Abstract
Aggrecan is an extracellular matrix molecule that contributes to the mechanical properties of articular cartilage and meniscal fibrocartilage, but the abundance and processing of aggrecan in these tissues are different. The objective of this study was to compare patterns of aggrecan processing by chondrocytes and meniscal fibrochondrocytes in tissue explants and cell–agarose constructs. The effects of transforming growth factor-beta 1 (TGF-β1) stimulation on aggrecan deposition and processing were examined, and construct mechanical properties were measured. Fibrochondrocytes synthesized and retained less proteoglycans than did chondrocytes in tissue explants and agarose constructs. In chondrocyte constructs, TGF-β1 induced the accumulation of a 120-kDa aggrecan species previously detected in mature bovine cartilage. Fibrochondrocyte-seeded constructs contained high-molecular-weight aggrecan but lacked aggrecanase-generated fragments found in native, immature meniscus. In addition, reflecting the lesser matrix accumulation, fibrochondrocyte constructs had significantly lower compression moduli than did chondrocyte constructs. These cell type–specific differences in aggrecan synthesis, retention, and processing may have implications for the development of functional engineered tissue grafts.
Introduction
The healthy articular cartilage extracellular matrix (ECM) is rich in aggrecan, a large, aggregating proteoglycan glycosylated with up to 100 negatively charged sulfated glycosaminoglycan (sGAG) chains.1 Aggrecan is also widely distributed and expressed in meniscal fibrocartilage,2,3 and the proteoglycan content of the knee menisci increases with age.4,5 In the bovine meniscus, aggrecan was found to be more highly processed than age- and species-matched articular cartilage,6 and aggrecanase-mediated aggrecan degradation was localized in the middle and outer zones of the immature meniscus (unpublished data). The functional significance of these tissue-specific patterns of aggrecan processing is unclear but may be related to the differences in ECM architecture and mechanical loading environment between cartilage and fibrocartilage. In addition, the development of engineered cartilage grafts with tissue-specific mechanical properties may hinge upon recapitulation of the native tissue's proteoglycan processing.
Poteolytic cleavage of the interglobular domain (IGD), exposure of the NITEGE392 C-terminal neoepitope, and release of sGAG characterize aggrecan degradation in degenerative articular cartilage. Proteases from the A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family, including ADAMTS-4 and -5 (aggrecanase-1 and -2), have been shown to mediate this “destructive” aggrecanolysis and the release of sGAG.7,8 In contrast, m-calpain-mediated aggrecan cleavage is marked by accumulation of the GVA719 neoepitope and is a product of “non-destructive” aggrecan processing thought to occur during normal aggrecan turnover.9 There are few reports describing the structure of aggrecan in engineered cartilages, but identifying mechanisms of aggrecan processing during de novo ECM assembly may provide clues on how to promote the maturation of functional cartilage and fibrocartilage grafts. Aggrecan in chondrocyte–alginate constructs was found primarily in high-molecular-weight forms,10 but aggrecan processing in fibrocartilage constructs has not been previously reported.
Members of the transforming growth factor beta (TGF-β) cytokine family, including TGF-β1, regulate ECM production in articular chondrocytes,11,12 and TGF-β1 is often used to promote chondrogenesis in chondrocyte- and progenitor cell–populated cartilage constructs.13,14 Paradoxically, TGF-β1 can also upregulate ADAMTS activity in chondrocytes.15 Meniscal fibrochondrocytes are a heterogeneous population of cells resembling, to various degrees, chondrocytes and fibroblasts.16 Like chondrocytes, the fibrochondrocytes also exhibit robust anabolic responses to TGF-β1 stimulation in explants,17,18 three-dimensional scaffolds,19,20 and monolayer.21 The concentration of TGF-β1 reported for synovial effusions from patients with osteoarthritis (3.8 ng/mL) and rheumatoid arthritis (10 ng/mL)22 falls in a range previously reported to stimulate meniscal explant sGAG synthesis and release.18 Tissue constructs would, if implanted for therapeutic purposes, presumably be exposed to a similar concentration of TGF-β1. However, the capacity for this cytokine to alter patterns of aggrecan processing in engineered cartilages has not been previously examined.
The aims of this study were to compare mechanisms of aggrecan turnover by chondrocytes and fibrochondrocytes and describe the cell type–dependent effects of TGF-β1 on proteoglycan accumulation and aggrecan processing. Tissue explants and isolated cells were harvested from immature bovine stifle joints, and engineered tissue constructs were prepared by suspending the cells in agarose. The results of this study highlight cell type–specific differences in the accumulation and retention of proteoglycans, mechanisms of aggrecanolysis, and mechanical properties of engineered cartilage and fibrocartilage. In addition, the results contribute to our understanding of ECM turnover and will aid in the development and assessment of engineered soft orthopedic tissues.
Methods
Materials and reagents
Immature bovine stifle joints were from Research 87 (Boylston, MA). High-glucose Dulbecco's modified Eagle medium (DMEM); antibiotic–antimycotic solution containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B; 1% non-essential amino acids (NEAAs), 1% N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid (HEPES), trypsin-ethylenediaminetetraacetic acid (EDTA), proteinase K, and Dulbecco's phosphate buffered saline (DPBS) were from Invitrogen (Carlsbad, CA). Ten percent neutral buffered formalin (NBF) was from VWR (West Chester, PA). Dr. John Sandy (Rush Medical University, Chicago, IL) provided antibodies to aggrecan G1, aggrecan G3, and the NITEGE and GVA neoepitopes. Dr. Amanda Fosang (University of Melbourne, Melbourne, Australia) provided the antibody to aggrecan G2. Dr. Larry Fischer (National Institute of Dental and Craniofacial Research, Bethesda, MD) provided the antibody LF-94 to bovine decorin. Guanidine hydrochloride, 4-morpholineethanesulfonic acid (MES), iodoacetic acid, sodium acetate, chondroitin sulfate, urea, protease-free chondroitinase ABC, keratinase I, agarase, agarose, and alkaline phosphatase–conjugated anti-rabbit secondary antibody were from Sigma (St. Louis, MO). ITST Premix containing insulin (6.25 μg/mL), transferrin (6.25 μg/mL), selenious acid (6.25 ng/mL), linoleic acid (5.35 μg/mL), and bovine serum albumin (1.25 mg/mL) was from BD Biosciences (San Jose, CA). Keratinase II was from Associates of Cape Cod (Falmouth, MA). Dimethylmethylene blue (DMMB) was from Polysciences (Warrington, PA). Recombinant human TGF-β1 was from R&D Systems (Minneapolis, MN). Protease inhibitor cocktail containing EDTA, 4-(2-aminoethyl)benzenesulfonylfluoride, leupeptin, E-64, and aprotinin was from Calbiochem (San Diego, CA). The chemifluorescent substrate ECF was from Amersham (Piscataway, NJ).
