Abstract
Cells derived from Wharton's jelly from human umbilical cords (called umbilical cord mesenchymal stromal cells herein) are a novel cell source for musculoskeletal tissue engineering. In this study, we examined the effects of different seeding densities on seeding efficiency, cell proliferation, biosynthesis, mechanical integrity, and chondrogenic differentiation. Cells were seeded on non-woven polyglycolic acid (PGA) meshes in an orbital shaker at densities of 5, 25, or 50 million cells/mL and then statically cultured for 4 weeks in chondrogenic medium. At week 0, initial seeding density did not affect seeding efficiency. Throughout the 4-week culture period, absolute cell numbers of the 25 and 50 million-cells/mL (higher density) groups were significantly larger than in the 5 million-cells/mL (lower density) group. The presence of collagen types I and II and aggrecan was confirmed using immunohistochemical staining. Glycosaminoglycan and collagen contents per construct in the higher-density groups were significantly greater than in the lower-density group. Constructs in the high-density groups maintained their mechanical integrity, which was confirmed using unconfined compression testing. In conclusion, human umbilical cord cells demonstrated the potential for chondrogenic differentiation in three-dimensional tissue engineering, and higher seeding densities better promoted biosynthesis and mechanical integrity, and thus a seeding density of at least 25 million cells/mL is recommended for fibrocartilage tissue engineering with umbilical cord mesenchymal stromal cells.
Introduction
Fibrocartilage is a primarily avascular tissue that is distinctly different from fibrous tissues or hyaline cartilage.1,2 For example, fibrocartilage contains more type I collagen and less type II collagen and proteoglycans than hyaline cartilage.1 Tissue engineering of fibrocartilage, aiming to create a functional replacement, offers promise for regenerative medicine using fibrocartilage defects. Insufficient cell availability and the loss of cell phenotype during in vitro expansion limit the approach of using differentiated adult cells.3–6 Tissue engineering with mesenchymal stromal cells (MSCs) is attractive because of their expansion ability (self-renewal ability) and their ability to differentiate into cartilage, bone, or other tissues in vitro.
Bone marrow–derived MSCs (BMSCs) are extensively used in musculoskeletal tissue engineering. They differentiate into multiple cell lineages, including bone, cartilage, and other mesenchymal tissues, when exposed to specific growth factors.7–11 However, BMSCs have limited self-renewal ability and an age-dependent decrease in cell availability and proliferation. Furthermore, isolation of BMSCs requires an invasive and painful surgical procedure. Recently, umbilical cord MSCs (UCMSCs) have been shown to have extensive in vitro expansion capability and the ability to differentiate into mesenchymal cell lineages such as osteogenic, chondrogenic, myogenic, and adipogenic.12–16 These cells are extracted through enzyme digestion or explant culture method from Wharton's jelly of umbilical cords.17 The extraction yields 10 × 103 to 50 × 103 cells per cm of cord,15,18 which can be expanded 300-fold for more than seven passages without the loss of differentiation potential.15 UCMSCs have the appearance of myofibroblasts in terms of positive staining for the expression of vimentin, desmin, and/or alpha-smooth muscle actin in native tissue or in vitro cultured cells.19–21 UCMSCs possess markers found in BMSCs, including CD29, CD44, CD90, CD105, and CD166.15,18 Moreover, the presence of octamer (Oct)-4, Nanog, and Sox-2 transcription factors indicated that a subpopulation of UCMSCs might share some properties of embryonic and non-embryonic stem cells.22
Therefore, with the advantages of being easily obtained from a normally discarded tissue, abundant supply, no donor site morbidity, and expansion ability before senescence, UCMSCs are an attractive alternative for tissue engineering and regenerative medicine.
Recently, human UCMSCs (hUCMSCs) have been proposed for use in musculoskeletal tissue engineering.23 The chondrogenic ability of hUCMSCs has been indicated using Alcian blue staining14,16 and toluidine blue staining for glycosaminoglycans (GAGs),15 picrosirius red staining and Heidenhain's azan staining for collagen,15,16 and immunohistochemical staining for type I and II collagen.14,15 Our previous study compared hUCMSCs and mandibular condylar cartilage cells for tissue engineering mandibular condylar cartilage, the first full study with hUCMSCs in three-dimensional (3D) musculoskeletal tissue engineering.23 Both types of cells were seeded on non-woven polyglycolic acid (PGA) meshes using spinner flasks and then statically cultured for 4 weeks in well plates containing chondrogenic or control medium. The results demonstrated that hUCMSCs can be induced to produce type I collagen, chondroitin sulfates, and other GAGs. hUCMSC constructs had more cells and higher GAG production then the tissue-engineered constructs using condylar cartilage cells.
For tissue engineering using hUCMSCs, the initial cell density is critical for cell growth and extracellular matrix synthesis.24–26 A previous study with fibrocartilage cells demonstrated that a lower cell density led to a loss of mechanical integrity, whereas a higher density benefited collagen production.25 The goal of the current study was to demonstrate the fibrochondrogenic differentiation of hUCMSCs on non-woven PGA scaffolds and evaluate the effects of initial cell seeding density on cell number, biosynthesis, and biomechanical properties. We hypothesized that hUCMSCs would form a fibrocartilage-like tissue with types I and II collagen and aggrecan and that a higher seeding density would maintain scaffold integrity and increase matrix synthesis per construct and per cell.
