Abstract
Because articular cartilage has a poor regeneration capacity, numerous cell-based approaches to therapy are currently being explored. The present study involved the use of gene transfer as a means to provide sustained delivery of chondrogenic proteins to primary mesenchymal stem cells (MSCs). In previous work, we found that adenoviral-mediated gene transfer of transforming growth factor-beta1 (TGF-β1) and bone morphogenetic protein 2 (BMP-2), but not insulin-like growth factor 1 (IGF-1), could be used to induce chondrogenic differentiation of MSCs in an aggregate culture system. In the present study, we examined the effects on chondrogenesis of these transgenes when delivered in combination. Cultures of bone marrow–derived MSCs were infected with 2.5 × 102 or 2.5 × 103 viral particles/cell of each adenoviral vector individually, or in combination, seeded into aggregates, and cultured for 3 weeks in a defined serum-free medium. Levels of transgene product in the medium were initially high, approximately 100 ng/mL TGF-β1, 120 ng/mL BMP-2, and 80 ng/mL IGF-1 at day 3, and declined thereafter. We found that co-expression of IGF-1 and TGF-β1, BMP-2, or both at low doses resulted in larger aggregates, higher levels of glycosaminoglycan synthesis, stronger staining for proteoglycans and collagen type II and X, and greater expression of cartilage-specific marker genes than with either transgene alone. Gene-induced chondrogenesis of MSCs using multiple genes that act synergistically may enable the administration of reduced viral doses in vivo and could be of considerable benefit for the development of cell-based therapies for cartilage repair.
Introduction
Because of the inability of articular cartilage to effectively self-repair, numerous cell-based approaches to therapy are being explored.1–4 Adult mesenchymal stem cells (MSCs) have the capacity to differentiate into various mesenchymal lineages5 and are readily obtained from multiple tissue sources, including bone marrow,6 trabecular bone,7 and fat,8 making them an important autogenous cell source for regenerative medicine.2–4 Although potentially useful as alternative cell source to chondrocytes, methods to effectively stimulate proliferation and subsequent chondrogenic differentiation of MSCs are needed to further develop the use of these cells for cartilage repair.1
Chondrogenesis of MSCs in vitro is a finely regulated process that requires high-density culture in the presence of specific media supplements, including dexamethasone and certain growth factors.2,3,9–16 Under these conditions, numerous studies have shown that exogenous administration of transforming growth factor-beta1 (TGF-β1) and bone morphogenetic protein-2 (BMP-2) efficiently stimulate chondrogenic differentiation.11,12,17,18 Related studies have identified the chondrogenic potential of other growth factors, including insulin-like growth factor-1 (IGF-1), TGF-β2, TGF-β3, BMP-4, and BMP-7.13–16,19 However, the delivery of these agents together with MSCs into cartilaginous lesions in vivo has not yet resulted in sustained regeneration of articular cartilage.1,2,3,9 One possible problem may be that the stimulation provided by a single bolus of soluble growth factor is insufficient to drive differentiation of the implanted cells in vivo.1,20,21 To overcome this, gene transfer approaches, which have been already explored clinically for the treatment of arthritis,22 might be adopted to provide sustained synthesis of growth and differentiation factors, which can promote chondrogenic differentiation of MSCs and increased matrix synthesis within cartilage lesions and ultimately lead to improved repair.21,23–25
We have previously shown that after adenoviral delivery of individual complementary DNAs (cDNAs) encoding certain growth factors into primary MSCs, the resulting expression is capable of inducing chondrogenesis in culture.26,27 However, it is likely that more-sophisticated strategies of gene transfer will be necessary to fulfill the potential of stem cell–based therapies for cartilage repair.25 These may include the use of regulatable systems for control of transgene expression and the co-delivery and expression of multiple genes. For example, the co-administration of recombinant IGF-1 and TGF-β1 has been shown to effectively increase matrix synthesis of chondrogenic cells.14,28,29 Thus, combined administration of these growth factors may improve a cartilaginous repair response by enhancing chondrogenesis of MSCs and the long-term maintenance of the articular cartilage phenotype. This may be achieved through greater synthesis of a stable cartilage extracellular matrix, expansion of chondrogenic cell populations, and the maintenance of chondrocytes at a pre-hypertrophic stage of differentiation.1–3,9,21,25
In the present study, using adenoviral-mediated gene transfer, we analyzed the effects of co-expression of IGF-1, TGF-β1, and BMP-2 cDNAs on chondrogenesis of primary MSCs in vitro. We found that co-expression of IGF-1 and TGF-β1 or BMP-2 at low doses enhanced the expression of articular cartilage marker genes and the deposition of cartilage-specific matrix rich in proteoglycans and collagen type II and X. The specific transgenes, the vector dose, and in turn, the level and duration of transgene expression directly influenced the extent of chondrogenesis.
Materials and Methods
Preparation of recombinant adenoviral vectors
First-generation, E1-, E3-deleted, serotype 5 adenoviral vectors carrying the cDNAs for human IGF-1, BMP-2, TGF-β1, firefly luciferase (Luc), or green fluorescent protein (GFP) were constructed using cre-lox recombination as described earlier.30 The resulting vectors were designated Ad.IGF-1, Ad.BMP-2, Ad.TGF-β1, Ad.Luc, and Ad.GFP; propagated using amplification in 293/Cre8 cells; and purified over successive cesium chloride gradients. After dialysis against 10mM Tris-hydrochloric acide, pH 7.8, 150 mM sodium chloride (NaCl), 10 mM magnesium chloride, and 4% sucrose,31 viral titers were estimated to be between 1012and 1013 particles/mL according to optical density and standard plaque assay.
