDNA3-HA-p73α and pcDNA3-HA-p73β encode epitope-tagged p73 proteins (12). The pcDNA3-HA-p73α derivatives, p73TADB and p73TA, were made by amplifying segments of the p73 coding region by using the primer 5′-CTTGGATCCAAGCTTATGGCCCAGTCCACCACC-3′, together with either of two second primers: 5′-TAAGAATTCTCAATTCAAGGCCTGCTGCTCC-3′ or 5′-TAAGAATTCTCAAGTGACCTCGAAGTGGTGG-3′. The PCR-amplified fragments were inserted into the EcoRI/HindIII site of pcDNA3. To generate p73αOL and p73βOL, EcoRI/XbaI fragments from pcDNA3-HA-p73α or pcDNA3-HA-p73β were cloned into EcoRI/XbaI-cleaved pcDNA3. Baby rat kidney cells transformed with the adenovirus E1A and E1B genes (XhoIC) or E1A, E1B, and E4orf6 genes (A2) have been described (7). H1299 and Saos-2 cells, which do not express endogenous p53 (17, 18), were obtained from the American Type Culture Collection. To produce derivatives expressing the E4orf6 protein (SaosE4#5 and SaosE4#11), Saos-2 cells were transfected with pCMVE4orf6 (3), and plasmid-containing clones were selected in medium containing 300 μg/ml G418.
Protein Interaction Assays.
To search for proteins that interact with E4orf6, transformed rat cells (7) were labeled with [35S] methionine and lysed in buffer (19) containing 250 mM NaCl/50 mM Hepes, pH 7.0/0.1% Nonidet P-40/10 μg/ml aprotinin/10 μg/ml leupeptin/1 μg/ml pepstatin/0.1 mM phenylmethylsulfonyl fluoride/5 mM EDTA. After preclearing, proteins were immunoprecipitated with the E4orf6-specific RSA3 monoclonal antibody (20) and subjected to electrophoresis in an SDS-containing polyacrylamide gel. To test for interactions between E4orf6 and selected viral oncoproteins, H1299 cells were transfected with various expression plasmids (15 μg) by using lipofectamine (GIBCO/BRL). After 48 h, the cells were lysed as described above, proteins were immunoprecipitated with E4orf6-specific RSA3 (17), E1B 55-kDa-specific 2A6 (21), or SV40 T antigen-specific PAb416 (22) monoclonal antibody and subjected to electrophoresis in an SDS-containing polyacrylamide gel. Proteins carrying an HA tag were detected by Western blot by using the 12CA5 antibody and a chemiluminescence detection system (New England Nuclear). To test for the interaction of E4orf6 with p73 and p73 derivatives, in vitro-translated products (TNT reticulocyte system, Promega) were mixed at 4°C for 30 min, the proteins were immunoprecipitated by using RSA3 monoclonal antibody or normal rabbit serum, subjected to electrophoresis in an SDS-containing polyacrylamide gel, and protein bands were visualized by fluorography. In similar experiments, in vitro-translated E1B 55-kDa protein or T antigen produced in baculovirus-infected cells and immunoaffinity purified (23) was mixed with in vitro-translated p73, and proteins were precipitated with E1B-specific 2A6 antibody or T antigen-specific PAb416 antibody, respectively.
Luciferase Assay.
H1299 (1 × 106) cells received a total of 7 μg DNA (2 μg of reporter plasmid and various amounts of effector plasmids plus pUC118, which was used as carrier DNA) by transfection by using lipofectamine (GIBCO/BRL), and luciferase assays were performed 24 h later.
Growth Suppression Assay.
Effector plasmids (2.5 μg) plus pBabe-puro (0.5 μg) were introduced into Saos-2, SaosE4#5, and SaosE4#11 cells by using the FuGENE6 transfection reagent (Boehringer Mannheim). After 48 h, the cell growth medium was supplemented with 0.5 μg/ml puromycin (Sigma) and, 2 weeks later, the cells were fixed, stained with crystal violet, and puromycin-resistant colonies were counted (24).