Materials and Methods
Cell harvest
The Kansas State University human subject board approved human umbilical cord collection and cell harvests (institutional review board approval no. 3966). Cells were isolated using enzyme digestions as described by Weiss et al.18 with minor modifications. In brief, human umbilical cords were collected and cut into 3- to 5-cm pieces, and then vessels were removed from cord segments. Cord segments were incubated in hyaluronidase (1 mg/mL; Catalog# H2126; Sigma, St. Louis, MO) and collagenase type I (300 units/mL; Catalog# 17100-017; Invitrogen, Carlsbad, CA) for 1 h at 37°C. After a 1-h incubation, the tissue pieces were crushed with tweezers to release cells from Wharton's jelly. The remaining segments were moved to new centrifuge tubes containing 0.1% trypsin/ethylenediaminetetraacetic acid for another 30 min of incubation at 37°C and were squeezed again to release additional cells from Wharton's jelly. Both vials containing cells from Wharton's jelly were combined and centrifuged at 250 × g for 5 min immediately after the second digestion. The cells were resuspended and plated in 6-well plates containing a low-serum medium at a density of 10,000 cells/cm2. The medium was composed of low-glucose Dulbecco's modified Eagle medium low-glucose Dulbecco's modified Eagle medium (DMEM; Catalog# 11885-092; Invitrogen) and MCDB-201 medium (Catalog# 045k8310; Sigma) supplemented with 1× insulin-transferrin-selenium (Catalog# 51300-044; Invitrogen), 0.15% lipid-rich bovine serum albumin (Catalog# 11020-021; Albumax, Invitrogen), 0.1 nM dexamethasone (Catalog# D2915; Sigma), 10 μM ascorbic acid-2-phosphate (Catalog# A-8960; Sigma), 1× penicillin/streptomycin (Catalog# 30-001-CI; Fisher Scientific, Pittsburgh, PA), 2% fetal bovine serum (FBS; Catalog# 16141079; Invitrogen), 10 ng/mL recombinant human epidermal growth factor (Catalog# 13247-051; Invitrogen), and 10 ng/mL human platelet-derived growth factor BB (Catalog# 220-BB-050; R&D Systems, Minneapolis, MN). Cells in the well plates were recorded as passage 0 (P0) and were fed every 2 to 3 days and maintained in a cell culture incubator (NuAire, Autoflow, 5% carbon dioxide, 37°C, and 90% humidity). When cells had reached 80% to 90% confluence, they were detached and plated into 25-cm2 flasks. At P1, cells were resuspended at a density of 1 million cells/mL of freezing medium, composed of 90% FBS and 10% dimethyl sulfoxide (DMSO; Catalog# 61097-1000; Fisher Scientific). The cell suspension was transferred into cryotubes (Catalog# 5000-1020; Nalgene Labware, Rochester, NY), which were stored in Mr. Frosty freezing containers (Catalog# 5000-0001; Nalgene) at −80°C overnight and transferred to a liquid nitrogen cryogenic storage system at −196°C for future use.
Cell seeding
Cells were thawed and expanded to P5 in the complete medium containing low-glucose DMEM, 10% FBS (Catalog# 6472; StemCell Technologies), 1% penicillin/streptomycin (Catalog# 15140-122; Invitrogen), and 1% non-essential amino acids (Catalog# 11140-050; Invitrogen). Non-woven PGA meshes (Concordia Manufacturing, Coventry, RI) were punched to round-shape scaffolds with a 5-mm diameter and 1.5-mm thickness and then sterilized with ethylene oxide. After sterilization, the scaffolds were aired under a fume hood for 1 day and then wetted with sterile filtered ethanol and two washes of sterile phosphate buffered saline (PBS). The scaffolds were then soaked in complete medium for 1 day and then removed for cell seeding. P5 hUCMSCs were seeded at 5, 25, and 50 million cells/mL of scaffold using orbital shakers onto PGA scaffolds at 150 rpm for 24 h and allowed to attach in the culture medium for another day. Finally, the complete medium was replaced by 2 mL of chondrogenic medium including high-glucose DMEM (Catalog# 10566-016; Invitrogen), 1% non-essential amino acids, 1× insulin-transferrin-selenium premix (Catalog# 354350; BD Biosciences, San Jose, CA), 10 ng/mL transforming growth factor beta-1 (TGF-β1; Catalog# 100-21C; PeproTech, Rocky Hill, NJ), 100 nM dexamethasone (Catalog# D4902; Sigma), 50 μg/mL ascorbic acid 2-phosphate (Catalog# A-8960; Sigma), 100 mM sodium pyruvate (Catalog# SH3023901; Fisher Scientific), and 40 μg/mL L-proline (Catalog# P5607-25G; Sigma). This time point was recorded as week 0. Medium was changed every other day for 4 weeks.
Biochemical analysis
At weeks 0, 2, and 4, constructs (n = 4) were digested by adding 1.1 mL papain solution (120 μg/mL) at 60°C overnight, and then constructs were stored at −20°C for biochemical assays. Cell number was determined by measuring DNA content, which was accomplished according to a reaction between PicoGreen and DNA using a kit with provided DNA standards (Kit# P7589; Invitrogen). A conversion factor of 8.5 pg DNA/cell was determined in preliminary studies. Biosynthesis was evaluated by measuring total GAG and collagen content. GAG content was measured using a dimethylmethylene blue (DMMB) dye binding assay kit (Kit# B1500; Biocolor; Newtownabbey, Northern Ireland). Chondroitin sulfate provided with the kit was used as the GAG standard. From each sample, 100 μL was added to 1 mL of DMMB and allowed 30 min to bind. Solutions were then centrifuged, supernatant was discarded, and the pellet was resuspended and read at 656 nm. Hydroxyproline content was determined using a modified hydroxyproline assay.27 Briefly, 400 μL of each sample was hydrolyzed with an equal volume of 4N sodium hydroxide at 121°C for 30 min, neutralized with an equal volume of 4N hydrochloric acid, and then titrated to an approximate pH range between 6.5 and 7.0. One mL of this solution was combined with 0.5 mL chloramine-T (14.1 g/L) in the buffer (50 g/L citric acid, 120 g/L sodium acetate trihydrate, 34 g/L sodium hydroxide, and 12.5 g/L acetic acid). The resulting solution was then combined with 0.5 mL of 1.17 mM p-dimethylaminobenzaldehyde in perchloric acid and read at 550 nm.