Culture of marrow-derived MSCs and adenoviral transduction
Bone marrow–derived MSCs were harvested from the femora and tibiae of ten 3- to 4-month-old bovine calves (Research 87, Inc., Marlborough, MA) in 10 independent preparations, as described previously.7 The collected cells were pelleted using centrifugation at 1,000 rpm for 10 min, resuspended in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (all Invitrogen, Carlsbad, CA). The cells were counted and seeded at 4 × 107 to 6 × 107 nucleated cells per 75-cm2 flask (Falcon, Beckton Dickinson Labware, Franklin Lakes, NJ). Nonadherent cells were removed after 3 days. The remaining attached cells were washed with PBS and cultured in DMEM with 10% FBS at 37°C, 5% carbon dioxide (CO2) with medium changes every 3 to 4 days. After 14 to 21 days, adherent colonies were trypsinized and replated in several 25-cm2 tissue culture flasks. At confluence (approximately 9 × 105 cells/25-cm2 flask), the cultures were infected in 250 μL of serum-free DMEM for 2 h with 2.5 × 102 vp/cell of Ad.TGF-β1, Ad.BMP-2, or Ad.IGF-1 alone, in combinations, or co-infected with a high dose of 2.5 × 103 vp/cell of Ad.IGF-1, as indicated in the respective experiments. Control cultures were similarly infected with Ad.GFP or Ad.Luc at 2.5 × 102 vp/cell, remained uninfected, or were maintained in the presence of 10 ng/mL TGF-β1. After viral infection, the supernatant was aspirated and replaced with 5 mL of complete DMEM.
Aggregate culture and transgene expression
At 24 h post-infection, the MSC cultures were trypsinized, and 3 × 105 cells in 1 mL of complete DMEM in 15-mL polypropylene conical tubes (Falcon) were centrifuged at 500 × g for 5 min to promote aggregate formation. The FBS-containing medium was then replaced with 0.5 mL of a defined serum-free medium containing 1 mM pyruvate, 1% insulin-transferrin-selenium + Premix, 37.5 mg/mL ascorbate-2-phosphate, and 10−7 M dexamethasone (all Sigma, St. Louis, MO); the recombinant protein control group was supplemented with anadditional 10 ng/mL TGF-β1 (R&D Systems, Minneapolis, MN).12 The cell pellets were cultured at 37°C, 5% CO2 and formed spherical aggregates within 24 h.
Media were changed every 2 to 3 days. The aggregates were harvested at various time points for reverse transcriptase polymerase chain reaction (RT-PCR) analyses or after 21 days for histologic and biochemical analyses. Media conditioned using the aggregates were collected at various time points and assayed for the respective growth factors using the appropriate commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) for human TGF-β1, BMP-2, and IGF-1.
Quantitative GAG and DNA content analyses
After 21 days, certain aggregates were harvested for quantitation of sulfated glycosaminoglycans (GAGs) and DNA content. The aggregates were washed with PBS, digested with 200 μL of proteinase K digest solution (1 μg/mL, Sigma), and incubated for 16 h at 65°C. Total GAG content was measured by reaction with 1,9-dimethylmethylene blue (Sigma)32 using shark chondroitin sulfate (Sigma) as the standard. For DNA analysis, an aliquot of the proteinase K digest was read fluorometrically (Hoefer Scientific Instruments, San Francisco, CA) using Hoechst dye no. 33258 (Sigma) dissolved in 2 mL of Tris–ethylenediaminetetraacetic acid –NaCl buffer. The DNA concentration was determined from a standard curve of calf thymus DNA (Sigma).
Histological and immunohistochemical analyses
Before tissue processing, representative aggregates of each group were photographed using a digital camera (Model DC290; Kodak, Rochester, NY). For histological analyses, aggregates were fixed in 10% buffered formalin for 1 h and embedded in 0.5% agarose before tissue processing. After dehydration in graded alcohols, the aggregates were paraffin embedded and sectioned to 5 μm. Representative sections were stained using toluidine blue (Sigma) for the detection of matrix proteoglycan, and alternate sections were used for immunohistochemistry.
For immunohistochemical analyses, sections were washed for 20 min in Tris-buffered saline (TBS) and incubated in 5% bovine serum albumin (BSA, Sigma). After washing in TBS, sections were pretreated with 40 mU/mL chondroitinase ABC (Sigma) (1 g/L) for 30 min at 37°C and then incubated with 5 μg/mL monoclonal anti-collagen type II (COL II), monoclonal anti-collagen type I (COL I) (both Rockland Immunochemicals, Inc., Gilbertsville, PA), or polyclonal anti-collagen type X (COL X) antibodies (a kind gift from Gary Gibson, Henry Ford Hospital, Detroit, MI). Washed sections were then incubated with the secondary antibody, anti-goat immunoglobulin (Ig)G crystalline fluorescein isothiocyanate (FITC) conjugate (Sigma). Sections were analyzed using fluorescence and light microscopy. Negative controls were incubated with non-immune serum before incubation with the FITC-labeled secondary antibody. To quantify aggregate sizes and relative toluidine blue or immunofluorescent staining, digital images of three individual mid-sections for each aggregate were taken, and the smallest and largest diameters, as well as the mean density of staining across the entire section, were determined using the Scion Image program version 1.63 (Scion, Frederick, MD). For the respective methods, sections of aggregates cultured in the absence of specific growth factor stimulation served as negative controls and were used to establish baseline levels of staining against which the sections from the experimental groups were evaluated. Aggregates maintained after single Ad.TGF-β1 or BMP-2 gene transfer at 2.5 × 102 vp/cell served as positive controls against which relative staining intensities were semi-quantitatively expressed.
Total RNA extraction and semi-quantitative RT-PCR
RNA was prepared from primary chondrocyte or MSC cultures and from subsequent MSC aggregate cultures using a modification of the method of Chomczynski and Sacci.33 For the monolayer cultures, the cells were trypsinized, centrifuged at 500 × g for 5 min, and lysed in 1 mL of TRI-reagent (Sigma). For aggregates, at each time point, 10 pellets per group were pooled and homogenized using a pellet pestle and repeated trituration in 1 mL of TRI-reagent. After incubation for 5 min in TRI-reagent, 200 μL of chloroform was added to each group and mixed using repeated inversion. After centrifugation at 12,000 × g for 10 min, the aqueous phase was collected, and RNA was precipitated using isopropanol. After centrifugation at 12,000 × g for 15 min, the RNA pellets were washed with 70% ethanol, allowed to air dry, and then resuspended in diethylpyrocarbonate-treated water (Invitrogen). RNA concentration and purity of each sample was estimated using OD260/280 ratios.