RESULTS
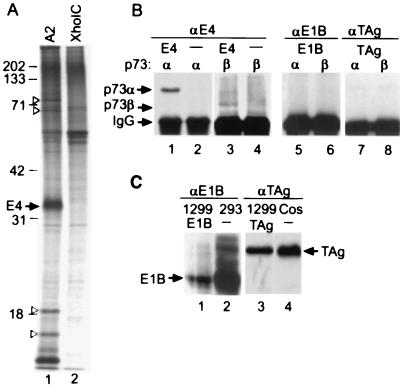
We first suspected that the adenovirus E4orf6 protein might bind to p73 when we immunoprecipitated the viral protein from 35S-labeled extracts of transformed rat cells. Immunoprecipitates from baby rat kidney A2 cells transformed with adenovirus E1A, E1B, and E4orf6 contained several proteins that were not detected in extracts of baby rat kidney XhoIC cells transformed with E1A and E1B alone (Fig. 1A). The proteins migrating at 73, 65, 18, and 13 kDa relative to markers failed to coprecipitate with antibody to E4orf6 after disruption of protein interactions with SDS (data not shown), identifying them as candidates for proteins that might be coprecipitated as part of a complex with E4orf6.
Figure 1.
Coimmunoprecipitation of p73α and p73β with E4orf6 from extracts of transformed baby rat kidney cells and transfected H1299 cells. (A) Coimmunoprecipitation of rat cell proteins with E4orf6. 35S-labeled extracts of baby rat kidney A2 cells expressing E1A, E1B, and E4orf6 or XhoIC cells expressing E1A and E1B were subjected to immunoprecipitation by using antibody to E4orf6. The positions at which marker proteins migrated are indicated by their molecular weights, the E4orf6 band is identified, and arrowheads mark bands corresponding to proteins that are evident in the precipitate from A2 but not XhoIC extracts, and, therefore, might associate with E4orf6. (B) Coimmunoprecipitation of p73 with E4orf6 but not with E1B 55-kDa or T antigen. H1299 cells were transfected with plasmids expressing the viral oncoproteins (E4, E1B, or TAg) plus p73 variants (α or β) indicated above the autoradiogram, and extracts were prepared and immunoprecipitated with antibodies to viral oncoproteins (αE4, αE1B, αTAg). Epitope-tagged p73α and p73β proteins were identified in the immunoprecipitates by Western blot by using antibody to the flu epitope. Arrows identify p73 variants and IgG, which interacts with the secondary antibody in the western blot. (C) Western blot demonstrating expression of E1B 55 kDa in transfected H1299 cells and in 293 cells, which constitutively express the protein, and SV40 large T antigen in transfected H1299 cells and in COS1 cells, which constitutively express the protein. The proteins were detected by using antibodies to the viral oncoproteins and the E1B 55-kDa (E1B) and T antigen (TAg)-specific bands are identified.
The size of the 73-kDa band and our earlier demonstration that E4orf6 binds to p53 (3) led us to test whether E4orf6 can interact with the p53 relative, p73. H1299 cells, which do not express endogenous p53 (17), were transfected with plasmids expressing E4orf6, p73α, and p73β. The two forms of p73 arise from alternative splicing, and they differ at their carboxy termini (12). Extracts were prepared 48 h after transfection, proteins were immunoprecipitated with a monoclonal antibody to E4orf6, and coprecipitated p73 proteins were detected by Western blot by using an antibody to the epitope (flu) tag appended to their amino termini. A band corresponding to p73α was precipitated from an extract of cells transfected with plasmids expressing E4orf6 plus p73α, but not from an extract of cells that received the p73α expression plasmid alone (Fig. 1B, lanes 1, 2). Much less p73β was coprecipitated with E4orf6 in a similar experiment (Fig. 1B, lanes 3, 4), even though the two p73 variants accumulated to similar levels within transfected cells (data not shown). E4orf6 and p73 are present in the same complex, and it is likely that E4orf6 binds directly to p73α and p73β because both contain a carboxyl-terminal domain homologous to the domain in p53 that binds to the viral protein.
