Abstract
Objective
This study investigated if central sensitization is induced in the trigeminal subnucleus caudalis (also termed the medullary dorsal horn) and C1 and C2 dorsal horns by noxious stimulation of deep upper cervical paraspinal tissues in a preparation relatively free of surgical trauma.
Methods
Adult male Sprague-Dawley rats (275–450 g) were anesthetized intraperitoneally. Animals were then placed in a stereotaxic frame; a small cutaneous incision was made 3 to 4 mm near the bregma in the midline, and an opening into the skull was prepared by a 1/32-inch drill, 1 mm to the left from the midline. An epoxylite-coated tungsten microelectrode was introduced at an 18° angle to enter this small opening on the skull and was then carefully advanced about 16 mm through cortex, cerebellum, and brainstem to reach subsequently histologically confirmed sites in the Vc and upper cervical (C1 and C2) dorsal horn region. Thirty-three, 27, and 15 neurons recorded in medullary, C1, and C2 dorsal horns, respectively, of chloralose/urethane-anesthetized rats were activated by noxious stimulation of mechanoreceptive fields involving V1, V2, and/or V3 trigeminal nerve territories. The inflammatory irritant mustard oil was injected into the deep paraspinal tissues at the level of the left C1-C2 joint. Pre and postinjection receptive field (RF) sizes were mapped by nonnoxious mechanical stimuli and noxious mechanical and heat stimuli.
Results
A 30- to 50-minute increase (mean, 165% ± 38.1%) in RF size postinjection for 62% of neurons tested was demonstrated, suggesting central sensitization; for most (>70%) neurons, the RF expanded caudally into cervically innervated tissues.
Conclusions
These findings provide the first documentation that deep cervical nociceptive inputs can induce central sensitization in medullary and C1/C2 dorsal horns and suggest that these effects may reflect mechanisms contributing to deep cervical pain and its referral.
Key Indexing Terms: Dorsal Horn Cells, Trigeminal Caudal Nucleus, Neck Muscles, Headache
The role of cervical structures in headache is unclear although upper cervical joint pain has been identified as a possible source of cervicogenic headache,1–4 whereas cervical muscular pain or tenderness is thought to contribute to tension-type headache.5,6 The notion of cervically induced headache is supported by findings of projections of upper cervical afferents to the trigeminal brainstem subnucleus caudalis (Vc; also termed the medullary dorsal horn) as well as to upper cervical dorsal horns C1 and C2 and that stimulation of upper cervical afferents as well as trigeminal afferents can activate nociceptive neurons in Vc and C1/C2 dorsal horns.7–19 In addition, studies using animal models of deep upper cervical pain have demonstrated that algesic chemical stimulation of deep cervical paraspinal tissues evokes reflex effects in rats, including prolonged increased electromyographic (EMG) activity in the ipsilateral jaw muscles and bilateral deep and superficial upper cervical muscles20–24 as well as alterations in the jaw-opening reflex.25 Similar findings of increased neck and jaw muscle EMG activity have been reported in rats for algesic stimulation of the posterior meninges.26 These effects induced by nociceptive afferent inputs into the central nervous system are suggestive of central sensitization that is thought to be a crucial process underlying the development and maintenance of referred pain and chronic pain states.27–30 Central sensitization is reflected in an expansion of the cutaneous and deep neuronal receptive fields (RFs) and other changes in the properties of neurons in central trigeminal and spinal nociceptive pathways.27–39
It would be of considerable interest for understanding the mechanisms underlying cervical pain and headache to investigate the effects of such nociceptive inputs on the properties of nociceptive neurons identified in single unit extracellular recordings. The standard methodology for such neuronal investigations has typically involved the surgical exposure of the brainstem or spinal dorsal horn by incision of the skin and removal of muscular and other subcutaneous tissues as well as bone overlying the brainstem or spinal cord, followed by stereotactically guided placement of a microelectrode to record the activity of single brainstem (eg, Vc) or spinal dorsal horn neurons. Such a surgical approach has the disadvantages of (1) damaging or removing those deep muscle tissues, from which nociceptive afferent input-induced effects might be tested, and (2) potentially contributing to or influencing any subsequent central sensitization induced by the nociceptive afferent inputs. Indeed, previous studies have shown that surgical trauma can influence the excitability of Vc and other trigeminal brainstem neurons.38,40 In the present investigation, we used a novel method that allows for single neuron recording in Vc and upper cervical dorsal horn in a preparation free of such extensive surgical trauma, to test in this model if central sensitization can be induced in these neurons by application of the inflammatory irritant mustard oil to deep upper cervical paraspinal tissues.
Methods
This study was carried out on adult male Sprague-Dawley rats (275–450 g) anesthetized intraperitoneally with a mixture of chloralose (50 mg/kg) and urethane (1 g/kg); each animal also received atropine (0.02 mg/kg) to reduce tracheal secretions. Adequacy of anesthesia was determined periodically by noting the lack of spontaneous movements by the animal as well as lack of autonomic responses (eg, heart rate increase to pinching the paw and the presence of a constricted pupil); a supplementary anesthetic dose (5 mg/kg of chloralose, 100 mg/kg of urethane, intraperitoneally) was administered if necessary. Heart rate and expired percentage of carbon dioxide were continuously monitored, and rectal temperature was maintained at 37.5°C. Tracheal and femoral venous cannulae were inserted with minimal surgical procedures, and the animals were paralyzed with gallamine triethiodide and artificially ventilated with an air/oxygen mixture. All surgeries and procedures were approved by the University of Toronto Animal Care Committee in accordance with the Ontario Animal Research Act (Canada).