RNA from primary and aggregate cultures (2 μg each) was used for random hexamer primed cDNA synthesis using Moloney murine leukemia virus reverse transcriptase (Invitrogen). Equal amounts of each cDNA synthesized (100 ng) were used as templates for PCR amplification in a 50-μL reaction volume using Taq DNA polymerase (Invitrogen) and 50 pmol of gene-specific primers, which were designed based on the respective GenBank sequence for the gene examined. The sequences, annealing temperatures, and product sizes of forward and reverse primers for COL II, aggrecan (AGC), biglycan (BGC), COL X, osteopontin (OP), COL I, and glyceraldehyde-3-phosphate dehydrogenase are listed in Table 1. Amplifications were performed for 35 cycles, and RT-PCR products were visualized on 1.5% agarose gels containing 0.1 μg/mL ethidium bromide and semi-quantified using Biocapt Image software (Vilber Lourmat, Eberhardzell, Germany).
Table 1.
Primer Sequences and Product Sizes for Semi-Quantitative Reverse Transcriptase Polymerase Chain Reaction
| Gene | Reverse Transcriptase-Polymerase Chain Reaction Primer Sequences (5’-3’) | Product size (bp) | Annealing temperature (°C) | Accession Number (GenBank)/Source |
|---|---|---|---|---|
| Collagen II | Sense: AACGGTGGCTTCCACTTCAG | 580 | 59 | [X02420]50 |
| Antisense: TGCCCAGTTCAGGTCTCTTAGAG | ||||
| Aggrecan | Sense: ACCGATACGAGATCAATG | 153 | 53 | [U76615]50 |
| Antisense: CTGTAGTCTGCGCTTGTA | ||||
| Biglycan | Sense: AGGCCCTCGTCCTGGTGAACA | 165 | 63 | [S82652]51 |
| Antisense: GGATGCGGTTGTCGTGGATGC | ||||
| Collagen X | Sense: GCAACAGCATTATGACCCA | 340 | 55 | [NM174634] |
| Antisense: CACCAAAGGAAGCCATCG | ||||
| Osteopontin | Sense: CCGAGGTGATAGTGTGGCTTAC | 464 | 55 | [AF492837]52 |
| Antisense: TTCATATTGTCTCCCACCCTG | ||||
| Collagen I | Sense: GCCGAGACTTGAGACTCAGCCACCCA | 537 | 68 | [AB008683]50 |
| Antisense: GATAGGCAGGCGAGATGGCTTGTT TG | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | Sense: AACTCCCTCAAGATTGTCAGCA | 553 | 59 | [U85042]53 |
| Antisense: TCCACCACCCTGTTGCTGTA |
Statistical analysis
Data from ELISA, aggregate diameter, and GAG/DNA content analyses for each marrow preparation were performed on three pellets per group, time-point, and experiment described, with the entire study being repeated on at least three and up to 10 different marrow preparations (m = 3-10) as indicated in the respective experiments. Semi-quantitative RT-PCR analyses were performed on RNAs extracted from 10 aggregates that were pooled for each group, time-point, and marrow preparation, with the entire study being repeated on three different marrow preparations. All data are expressed as means ± standard deviations. Statistical significance between groups for the aggregate diameter and GAG/DNA data was determined using one-way analysis of variance (ANOVA) and Dunnett's post hoc t-test. A level of p < 0.05 was considered significant.
Results
Transgene expression patterns of MSC aggregates genetically modified to express multiple growth factors
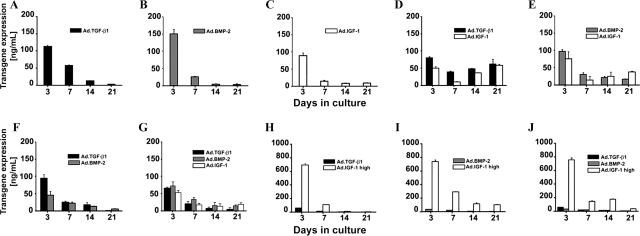
To determine whether adherent bone marrow–derived MSCs can be genetically modified to functionally co-express 2 anabolic transgenes in high-density aggregate culture, first-passage monolayer cultures were infected with recombinant Ad.TGF-β1, Ad.BMP-2, or Ad.IGF-1 alone or in combination at viral doses of 2.5 × 102 vp/cell or 2.5 × 103 vp/cell (high) as indicated in the respective experiments. Control groups consisted of naïve and infected MSC cultures with equivalent doses of adenoviral vectors encoding Ad.GFP or Ad.Luc. Twenty-four-h post-infection, the cells were seeded into aggregate cultures, and secreted transgene products were measured in medium conditioned over a 24-h period at days 3, 7, 14, and 21.
Consistent with previous findings,26 cultures infected with Ad.TGF-β1, Ad.BMP-2, or Ad.IGF-1 alone at 2.5 × 102 vp/cell generated approximately 50 to 150 ng/mL of gene product per 24 h at day 3 post-infection (Fig. 1A–C). The amount of respective transgene product steadily decreased thereafter and was near background levels at day 21 (Fig. 1A–C). Levels of TGF-β1, BMP-2, and IGF-1 in medium conditioned using Ad.GFP- or Ad.Luc-infected cultures were not detected above the levels observed in the naïve controls (<100 pg/mL; data not shown).
FIG. 1.
Transgene expression profiles from mesenchymal stem cell (MSC) aggregates after adenoviral-mediated gene transfer of transforming growth factor beta 1 (TGF-β1), bone morphogenetic protein 2 (BMP-2), and insulin-like growth factor (IGF) alone and in combination. Values represent levels of transgene product expressed in ng/mL in the conditioned medium over a 24-h period at days 3, 7, 14, and 21. (A–C) MSC aggregates singly infected with (A) adenoviral-mediated gene transfer of TGF-β1 (Ad.TGF-β1), (B) Ad.BMP-2, or (C) Ad.IGF-1 at 2.5 × 102 vp/cell. MSC aggregates infected dually (D–F) or with all three transgenes (G) at low viral doses with 2.5 × 102 vp/cell for each vector as indicated. (H–J) MSC aggregates after co-infection with Ad.IGF-1 at high dose (Ad.IGF-1 high; 2.5 × 103 vp/cell) and (H) Ad.TGF-β1, (I) Ad.BMP-2, or (J) both at 2.5 × 102 vp/cell. The data are represented as means ± standard deviations of three pellets per condition from one marrow preparation; similar trends were observed in independent experiments. To indicate differences between marrow samples, the mean values of three pellets per group and time-point for each of three different marrow preparations tested are shown in Table 2.