Tissue culture
Articular cartilage and meniscal fibrocartilage were harvested from calf stifle joints under aseptic conditions. Cartilage explants (n = 84) were taken from the patellar groove and femoral condyles using a 4-mm diameter biopsy punch, and fibrocartilage explants (n = 84) were taken from the middle regions of medial and lateral menisci. Surface zone tissue was trimmed away, and explants were cut to 2 mm thick using a custom cutting block. The explants were moved to 48-well plates and cultured overnight in 0.5 mL of basal medium consisting of high-glucose DMEM, ITS+, 0.1 mM NEAA, 10 mM HEPES, 82 μg/mL L-ascorbic acid-2-PO4, and antibiotic–antimycotic (100 U penicillin/mL, 100 μg streptomycin/mL, 0.25 μg amphotericin B/mL). The following day, some explants were removed as day 0 samples (n = 6/tissue type) and stored at −20°C in DPBS with protease inhibitors. Remaining explants were cultured for 10 days in basal medium or with 5 ng/mL TGF-β1. Media were collected, replaced, and prepared with fresh cytokine every 48 h; conditioned media were stored at −20°C for biochemical analysis. Day 10 explants were stored at −20°C in DPBS with protease inhibitors.
Cell–agarose constructs were prepared using articular chondrocytes and meniscal fibrochondrocytes isolated from calf stifle joints (different than those from which the explants were harvested). Articular cartilage from the patellar groove and femoral condyles and fibrocartilage from the medial and lateral menisci were minced to approximately 1-mm3 pieces, weighed, and moved to T75 flasks. After a 30-min digestion at 37°C with 0.025% trypsin-EDTA in Ca++/Mg++-free DPBS, the tissues were digested in 0.4% collagenase/DMEM at 37°C on a shaker plate. After 48 h, the tissue digests were filtered through a sterile 37-μm-pore-size nylon mesh, and cell viability and counts were quantified using an automated viability counter (ViCell XR, Beckman Coulter, Fullerton, CA). Cell–agarose constructs were prepared by mixing equal volumes of 45°C 3% (w/v) agarose/DPBS and cells in DMEM at 2 × 107 cells/mL. The molten cell–agarose suspensions were thoroughly mixed and cast between two parallel glass plates approximately 2.5 mm apart. The cell–agarose slab was allowed to polymerize for 20 min at 4°C, and cylindrical samples were cut from the slab using a 6-mm-diameter biopsy punch (n = 48/cell type). Cell–agarose constructs were equilibrated overnight in basal medium. Day 0 constructs (n = 6/cell type) were moved to DPBS with protease inhibitors and stored at 4°C before undergoing mechanical tests within 72 h. The remaining constructs were cultured for 16 days in basal medium or basal medium with 10 ng/mL TGF-β1. Media were collected, replaced, and prepared with fresh cytokine every 48 h; collected media were stored at −20°C. At the end of each experiment, cell–agarose constructs were fixed in 10% NBF for 4 h at 4°C and embedded in paraffin for histochemical analysis (n = 2/treatment/cell type) or stored in DPBS with protease inhibitors at 4°C before mechanical testing.
Mechanical testing
The cell–agarose constructs were tested in oscillatory unconfined compression. Before testing, each construct was weighed, and the thickness and diameter were measured in three locations using digital calipers (Series 500, Mitutoyo America, Aurora, IL). Each construct was positioned between impermeable platens on an ELF3100 mechanical test frame (Bose Electroforce, Eden Prairie, MN) and brought into contact with the platens by application of a 10-mN preload. After compression by 10% at 0.1 mm/min and relaxation for 1200 s, each construct was loaded in oscillatory compression at 0.05, 0.1, 0.5, and 1 Hz and 1.5% strain amplitude. The dynamic compression moduli (E*) were calculated as the ratio of the magnitude of the stress and strain waveforms at the fundamental frequency. After testing, constructs were frozen and lyophilized before biochemical analysis.
Biochemistry
Conditioned media were assayed for released sGAG by the DMMB assay23 using chondroitin sulfate standards diluted in basal medium. After mechanical testing and lyophilization, constructs were weighed and extracted in 1 mL of extraction buffer (4M guanidine hydrochloride, 10 mM of MES, 50 mM of sodium acetate, 5 mMof iodoacetic acid, protease inhibitors, pH 6.5) for 48 h at 4°C on a rocker. Lyophilized explants were extracted similarly. Extracts were assayed for sGAG using the DMMB assay using chondroitin sulfate standards diluted in extraction buffer. After extraction, explant and construct residues were frozen, lyophilized, weighed, and digested in 500 μL of 300-μg/mL proteinase K/100 mM of ammonium acetate overnight at 60ºC. Cell–agarose residues were melted at 100ºC for 10 min and further digested with 3 U (15 μL) of agarose overnight at 43°C. Explant and construct residue digests were assayed for sGAG content using the DMMB assay. The sGAG release and retention results were consistent between two (for explants) or three (for constructs) independent experiments. Construct extracts and residue digests were assayed for DNA content using the Hoechst 33258 dye method.24
Equal volumes of explant or construct extracts were pooled from six condition-matched samples and added to 3 volumes of ice cold ethanol/5 mM of sodium acetate for overnight precipitation of proteoglycans at −20°C. Precipitates were centrifuged at 21 k × g for 30 min, the supernatants were removed, and the pellets were dried for 0.5 to 2 h at room temperature. Pellets were resuspended in buffer containing 50 mM of Tris hydrochloride, 50 mM of sodium acetate, 10 mM of EDTA, and protease inhibitors, at pH 7.5, and assayed for sGAG. Precipitations typically yielded 80% recovery of sGAG from guanidine extracts. Samples were deglycosylated using incubation with chondroitinase ABC, keratinase I, and keratinase II overnight at 37°C. Samples were then frozen, lyophilized, and resuspended in sample buffer containing 3 M of urea, tris-glycine sample buffer, and dithiothreitol. Extracts of chondrocyte constructs and articular cartilage explants were resuspended in 5 and 10 times greater volumes of sample buffer, respectively, than fibrochondrocyte construct and fibrocartilage explant extracts to accommodate higher sGAG concentrations.