In contrast to E4orf6, two additional viral oncoproteins that bind to p53, the adenovirus E1B 55-kDa protein (25) and SV40 T antigen (26, 27), did not interact with the p73 variants in our assay. Although the two viral oncoproteins were efficiently expressed within transfected cells (Fig. 1C), antibodies to E1B 55-kDa (Fig. 1B, lanes 5, 6) or SV40 T antigen (Fig. 1B, lanes 7, 8) failed to coimmunoprecipitate detectable quantities of p73α or p73β from extracts of cells cotransfected with a p73 variant plus an oncoprotein.
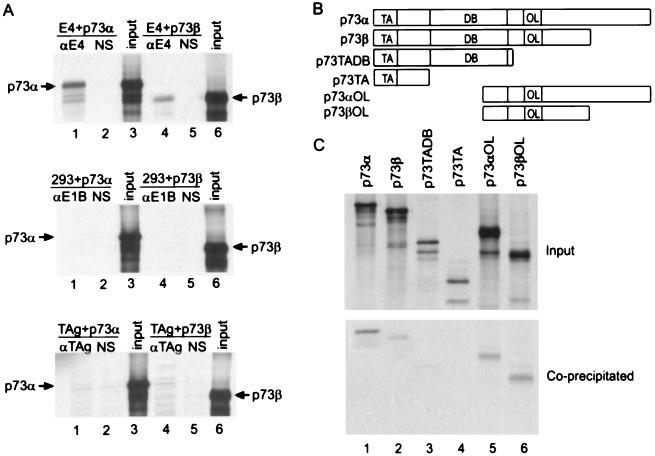
We confirmed the interaction of E4orf6 with p73 using proteins that were translated in vitro. When unlabeled E4orf6 was mixed with 35S-labeled p73α or p73β, the p73 proteins were coimmunoprecipitated with antibody to E4orf6 but not with normal rabbit serum (Fig. 2A Top). In contrast, p73 did not interact at a detectable level with either E1B 55-kDa or T antigen (Fig. 2A Middle and Bottom), although both of these viral proteins interacted with p53 in this assay (data not shown). This experiment was repeated by using deleted derivatives of the p73 proteins (Fig. 2B) that were efficiently synthesized in the in vitro reaction (Fig. 2C Upper). Antibody to E4orf6 coimmunoprecipitated carboxyl-terminal segments of p73α and p73β with E4orf6 (Fig. 2C Lower, lanes 5, 6). An amino-terminal segment of p73 containing the domain homologous to the p53 transactivation domain was not coprecipitated, whereas an amino-terminal segment including the domain homologous to the p53 DNA-binding domain was coprecipitated to a limited extent with E4orf 6 (Fig. 2C Lower, lanes 3, 4). It appears that the E4orf6-p73 interaction occurs primarily through the carboxyl-terminal domain of p73, consistent with our earlier work showing that E4orf6 binds to amino acids 318–360 within the carboxyl-terminal domain of p53 (3).
Figure 2.
Coimmunoprecipitation of in vitro-translated p73 and p73 fragments with E4orf6. (A) Coimmunoprecipitation of p73α and p73β with E4orf6-specific antibody. 35S-labeled p73α or p73β variants were mixed with unlabeled E4orf6 protein (E4) (Top), E1B 55-kDa protein (E1B) (Middle) or T antigen (TAg) (Lower) and then subjected to immunoprecipitation by using antibody to the unlabeled protein (αE4, αE1B, αTAg) or normal rabbit serum (NS). Lanes marked “input” received 20% of the amount of in vitro-translated p73α or p73β that was added to binding reactions. The position of bands corresponding to p73 variants is indicated. (B) Diagrammatic representation of p73α, p73β, and deleted derivatives of the proteins. TA, DB, and OL designate domains in the p73 proteins that correspond to the transcriptional activation, DNA-binding, and oligomerization domains of p53, respectively. (C) Coimmunoprecipitation of p73 and deleted p73 derivatives with E4orf6. (Upper) (input) In vitro-translated, 35S-labeled p73 variants (5% of amount added to binding reactions). (Lower) (coprecipitated) Immunoprecipitation by antibody to E4orf6 after p73 variants were mixed with unlabeled E4orf6.