For the cultures dually infected at 2.5 × 102 vp/cell, the transgene expression profiles were somewhat different (Fig. 1D-F). At day 3, the levels of secreted product were moderately lower for each transgene thanfor the singly infected cultures but were still near the 50- to 100-ng/mL range. Combinations of Ad.IGF-1 with Ad.TGF-β1 or Ad.BMP-2 maintained expression of at least 38 ng/mL of TGF-β1, 18 ng/mL of BMP-2, and 13 of ng/mL IGF-1 through the 21-day culture period (Fig. 1D, E). Moreover, in Ad.TGF-β1 and Ad.IGF-1 co-infected cultures, transgene expression was as high at day 21 as at day 7 (Fig. 1D). This pattern was not observed with co-delivery of Ad.TGF-β1 and Ad.BMP-2 (Fig. 1F), although when IGF-1 was co-delivered together with Ad.TGF-β1 and Ad.BMP-2 at 2.5 × 102 vp/cell, each expression of at least 5 ng/mL of TGF-β1, 12 ng/mL of BMP-2, and 18 ng/mL of IGF-1 was maintained through the 21-day culture period (Fig. 1G).
To further explore the effect of Ad.IGF-1 co-delivery on transgenic expression, we incubated monolayer cultures of MSCs with Ad.TGF-β1 or Ad.BMP-2 at 2.5 × 102 vp/cell and co-infected each with Ad.IGF-1 at a 10 times higher dose of 2.5 × 103 vp/cell (Ad.IGF-1 high). As above, the cultures were seeded into aggregates, and transgene expression was followed over time. Under these conditions, as seen in Figure 1H–J, exceptionally high levels of IGF-1 (>700 ng/mL) were generated at day 3 but then had dropped at least 70% at day 7. Expression of the TGF-β1 and BMP-2 transgenes was in the range of 20 to 80 ng/mL at day 3 and was not prolonged.
The data in Figure 1 represent means ± standard deviations of three pellets per condition from one marrow preparation; similar trends were observed in independent experiments with three different marrow preparations as shown in Table 2.
Table 2.
Variations of Transgene Expression Between Marrow Samples
| |
Mean transgene expression values per marrow sample (n = 3) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Days in aggregate culture |
|||||||||||||||||||
| Donor cow | 3 | 7 | 14 | 21 | 3 | 7 | 14 | 21 | 3 | 7 | 14 | 21 | 3 | 7 | 14 | 21 | 3 | 7 | 14 | 21 |
| TGF-β1 | TGF-β1 | TGF-β1 | TGF-β1 | BMP-2 | BMP-2 | BMP-2 | BMP-2 | IGF-1 | IGF-1 | IGF-1 | IGF-1 | TGF-β1 + IGF-1 low | TGF-β1 + IGF-1 low | TGF-β1 + IGF-1 low | TGF-β1 + IGF-1 low | BMP-2 + IGF-1 low | BMP-2 + IGF-1 low | BMP-2 + IGF-1 low | BMP-2 + IGF-1 low | |
| 1 | 112.8 | 57.3 | 12.1 | 3.3 | 152.7 | 26.3 | 5.6 | 5.0 | 84.4 | 18.2 | 7.7 | 8.8 | 49.7 | 9.8 | 36.8 | 58.2 | 76.7 | 15.3 | 25.8 | 39.0 |
| 80.3 | 38.8 | 48.0 | 62.4 | 98.5 | 31.8 | 22.5 | 17.9 | |||||||||||||
| 2 | 83.8 | 18.8 | 12.3 | 1.9 | 73.3 | 24.8 | 3.2 | 1.1 | 68.3 | 23.7 | 13.3 | 9.8 | 71.7 | 25.4 | 25.0 | 26.8 | 43.4 | 18.9 | 26.3 | 32.9 |
| 120.0 | 21.4 | 42.6 | 43.3 | 34.7 | 13.3 | 11.8 | 26.7 | |||||||||||||
| 3 | 94.2 | 36.0 | 20.6 | 6.2 | 144.1 | 34.9 | 17.4 | 6.9 | 90.6 | 18.2 | 3.2 | 4.2 | 94.2 | 20.61 | 35.9 | 31.4 | 49.1 | 33.4 | 35.6 | 47.1 |
| 68.3 | 19.7 | 26.6 | 47.4 | 48.1 | 4.6 | 14.4 | 14.2 | |||||||||||||
| TGF-β1 + BMP-2 | TGF- β1 + BMP-2 | TGF- β1 + BMP-2 | TGF- β1 + BMP-2 | TGF- β1 + BMP-2 + IGF-1 low | TGF- β1 + BMP-2 + IGF-1 low | TGF- β1 + BMP-2 + IGF-1 low | TGF- β1 + BMP-2 + IGF-1 low | TGF-β1 + IGF-1 high | TGF-β1 + IGF-1 high | TGF-β1 + IGF-1 high | TGF-β1 + IGF-1 high | BMP-2 + IGF-1 high | BMP-2 + IGF-1 high | BMP-2 + IGF-1 high | BMP-2 + IGF-1 high | TGF- β1 + BMP-2 + IGF-1 high | TGF- β1 + BMP-2 + IGF-1 high | TGF- β1 + BMP-2 + IGF-1 high | TGF- β1 + BMP-2 + IGF-1 high | |
| 1 | 93.0 | 24.5 | 17.1 | 0.5 | 66.7 | 23.0 | 8.7 | 6.3 | 60.5 | 15.0 | 1.7 | 0.7 | 28.1 | 22.4 | 6.1 | 4.6 | 59.1 | 18.0 | 12.1 | 3.3 |
| 44.31 | 21.7 | 12.7 | 5.4 | 71.4 | 33.3 | 15.4 | 15.1 | 683.6 | 108.3 | 6.2 | 2.7 | 749.9 | 293.2 | 102.3 | 94.2 | 32.8 | 14.7 | 15.4 | 5.6 | |
| 53.2 | 18.3 | 23.5 | 19.8 | 761.4 | 137.6 | 173.4 | 39.3 | |||||||||||||
| 2 | 119.7 | 57.3 | 21.0 | 4.0 | 139.5 | 44.9 | 49.8 | 46.8 | 37.5 | 7.64 | 3.1 | 1.7 | 37.4 | 18.8 | 8.4 | 2.1 | 47.1 | 16.1 | 8.2 | 6.9 |
| 25.6 | 13.8 | 3.9 | 3.8 | 46.9 | 27.0 | 14.1 | 27.6 | 450.3 | 75.5 | 18.1 | 3.8 | 909.9 | 202.3 | 294.2 | 209.3 | 54.6 | 9.6 | 1.9 | 3.7 | |
| 81.5 | 54.6 | 36.7 | 24.9 | 357.4 | 138.1 | 91.2 | 68.9 | |||||||||||||
| 3 | 49.9 | 18.9 | 9.3 | 3.8 | 88.4 | 28.2 | 33.6 | 24.7 | 41.9 | 9.4 | 6.1 | 6.9 | 31.4 | 19.8 | 19.1 | 7.8 | 36.7 | 7.9 | 6.2 | 2.9 |
| 58.8 | 33.17 | 3.7 | 6.2 | 61.6 | 33.1 | 36.5 | 58.5 | 240.0 | 86.6 | 28.1 | 4.8 | 291.2 | 90.0 | 111.8 | 46.5 | 35.4 | 5.5 | 1.7 | 2.0 | |
| 45.6 | 14.5 | 13.5 | 8.5 | 455.2 | 118.7 | 123.5 | 41.6 | |||||||||||||
TGF-β1 transgene expression: normal font; BMP-2 transgene expression: italic font; IGF-1 transgene expression: bold font.