Before electrophoretic separation, samples were melted at 110ºC for 5 min and loaded into 4% to 12% (for anti-G1, -G2, -G3, and -NITEGE blots) or 4% (for anti-GVA blots) Tris-glycine gels. The samples were separated at 200 V and transferred to nitrocellulose at 50 to 100 V. Membranes were blocked in 1% nonfat dry milk for 30 min at room temperature and incubated with primary antibodies diluted 1:1000 to 1:5000. After incubation with secondary antibody (1:10,000) for 1 h at room temperature, membranes were developed in ECF for 5 min and imaged on an FLA3000 phosphorimager (Fuji, Tokyo, Japan). Membranes initially probed with anti-NITEGE antisera (1:1000) were subsequently stripped in 1 × Re–Blot reagent (Chemicon, Temecula, CA) for 10 min at room temperature and reprobed with anti-G1 antisera (1:5000). Anti-G2 was used at 1:4000, and anti-GVA was used at 1:1000. Membranes initially probed with the anti-G3 antisera were stripped and reprobed with the LF-94 anti-decorin antisera (1:5000).
Statistics
Data were analyzed using analysis of variance and the general linear model in MINITAB Release 14 (Minitab, Inc, State College, PA). Differences were deemed significant at p < 0.05.
Results
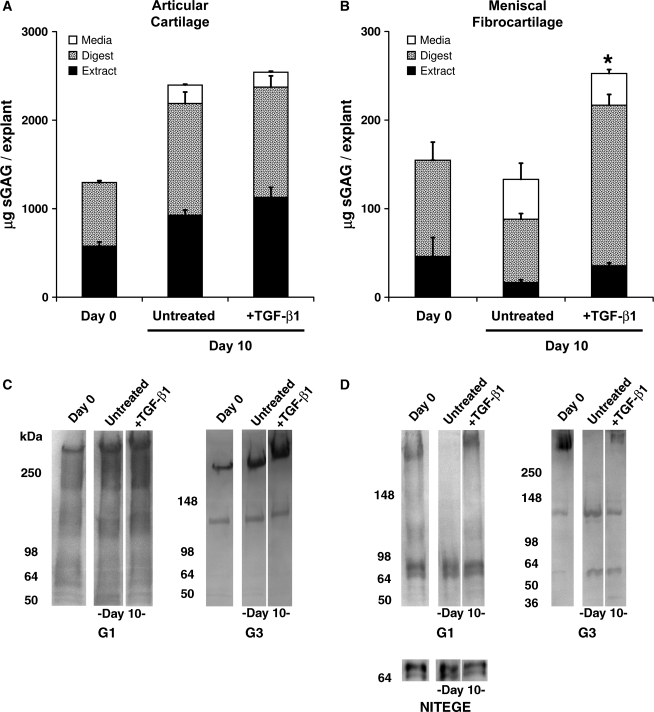
Cell type–specific patterns of proteoglycan deposition were first examined in explanted immature bovine cartilage and meniscal fibrocartilage. Quantitative measurements of sGAG in conditioned media, explant extracts, and residue digests (Fig. 1A, B) were coupled with sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of aggrecan extracted from the explants (Fig. 1C, D). After 10 days of culture with or without TGF-β1 stimulation, articular cartilage explants had tissue (extract + digest) sGAG much greater than that of day 0 explants (Fig. 1A), indicating that chondrocyte proteoglycan synthesis is maintained under the basal culture conditions used in this study. TGF-β1-stimulated cartilage exhibited tissue sGAG similar to untreated controls, although sGAG release was significantly reduced by 23% (p < 0.05). In contrast, untreated meniscal fibrocartilage explants exhibited a marked loss (33%) of tissue sGAG to the medium (Fig. 1B). TGF-β1-stimulated fibrocartilage explants had significantly higher total sGAG (explant content + media) than untreated controls (p < 0.05). Fibrocartilage explants exhibited a lower capacity to incorporate sGAG than cartilage, releasing more than 37% of newly synthesized sGAG to the medium (<14% was released from similarly treated cartilage), and TGF-β1 had little effect on this sGAG release. The fraction of extractable proteoglycans also varied with cell type and culture condition and was consistently lower in fibrocartilage (16-24%) than in articular cartilage explants (43–47%).
FIG. 1.
Articular cartilage (A,C) and meniscal fibrocartilage (B,D) explants exhibit distinct patterns of proteoglycan distribution (A,B) and aggrecan processing in vitro. *Indicates total construct sGAG (extract + digest) is different from untreated day 10 controls (p < 0.05). Note 10-fold difference in scale between A and B. Data are mean + SEM, with n = 6. In C & D, equal portions of explant extracts (pooled from 6 samples) were loaded in each lane. Articular cartilage extracts were diluted 10-fold over fibrocartilage extracts to account for the substantially higher sGAG content. Numbers to the left ofeach blot indicate the migration of molecular weight markers in kDa. sGAG, sulfated glycosaminoglycan; TGF-β1, transforming growth factor-beta 1.
Extracts of articular cartilage and meniscal fibrocartilage were reactive with antibodies to the N- and C-terminal globular domains (G1 and G3, respectively) of the aggrecan core protein. Cartilage explants retained primarily high-molecular-weight G1- and G3-bearing fragments (i.e., full-length aggrecan), several minor G1 fragments, and a 120-kDa G3 fragment (Fig. 1C). TGF-β1 stimulation did not appear to alter the baseline pattern of aggrecan processing in cartilage. In contrast, extracts of fibrocartilage explants contained full-length aggrecan and a 65- to 70-kDa G1 doublet (Fig. 1D). Whereas the doublet was retained in the explants regardless of culture condition, TGF-β1 stimulation was required to retain full-length aggrecan. The doublet was identified as the aggrecanase-generated fragment bearing the NITEGE neoepitope (Fig. 1D, lower panel), and this fragment was not detected in articular cartilage extracts. Similar patterns of aggrecan G1 bands were observed in extracts from a second, independent experiment. An approximately 55-kDa G3 fragment was also uniquely detected in cultured fibrocartilage explants. Collectively, these data show important baseline and TGF-β1-sensitive differences in sGAG accumulation and aggrecan processing between chondrocytes and meniscal fibrochondrocytes in their native ECMs.