Because p73 can activate the transcription of p53-responsive genes (12, 13), we tested whether E4orf6 can antagonize p73-mediated activation, as it does for p53 (3). A luciferase reporter construct whose promoter includes the p53 binding site from the p21waf gene (28) was activated by a factor of 6 to 7 when it was cotransfected into H1299 cells with effector plasmids expressing p73α or p73β (Fig. 3A). Inclusion of a second effector plasmid expressing E4orf6 protein completely blocked the induction of the reporter by p73α and reduced the p73β-mediated induction by a factor of 4, whereas the parental effector plasmid without an E4orf6-specific insert had no effect (Fig. 3A). The magnitude of the inhibition correlated with the amount of the E4orf6-expressing plasmid added to cells (Fig. 3B).
Figure 3.
Effect of viral oncoproteins on p73-mediated transcriptional activation. H1299 cells were transfected with the indicated plasmids and luciferase activity was assayed 24 h later. The bar graphs report the fold activation of the luciferase reporter, pWWP-GL2 (2 μg per transfection), by various effector plasmids relative to its basal expression. The amount of DNA (μg) added to transfection mixtures is indicated below the bars. Two plasmids were included to control for nonspecific effects of the expression vectors: pcDNA3 is the vector used to express p73 variants and pCMVneo carries the neomycin-resistance gene in the same expression vector as pCMVE4orf6. The mean and standard deviation for three independent determinations are reported. (A) Inhibition of p73-mediated activation by E4orf6. (B) Dose-dependent inhibition of p73-mediated activation by E4orf6.
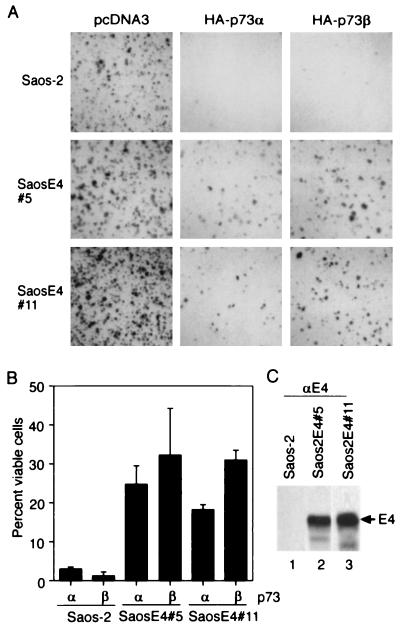
Like p53, p73 has been shown to induce apoptosis when overexpressed (12). To evaluate the effect of E4orf6 on p73-mediated cell killing, we tested the effect of p73α and p73β on colony formation using Saos-2 cells, which lack the p53 gene (18) and fail to express detectable levels of p73 (13), and on derivatives of Saos-2 cells expressing the E4orf6 protein (Fig. 4C). Cells were transfected with a plasmid encoding a puromycin-resistance marker plus the vector expressing p73α or p73β or, as a control, the vector used to express p73 with no insert. Expression of p73α or p73β almost completely inhibited the formation of drug-resistant colonies in Saos-2 cells (Fig. 4A, B). In contrast, two independently derived derivatives of Saos-2 cells expressing E4orf6 protein were partially resistant to p73-mediated killing, producing 19–32% of the total number of colonies that were generated by transfection with the drug-resistance marker plus the empty expression vector (Fig. 4A, B). We conclude that the E4orf6 protein can protect cells from p73-mediated toxicity. We assume that it is blocking p73-mediated apoptosis, because overexpression of p73 has been shown previously to induce apoptosis in Saos-2 cells (12).
Figure 4.
Inhibition of p73-mediated cell killing by E4orf6. (A, B) Saos-2 cells and derivatives of Saos-2 cells expressing E4orf6 protein (SaosE4#5 and SaosE4#11) were cotransfected with a plasmid expressing the puromycin-resistance marker plus a plasmid expressing p73α, p73β, or the expression plasmid with no insert. Puromycin-resistant colonies were counted 14 days later. Photographs of colonies (A) and the percentage of cells giving rise to colonies (B) are presented. (C) Western blot assay using antibody to E4orf6 protein (αE4) demonstrating expression of the E4orf6 protein (E4) in derivatives of Saos-2 cells that contain the E4orf6 gene.