Immunohistochemistry for types I and II collagen and aggrecan
Immunohistochemical analysis was performed in a BioGenex i6000 autostainer (BioGenex, San Ramon, CA). Frozen sections of the 3D constructs (10 μm) (n = 2) were rehydrated with PBS for 5 min, and endogenous peroxidase activity was inhibited using 1% hydrogen peroxide in methanol for 30 min. The sections were then blocked in 3% horse serum for 20 min and incubated with a primary antibody for 1 h. Primary antibodies used in this study included the mouse monoclonal immunoglobulin (IgG) anti-collagen I (1:1500 dilution; Catalog# BYA6520-1; Accurate Chemical and Scientific, Westbury, NY), mouse monoclonal IgG anti-collagen II (1:1000 dilution; Catalog# 7005; Chondrex, Redmond, WA), and mouse monoclonal IgG anti-aggrecan (1:50 dilution; Catalog# ab3778-1; Abcam, Cambridge, MA). After primary antibody incubation, the sections were incubated with a streptavidin-linked horse anti-mouse IgG secondary antibody (Kit# PK-6102; Vector Laboratories, Burlingame, CA) for 30 min. After secondary antibody incubation, the sections were incubated with avidin-biotinylated enzyme complex (Kit# PK-6102; ABC complex; Vector Laboratories) for 30 min, and then VIP substrate (purple color) (Catalog# SK-4600; Vector Laboratories) was applied on sections for 4 min. Protocols run with the primary antibody omitted served as negative controls.
Mechanical integrity
At week 4, unconfined compression tests were performed using a uniaxial testing apparatus (Instron 5848, Norwood, MA). Hydrated samples (n = 4) were placed on the testing platen in a custom-made bath, and a tare load of 0.01 N was applied. The bath was then filled with 0.1 M PBS at 37°C to equilibrate under the tare load for 5 min. A 20% ramp strain was then applied at 1 mm/min, followed by stress relaxation for 1.5 h. Compressive elastic moduli were determined from the strain–stress curve. The stress relaxation after the ramp strain was fitted by the second-order generalized Kelvin model as described by Fung:28
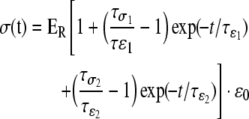 |
where ER is the equilibrium modulus, σ(t) is the stress profile, ɛo is the ramp strain, and τσ and τɛ are the creep and stress relaxation time constants, respectively.
Statistical analysis
All data were expressed as means ± standard deviations and analyzed using analysis of variance (ANOVA) followed by Tukey's honestly significant difference post hoc tests. Two-way ANOVAs with interaction were used to determine whether there were differences between time points or seeding densities. In addition, one-way ANOVAs were performed specifically to compare the differences between groups at specific time points or at specific densities. A statistical threshold of p < 0.05 was used to indicate whether there were statistically significant differences between groups.
Results
Scaffold morphology
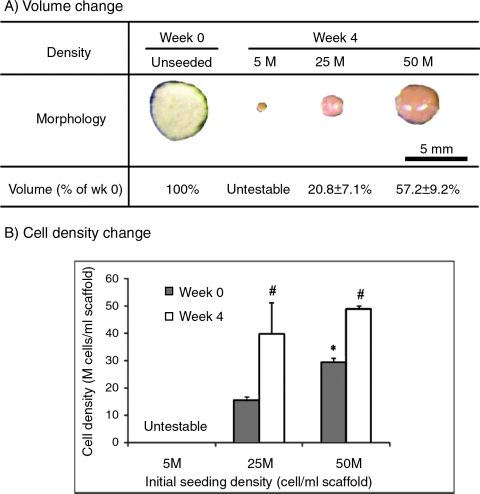
After 4 weeks of culture, the dimensions of the scaffolds had decreased to 20.8 ± 7.1% of the original volume at the medium density and 57.2 ± 9.2% at the high density (Fig. 1A). It was not possible to record accurate dimensions for the low-density group because of the formation of tiny cell pellets with irregular shapes and poor mechanical integrity. The volume loss corresponded to an increase in the cell density of the scaffolds (Fig. 1B). Cell densities in the medium- and high-density groups each increased significantly from week 0 to week 4 (p < 0.05).
FIG. 1.
Scaffold size (A) and cell density (B, cells per scaffold volume) (n = 4). *Statistically significant difference between the medium- and high-density groups. #Statistically significant difference between weeks 0 and 4. Error bars represent standard deviations. Color images available online at www.liebertonline.com/ten.