Subsequent procedures for animal preparation, stimulation, and neuronal recording and classification were similar to those previously described in detail,9,12,31,34 except for the following surgical procedure to minimize surgically induced trauma to cervical tissues. The animal was placed in a stereotaxic frame; a small cutaneous incision was made 3 to 4 mm near the bregma in the midline, and an opening into the skull was prepared by a 1/32-inch drill, 1 mm to the left from the midline. An epoxylite-coated tungsten microelectrode was introduced at an 18° angle to enter this small opening on the skull and was then carefully advanced about 16 mm through cortex, cerebellum, and brainstem to reach subsequently histologically confirmed sites in the Vc and upper cervical (C1 and C2) dorsal horn region (see later). Thus, unlike previous Vc and upper cervical dorsal horn electrophysiologic studies by ourselves and others in rats,12,15,19,31–36 the cutaneous as well as deep structures around the neck region were intact, except for a small incision in the anterior neck to allow for the placement of the tracheal cannula. With this approach, the activity of Vc or C1/C2 dorsal horn nociceptive neurons could be recorded in 53 of 77 rats, and 34 of these rats were injected with mustard oil (20 μL, 80% mineral oil and 20% allyl isothiocyanate; Aldrich Chemicals, St. Louis, MO) into the deep paraspinal tissues20,24 to test for indications of central sensitization in nociceptive neurons in Vc and C1 and C2 dorsal horns (see later). Mineral oil (as vehicle control) was not used because we have previously documented that its injection into craniofacial or cervical tissues does not induce any evidence of central sensitization.20,32,41
Neuronal activity was amplified and displayed on a digitizing oscilloscope, to allow for discrimination of single units.12,31 The spontaneous or evoked activity could also be led to a window discriminator and to an electronic counter to measure neuronal firing rates. Neuronal activity was also stored on a PC computer and interface system (CED 1401/Spike2, Cambridge Electronic Design, Cambridge, United Kingdom) for subsequent off-line computer-assisted analysis of the data, including a dot raster display of evoked responses.
We initially searched for spontaneous neuronal activity and neuronal responses evoked by search stimuli that included brushing and air puffs delivered to the shaved facial skin and neck and a blunt probe applied to the craniofacial, neck, shoulder, trunk, and limb tissues. If the craniofacial or cervical stimuli evoked responses that indicated the location of the microelectrode in the Vc or C1 or C2 dorsal horn,12,19 then the microelectrode was slowly advanced to search for nociceptive neurons. Immediately after isolation of a neuron, a 2-minute nonstimulation period was used to determine if the neuron had any spontaneous activity. Then, if responses of the neuron could be reproducibly evoked by the search stimuli, further stimuli were applied to determine the response properties of the neuron, its RF extent and border, and its classification. These stimuli included air puffs, muscle palpation and stretching, pressure and pinch stimuli applied by calibrated forceps, von Frey filaments, and noxious radiant heat stimulation (51°C–53°C) applied by a focused projector bulb, as well as electrical stimuli (0.1–2 millisecond, 0.5–5 mA, constant current single pulses) applied within the delineated RF so as to determine whether the neuron was receiving inputs from A or C fiber inputs8,9,31 (see also later). On the basis of its cutaneous RF characteristics, minimal response latency and activity evoked by graded electrical stimuli, each unit could be classified as a primary afferent or as 1 of 3 types of cutaneous nociceptive or nonnociceptive neuron, as previously described.8,12,31 The 3 classes of neurons included low-threshold mechanoreceptive neurons that responded to hair movement or to light tactile stimulation and showed no increase in discharge with more intense stimuli. Wide dynamic range (WDR) neurons responded to low-threshold mechanical stimuli (von Frey filament force < 500 mg) but, in contrast to low-threshold mechanoreceptive neurons, increased their firing as the mechanical stimulus intensity was increased into the noxious range, that is, intensities that produced frank pain when applied to the experimenter’s skin. If the neuron did not respond to these tactile stimuli but responded only to pressure and pinch or only pinch, it was classified as nociceptive-specific (NS).
Only NS and WDR neurons were studied in detail. Their peak firing rate evoked by brushing, pressure, and pinch was displayed on the electronic counter and recorded. The threshold for electrically induced activation from within the RF was also determined for most neurons, and suprathreshold stimuli were then applied to determine the minimal latency of the first spike consistently evoked by each of 5 to 10 suprathreshold electrical stimuli delivered at 0.5 to 1 Hz. For each NS and WDR neuron, in addition to the standard electrical stimulus parameters (see previously), a 5-mA 2-millisecond electrical stimulus was also applied within and outside the RF to determine if the neuron received a C-fiber afferent input. A discharge consistently evoked at a latency greater than 30 millisecond was attributed to C-fiber excitatory inputs on the basis of a 20- to 30-mm conduction path and allowances of 1 millisecond for peripheral activation time, central narrowing of the afferents, and synaptic delay.8,12,31
As previously described,20 mustard oil was injected from a dorsal approach into the deep paraspinal tissues of the left side of the neck, 0.5 cm lateral to the midline and 1.5 cm behind the occipital ridge; the injection was placed 1 cm below the skin surface, within the paraspinal tissues around the C1 through C3 vertebrae. Only one neuron was studied with mustard oil in each experiment, and, after a nociceptive neuron was identified by mechanical, thermal, and electrical stimuli, its spontaneous activity and RF were determined and served as baseline values. Subsequently, the spontaneous activity and RF were determined at 5- to 10-mm intervals for the subsequent 50-minute observation period after the mustard oil injection.