DISCUSSION
The 73-kDa protein band that coimmunoprecipitates with E4orf6 protein from extracts of transformed baby rat kidney cells (Fig. 1A) is comprised, at least in part, of p73. E4orf6 protein can form a complex with p73α and p73β (Fig. 1B and Fig. 2 A, C), and inhibit p73-mediated transcriptional activation (Fig. 3) and toxicity (Fig. 4). Roth et al. (29) have recently reported that E4orf6 protein does not influence transcriptional activation by p73β. E4orf6 appears to bind and inhibit p73α more efficiently than p73β (Figs. 1–3), and Roth et al. (29) tested p73β. Nevertheless, we observe both a physical and a functional interaction between E4orf6 and p73β. As yet, we cannot explain our different results.
The inactivation of p73 function by the E4orf6 protein suggests that its inhibition might facilitate adenovirus replication. Perhaps a block to the function of p73 in addition to p53 prevents activation of p53/p73-responsive genes that could interfere with viral replication. For example, the failure to activate p73-responsive genes might help to protect infected cells from undergoing apoptosis, preventing premature cell death and facilitating the production of an optimal yield of progeny virus. Alternatively, the E4orf6 protein might modulate p73 function, modifying its activity to benefit the virus.
The adenovirus E1B 55-kDa protein and SV40 T antigen failed to interact at a detectable level with p73 (Fig. 1B and Fig. 2A). The E1B 55-kDa protein binds to the amino-terminal transcriptional activation domain of p53 (4–6) and T antigen interacts with its DNA-binding domain (30–32). Apparently, their binding sites are not conserved or are not accessible within the corresponding domains on p73, as is the case for the carboxyl-terminal site on p73 through which E4orf6 interacts to form a complex. Indeed, two residues in the amino-terminal domain of p53 that have been shown to be important for its interaction with E1B 55 kDa, Lys-24, and Pro-27 (6), are not conserved in p73. Consistent with our result, two recent reports failed to detect an interaction of the adenovirus E1B 55-kDa protein (29, 33), T antigen (33), or human papillomavirus E6 protein (33) with p73. Thus, in contrast to the precedent set by the interaction of the adenovirus E1A protein, SV40 T antigen and papillomavirus E7 protein with all members of the retinoblastoma protein family, DNA tumor virus oncoproteins do not appear to recognize all members of the p53 family.
It is noteworthy that the adenovirus E4 transcription unit encodes a variety of proteins with oncogenic potential. The E4orf1 protein induces estrogen-dependent mammary tumors in female rats and transforms rat cells (34), and its transforming domain binds to a cytoplasmic PDZ-domain-containing protein (35, 36). The E4orf3 protein cooperates with E1A to transform rodent cells (37), and it localizes within POD/ND10 nuclear structures (38). The E4orf6/7 protein competes with the retinoblastoma protein for binding to E2F family members (39), and E2F stimulates progression into the S phase of the cell cycle once freed of its association with the retinoblastoma protein (40). E4orf6 not only cooperates with E1A to transform cells (7, 8) but also modifies the phenotype of cells transformed by the adenovirus E1A and E1B proteins (7, 9). Inclusion of E4orf6 markedly enhanced the ability of transformed rat and human cells to form tumors in nude mice (7). Although the difference in growth potential could result from its effects on p53, the E1B 55-kDa and E1B 19-kDa proteins, which are also present in the transformed cells, both antagonize p53 functions. Perhaps, then, the additional effect of E4orf6 on p73 or, conceivably, on other p53 family members is responsible for the enhanced tumorigenic potential observed for cells expressing the oncoprotein.
Acknowledgments
We thank W. Kaelin for p73 expression plasmids, J. Roth for pWWP-GL2, and S. J. Flint for critical reading of the manuscript. This work was supported by a grant from the National Cancer Institute (CA41086). F.H. received postdoctoral fellowship support from the Naito Foundation. T.S. is an American Cancer Society Professor and an Investigator of the Howard Hughes Medical Institute.