Cell number
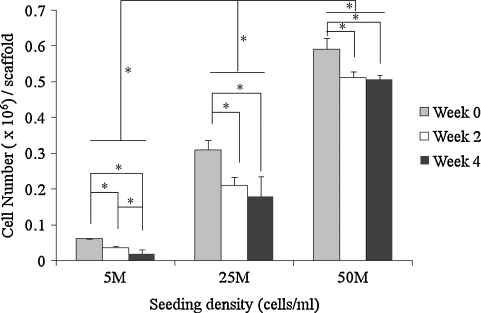
There were no significant differences between the seeding efficiencies, with 56.6 ± 0.9% at the low density, 57.3 ± 5.0% at the medium density, and 54.7 ± 2.8% at the high density. The cell numbers in all groups had moderate or significant decreases over the 4-week culture time (Fig. 2), despite the increase in cell density. All groups had a significant decrease in cell number between week 0 and week 2. Although the low-seeding-density group had a significant decrease from week 2 to week 4, there was no significant decrease in the medium-seeding-density or high-seeding-density group between weeks 2 and 4. As expected, the high-density group maintained higher cellularity than the medium-density group over the culture period (p < 0.05) (Fig. 2). At week 2, the high-density group had 2.4 and 14.1 times as many cells as the medium- (p < 0.05) and low-(p < 0.05) density groups, respectively. At week 4, the high-density group had 1.9 and 25.1 times as many cells as the medium- (p < 0.05) and low-(p < 0.05) density groups, respectively.
FIG. 2.
Cell number per construct with low, medium, and high cell seeding density derived from DNA content (n = 4). *Statistically significant difference. Error bars represent standard deviations.
GAG and hydroxyproline content
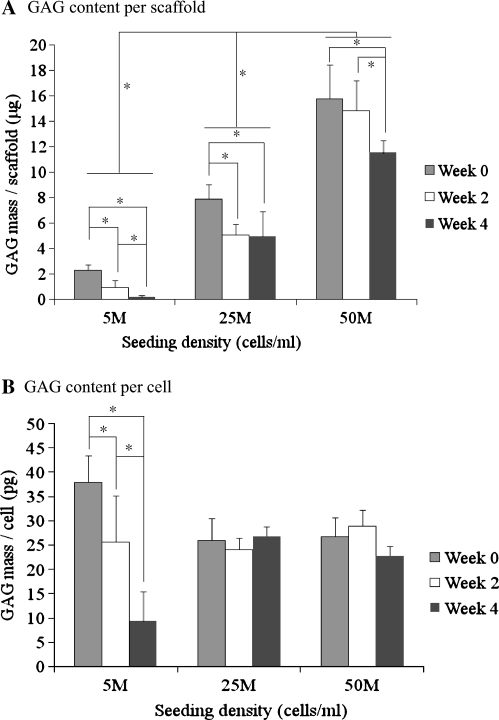
There was a significant decrease in GAG content per construct with all density groups from week 0 to week 4 (Fig. 3A). The low-density group at week 4 lost almost all GAG content (<1 μg), and GAG content per cell in the low-density group fell as well (Fig. 3B). GAG content per cell in the low-density group at week 0 was significantly higher than in the medium- and high-density groups. However, GAG content per construct of the medium- and high-density groups at week 4 were 62.7% and 72.9% of their values at week 0, respectively, whereas there was no significant difference in GAG content per cell between these two groups at any time point. The high-density group possessed a higher GAG content per construct than the low- and medium-density groups at every time point (p < 0.05). At week 2, the constructs in the high-density group had 2.9 and 15.4 times as many GAGs as the medium- (p < 0.05) and low-(p < 0.05) density groups, respectively. At week 4, the high-density group had 2.3 and 59.6 times as many GAGs as the medium- (p < 0.05) and low-(p < 0.05) density groups, respectively.
FIG. 3.
Glycosaminoglycan (GAG) content per construct (A) and per cell (B) with low, medium and high cell seeding density (n = 4). *Statistically significant difference. Error bars represent standard deviations.
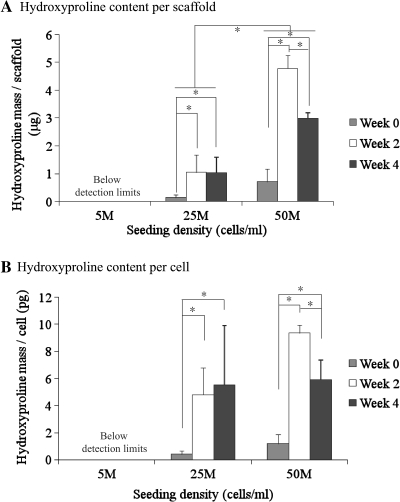
Hydroxyproline content per construct and per cell in the medium- and high-density groups increased significantly after 4 weeks of culture, but there was no detectable hydroxyproline in the low-density group (Fig. 4). Hydroxyproline content of the medium- and high-density groups increased from week 0 to week 2 (p < 0.05). There was no difference in hydroxyproline content for the medium-density group between weeks 2 and 4. Hydroxyproline content in the high-density group decreased 37.4% from week 2 to week 4 (p < 0.05), and hydroxyproline content per cell also fell significantly. The high-density group maintained a higher hydroxyproline content than the medium-density group at every culture time point (p < 0.05). At week 0, the high-density group had 5.2 times as much hydroxyproline per construct as the medium-density group. At week 4, the high-density group had 2.9 times as much hydroxyproline per construct as the medium-density group (p < 0.05). but there was no significant difference in collagen density (content per mL of scaffold) between the low- and high-density groups at week 4. A conversion factor of 11.5 can be used to convert hydroxyproline mass to collagen mass, based on our preliminary studies (unpublished data).
FIG. 4.
Hydroxyproline content per construct (A) and per cell (B) with low, medium, and high cell seeding density (n = 4). *Statistically significant difference. The high-density group had significantly higher hydroxyproline content per construct than the medium-density group. Error bars represent standard deviations. For collagen content, multiply values by a conversion factor of 11.5.