Previous studies in our laboratories20,42 have shown that the visual localization of the mustard oil-induced Evans Blue dye extravasation in deep paraspinal or craniofacial tissues correlates well with the spectrophotometric detection of its presence and histologic parameters of inflammation. This was confirmed in 5 rats in which, at the end of the experiment, 40 minutes after the injection of mustard oil, Evans blue (0.2 mL, 1%) was injected into the right external jugular vein; 20 minutes later, the animal was given a lethal injection (T61, Hoechst, Montreal, Quebec, Canada). The mustard oil injection site was visually localized by the appearance of extravasated dye and was then outlined on a standard drawing of the neck region of the rat; the tissues were also examined histologically to confirm the presence of inflammation.
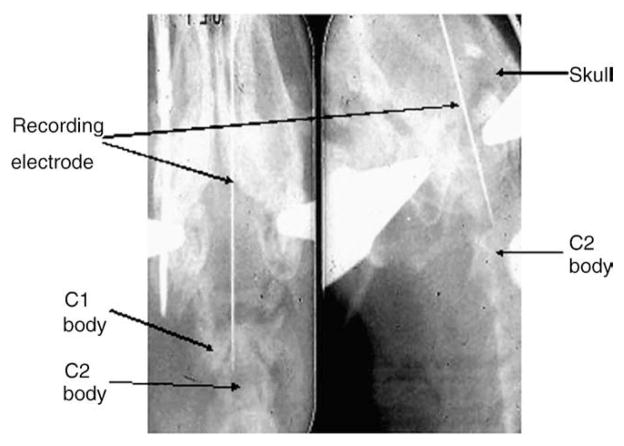
Electrolytic lesions were made at selected recording loci so that verification of the loci could be made, after sacrifice of the animal, from histologic sections of the Vc or upper cervical spinal cord and microdrive readings of microelectrode depth; an x-ray providing dorsal and sagittal views of the cranium and neck (Fig 1) also assisted in the assessment of microelectrode location. The reconstructed histologic data were based upon camera lucida drawings12,19,31 and transferred onto a diagram of the Vc, C1, and C2 spinal cord as outlined and defined by Molander et al.43
Fig 1.
X-ray photograph of the penetration course and final location of the recording electrode (see Methods). Left, anteroposterior view of the skull and cervical spine; right, lateral view.
Data were statistically analyzed using the Student t test for continuous data and the Mann-Whitney U test for nonparametric data.
Results
General Features
A total of 81 nociceptive neurons (42 WDR and 39 NS) were recorded in this minimal surgical preparation, which provided for stable recordings at Vc and C1/C2 dorsal horn sites. Spontaneous activity was present in 24% of the 42 WDR and 5% of the 39 NS neurons and ranged from 20 spikes per second to 3 spikes per minute. Based on their RF location and histologic reconstruction of recording sites, 33 neurons were recorded in Vc, 27 neurons in C1 dorsal horn, and 15 neurons in C2 dorsal horn; the location of the remaining 6 neurons could not be confirmed. The WDR neurons could be activated by low-threshold as well as high-intensity mechanical stimuli, whereas the NS neurons were activated only by noxious mechanical stimuli (Figs 2, 3, 4); 26 WDR and 31 NS neurons tested could also be activated by noxious heat stimulation of the RF. An RF could not be fully delineated with mechanical stimulation in 9 of the 81 neurons. In 72 of the 81 neurons, an RF was fully delineated and varied greatly in size; the mean (± SE) RF sizes of WDR neurons (825 ± 217.2 mm2) and NS neurons (541.6 ± 120.6 mm2) were not significantly different (P > .05, t test). Their RFs were located on the face, with 13, 11, and 5 neurons having their RF located exclusively in the V1, V2, or V3 trigeminal nerve territory, respectively, and 20, 12, and 11 neurons having their RF involving both V1 and V2; V2 and V3; and V1, V2, and V3, respectively.
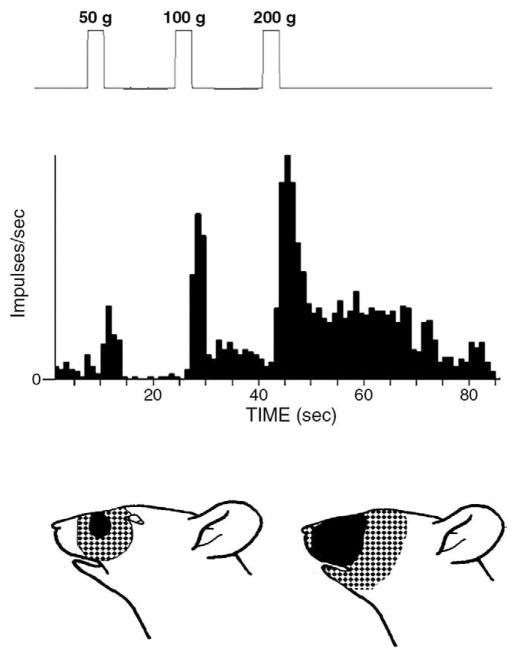
Fig 2.