References
- 1.Levine A J. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 2.Nevins J R, Vogt P K. In: FieldsVirology. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott; 1996. pp. 2111–2148. [Google Scholar]
- 3.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 4.Kao C C, Yew P R, Berk A J. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite A W, Blair G E, Nelson C C, McGovern J, Bellett A J. Oncogene. 1991;6:781–787. [PubMed] [Google Scholar]
- 6.Lin J, Chen J, Elenbaas B, Levine A J. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 7.Moore M, Horikoshi N, Shenk T. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevels, M., Spruss, T., Wolf, H. & Dobner, T., Oncogene, in press. [DOI] [PubMed]
- 10.Goodrum F D, Shenk T, Ornelles D A. J Virol. 1996;70:6323–6335. doi: 10.1128/jvi.70.9.6323-6335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbelstein M, Roth J, Kimberly W T, Levine A J, Shenk T. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jost C A, Marin M C, Kaelin W G., Jr Nature (London) 1997;389:191–194. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 13.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan J-C, Valent A, Minty A, Chalon P, Lelias J M, Dumont X, et al. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 14.Trink B, Okami K, Wu L, Sriuranpong V, Jen J, Sidransky D. Nat Med. 1998;4:747–748. doi: 10.1038/nm0798-747. [DOI] [PubMed] [Google Scholar]
- 15.Osada M, Ohba M, Kawahara C, Ishioka C, Kanamaru R, Katoh I, Ikawa Y, Nimura C, Nakagama A, Obinata M, et al. Nat Med. 1998;4:839–843. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- 16.Yang A, Kaghad M, Wang Y, Gillett E, Fleming M D, Dotsch V, Andrews N C, Caput D, McKeon F. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 17.Chiba I, Takahashi T, Nau M M, D’Amico D, Curiel D T, Mitsudomi T, Buchhagen D L, Carbone D, Piantadosi S, Koga H, et al. Oncogene. 1990;5:1603–1610. [PubMed] [Google Scholar]
- 18.Chandar N, Billig B, McMaster J, Novak J. Br J Cancer. 1992;65:208–214. doi: 10.1038/bjc.1992.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow E, Whyte P, Franza R, Schley C. Mol Cell Biol. 1986;6:1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marton M J, Baim S B, Ornelles D A, Shenk T. J Virol. 1990;64:2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarnow P, Sullivan C A, Levine A J. Virology. 1982;120:510–517. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- 22.Harlow E, Crawford L V, Pim D C, Williamson N, M. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellino A M, Cantalupo P, Marks I M, Vartikar J V, Peden K W C, Pipas J M. J Virol. 1997;71:7549–7559. doi: 10.1128/jvi.71.10.7549-7559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin X Q, Chittenden T, Livingstone D M, Kaelin W. Genes Dev. 1992;6:953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- 25.Sarnow P, Ho Y S, Williams J, Levine A J. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 26.Lane D P, Crawford L V. Nature (London) 1979;278:261–263. doi: 10.1038/278261a0. (1979). [DOI] [PubMed] [Google Scholar]
- 27.Linzer D I, Levine A J. Cell. 1979;17:43–52. doi: 10.1016/0092-8674(79)90293-9. (1979). [DOI] [PubMed] [Google Scholar]
- 28.El Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 29.Roth J, Konig C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. J Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmieg F I, Simmons D T. Virology. 1988;164:132–140. doi: 10.1016/0042-6822(88)90628-9. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Fields S. FASEB J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- 32.Ruppert J, Stillman B. Mol Cell Biol. 1993;13:3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marin M C, Jost C A, Irwin M S, DeCaprio J A, Caput D, Kaelin W G., Jr Mol Cell Biol. 1998;18:6316–6324. doi: 10.1128/mcb.18.11.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javier R, Raska K, Jr, Shenk T. Science. 1992;127:1267–1271. doi: 10.1126/science.1519063. [DOI] [PubMed] [Google Scholar]
- 35.Lee S S, Weiss R S, Javier R T. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss R S, Javier R T. J Virol. 1997;71:7873–7880. doi: 10.1128/jvi.71.10.7873-7880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nevels, M., Taeuber, B., Kremmer, E., Spruss, T, Wolf, H. & Dobner, T. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 38.Doucas V, Ishov A M, Romo A, Juguilon H, Weitzman M D, Evans R M, Maul G G. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 39.O’Connor R J, Hearing P. J Virol. 1994;68:6848–6862. doi: 10.1128/jvi.68.11.6848-6862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowalik T F, DeGregori J, Schwarz J K, Nevins J R. J Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]