Immunohistochemical results
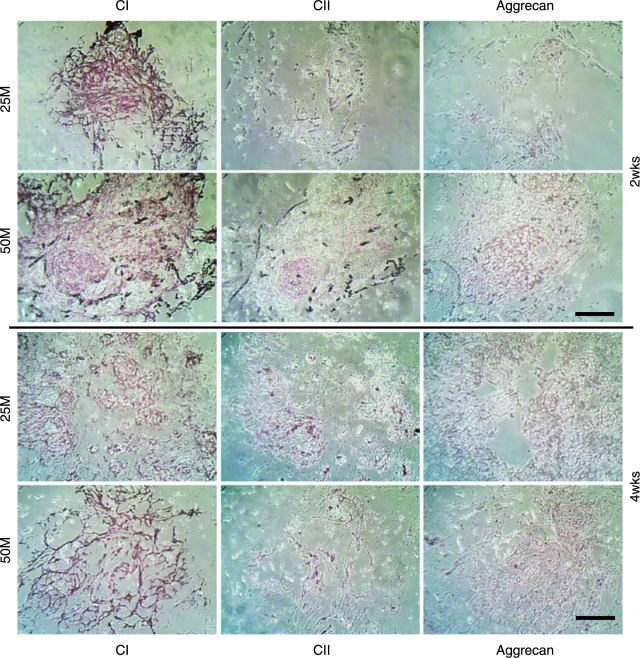
Immunohistochemistry revealed positive staining for types I and II collagen and aggrecan through the 4-week culture (Fig. 5). At week 2, immunohistochemical analysis demonstrated intense staining of type I collagen in the medium- and high-density groups. The high-density group had moderate amounts of type II collagen and aggrecan, whereas the medium-density group had no type II collagen and a minute amount of aggrecan. At week 4, the medium- and high-density groups had strong staining of type I collagen and moderate staining of type II collagen and aggrecan. In comparison, there was more-intense type I collagen staining in the medium- and high-density groups at week 2 than at week 4 and more-intense type II collagen and aggrecan staining at week 4 than at week 2. Moreover, the high-density group appeared to have stronger staining of types I and II collagen and aggrecan than the medium-density group.
FIG. 5.
Immunohistochemical staining for types I (CI) and II collagen (CII) and aggrecan. The scale bar is 100 μm. Moderate type II collagen and aggrecan staining indicates the chondrogenic differentiation of human umbilical cord matrix mesenchymal stromal cells. Color images available online at www.liebertonline.com/ten.
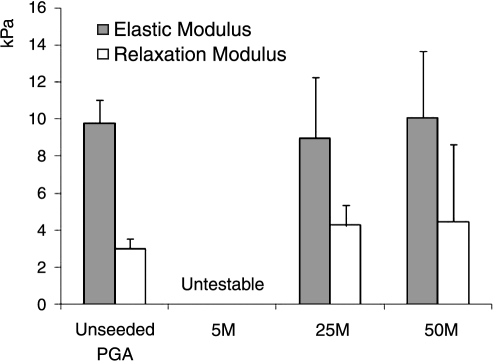
Mechanical properties
There were no significant differences in compressive elastic moduli or relaxation moduli between unseeded PGA scaffolds at week 0 and the medium- and high-density groups at week 4 (Fig. 6). Mechanical tests could not be performed on the low-density group because of the lack of mechanical integrity.
FIG. 6.
Mechanical testing of unseeded polyglycolic acid at week 0 and seeded constructs at week 4. Compressive elastic modulus and relaxation modulus (n = 4). Error bars represent standard deviations.
Discussion
hUCMSCs are fetus-derived cells and are more primitive than adult-derived stem cells based upon their expansion ability in vitro and their tissue origin.17 hUCMSCs have the ability to differentiate toward mesenchymal lineage cells such as cartilage, bone, adipose, and possibly neural cells of the ectodermal lineage.12–16,18,21,23,29–33 Moreover, there have been studies in the past 3 years that have focused on cardiovascular tissue engineering using hUCMSCs and umbilical cord vein cells.34–39 Here we explored chondrogenic differentiation in 3D PGA scaffolds and the effects of initial cell seeding density of hUCMSCs for fibrocartilage engineering.
Seeding with orbital shakers demonstrated consistent efficiency (57% at low density, 57% at medium density, and 55% at high density). In a parallel study, we found that higher rotation speeds improved efficiency (data not shown here). Cell numbers fell in all groups between week 0 and week 2. At low density, cell numbers also fell significantly from week 2 and week 4. Degradation of the PGA scaffolds might explain these decreases in cell number. In a previous study, quenched PGA demonstrated a rapid decrease in mass and an increase in water content after 10 days in PBS at 37°C.40 In a previous comparison with poly(lactic-co-glycolic acid) (PLGA; 82:18) and poly-L-lactic acid (PLLA), PGA fibers degraded faster, losing their integrity and becoming fiber fragments in the cell culture medium within 2 weeks.41 Ideally, extracellular matrix created by hUCMSCs will fill the space created by scaffold degradation. In the low-density group, insufficient extracellular matrix was produced because of the low cell number, leading to “contraction” (inward collapse) of the scaffolds. As observed under the microscope during culture, many cells and PGA debris fell into the cell culture medium, and scaffolds lost their mechanical integrity in the low-seeding-density cultures. In a future experiment, cell number and matrix content in the medium can be measured to correlate the relationship between scaffold degradation and the loss of cells and matrix. The medium and high seeding densities better maintained mechanical properties because of greater matrix production. Therefore, the medium and high cell seeding densities are recommended for future work with rapidly degrading scaffolds based on cell number and matrix production demands.