Chondrogenic differentiation of MSCs after adenoviral delivery of TGF-β1 and IGF-1
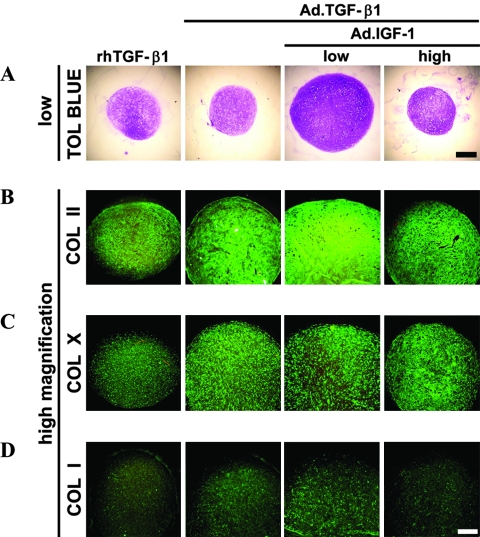
Because primary MSCs were shown to be capable of sustained expression of two different anabolic transgenes after adenoviral-mediated transduction, we analyzed the effects of growth factor co-expression on in vitro chondrogenesis of MSC aggregates. As seen previously,26 medium supplementation with 10 ng/mL of recombinant TGF-β1 protein and infection of MSCs with Ad.TGF-β1 using viral doses sufficient to generate between 50 and 150 ng of transgene product at day 3 (Fig. 1A) induced a significant chondrogenic response in the respective aggregate cultures. Strong production of COL II and proteoglycans, as indicated by immunohistochemistry and metachromatic staining with toluidine blue, demonstrated this (Fig. 2A, B).
FIG. 2.
Chondrogenesis of mesenchymal stem cells (MSCs) in aggregate culture after adenoviral-mediated gene transfer of transforming growth factor beta 1 (TGF-β1) alone or in combination with insulin-like growth factor 1 (IGF-1). Monolayer cultures of MSCs were infected with adenoviral-mediated gene transfer of TGF-β1 (Ad.TGF-β1) alone at 2.5 × 102 vp/cell as indicated, and certain cultures were co-infected with Ad.IGF-1 at 2.5 × 102 or 2.5 × 103 vp/cell (low and high doses, respectively, as indicated). At 24 h after infection, the cells were seeded into aggregates and cultured for 21 days, and representative sections are shown. Control cultures maintained in the presence of 10 ng/mL recombinant human TGF-β1 are also shown. (A) Toluidine blue staining for detection of matrix proteoglycan (50 × magnification, bar = 400 μm). (B) Immunostaining for the presence of collagen type II (COL II), (C) COL X, and (D) COL I (100 × magnification, bar = 200 μm). Regions of positive staining show green fluorescence. Color images available online at www.liebertonline.com/ten.
Aggregates that received Ad.TGF-β1 together with Ad.IGF-1 at a low dose (2 × 102 vp/cell) showed significantly greater chondrogenesis than aggregates receiving Ad.TGF-β1 alone. Co-delivery of IGF-1 led to larger aggregate size, greater cellularity, and greater deposition of COL II and proteoglycan (Fig. 2A, B). Semi-quantitative analyses of staining performed on six independent marrow preparations with three aggregates per group per marrow preparation being analyzed indicated an increase of mean toluidine blue and COL II staining densities of 1.5 and 1.3 times greater, respectively, in the Ad.TGF-β1 and low-dose Ad.IGF-1 co-infected aggregates than aggregates transduced with Ad.TGF-β1 alone (Fig. 2A, B). Phenotypic evidence of chondrogenesis was not greater when Ad.IGF-1 was used to co-infect the aggregate cultures at the high dose (2 × 103vp/cell) (Fig. 2A, B).
We used immunohistochemistry to stain for COL X as a marker for chondrocyte hypertrophy. Staining was primarily pericellular in the aggregates infected with Ad.TGF-β1 or co-infected with Ad.TGF-β1 and Ad.IGF-1 (Fig. 2C). Low but detectable immunostaining for COL I was observed in all groups (Fig. 2D); no significant differences were noted between the aggregates.