Reponses and mechanoreceptive field of a WDR neuron. The neuron could be activated by brushing and by a 50-g mechanical stimulus applied to its RF but showed greater responses to 100-g and 200-g stimuli. The neuron continued to be active for several seconds after the 100-g and 200-g stimuli. The tactile component (solid area) and pinch component (stippled areas) of its RF are shown in the face figurine (lower left); the lower right face figurine shows the expansion of these 2 components at 10 minutes after mustard oil was injected into the deep cervical paraspinal tissues.
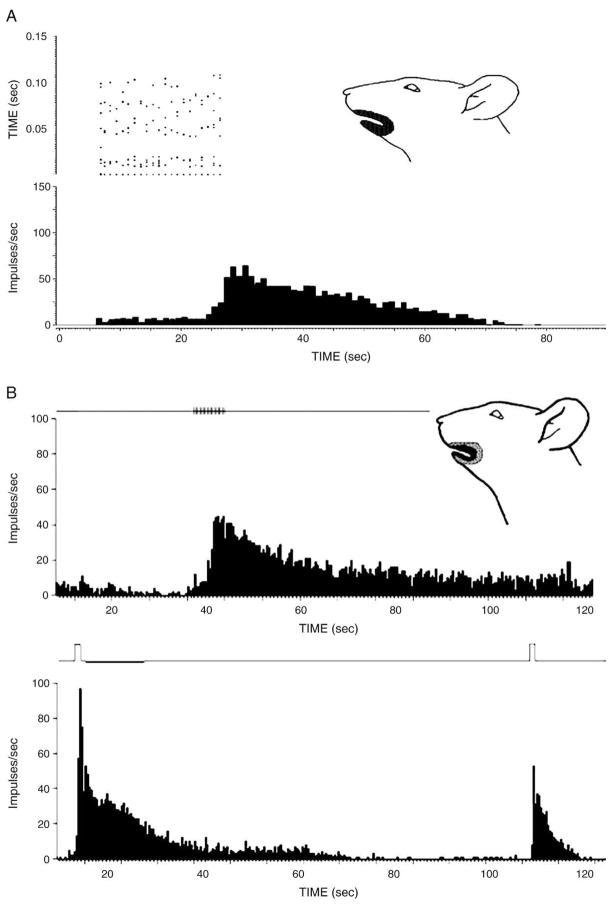
Fig 3.
Reponses of nociceptive neurons to stimulation of their mechanoreceptive field. The neuron shown in A was an NS neuron that responded to noxious mechanical stimulation of its RF. The top left panel shows the dot raster display of A-afferent and C-afferent related discharges evoked by electrical stimulation (each 5 mA, 2 milliseconds) applied to the RF shown in the face figurine. After the offset of the series of electrical stimuli, the neuron showed increased activity that lasted more than 45 seconds. The neuron shown in B was a WDR neuron activated by tactile and noxious mechanical and heat stimulation of the RF shown in the face figurine. The neuron’s responses to electrical (2.5 mA, sixteen 2-millisecond pulses, shown by vertical marks on the line in the top panel) stimulation progressively increased after the first few pulses, and the increased activity outlasted the series of pulses by approximately 200 seconds. The neuron’s responses to noxious mechanical stimulation (shown by vertical marks on the line in the bottom panel, left) and to noxious heat (shown by the vertical mark on the line in the bottom panel, right) also outlasted these stimuli by approximately 120 and 25 seconds, respectively.
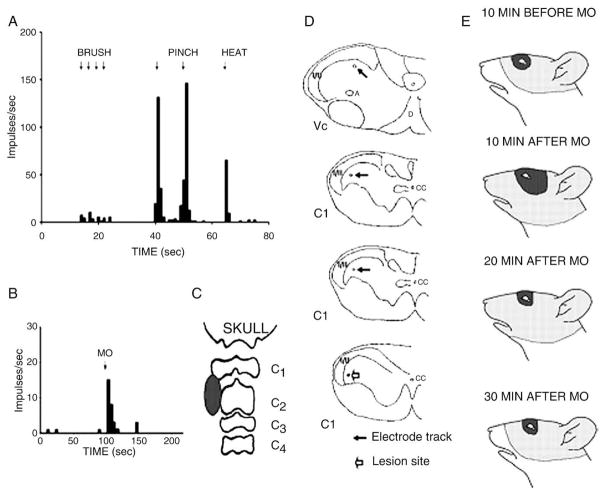
Fig 4.
Responses of a WDR neuron located in the C1 dorsal horn region. A shows a rate histogram depicting the neuron’s responses to 4 brushes, 2 noxious mechanical (pinch), and 1 noxious heat stimuli applied to its RF shown in E (top face figurine), and B illustrates its response to mustard oil (MO) injected into the deep cervical paraspinal tissues; the injection site shown in C was visualized by the extent of extravasated Evans blue dye. D indicates the reconstruction of the microelectrode tract (solid arrows) in Vc and C1 dorsal horn region; note the electrolytic lesion site (open arrow) in the bottom figurine. E indicates the neuron’s RF before and after MO was injected into the paraspinal tissues. Note the RF expansion for both tactile (solid area) and pinch (stippled region) components of the RF at 10 minutes after the MO injection and that the tactile RF had returned to its original size 20 minutes after the MO injection and that the pinch RF had returned to its original size 30 minutes after the MO injection.