In this study, the high-density group maintained a higher GAG content throughout the 4-week culture period, although all groups had lower GAG contents at week 4 than at week 0. Some tissue-engineering studies using mature chondrocytes have reported the same phenomenon of a GAG decrease in hyaluronic acid scaffolds with swine chondrocytes,42 in poly(ethylene glycol)-co-PLLA hydrogels with calf chondrocytes,43 and in PGA scaffolds with calf chondrocytes in a perfusion chamber.44 In cartilage explant culture, medium-molecular-weight GAGs such as chondroitin and keratan sulfates have been shown to leach out.45,46 Thus, it is possible that the drop in GAG content in the current study could in part be attributed to some leaching out of GAGs. Scaffold degradation also may contribute to the decrease in GAG content as discussed in the above section. Extensive PGA scaffold degradation in the low-density group not only influenced direct GAG loss into the culture medium, but also decreased GAG content indirectly by the loss of cells (Fig. 3). In the medium- and high-density groups, it is likely that the GAG decrease was due to cell loss, because GAG content per cell remained constant. After the 2-day seeding period, GAG content per cell in the medium- and high-density groups achieved an equilibrium over 4 weeks of culture. The dynamic environment in the orbital shaker might promote GAG synthesis during the seeding periods.47–49 It must be noted that the highest GAG content at week 0 does not necessarily indicate the presence of GAGs specific to cartilage proteoglycans.
This study also demonstrated a significant increase in collagen content with the medium- and high-density groups during the 4-week culture period. At low density, there was no detectable collagen according to hydroxyproline assays because of the dissociation of PGA scaffolds. The rapid increases in hydroxyproline content occurred between weeks 0 and 2, which helped to maintain the mechanical integrity. There was the same and less hydroxyproline content with the medium and high density from week 2 to week 4, respectively. During the first 2 weeks, there was enough space in the scaffolds for collagen synthesis; however, the scaffold shrinkage50 led to a denser packing of cells (Fig. 1). The highly packed cell and extracellular matrix (especially evident with the high-density group at week 2) may have limited nutrient diffusion and provided limited room for extracellular matrix synthesis, perhaps affecting the balance of collagen metabolic activity in which collagen catabolism led to the loss of collagen. In native cartilage tissues, collagen contributes primarily to tensile properties, whereas GAGs (when associated with aggrecan) contribute to compressive integrity. No such positive correlation was found between matrix content (GAG and collagen) and compressive stiffness, although high collagen content helped retain overall construct integrity. A weak correlation between GAG and collagen contents and mechanical integrity was previously observed in tissue-engineered constructs using mature hyaline cartilage cells and fibrocartilage cells.25,51 It was not surprising that statistically significant differences in moduli were not observed between groups, because differences would only be expected with much larger increases in matrix content and organization over a longer period of time. Moreover, the greater matrix content in the higher-density group was distributed over a larger volume, so it was also not surprising that the modulus variations between the medium- and high-density groups were minimal.
hUCMSCs demonstrated better performance than mature temporomandibular joint (TMJ) condylar cells in a prior study, with two times as many cells and four times as many GAGs.23 However, the contraction was still observed with the highest density of 50 million cells/mL in the current study, along with a commensurate loss of cells and matrix, as discussed earlier. More slowly degrading scaffolds such as PLGA or PLLA should be investigated in the future. In fact, PLLA has demonstrated its potential to maintain scaffold integrity with ample matrix production in fibrocartilage tissue engineering with TMJ disc cells.52 On the other hand, improvement in seeding technique and culture conditions can aid tissue genesis in PGA scaffolds. Highly packed cells and matrix might block the pathway of nutrition and waste exchange, delaying tissue genesis inside of the scaffolds and contributing to the contraction of PGA scaffolds. Perfusion bioreactors might be used to provide more-homogenous cell distribution throughout scaffolds, assist in mass transport, and stimulate cell growth mechanically.44,53,54 Moreover, bioactive signals such as insulin-like growth factor-I and TGF-β1 can enhance biosynthesis to help maintain the original shape of scaffolds.52
In the past 4 years, only four previous studies have demonstrated the chondrogenic differentiation of hUCMSCs in cell pellet culture14–16 or PGA scaffolds.23 Larger pellets were observed in hUCMSC groups than in BMSCs, with a better filamentous extracellular matrix.15,16 hUCMSC pellets had more-intense collagen staining than BMSC groups. In a parallel tissue-engineering study (unpublished data), hUCMSCs also had a higher cell number, GAG content, and collagen content than hBMSCs after 6 weeks of culture on PGA scaffolds. Slight type I collagen and plentiful of type II collagen were observed with immunohistochemical staining in hUCMSC pellets, whereas only a trace amount of type II collagen was observed in BMSC pellets.15 Here, intense type I collagen staining and moderate collagen type II and aggrecan staining was shown; this is similar to native fibrocartilage. The decrease in type I collagen staining and increase in type II collagen and aggrecan staining from week 2 to week 4 suggests that further chondrogenic differentiation occurred during this period. Despite the smaller amount of type II collagen produced in this study than with hBMSCs in the literature,55 hUCMSCs may progress further down a chondrogenic lineage, with more collagen II production, with the investigation of a slightly modified set of signals to provide for optimal chondrogenesis. The identification of these signals, with further validation via analysis of gene expression, will be an important area of future investigation.