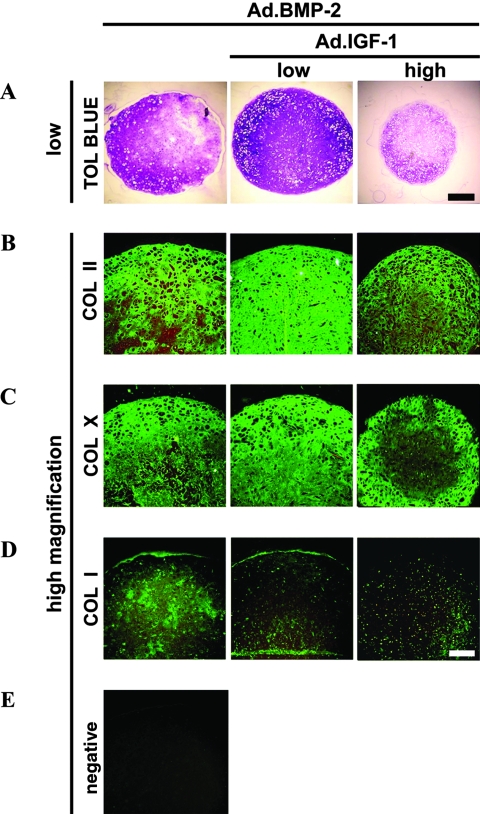
Chondrogenic differentiation of MSCs after adenoviral delivery of BMP-2 and IGF-1
Transduction of MSCs with Ad.BMP-2 using viral doses sufficient to generate between 50 and 150 ng of transgene product at day 3 (Fig. 1B) induced chondrogenesis in aggregate cultures, as demonstrated by strong production of COL II and proteoglycans, as indicated by immunohistochemistry and metachromatic staining with toluidine blue (Fig. 3A, B). In agreement with previous work,26 under these conditions, delivery of BMP-2 resulted in larger aggregates with more-intense staining for proteoglycan and COL II than TGF-β1 (Fig. 2A, B). Aggregates that received Ad.BMP-2 together with Ad.IGF-1 at a low dose (2 × 102 vp/cell) showed significantly greater chondrogenesis than aggregates receiving Ad.BMP-2 alone. Co-delivery of IGF-1 increased aggregate size and cellularity and enhanced deposition of COL II and proteoglycan in these cultures (Fig. 3A, B), with semi-quantitative estimates indicating 1.3 times greater mean toluidine blue and COL II staining densities in the Ad.BMP-2 and low-dose Ad.IGF-1 co-infected aggregates than in aggregates transduced with Ad.BMP-2 alone (n = 3; m = 10). Chondrogenesis was not greater when Ad.IGF-1 was used to co-infect the aggregate cultures at the high dose (2 × 103vp/cell) (Fig. 3A, B).
FIG. 3.
Chondrogenesis of mesenchymal stem cells (MSCs) in aggregate culture after adenoviral-mediated gene transfer of bone morphogenetic protein 2 (BMP-2) alone, and in combination with insulin-like growth factor 1 (IGF-1). Monolayer cultures of MSCs were infected with adenoviral-mediated gene transfer of BMP-2 (Ad.BMP-2) at 2.5 × 102 vp/cell, and certain cultures were co-infected with Ad.IGF-1 at 2.5 × 102 or 2.5 × 103 vp/cell (low and high doses, respectively, as indicated). At 24 h after infection, the cells were seeded into aggregates and cultured for 21 days. Representative sections from a series of three experimental replicates with three pellets per group per replicate are shown. (A) Toluidine blue staining for detection of matrix proteoglycan (50 × magnification, bar = 400μm). (B) Immunostaining for the presence of collagen II (COL II), (C) COL X, and (D) COL I (100 × magnification, bar = 200 μm). (E) Negative control performed with non-immune serum instead of specific primary antibody. Regions of positive staining show green fluorescence. Color images available online at www.liebertonline.com/ten.
In contrast to the TGF-β1–expressing cultures (Fig. 2C), aggregates transduced with Ad.BMP-2 showed more-abundant staining for COL X throughout the extracellular matrix (Fig. 3C); staining was most extensive in aggregates co-infected with low-dose Ad.IGF-1 (Fig. 3C). Correspondingly, the phenotype of the Ad.BMP-2–infected aggregates appeared more hypertrophic in that the cells were rounder with greater cytoplasmic volume (Fig. 3). Positive immunostaining for COL I was observed in all groups (Fig. 3D), but no significant differences could be detected between the aggregates (n = 3; m = 10).
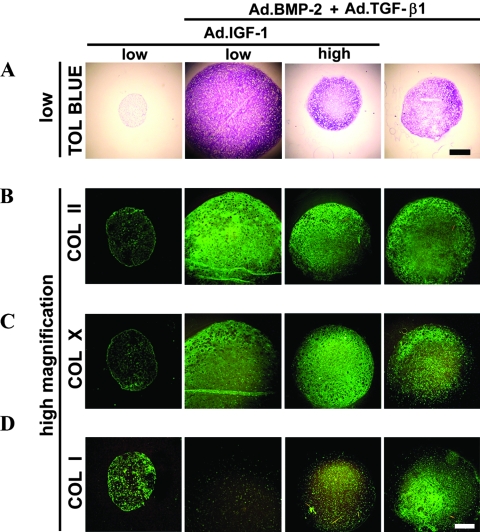
Influence of adenoviral delivery of IGF-1, or TGF-β1 and BMP-2, or TGF-β1 and BMP-2 and IGF-1 on MSC chondrogenesis
Aggregates of cells transduced with Ad.IGF-1 alone (Fig. 4A, B) or Ad.GFP or Ad.Luc as control (data not shown) showed no evidence of chondrogenesis, confirming previous observations.26 Aggregates that received Ad.TGF-β1 together with Ad.BMP-2 at a low dose (2 × 102 vp/cell) showed a chondrogenic phenotype (Fig. 4A, B), but the staining intensities for toluidine blue and COL II were not greater than in aggregates receiving Ad.TGF-β1 (Fig. 2A, B) or Ad.BMP-2 (Fig. 3A, B) alone. However, when IGF-1 was co-delivered with Ad.TGF-β1 and Ad.BMP-2 at a low dose (2 × 102 vp/cell) for each vector, higher levels of chondrogenesis were observed than with any other groups shown in Figure 4 or than the single TGF-β1– (Fig. 2A, B) or BMP-2– (Fig. 3A, B) transduced cultures. Co-delivery of IGF-1 at a low dose together with TGF-β1 and BMP-2 increased deposition of COL II and proteoglycan to levels comparable with the other IGF-1 co-expressing cultures at a low dose approximately 1.3 times for both stains relative to the Ad.TGF-β1 or Ad.BMP-2 only transduced groups (Fig. 2 and 3; n = 3, m = 3-6). In contrast, chondrogenesis was not enhanced when Ad.IGF-1 was used to co-infect the aggregate cultures at the high dose (2 × 103 vp/cell) together with Ad.TGF-β1 and Ad.BMP-2 at 2 × 102 vp/cell for each vector (Fig. 4A, B; n = 3, m = 3-6). Immunostaining for COL X was negative in the Ad.IGF-1 alone (Fig. 4C), or Ad.GFP or Ad.Luc (data not shown) infected aggregate cultures. Staining patterns for COL X in the aggregate cultures receiving Ad.TGF-β1 and Ad.BMP-2, or Ad.TGF-β1 and Ad.BMP-2 and Ad.IGF-1 (Fig 4C) resembled that of the single Ad.BMP-2 infected cultures (Fig. 3C), with abundant staining for COL X throughout the extracellular matrix, and levels were not enhanced compared to these cultures (n = 3; m = 3). Immunostaining for COL I was detectable at low levels in all groups (Fig. 4D).