Among those neurons with clearly discriminated A and/or C-afferent fiber inputs (n = 60; see Methods), WDR neurons had significantly shorter latencies (P < .001, Mann-Whitney U test) related to A-afferent inputs (n = 32; 4.26 ± 0.23 milliseconds) compared to NS neurons (n = 28; 6.4 ± 0.48 milliseconds). Thirty-one of the 42 WDR neurons and 17 of the 39 NS neurons had C-afferent as well as A-afferent inputs, and the latencies related to C-afferent fiber inputs were similar for both WDR and NS neurons (54.0 ± 3.0 and 53.9 ± 2.14 milliseconds, respectively). The remaining neurons had either A- or C-afferent input. Whereas most neurons showed a progressively increasing C-fiber related discharge to a train of 10 electrical stimuli (1 Hz) that quickly (within 100 milliseconds) subsided after stimulus offset, consistent with earlier studies12,44,45 documenting such “windup” in Vc or C1/C2 nociceptive neurons, 3 WDR and 1 NS neurons showed a novel type of response. These 3 neurons had a sustained discharge that started to develop before stimulus train offset and then continued for approximately another 300 milliseconds (Fig 3). Sustained discharges that continued for several seconds after stimulus offset were also apparent in these neurons’ responses to noxious mechanical or heat stimuli (Fig 3).
Effects of Mustard Oil
Consistent with our previous findings,20 we found that mustard oil induced Evans blue dye extravasation and an inflammatory reaction in the deep upper cervical paraspinal tissues (Fig 4). A total of 34 nociceptive neurons (21 WDR and 13 NS) were tested with mustard oil injected into the paraspinal tissues. After the mustard oil injection, 12 neurons (35%) showed an evoked discharge that typically lasted less than 5 minutes (Fig 4), but 1 nociceptive neuron had evoked activity lasting approximately 1200 seconds after the injection of mustard oil; 1 NS neuron showed a mustard oil-evoked inhibition of its spontaneous activity. Twenty-one (62%) of the neurons tested with mustard oil showed a RF expansion (Figs 2, 4), whereas the remaining neurons did not show any RF change or were lost before a definitive response to the mustard oil injection could be determined. Most (>70%) of these 21 neurons (WDR = 16; NS = 5) showed RF expansion caudally into cervically innervated areas (Figs 2 and 4). Of the 21 neurons showing RF expansion, 35% were recorded in Vc, 35% in C1, and 30% in C2. Their pinch RF increased (mean, 165.4% ± 38.1%) above the original (premustard oil injection) size, and 4 WDR neurons showed also expansion (242.0% ± 86.1%) of the tactile component of their RF. The duration of the RF expansion varied between 30 and 50 minutes after the mustard oil injection (Fig 4).
Discussion
This study provides the first documentation that central sensitization can be induced in functionally identified single nociceptive neurones in the Vc and C1/C2 dorsal horns by inflammatory irritation of deep upper cervical paraspinal tissues. Furthermore, this study has introduced a new electrophysiologic recording technique that enabled investigation of the effect of inflammatory irritation of deep upper cervical tissues produced by mustard oil on these neurons in a preparation that was relatively free of surgical trauma. This preparation provided for stable recordings of nociceptive neurons from approximately 70% of the rats used in this study and also minimized the possibility of a baseline level of central sensitization or nociceptive inputs that conceivably may have influenced the effects of mustard oil on Vc and C1/C2 dorsal horn neurons. Such a possibility is not, however, readily apparent from most of the baseline neuronal properties or central sensitization revealed in the present study compared to previous studies in preparations with more extensive surgical trauma. For example, several of the baseline properties of the rat Vc and C1/C2 dorsal horn nociceptive neurons in this novel preparation were comparable to those previously described in other anesthetized rat preparations in our laboratory under otherwise similar experimental conditions12,16,19,31–34,38,39; these included the proportions of WDR vs NS neurons; the proportions of these neurons displaying spontaneous activity or heat-evoked responses; the mean latencies of evoked A-fiber and C-fiber related responses; the shorter mean latency of these responses in WDR neurons compared to NS neurons; and the localization of the RF to V1, V2, and/or V3 trigeminal nerve territories. It is possible that the microelectrode, in its penetration through the suprabulbar and brainstem structures, caused some damage that affected the properties of the neurons we recorded, but this is unlikely because these neuronal properties were generally similar to those recorded in previous studies by ourselves12,16,19,31–34,38,39 where direct visual access was made to the medulla and upper cervical cord without the passage of the microelectrode through higher brain structures.
Also consistent with the previous studies was our finding that most nociceptive neurons showed a progressively increasing C-fiber related discharge to a train of electrical stimuli that quickly dissipated after stimulus offset. Nonetheless, a novel finding in a small number of nociceptive neurons was a sustained discharge outlasting high-intensity electrical stimuli as well as noxious mechanical and heat facial stimuli, suggesting that a hyperexcitable state had been induced by these stimuli in these nociceptive neurons. It is possible that the sustained activity is not readily observable in preparations with more extensive surgical trauma due to the presence of nociceptive afferent inputs producing a level of diffuse noxious inhibitory control that suppresses such activity in these preparations.12,38,40,46
In the case of central sensitization, although the present study is the first to demonstrate that noxious stimulation of deep paraspinal cervical tissues induces changes in functionally identified nociceptive neurons in Vc and C1/C2 dorsal horn that are consistent with central sensitization, our findings are also consistent with earlier studies showing central sensitization in Vc or C1/C2 dorsal horn nociceptive neurons resulting from noxious stimulation of craniofacial tissues.29–39 The magnitude and duration of the RF changes and the proportion of nociceptive neurons demonstrating such changes in the present study are consistent with these earlier findings and suggest that the surgical trauma produced in the previous studies may not have significantly interfered with subsequent central sensitization induced by the experimental noxious stimulation. The RF expansion is a well-documented parameter of central sensitization in Vc and spinal dorsal horn,15,27,29–39 and our findings indicate that RF expansion of Vc and C1/C2 nociceptive neurons may occur in response to mustard oil-induced inflammatory irritation of the deep upper cervical paraspinal tissues. Earlier studies have provided indirect evidence of such central sensitization by their findings of prolonged reflex effects of inflammatory irritation of deep upper cervical paraspinal tissues; the ipsilateral and contralateral upper cervical muscles and ipsilateral jaw muscles demonstrated reflexly induced patterns of prolonged activation,20–24 and the jaw-opening reflex also manifested long-term potentiation.25 In addition, Panfil et al17 have demonstrated up-regulation of c-fos in brainstem nuclei and C1/C2 dorsal horn neurons in response to algesic stimulation of cervical muscles, providing another indirect demonstration of nociceptive-induced changes consistent with central sensitization.