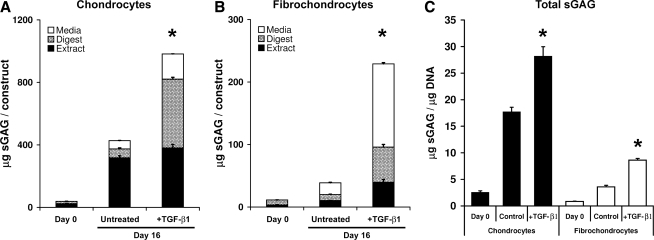
To examine the fidelity with which engineered cartilages recapitulate the cell type–specific patterns of proteoglycan processing observed in tissue explants, chondrocyte– and fibrochondrocyte–agarose constructs were prepared. The accumulation of sGAG within the construct and the medium were measured (Fig. 2A, B). Over 16 days, chondrocyte constructs synthesized approximately four times the total sGAG of similarly treated fibrochondrocyte constructs. Although fibrochondrocyte constructs contained 15% to 20% fewer cells than the chondrocyte constructs (depending on culture condition), the cell-type differences in sGAG accumulation were also evident after normalizing sGAG content to DNA content (Fig. 2C). TGF-β1-stimulated chondrocytes and fibrochondrocytes exhibited significantly higher total construct sGAG (the sum of construct extract and digest) than untreated controls, consistent with the expected anabolic responses to TGF-β1. A striking feature of these data, consistent with the observations of explants, is that the proportion of newly synthesized sGAG released to the medium was substantially higher for fibrochondrocyte constructs (∼50%) than for chondrocyte constructs (∼15%). The absolute amounts of sGAG released to the medium from chondrocyte and fibrochondrocyte constructs were comparable and increased with TGF-β1 stimulation. Although the fraction of guanidine-extractable sGAG was generally higher in constructs than in explants, the fraction was lower in fibrochondrocyte constructs (28–52%) than in chondrocyte constructs (46–85%).
FIG. 2.
Chondrocyte-agarose (A) and meniscal fibrochondrocyte-agarose (B) constructs exhibit distinct patterns of proteoglycan distribution. Total sGAG (retained in constructs + released to the media) was normalized by DNA content in C. *Indicates a statistically significant difference between TGF-β1-stimulated and untreated day 16 constructs (p < 0.05). Note 4-fold difference in scale between A and B. Data are mean + SEM, with n = 6.
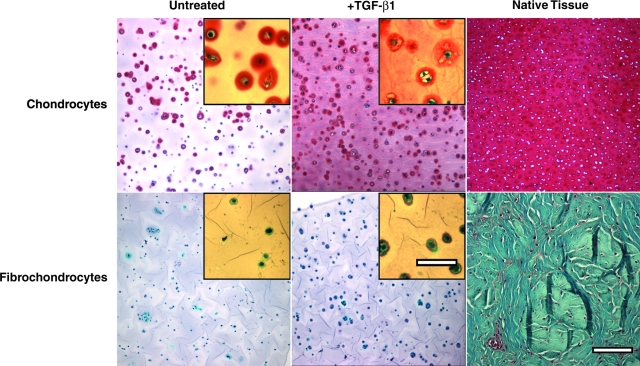
Safranin-O staining of day 16 constructs (Fig. 3) revealed cell type–specific spatial distributions of sGAG accumulation. Untreated chondrocytes assembled an sGAG-rich pericellular ECM, and TGF-β1 stimulation promoted sGAG accumulation in the further-removed matrix. In contrast, the fibrochondrocyte constructs appeared to be largely devoid of sGAG. TGF-β1-stimulated fibrochondrocytes appeared to deposit more sGAG in the pericellular compartment than controls (Fig. 3, lower panel insets). The fast green counterstain in these images highlights pericellular accumulation of protein in the fibrochondrocyte constructs and suggests that the fibrochondrocytes are assembling ECM, albeit low in sGAG content. Stained sections of immature bovine articular cartilage and meniscal fibrocartilage (right panels) demonstrate the unique ECM composition and structure of each tissue that appear to be partially recapitulated in the cell–agarose tissue constructs.
FIG. 3.
Cell type differences in deposition of newly synthesized sGAG are illustrated by Safranin-O staining. Cell-agarose constructs treated with (middle panels) or without (left panels) TGF-β1 for 16 days were compared with sections of native articular cartilage or midstubstance meniscal fibrocartilage (right panels). Sections were counterstained with fast green and hematoxylin. Scale bar = 100 μm; inset scale bar = 25 μm.
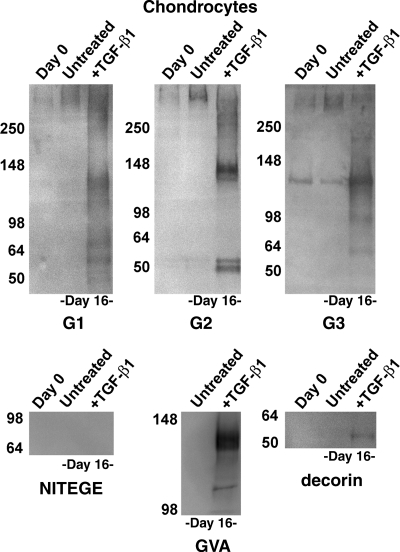
Western blots of chondrocyte construct extracts showed more-extensive aggrecan processing in TGF-β1-stimulated constructs than in untreated controls (Fig. 4). Aggrecan G1, G2, and G3 blots demonstrated the presence of full-length aggrecan in the controls, whereas TGF-β1 induced generation of a G1- and G2-bearing species migrating at 140 kDa and a G2-bearing doublet at approximately 50 kDa. The 140-kDa fragment was subsequently shown to bear the GVA C-terminal neoepitope. A G3 fragment migrating at approximately 120 kDa was detected in all lanes, and minor bands at approximately 98 kDa and 60 kDa were found only in extracts of TGF-β1-stimulated constructs. Day 0 constructs contained aggrecan that, presumably, was retained in the intra- or peri-cellular compartments during isolation from the tissue. Destructive aggrecanolysis, typically marked by generation of the NITEGE neoepitope, was absent under all treatment conditions. TGF-β1 stimulation also increased accumulation of the small proteoglycan decorin over untreated chondrocyte constructs.