FIG. 4.
Chondrogenesis of mesenchymal stem cells (MSCs) in aggregate culture after adenoviral-mediated gene transfer of insulin-like growth factor 1 (IGF-1) (Ad.IGF-1) alone, bone morphogenetic protein 2 (BMP-2) and transforming growth factor (TGF-β1), or BMP-2 and TGF-β1 and IGF-1. MSC aggregates modified with Ad.IGF-1 alone at 2.5 × 102 vp/cell (low dose), Ad.BMP-2 and Ad.TGF-β1 at 2.5 × 102 vp/cell, or Ad.BMP-2 and Ad.TGF-β1 at 2.5 × 102 vp/cell together with Ad.IGF-1 at 2.5 × 102 vp/cell (low dose) or 2.5 × 103 vp/cell (high dose) and representative sections after 21 days of culture are shown. (A) Toluidine blue staining for detection of matrix proteoglycan (50x magnification, bar = 400 μm). (B) Immunostaining for the presence of collagen II (COL II), (C) COL X, and (D) COL I (100x magnification, bar = 200 μm). Regions of positive staining show green fluorescence. Color images available online at www.liebertonline.com/ten.
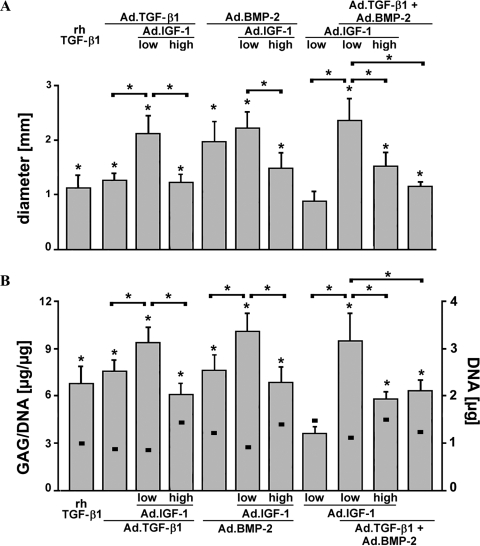
Aggregate size and DNA and GAG synthesis
Aggregate sizes estimated using mean aggregate diameters from three aggregates per group from up to 10 different MSC populations (m = 3-10) are presented in Figure 5A. As reflected in Figure 5A, aggregates co-infected with low-dose Ad.IGF-1 were larger than those transduced to express only a single growth factor. To quantitatively compare extracellular matrix synthesis between treatment groups, GAG levels in the aggregates after 21 days in culture were determined and normalized to DNA content. All aggregates infected with Ad.TGF-β1 or Ad.BMP-2, alone or in combination, showed significantly greater GAG production than those receiving Ad.IGF-1 alone (Fig. 5B), Ad.GFP or Ad.Luc (not shown), aggregates that showed no phenotypic evidence of chondrogenesis. Furthermore, aggregates co-infected with low-dose Ad.IGF-1 and Ad.TGF-β1 or Ad.BMP-2 accumulated significantly more GAG than the other treatment groups (Fig. 5B). These findings correspond with the respective aggregate sizes shown above and correlate with the respective histological and immunohistochemical findings (Fig. 2–4).
FIG. 5.
Aggregate size, DNA, and glycosaminoglycan (GAG) synthesis of genetically modified mesenchymal stem cell (MSC) aggregates. (A) Mean diameters of genetically modified MSC aggregates described in Figures 2-4. (B) GAG content per μg of DNA (bars) was measured for each treatment group after 21 days as described in Materials and Methods. Squares indicate the DNA content values for each group. Diameters and GAG/DNA ratios are expressed as means ± standard deviations with three pellets per group per marrow preparation; m = 3-10 marrow preparations were tested, depending on group and time-point. Asterisks indicate values that were statistically significant for each group compared with the non-chondrogenic adenoviral-mediated gene transfer of IGF-1 group or between groups as indicated (p < 0.05).
Time course of chondrocyte marker gene expression
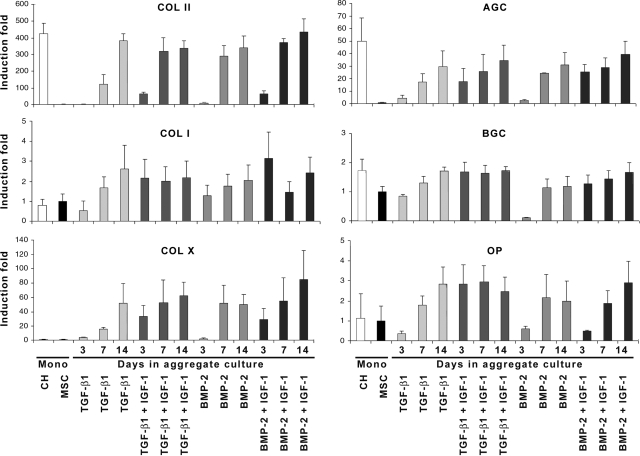
To further examine the apparent synergistic effects of co-delivery of IGF-1 with TGF-β1 or BMP-2 in the aggregate cultures, we analyzed the temporal expression profiles of genes associated with chondrogenic and osteogenic differentiation. As before, aggregates were infected with Ad.TGF-β1 or Ad.BMP-2 with or without low-dose Ad.IGF-1. At days 3, 7, and 14, aggregates (n = 10) from each group were harvested, pooled, and analyzed using RT-PCR (Fig. 6). Monolayer cultures of primary chondrocytes (lane CH) and undifferentiated MSCs (lane MSC) served as controls.
FIG. 6.