Our findings in Vc and C1/C2 dorsal horn nociceptive neurons, as well as these earlier findings related to central sensitization manifested in RF expansion, also bear on possible mechanisms of some headaches. As noted above, earlier studies have shown that nociceptive inputs from cervical tissues evoke prolonged changes in EMG activity of cranial and cervical muscles.20–25 Such motor changes could conceivably contribute to processes underlying some forms of headache, through activation of craniocervical muscles, which may then lead to local and referred muscular pain. Indeed, some investigators14,26,47–50 suggest that tension-type headaches may, in part, arise from such mechanisms. The mechanisms of cervically induced headache, however, may involve not only motor changes resulting in increased craniocervical muscular activity but also somatosensory changes in Vc and C1/C2 nociceptive neurons that result in referred cervicocranial pain. The RF expansion in nociceptive neurons after peripheral tissue inflammation or nerve injury is considered a central sensitization mechanism that contributes to pain spread and referral in such conditions, as is reviewed in a number of studies.15,27,28,30,37,51 A feature of the present findings was that noxious stimulation of deep cervical tissues supplied by C2 spinal afferents7,13–15,18 can evoke central sensitization, reflected in an RF expansion into cervically innervated areas, in many Vc and C1 as well as C2 nociceptive neurons having a baseline craniofacial RF. These findings may reflect mechanisms contributing to deep cervical pain and to the development of referred pain arising from cervical tissues.
Conclusion
This study has provided the first documentation that central sensitization can be induced in medullary and C1 and C2 dorsal horns by noxious stimulation of deep upper cervical paraspinal tissues in a preparation relatively free of surgical trauma. The findings of an increase in orofacial RF size that typically expanded caudally into cervically innervated tissues after the noxious cervical stimulation also suggest that these effects may reflect mechanisms contributing to deep cervical pain and its referral.
Acknowledgments
The authors gratefully acknowledge the assistance in some of the experiments or data analyses of Dr J Hu and the secretarial assistance of F Yuen.
Funding Sources and Potential Conflicts of Interest
No conflict of interest were reported for this study. This study received funding support from the Canadian Memorial Chiropractic College, National Institutes of Health grant DE 04786 and Canadian Institutes of Health Research grant MT-4918.
References
- 1.The International Classification of Headache Disorders. Headache classification subcommittee of the International Headache Society. Cephalalgia. 2004;24(Suppl 1):8–152. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Bogduk N. Cervicogenic headache. Cephalalgia. 2004;24:819–20. doi: 10.1111/j.1468-2982.2004.00774.x. [DOI] [PubMed] [Google Scholar]
- 3.Bogduk N. The neck and headaches. Neurol Clin. 2004;22:151–71. doi: 10.1016/S0733-8619(03)00100-2. [DOI] [PubMed] [Google Scholar]
- 4.Sjaastad O, Fredriksen TA, Petersen H, Bakketeig L. Features indicative of cervical abnormality. A factor to be reckoned with in clinical headache work and research? Funct Neurol. 2003;18:195–203. [PubMed] [Google Scholar]
- 5.Ashina M, Bendtsen L, Jensen R, Sakai F, Olesen J. Muscle hardness in patients with chronic tension-type headache: relation to actual headache state. Pain. 1999;79:201–5. doi: 10.1016/s0304-3959(98)00167-5. [DOI] [PubMed] [Google Scholar]
- 6.Vandenheede M, Schoenen J. Central mechanisms in tension-type headaches. Curr Pain Headache Rep. 2002;6:392–400. doi: 10.1007/s11916-002-0082-x. [DOI] [PubMed] [Google Scholar]
- 7.Kerr FW. Structural relation of the trigeminal spinal tract to upper cervical roots and the solitary nucleus in the cat. Exp Neurol. 1961;4:134–48. doi: 10.1016/0014-4886(61)90036-x. [DOI] [PubMed] [Google Scholar]
- 8.Price DD, Dubner R, Hu JW. Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal and nociceptive stimulation of the monkey’s face. J Neurophysiol. 1976;39:936–53. doi: 10.1152/jn.1976.39.5.936. [DOI] [PubMed] [Google Scholar]
- 9.Hu JW, Dostrovsky JO, Sessle BJ. Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). I. Responses to oral-facial noxious and nonnoxious stimuli and projections to thalamus and subnucleus oralis. J Neurophysiol. 1981;45:173–92. doi: 10.1152/jn.1981.45.2.173. [DOI] [PubMed] [Google Scholar]
- 10.Gobel S, Hockfield S, Ruda MA. Anatomical similarities between medullary and spinal dorsal horns. In: Kawamura Y, Dubner R, editors. Oral-facial sensory and motor functions. Tokyo, Japan: Quintessence; 1981. pp. 211–23. [Google Scholar]