FIG. 4.
Highly processed aggrecan accumulates in TGF-β1-stimulated chondrocyte-agarose tissue constructs. Blots are of extracts of freshly-prepared constructs (Day 0) and constructs cultured for 16 days with or without TGF-β1. Aggrecan fragments bearing the G1, G2, and G3 domains or the NITEGE or GVA neoepitopes were examined. Extracts were also probed for the small proteoglycan, decorin. Numbers to the left of each blot indicate the migration of molecular weight markers (in kDa). Equal volumes of extracts pooled from 6 constructs were loaded in each lane.
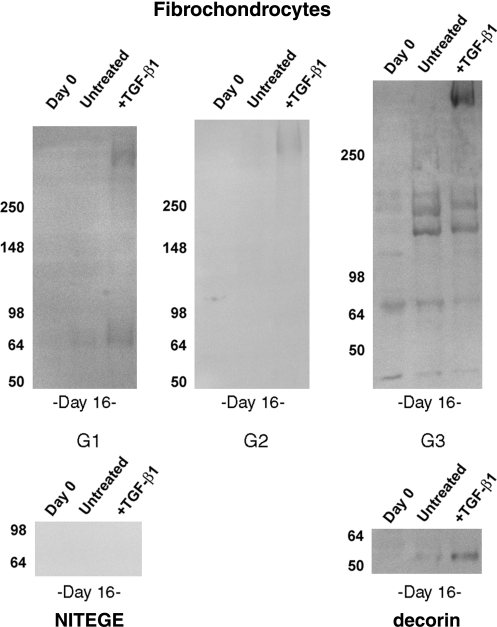
Fibrochondrocyte constructs accumulated limited amounts of highly processed aggrecan (Fig. 5). Aggrecan G1- and G2-containing fragments were relatively low in abundance, and, consistent with the low sGAG content of these constructs, there was little evidence of sGAG-bearing aggrecan, as indicated by weak staining in the high-molecular-weight regions of G1, G2, and G3 blots. TGF-β1 modestly promoted accumulation of full-length aggrecan in the constructs to within the detection limit of the assays. Smaller fragments migrating at 65 to 80 kDa were also detected in the TGF-β1-stimulated constructs, but there was no evidence of the 140-kDa G1- and G2-containing aggrecan observed in chondrocyte constructs. Blots for G3 revealed the clear presence of several fragments, including a 120-kDa band similar to that observed in chondrocyte constructs and bands at 140 kDa, 65 kDa, and 40 kDa that were apparently unique to the fibrochondrocyte constructs. These fragments accumulated independent of treatment group. The 65-kDa and 40-kDa fragments were evident in day 0 constructs, and were presumably carried in intracellular or pericellular compartments during isolation. As with the chondrocyte cultures, aggrecan-NITEGE was not detected in fibrochondrocyte constructs, and accumulation of decorin increased with TGF-β1 stimulation.
FIG. 5.
Fibrochondrocyte constructs stimulated with TGF-β1 accumulate full length aggrecan. Blots are of extracts of freshly-prepared constructs (Day 0) and constructs cultured for 16 days with or without TGF-β1. Aggrecan fragments bearing the G1, G2, and G3 domains or the NITEGE neoepitope were examined. Extracts were also probed for the small proteoglycan, decorin. Numbers to the left of each blot indicate the migration of molecular weight markers (in kDa). Equal volumes of extracts pooled from 6 constructs were loaded in each lane.
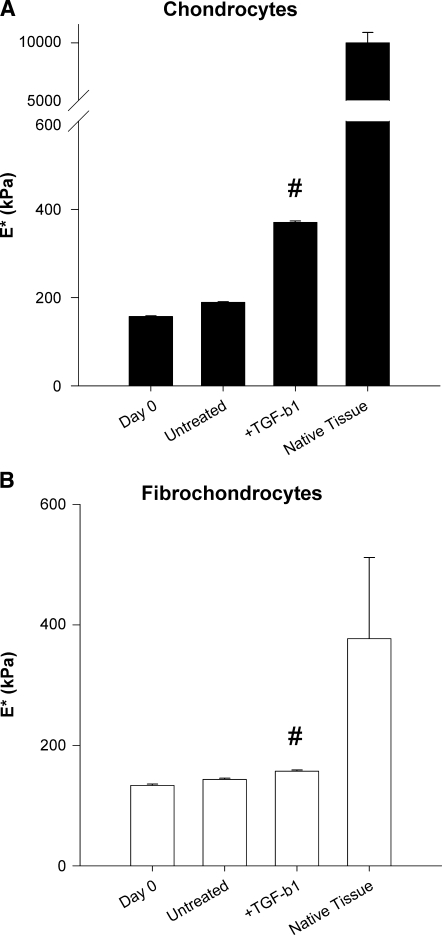
Oscillatory compression tests revealed cell type–dependent differences in the mechanical properties of engineered cartilage and fibrocartilage. Chondrocyte and fibrochondrocyte constructs cultured in basal medium for 16 days exhibited higher dynamic compression moduli than the respective freshly prepared (day 0) constructs, indicating that the encapsulated cells assembled a load-bearing ECM (Fig. 6). TGF-β1 stimulation resulted in significantly greater dynamic compression moduli of chondrocyte and fibrochondrocyte constructs than in untreated controls (89% and 7%, respectively). This was consistent across frequencies. (Liftoff was observed at the 1 Hz frequency for some samples, primarily TGF-β1-treated articular chondrocyte constructs.) Despite the cytokine-induced gains in construct stiffness, the compression moduli of immature bovine articular cartilage and meniscal fibrocartilage explants (at 0.1 Hz) were 25 and 2 times higher than the stiffest chondrocyte and fibrochondrocyte constructs, respectively.