In conclusion, hUCMSCs exhibited characteristics of differentiation along a fibrocartilaginous lineage. The production of an abundance of type I collagen and a moderate amount of type II collagen and aggrecan suggest that hUCMSCs may be a suitable cell source for fibrocartilage tissue engineering. A concentrated effort to drive hUCMSCs down an exclusively chondrogenic lineage (collagen II expression and production in lieu of collagen I) will be an exciting area of future investigation. As hypothesized, the constructs in the medium- and high-density groups had more cells and extracellular matrix (per construct and per cell) than the low-density group over 4 weeks of culture and, more importantly, retained their mechanical integrity, unlike the low-density group. Thus, it is recommended that the cell seeding density in related future studies be not less than 25 million hUCMSCs/mL.
Acknowledgments
This work was supported by the Arthritis Foundation (MD) and the NIH NS340160 (MLW) and a State of Kansas legislature direct appropriation. Dr. S. Bennett and the nursing staff on the OB/GYN ward at Mercy Medical Center are thanked for their assistance with umbilical cord collection. The anonymous tissue donors are thanked for supplying tissues for evaluation.
Conflicts of interest statement: MLW is a paid consultant of Toucan Capital Inc and The Regenerative Medicine Institute. MLW's lab has received research support for Toucan Capital (not the work presented here).
References
- 1.Benjamin M. Evans E.J. Fibrocartilage. J Anat. 1990;171:1. [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M. Ralphs J.R. Biology of fibrocartilage cells. Int Rev Cyctol. 2004;233:1. doi: 10.1016/S0074-7696(04)33001-9. [DOI] [PubMed] [Google Scholar]
- 3.Darling E.M. Athanasiou K.A. Retaining zonal chondrocyte phenotype by means of novel growth environments. Tissue Eng. 2005;11:395. doi: 10.1089/ten.2005.11.395. [DOI] [PubMed] [Google Scholar]
- 4.Darling E.M. Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 6.von der Mark K. Gauss V. von der Mark H. Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P. Riminucci M. Gronthos S. Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 8.Mackay A.M. Beck S.C. Murphy J.M. Barry F.P. Chichester C.O. Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Owen M. Friedenstein A.J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 11.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 12.Eblenkamp M. Aigner J. Hintermair J. Potthoff S. Hopfner U. Jacobs V. Niemeyer M. Wintermantel E. [Umbilical cord stromal cells (UCSC). Cells featuring osteogenic differentiation potential] Orthopade. 2004;33:1338. doi: 10.1007/s00132-004-0730-4. [DOI] [PubMed] [Google Scholar]
- 13.Sarugaser R. Lickorish D. Baksh D. Hosseini M.M. Davies J.E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 14.Wang H.S. Hung S.C. Peng S.T. Huang C.C. Wei H.M. Guo Y.J. Fu Y.S. Lai M.C. Chen C.C. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 15.Karahuseyinoglu S. Cinar O. Kilic E. Kara F. Akay G.G. Demiralp D.O. Tukun A. Uckan D. Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 16.Baksh D. Yao R. Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 17.Can A. Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 18.Weiss M.L. Medicetty S. Bledsoe A.R. Rachakatla R.S. Choi M. Merchav S. Luo Y. Rao M.S. Velagaleti G. Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 19.Nanaev A.K. Kohnen G. Milovanov A.P. Domogatsky S.P. Kaufmann P. Stromal differentiation and architecture of the human umbilical cord. Placenta. 1997;18:53. doi: 10.1016/s0143-4004(97)90071-0. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K. Kubota T. Aso T. Study on myofibroblast differentiation in the stromal cells of Wharton's jelly: expression and localization of alpha-smooth muscle actin. Early Hum Dev. 1998;51:223. doi: 10.1016/s0378-3782(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell K.E. Weiss M.L. Mitchell B.M. Martin P. Davis D. Morales L. Helwig B. Beerenstrauch M. Abou-Easa K. Hildreth T. Troyer D. Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 22.Carlin R. Davis D. Weiss M. Schultz B. Troyer D. Expression of early transcription factors Oct4, Sox2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey M.M. Wang L. Bode C.J. Mitchell K.E. Detamore M.S. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 24.Seghatoleslami M.R. Tuan R.S. Cell density dependent regulation of AP-1 activity is important for chondrogenic differentiation of C 3 H 10 T 1/2 mesenchymal cells. J Cell Biochem. 2002;84:237. doi: 10.1002/jcb.10019. [DOI] [PubMed] [Google Scholar]
- 25.Almarza A.J. Athanasiou K.A. Effects of initial cell seeding density for the tissue engineering of the temporomandibular joint disc. Ann Biomed Eng. 2005;33:943. doi: 10.1007/s10439-005-3311-8. [DOI] [PubMed] [Google Scholar]
- 26.Kavalkovich K.W. Boynton R.E. Murphy J.M. Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 457:38. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Edwards C.A. O'Brien W.D. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 28.Fung Y. New York: Springer; 1993. Biomechanics: Mechanical Properties of Living Tissues. [Google Scholar]
- 29.Lu L.L. Liu Y.J. Yang S.G. Zhao Q.J. Wang X. Gong W. Han Z.B. Xu Z.S. Lu Y.X. Liu D. Chen Z.Z. Han Z.C. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017. [PubMed] [Google Scholar]