- 11.Sessle BJ, Hu JW, Amano N, Zhong G. Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and non-nociceptive neurones in the trigeminal subcaudalis nucleus (medullary dorsal horn) and its implications for referred pain. Pain. 1986;27:219–35. doi: 10.1016/0304-3959(86)90213-7. [DOI] [PubMed] [Google Scholar]
- 12.Hu JW. Response properties of nociceptive and non-nociceptive neurons in the rat’s trigeminal subnucleus caudalis (medullary dorsal horn) related to cutaneous and deep craniofacial afferent stimulation and modulation by diffuse noxious inhibitory controls. Pain. 1990;41:331–45. doi: 10.1016/0304-3959(90)90010-B. [DOI] [PubMed] [Google Scholar]
- 13.Angus-Leppan H, Lambert GA, Michalicek J. Convergence of occipital nerve and superior cervical sinus input in the cervical cord of the cat. Cephalagia. 1997;17:625–30. doi: 10.1046/j.1468-2982.1997.1706625.x. [DOI] [PubMed] [Google Scholar]
- 14.Vernon HT, Hu J. Neuroplasticity of neck/craniofacial pain mechanisms: a review of basic science studies. J Neuromusculoskel Syst. 1999;7:51–64. [Google Scholar]
- 15.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurons to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–13. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 16.Hu JW, Sun KQ, Vernon H, Sessle BJ. Craniofacial inputs to upper cervical dorsal horn: implications for somatosensory information processing. Brain Res. 2005;1044:93–106. doi: 10.1016/j.brainres.2005.03.004. [E-pub 2005 Apr 7] [DOI] [PubMed] [Google Scholar]
- 17.Panfil C, Makowska A, Ellrich J. Brainstem and cervical spinal cord Fos immunoreactivity evoked by nerve growth factor injected into neck muscles in mice. Cephalalgia. 2005;26:128–35. doi: 10.1111/j.1468-2982.2005.01005.x. [DOI] [PubMed] [Google Scholar]
- 18.Le Doare K, Akerman S, Holland PR, Lasalandra M-P, Bergerot A, Classey JD, Knight YE, Goadsby PJ. Occipital afferent activation of second order neurons in the trigeminocervical complex in the rat. Neurosci Let. 2006;403:73–7. doi: 10.1016/j.neulet.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Mørch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci. 2007 Jul;26:142–54. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu JW, Yu X-M, Vernon H, Sessle BJ. Excitatory effects on neck and jaw muscle activity of inflammatory irritant injections into cervical paraspinal tissues. Pain. 1993;55:243–350. doi: 10.1016/0304-3959(93)90153-G. [DOI] [PubMed] [Google Scholar]
- 21.Hu JW, Yu X-M, Sunakawa M, et al. In: Gebhart GF, Hammond DL, Jensen TS, editors. Electromyographic and trigeminal brainstem neuronal changes associated with inflammatory irritation of superficial and deep craniofacial tissues in rats; Proceedings of the 7th World Congress on Pain; Seattle (Wash): IASP Press; 1994. pp. 325–66. [Google Scholar]
- 22.Hu JW, Tatourian I, Vernon H. Opioid involvement in electromyographic (EMG) responses induced by injection of inflammatory irritant into deep neck tissues. Somatosens Mot Res. 1997;13:139–46. doi: 10.3109/08990229609051401. [DOI] [PubMed] [Google Scholar]
- 23.Hu JW, Tsai C-M, Bakke M, Seo K, Tambeli CH, Vernon H, Bereiter D, Sessle B. In: Jensen TS, Turner JA, Wiesenfield-Hallin Z, editors. Deep craniofacial pain: involvement of trigeminal subnucleus caudalis and modulation; Proceedings of the 8th World Congress on Pain; Seattle (Wash): IASP Press; 1997. [Google Scholar]
- 24.Shin P, Vernon H, Sessle BJ, Hu JW. Neck muscle length modulates nociceptive reflex evoked by noxious irritant application to rat neck tissues. Exp Brain Res. 2005;163:314–23. doi: 10.1007/s00221-004-2172-y. [E-pub, 2005, Jan 18] [DOI] [PubMed] [Google Scholar]
- 25.Makowska A, Panfil C, Ellrich J. Long-term potentiation of orofacial sensorimotor processing by noxious input from the semispinal neck muscle in mice. Cephalalgia. 2004;25:109–16. doi: 10.1111/j.1468-2982.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 26.Hu JW, Vernon HT, Tatourian I. Changes in neck electromyography associated with meningeal noxious stimulation. J Manipulative Physiol Ther. 1995;18:577–81. [PubMed] [Google Scholar]
- 27.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental studies. Pain. 1993;52:259–85. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 28.Arendt-Nielsen L, Laursen RJ, Drewes AM. Referred pain as an indicator of neural plasticity. Prog Brain Res. 2000;129:343–56. doi: 10.1016/s0079-6123(00)29026-2. [DOI] [PubMed] [Google Scholar]
- 29.Salter MW. Cellular neuroplasticity mechanisms mediating pain persistence. J Orofac Pain. 2004;18:318–24. [PubMed] [Google Scholar]
- 30.Sessle BJ. Trigeminal central sensitization. Rev Analg. 2005;8:85–102. [Google Scholar]