FIG. 6.
Chondrocytes (A) and fibrochondrocytes (B) assemble ECMs with different compressive properties. The dynamic compressive moduli (at 0.1 Hz) were determined by oscillatory unconfined compression tests. # indicates different from untreated day 16 controls (p < 0.05). Data are mean + SEM, with n = 6.
Discussion
The results of this study reveal important differences in the ways immature chondrocytes and meniscal fibrochondrocytes synthesize, accumulate, and process aggrecan. Proteoglycan synthesis in fibrochondrocyte constructs (as indicated by total sGAG production) was approximately 25% of that in articular chondrocyte–populated constructs. This result is consistent with previous reports and work in our laboratory using 35SO4 incorporation as an index of proteoglycan synthesis.17,18,25,26 sGAG synthesized by fibrochondrocytes was less likely to be retained within the constructs than sGAG synthesized by chondrocytes. In fibrochondrocyte cultures, 48% to 58% of the total synthesized sGAG was found in the medium, whereas chondrocytes only released 11% to 17%. For the same anabolic stimulus, fibrochondrocytes had lower proteoglycan synthesis and retained a smaller fraction of newly synthesized proteoglycans than articular chondrocytes. The proteoglycans in fibrochondrocyte cultures were more difficult to extract than those in chondrocyte cultures, suggesting the presence of a tightly bound pool of proteoglycans in the fibrocartilage explants and constructs that is absent or smaller in chondrocyte cultures. In vitro fibrocartilage degradation studies have also suggested the presence of such a proteoglycan population (unpublished data). These tissue-specific differences in proteoglycan deposition, consistent between two experimental systems, may be significant in the development and evaluation of functional engineered grafts.
The mechanisms regulating sGAG accumulation in these cell types are not well understood, and the results of this study indicate that aggrecanolysis is not a primary mechanism. Although the absence or presence of various aggrecan fragments and their neoepitopes demonstrated qualitative differences in aggrecan processing between the cell types, the two cell types may differentially express molecules involved in proteoglycan aggregation, including hyaluronan, link protein, and the hyaluronan receptor CD44, to regulate sGAG deposition. Versican, another large aggregating proteoglycan, is expressed during the maturation of articular cartilage27 and fibrocartilage,28 and differential expression of versican or other proteoglycans by these cell types may also contribute to distinct patterns of sGAG accumulation and retention. Decorin, detected in cartilage and fibrocartilage extracts (not shown) and in TGF-β1-stimulated chondrocyte and fibrochondrocyte–agarose constructs, may indirectly regulate sGAG accumulation by binding (and sequestering) TGF-β129 or altering collagen fibrillogenesis.30 Alternatively, chondrocytes may intrinsically assemble a collagen network that more effectively entangles proteoglycan aggregates than does the fibrochondrocyte-assembled ECM. It is also possible that changes in aggrecan glycosylation or sGAG sulfation patterns31,32 contributed to cell type– and culture condition–dependent differences in sGAG accumulation. Elucidation and manipulation of the phenotypic differences between cell types in proteoglycan synthesis, post-translational modification, and retention will be useful in the development of engineered cartilage and fibrocartilage.
Western analysis of chondrocyte construct extracts revealed that TGF-β1 induced differences in aggrecan processing. Chondrocytes suspended in alginate were previously shown to accumulate primarily (∼98%) full length aggrecan, and those experiments were performed in the presence of serum.10 Under the serum-free conditions used in the current study, chondrocytes also accumulated primarily full-length aggrecan. Surprisingly, TGF-β1-stimulated chondrocytes accumulated aggrecan bearing the neoepitope GVA719, known to be generated by m-calpain in vitro. A similar fragment was detected in low abundance in the “further removed matrix” of chondrocyte-alginate constructs,10 and several groups have documented the presence of this fragment in extracts of mature articular cartilage.6,9,33 Cartilage explant extracts contained 140-kDa aggrecan G1- and G2-positive fragments (Fig. 1), and we have immunolocalized m-calpain and aggrecan-GVA in thin sections of immature cartilage (unpublished observations), suggesting that this enzyme and aggrecan fragment also exist in native immature articular cartilage. Although other enzymes may contribute to generation of this fragment, metalloproteinases, including ADAMTS-4 and matrix metalloproteinase-3, were incapable of cleaving aggrecan at this site.9 m-Calpain, or an enzyme with equivalent activity on aggrecan, thus appears to play a role in non-destructive aggrecanolysis during TGF-β1-stimulated cartilage matrix remodeling.
Unlike the chondrocytes, fibrochondrocytes exhibited modest differences in aggrecan processing upon stimulation with TGF-β1. Anti-G1 and -G2 blots (Fig. 5), loaded with five times the extract volume of the blots of chondrocyte construct extracts (Fig. 4), indicated the low amounts of aggrecan in the fibrochondrocyte cultures. Anti-G3 blots, however, revealed the presence of aggrecan species in fibrochondrocyte constructs that were apparently absent in chondrocyte cultures. Based on their migration, these fragments are thought to be products of non-destructive ADAMTS-mediated trimming of the aggrecan core protein.34 The basis for retention of aggrecan G3 fragments within the constructs is unclear. Because these species lack a hyaluronan-binding domain, they are not expected to form proteoglycan aggregates. Rather, G1-lacking aggrecan fragments would be expected to emerge in the supernatants of these cultures. The conditioned media from both cell types contained primarily full-length aggrecan (not shown), suggesting that aggrecan degradation products are retained in the newly formed ECM. Aggrecan G3 contains lectin domains that can interact with other ECM molecules, such as tenascin- and fibulin-family proteins, and G3 fragments may be retained through these interactions.35 It is possible that aggrecan G3 fragments can modulate the ECM organization, material properties, and biosynthetic activity of engineered cartilages, and selective perturbation of fragment formation and retention may be useful in guiding tissue-specific construct maturation.