- 30.Wu K.H. Zhou B. Lu S.H. Feng B. Yang S.G. Du W.T. Gu D.S. Han Z.C. Liu Y.L. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 31.Fu Y.S. Shih Y.T. Cheng Y.C. Min M.Y. Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci. 2004;11:652. doi: 10.1007/BF02256131. [DOI] [PubMed] [Google Scholar]
- 32.Yang L.Y. Zheng J.K. Wang C.Y. Xu M.D. [Stromal cells from human Wharton's jelly differentiate into neural cells]. Sichuan da xue xue bao. Yi Xue Ban. 2005;36:13. [PubMed] [Google Scholar]
- 33.Fu Y.S. Cheng Y.C. Lin M.Y. Cheng H. Chu P.M. Chou S.C. Shih Y.H. Ko M.H. Sung M.S. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 34.Kadner A. Zund G. Maurus C. Breymann C. Yakarisik S. Kadner G. Turina M. Hoerstrup S.P. Human umbilical cord cells for cardiovascular tissue engineering: a comparative study. Eur J Cardiothorac Surg. 2004;25:635. doi: 10.1016/j.ejcts.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt D. Hoerstrup S.P. Tissue engineered heart valves based on human cells. Swiss Med Wkly. 2007;137 Suppl 155:80S. [PubMed] [Google Scholar]
- 36.Schmidt D. Mol A. Odermatt B. Neuenschwander S. Breymann C. Gossi M. Genoni M. Zund G. Hoerstrup S.P. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12:3223. doi: 10.1089/ten.2006.12.3223. [DOI] [PubMed] [Google Scholar]
- 37.Breymann C. Schmidt D. Hoerstrup S.P. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2:87. doi: 10.1007/s12015-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt D. Hoerstrup S.P. Tissue engineered heart valves based on human cells. Swiss Med Wkly. 2006;136:618. doi: 10.4414/smw.2006.11400. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt D. Asmis L.M. Odermatt B. Kelm J. Breymann C. Gossi M. Genoni M. Zund G. Hoerstrup S.P. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82:1465. doi: 10.1016/j.athoracsur.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 40.Hurrell S. Cameron R.E. Polyglycolide: degradation and drug release. Part I: changes in morphology during degradation. J Mater Sci. 2001;12:811. doi: 10.1023/a:1017925019985. [DOI] [PubMed] [Google Scholar]
- 41.Lu H.H. Cooper J.A., Jr. Manuel S. Freeman J.W. Attawia M.A. Ko F.K. Laurencin C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials. 2005;26:4805. doi: 10.1016/j.biomaterials.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 42.Chung C. Mesa J. Miller G.J. Randolph M.A. Gill T.J. Burdick J.A. Effects of auricular chondrocyte expansion on neocartilage formation in photocrosslinked hyaluronic acid networks. Tissue Eng. 2006;12:2665. doi: 10.1089/ten.2006.12.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryant S.J. Durand K.L. Anseth K.S. Manipulations in hydrogel chemistry control photoencapsulated chondrocyte behavior and their extracellular matrix production. J Biomed Mater Res. 2003;67:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 44.Seidel J.O. Pei M. Gray M.L. Langer R. Freed L.E. Vunjak-Novakovic G. Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorheology. 2004;41:445. [PubMed] [Google Scholar]
- 45.Campbell M.A. Handley C.J. Hascall V.C. Campbell R.A. Lowther D.A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys. 1984;234:275. doi: 10.1016/0003-9861(84)90350-3. [DOI] [PubMed] [Google Scholar]
- 46.Bolis S. Handley C.J. Comper W.D. Passive loss of proteoglycan from articular cartilage explants. Biochim Biophys Acta. 1989;993:157. doi: 10.1016/0304-4165(89)90158-x. [DOI] [PubMed] [Google Scholar]
- 47.Bilgen B. Orsini E. Aaron R.K. Ciombor D.M. FBS suppresses TGF-beta1-induced chondrogenesis in synoviocyte pellet cultures while dexamethasone and dynamic stimuli are beneficial. 2007;1:436. doi: 10.1002/term.56. [DOI] [PubMed] [Google Scholar]
- 48.Vunjak-Novakovic G. Freed L.E. Biron R.J. Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE J. 1996;42:850. [Google Scholar]
- 49.Davisson T. Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res. 2002;20:842. doi: 10.1016/S0736-0266(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 50.Detamore M.S. Athanasiou K.A. Use of a rotating bioreactor toward tissue engineering the temporomandibular joint disc. Tissue Eng. 2005;11:1188. doi: 10.1089/ten.2005.11.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly D.J. Crawford A. Dickinson S.C. Sims T.J. Mundy J. Hollander A.P. Prendergast P.J. Hatton P.V. Biochemical markers of the mechanical quality of engineered hyaline cartilage. J Mater Sci. 2007;18:273. doi: 10.1007/s10856-006-0689-2. [DOI] [PubMed] [Google Scholar]
- 52.Allen K.D. Athanasiou K.A. Scaffold and growth factor selection in temporomandibular joint disc engineering. J Dent Res. 2008;87:180. doi: 10.1177/154405910808700205. [DOI] [PubMed] [Google Scholar]
- 53.Davisson T. Sah R.L. Ratcliffe A. Perfusion increases cell content and matrix synthesis in chondrocyte three-dimensional cultures. Tissue Eng. 2002;8:807. doi: 10.1089/10763270260424169. [DOI] [PubMed] [Google Scholar]
- 54.Boschetti F. Raimondi M.T. Migliavacca F. Dubini G. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J Biomech. 2006;39:418. doi: 10.1016/j.jbiomech.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 55.Yoo J.U. Barthel T.S. Nishimura K. Solchaga L. Caplan A.I. Goldberg V.M. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg. 1998;80:1745. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]