- 31.Hu JW, Sessle BJ, Raboisson P, Dallel R, Woda A. Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brainstem neurones. Pain. 1992;42:53–60. doi: 10.1016/0304-3959(92)90131-T. [DOI] [PubMed] [Google Scholar]
- 32.Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998;80:2621–31. doi: 10.1152/jn.1998.80.5.2621. [DOI] [PubMed] [Google Scholar]
- 33.Chiang CY, Zhang S, Xie YF, Hu JW, Dostrovsky JO, Salter MW, Sessle BJ. Endogenous ATP involvement in mustard oil-induced central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 2005;94:1751–60. doi: 10.1152/jn.00223.2005. [DOI] [PubMed] [Google Scholar]
- 34.Yu XM, Sessle BJ, Hu JW. Differential effects of cutaneous and deep application of inflammatory irritant on mechanoreceptive field properties of trigeminal brain stem nociceptive neurons. J Neurophysiol. 1993;70:1704–7. doi: 10.1152/jn.1993.70.4.1704. [DOI] [PubMed] [Google Scholar]
- 35.Iwata K, Tashiro A, Tsuboi Y, Imai T, Sumino R, Morimoto T, Dubner R, Ren K. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol. 1999;82:1244–53. doi: 10.1152/jn.1999.82.3.1244. [DOI] [PubMed] [Google Scholar]
- 36.Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol. 2005;94:3815–25. doi: 10.1152/jn.00616.2005. [Epub 2005 Jul 27] [DOI] [PubMed] [Google Scholar]
- 37.Sessle BJ. Mechanisms of pain. In: Henry JL, Panju AA, Yashpal K, editors. Central post-stroke pain. Seattle: IASP Press; 2007. pp. 67–79. [Google Scholar]
- 38.Lam DK, Sessle BJ, Hu JW. Surgical incision can alter capsaicin-induced central sensitization in rat brainstem nociceptive neurons. Neurosci. 2008;156:737–47. doi: 10.1016/j.neuroscience.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 39.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res. 2009;1253:48–59. doi: 10.1016/j.brainres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 40.Clarke RW, Matthews B. The thresholds of the jaw-opening reflex and trigeminal brainstem neurons to tooth-pulp stimulation in acutely and chronically prepared rats. Neurosci. 1990;36:105–14. doi: 10.1016/0306-4522(90)90354-7. [DOI] [PubMed] [Google Scholar]
- 41.Sunakawa M, Chiang CY, Sessle BJ, Hu JW. Jaw electromyographic (EMG) activity induced by application of algesic chemicals to the rat tooth pulp. Pain. 1999;80:493–501. doi: 10.1016/S0304-3959(98)00241-3. [DOI] [PubMed] [Google Scholar]
- 42.Haas DA, Nakanishi O, MacMillan RE, Jordan RC, Hu JW. Development of an orofacial model of acute inflammation in the rat. Arch Oral Biol. 1992;37:417–22. doi: 10.1016/0003-9969(92)90026-5. [DOI] [PubMed] [Google Scholar]
- 43.Molander C, Xu C, Rivero-Melian C, Grant G. Cytoarchitectonic organization of the spinal cord in the rat: II. The cervical and upper thoracic cord. J Comp Neurol. 1989;298:375–85. doi: 10.1002/cne.902890303. [DOI] [PubMed] [Google Scholar]
- 44.Luccarini P, Sessle BJ, Woda A. Superficial and deep convergent nociceptive neurons are differentially affected by N-methyl-D-aspartate applied on the brainstem surface of the rat medullary dorsal horn. Neurosci. 2001;107:311–6. doi: 10.1016/s0306-4522(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 45.Coste J, Voisin DL, Luccarini P, Dallel R. A role for wind-up in trigeminal sensory processing: intensity coding of nociceptive stimuli in the rat. Cephalalgia. 2008;28:631–9. doi: 10.1111/j.1468-2982.2008.01568.x. [Epub 2008 Apr 16] [DOI] [PubMed] [Google Scholar]
- 46.Raboisson P, Dallel R, Clavelou P, Sessle BJ, Woda A. Effects of subcutaneous formalin on the activity of trigeminal brain stem nociceptive neurones in the rat. J Neurophysiol. 1995;73:496–505. doi: 10.1152/jn.1995.73.2.496. [DOI] [PubMed] [Google Scholar]
- 47.Jensen R. Peripheral and central mechanisms in tension-type headache: an update. Cephalalgia. 2003;23:49–52. doi: 10.1046/j.1468-2982.2003.00574.x. [DOI] [PubMed] [Google Scholar]
- 48.Lang AM. Botulinum toxin therapy for myofascial pain disorders. Curr Pain Headache Rep. 2002;6:355–60. doi: 10.1007/s11916-002-0076-8. [DOI] [PubMed] [Google Scholar]
- 49.Fernández-de-Las-Peñas C, Simons D, Cuadrado ML, Pareja J. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep. 2007;11:365–72. doi: 10.1007/s11916-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 50.Fernández-de-Las-Peñas C, Cuadrado ML, Pareja JA. Myofascial trigger points, neck mobility, and forward head posture in episodic tension-type headache. Headache. 2007;47:662–72. doi: 10.1111/j.1526-4610.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 51.Arendt-Nielsen L, Graven-Nielsen T. Translational aspects of musculoskeletal pain: From animals to humans. In: Graven-Nielsen T, Arendt-Nielsen L, Mense S, editors. Foundations of musculoskeletal pain. Seattle (Wash): IASP Press; 2008. pp. 347–66. [Google Scholar]