In contrast to age-matched meniscal midsubstance explants, the fibrochondrocyte constructs did not appear to generate or accumulate aggrecan fragments with the NITEGE neoepitope. The fibrochondrocytes were isolated from whole menisci, and whereas the middle and outer regions of the immature menisci contain abundant aggrecan-NITEGE, the inner region of the menisci has much less. In addition, cells from the inner meniscus are phenotypically more similar to articular chondrocytes (e.g., exhibit rounded morphology and express more aggrecan and collagen type II) than cells in the middle and outer regions of the menisci.21,36–38 Thus, agarose suspension may induce fibrochondrocytes to exhibit a less aggrecan–destructive phenotype characteristic of cells isolated from the inner meniscus; indeed, agarose suspension culture has demonstrated utility in maintaining the chondrocyte phenotype.39,40 The agarose scaffolds used in these experiments initially lack the barriers to diffusion (e.g., high concentrations of structural proteins) that are present in the explants, and agarose does not readily bind cytokines as native ECM can; these differences may underlie the differences in fibrochondrocyte-mediated aggrecan processing, retention, and release between the two experimental systems. Other aspects of the apparent phenotypic change, such as the types of collagen synthesized, were not examined in this study but will benefit from future investigation. The lower extractability of proteoglycans from fibrochondrocyte cultures (explants and constructs) or limited sensitivity of the western blot assays may have precluded detection of various aggrecan species, and more-aggressive extraction techniques may allow a more-complete analysis of aggrecan processing in these experimental systems.
Consistent with the biochemical data, chondrocytes and fibrochondrocytes exhibited differences in their capacity to assemble a compressive load–bearing ECM. Chondrocyte constructs exhibited robust increases in dynamic compressive modulus with time and TGF-β1 stimulation, whereas fibrochondrocyte constructs from all culture conditions showed only modest differences in compressive modulus from day 0 constructs. Immature articular cartilage is stiffer in compression than age- and species-matched meniscal fibrocartilage, and this difference in native tissue material properties may be related to intrinsic differences in the metabolic activity, ECM protein gene expression, and cell–cell interactions. The dynamic compressive modulus was chosen as the measure of construct mechanical function because we have found it to be more sensitive to changes in matrix composition than the equilibrium modulus,41 but alternative tests (e.g., tension or shear) might reveal other cell type–specific differences in the construct material properties. For example, scaffold-free constructs prepared with meniscal fibrochondrocytes exhibited higher tensile modulus and ultimate tensile strength than constructs prepared with chondrocytes.42 Fibrochondrocytes may, then, preferentially exclude sGAG-bearing aggrecan in favor of matrix components that more aptly bear tensile or shear loads. The results reported here support the idea that maintaining tissue-specific patterns of matrix remodeling promotes the formation of engineered tissues with appropriate, tissue-specific material properties.
The composition and structure of articular cartilage and fibrocartilage are related but distinct, and the cells residing in these tissues exhibit differences in gene expression, morphology, and their responses to cytokine stimulation. Development of functional engineered tissue grafts for these two tissues, then, is expected to benefit from recapitulation of the tissue-specific patterns of ECM production and processing. Identifying tissue-specific patterns of aggrecan degradation may also provide criteria for evaluating the chondrogenic or fibrochondrogenic potential of tissue-engineered cell sources. The results reported here underscore the functional implications of tissue-specific ECM remodeling in the maturation of engineered cartilage and fibrocartilage.
Acknowledgments
The authors thank Dr. Larry Fisher and Dr. Amanda Fosang for generously providing reagents. Dr. John Sandy contributed antisera and insightful discussion of the results. This work was supported by National Institutes of Health Grant AR052861 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Presented in part at the 2007 ASME Summer Bioengineering Conference, Keystone, Colorado.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hardingham T.E. Fosang A.J. The structure of aggrecan and its turnover in cartilage. J Rheumatol Suppl. 1995;43:86. [PubMed] [Google Scholar]
- 2.Valiyaveettil M. Mort J.S. McDevitt C.A. The concentration, gene expression, and spatial distribution of aggrecan in canine articular cartilage, meniscus, and anterior and posterior cruciate ligaments: a new molecular distinction between hyaline cartilage and fibrocartilage in the knee joint. Connect Tissue Res. 2005;46:83. doi: 10.1080/03008200590954113. [DOI] [PubMed] [Google Scholar]
- 3.Upton M.L. Chen J. Setton L.A. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- 4.Melrose J. Smith S. Cake M. Read R. Whitelock J. Comparative spatial and temporal localisation of perlecan, aggrecan and type I, II and IV collagen in the ovine meniscus: an ageing study. Histochem Cell Biol. 2005;124:225. doi: 10.1007/s00418-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 5.McAlinden A. Dudhia J. Bolton M.C. Lorenzo P. Heinegard D. Bayliss M.T. Age-related changes in the synthesis and mRNA expression of decorin and aggrecan in human meniscus and articular cartilage. Osteoarthritis Cartilage. 2001;9:33. doi: 10.1053/joca.2000.0347. [DOI] [PubMed] [Google Scholar]
- 6.Sandy J.D. Plaas A.H. Koob T.J. Pathways of aggrecan processing in joint tissues. Implications for disease mechanism and monitoring. Acta Orthop Scand Suppl. 1995;266:26. [PubMed] [Google Scholar]
- 7.Stanton H. Rogerson F.M. East C.J. Golub S.B. Lawlor K.E. Meeker C.T. Little C.B. Last K. Farmer P.J. Campbell I.K. Fourie A.M. Fosang A.J. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 8.Tortorella M.D. Malfait A.M. Deccico C. Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- 9.Oshita H. Sandy J.D. Suzuki K. Akaike A. Bai Y. Sasaki T. Shimizu K. Mature bovine articular cartilage contains abundant aggrecan that is C-terminally truncated at Ala719-Ala720, a site which is readily cleaved by m-calpain. Biochem J. 2004;382:253. doi: 10.1042/BJ20040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragan P.M. Chin V.I. Hung H.H. Masuda K. Thonar E.J. Arner E.C. Grodzinsky A.J. Sandy J.D. Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys. 2000;383:256. doi: 10.1006/abbi.2000.2060. [DOI] [PubMed] [Google Scholar]
- 11.Malemud C.J. Killeen W. Hering T.M. Purchio A.F. Enhanced sulfated-proteoglycan core protein synthesis by incubation of rabbit chondrocytes with recombinant transforming growth factor-beta 1. J Cell Physiol. 1991;149:152. doi: 10.1002/jcp.1041490119. [DOI] [PubMed] [Google Scholar]
- 12.Morales T.I. Transforming growth factor-beta 1 stimulates synthesis of proteoglycan aggregates in calf articular cartilage organ cultures. Arch Biochem Biophys. 1991;286:99. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- 13.Yaeger P.C. Masi T.L. de Ortiz J.L. Binette F. Tubo R. McPherson J.M. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 14.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 15.Moulharat N. Lesur C. Thomas M. Rolland-Valognes G. Pastoureau P. Anract P. De Ceuninck F. Sabatini M. Effects of transforming growth factor-beta on aggrecanase production and proteoglycan degradation by human chondrocytes in vitro. Osteoarthritis Cartilage. 2004;12:296. doi: 10.1016/j.joca.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Mauck R.L. Martinez-Diaz G.J. Yuan X. Tuan R.S. Regional multilineage differentiation potential of meniscal fibrochondrocytes: implications for meniscus repair. Anat Rec (Hoboken) 2007;290:48. doi: 10.1002/ar.20419. [DOI] [PubMed] [Google Scholar]
- 17.Collier S. Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3:127. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 18.Imler S.M. Doshi A.N. Levenston M.E. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Zaleskas J.M. Kinner B. Freyman T.M. Yannas I.V. Gibson L.J. Spector M. Growth factor regulation of smooth muscle actin expression and contraction of human articular chondrocytes and meniscal cells in a collagen-GAG matrix. Exp Cell Res. 2001;270:21. doi: 10.1006/excr.2001.5325. [DOI] [PubMed] [Google Scholar]
- 20.Pangborn C.A. Athanasiou K.A. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T. Fujii K. Kumagae Y. Comparison of biochemical characteristics of cultured fibrochondrocytes isolated from the inner and outer regions of human meniscus. Knee Surg Sports Traumatol Arthrosc. 1999;7:75. doi: 10.1007/s001670050125. [DOI] [PubMed] [Google Scholar]
- 22.Fava R. Olsen N. Keski-Oja J. Moses H. Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989;169:291. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y.J. Sah R.L. Doong J.Y. Grodzinsky A.J. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 25.Iwata H. Kaneko M. Kawai K. Kajino G. Nakagawa M. Uptake of glycosaminoglycan polysulfate by articular and meniscus cartilage: a biochemical and autoradiographic investigation. Clin Orthop. 1980:265. [PubMed] [Google Scholar]
- 26.Vanderploeg E.J. Imler S.M. Brodkin K.R. Garcia A.J. Levenston M.E. Oscillatory tension differentially modulates matrix metabolism and cytoskeletal organization in chondrocytes and fibrochondrocytes. J Biomech. 2004;37:1941. doi: 10.1016/j.jbiomech.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto K. Kamiya N. Suwan K. Atsumi F. Shimizu K. Shinomura T. Yamada Y. Kimata K. Watanabe H. Identification and characterization of versican/PG-M aggregates in cartilage. J Biol Chem. 2006;281:18257. doi: 10.1074/jbc.M510330200. [DOI] [PubMed] [Google Scholar]
- 28.Hellio Le Graverand M.P. Reno C. Hart D.A. Gene expression in menisci from the knees of skeletally immature and mature female rabbits. J Orthop Res. 1999;17:738. doi: 10.1002/jor.1100170518. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand A. Romaris M. Rasmussen L.M. Heinegard D. Twardzik D.R. Border W.A. Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302 (Pt 2):527. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown D.C. Vogel K.G. Characteristics of the in vitro interaction of a small proteoglycan (PG II) of bovine tendon with type I collagen. Matrix. 1989;9:468. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- 31.Lammi M.J. Inkinen R. Parkkinen J.J. Hakkinen T. Jortikka M. Nelimarkka L.O. Jarvelainen H.T. Tammi M.I. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304 (Pt 3):723. doi: 10.1042/bj3040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouw J.K. Case N.D. Guldberg R.E. Plaas A.H. Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Maehara H. Suzuki K. Sasaki T. Oshita H. Wada E. Inoue T. Shimizu K. G1-G2 aggrecan product that can be generated by M-calpain on truncation at Ala709-Ala710 is present abundantly in human articular cartilage. J Biochem. 2007;141:469. doi: 10.1093/jb/mvm052. [DOI] [PubMed] [Google Scholar]
- 34.Tortorella M.D. Pratta M. Liu R.Q. Austin J. Ross O.H. Abbaszade I. Burn T. Arner E. Sites of aggrecan cleavage by recombinant human aggrecanase-1 (ADAMTS-4) J Biol Chem. 2000;275:18566. doi: 10.1074/jbc.M909383199. [DOI] [PubMed] [Google Scholar]
- 35.Day J.M. Olin A.I. Murdoch A.D. Canfield A. Sasaki T. Timpl R. Hardingham T.E. Aspberg A. Alternative splicing in the aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J Biol Chem. 2004;279:12511. doi: 10.1074/jbc.M400242200. [DOI] [PubMed] [Google Scholar]
- 36.Hellio Le Graverand M.P. Ou Y. Schield-Yee T. Barclay L. Hart D. Natsume T. Rattner J.B. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scott P.G. Nakano T. Dodd C.M. Isolation and characterization of small proteoglycans from different zones of the porcine knee meniscus. Biochim Biophys Acta. 1997;1336:254. doi: 10.1016/s0304-4165(97)00040-8. [DOI] [PubMed] [Google Scholar]
- 38.Nakano T. Dodd C.M. Scott P.G. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res. 1997;15:213. doi: 10.1002/jor.1100150209. [DOI] [PubMed] [Google Scholar]
- 39.Elima K. Vuorio E. Expression of mRNAs for collagens and other matrix components in dedifferentiating and redifferentiating human chondrocytes in culture. FEBS Lett. 1989;258:195. doi: 10.1016/0014-5793(89)81651-5. [DOI] [PubMed] [Google Scholar]
- 40.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 41.Wilson C.G. Palmer A.W. Zuo F. Eugui E. Wilson S. Mackenzie R. Sandy J.D. Levenston M.E. Selective and non-selective metalloproteinase inhibitors reduce IL-1-induced cartilage degradation and loss of mechanical properties. Matrix Biol. 2007;26:259. doi: 10.1016/j.matbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aufderheide A.C. Athanasiou K.A. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]