Temporal patterns of gene expression in genetically modified mesenchymal stem cell (MSC) aggregate cultures. MSCs were transduced with 2.5 × 102 vp/cell of the respective adenoviral vectors as indicated, seeded into aggregates, and maintained in a defined serum-free medium for 3, 7, or 14 days. For each treatment group and time point indicated, RNA was extracted from 10 aggregates per marrow preparation, and expression of cartilage-specific marker genes was determined according to reverse transcriptase polymerase chain reaction (RT-PCR) from three different marrow preparations (m = 3). RT-PCR products from RNA isolated from primary chondrocyte (CH; lane 1) and primary MSCs (lane 2) cultures were used as comparative controls. The primer sequences, product sizes, and annealing temperatures for collagen II (COL II), aggrecan, biglycan, COL X, osteopontin, and COL I are listed in Table 1. Band intensities of RT-PCR products between groups and time points were normalized using the glyceraldehyde-3-phosphate dehydrogenase reaction products. Values are expressed as fold changes of means ± standard deviations of normalized raw data from band density measurements relative to the MSC controls.
Consistent with the preceding analyses, all of the aggregate cultures tested showed evidence of chondrogenic differentiation at the RNA level. The most notable difference was seen in the early time points, particularly at day 3, when the aggregates receiving Ad.TGF-β1 and Ad.IGF-1 showed earlier onset of expression of COL II and AGC and greater expression of BGC than aggregates transduced with TGF-β1 only(Fig. 6). Correspondingly, at day 3, expression of COL II, AGC, and BGC was greater in the aggregates co-transduced with BMP-2 and IGF-1 than in the cultures infected with BMP-2 only (Fig. 6).
Earlier onset of COL X and OP expression in the aggregates co-expressing IGF-1 and TGF-β1 at day 3, than in the aggregate cultures transduced with TGF-β1 only also reflected evidence of chondrocyte hypertrophy, with greater message at day 7 of culture. In the aggregates co-infected with BMP-2 and IGF-1, COL X expression was markedly greater at day 3 than with MSC aggregate cultures modified with single BMP-2 (Fig. 6). Co-infection with Ad.IGF-1 had no detectable effect on the transcription patterns of the genes analyzed at days 7 or 14. These results suggest that IGF-1 co-expression at low dose accelerates and enhances the process of chondrogenesis but does not delay hypertrophic differentiation.
Discussion
Gene transfer using adenoviral vectors provides a useful tool to examine and screen the chondrogenic potential of candidate transgenes in vitro, before their evaluation in experimental animals in vivo.21 We and others have shown previously that primary MSCs undergo chondrogenesis after genetic modification with Ad.BMP-2 or Ad.TGF-β1 in aggregate culture in vitro.26,27,34,35 Furthermore the BMP-2, TGF-β1, or IGF-1 transgenes stimulated cartilage repair when genetically modified cells were transplanted into chondral defects in vivo.36–38 In the present study, we adapted the MSC aggregate culture system to determine whether adenoviral delivery of multiple growth factor genes can lead to greater chondrogenesis in vitro.
We found that co-delivery of a low dose of Ad.IGF-1, capable of generating between 50 and 100 ng/mL of transgene expression at day 3 post-infection, accelerated and enhanced the chondrogenic response of MSCs to infection with Ad.TGF-β1, Ad.BMP-2, or both. This is consistent with the literature, with synergistic effects on chondrogenesis having been reported for IGF-1 and TGF-β1 in chondrocytes,14,37–40 MSCs14,28 and periosteal cells29 but also when IGF-1 is co-administered with other members of the TGF-β superfamily.39,41–43 In our experiments co-delivery of Ad.IGF-1 at low doses with Ad.TGF-β1, Ad.BMP-2, or both resulted in prolonged expression of the respective transgenes (Fig. 1D, E, J). Currently we have no clear explanation for this observation, because TGF-β1 and IGF-1 are both known to increase the proliferation rate of MSCs, but because the non-integrating adenoviral genomes have no mechanism to resegregate to daughter nuclei after mitosis, it is somewhat surprising that transgene expression would show greater persistence in the presence of stimulation from both growth factors. Temporal examination of endogenous gene expression patterns showed that IGF-1 co-expression also led to earlier transcription of genes associated with chondrocyte differentiation. However, this study was limited to the use of semi-quantitative PCR, and thus conclusions from gene expression analyses cannot be drawn with complete assurance.
At this point, we cannot determine whether the enhanced chondrogenic response is due to the apparent earlier onset of differentiation or the prolonged production of chondrogenic growth factors; it is most likely that both are important.29 Nonetheless, toward the long-term goal of effecting cartilage repair, these results clearly support the sustained co-administration of IGF-1 with TGF-β1, BMP-2, or both.
Our results differ somewhat from those reported by Hanada et al.,16 who found greater chondrogenesis and modulation of hypertrophy when MSC aggregates were cultured in the presence of recombinant TGF-β1 and BMP-2. We saw no evidence of greater chondrogenesis or additive effects from combined delivery of Ad.TGF-β1 and Ad.BMP-2. It is likely that this discrepancy is due to the different method of protein delivery, species, and tissue source of MSCs used. In our previous work, we found that gene transfer provided greater potency of growth factor activity than equivalent levels of recombinant protein.26 Furthermore, in the present study, we used conditions that provided maximal chondrogenic activity for each vector individually. Because intracellular signal transduction from TGF-β1 and BMP-2 receptor binding occurs via overlapping SMAD signalling pathways, it is likely that the chondroinductive activities of each were maximally activated individually such that an additional related stimulus had little observable effect. Cell signalling for IGF-1, however, follows different pathways involving the phosphoinositide 3-kinase and extracellular-signal-regulated kinase cascades and activates different subsets of genes and intracellular proteins.44 With respect to cartilage, IGF-1 activity is most frequently associated with chondrocyte proliferation and enhanced proteoglycan synthesis.29 Thus, with regard to the data presented here, it appears that the TGF-β superfamily members, TGF-β1 and BMP-2, provide the primary chondroinductive stimulus that IGF-1 in turn complements and enhances.
Consistent with our earlier data, co-administration of high doses of Ad.IGF-1 (2.5 × 103 vp/cell) with Ad.TGF-β1 or Ad.BMP2 showed no synergistic chondrogenic effects in the MSC aggregates (Fig. 2–4). We found previously that adenoviral infection was progressively inhibitory to chondrogenesis at doses exceeding 1000 vp/cell;26 thus, we attribute the decreased chondrogenesis to excessive viral load. This is in agreement with findings of Kawamura et al.35 who reported dose-dependent inhibition of chondrogenesis of marrow-derived primary MSCs in aggregate cultures when IGF-1 and TGF-β1 were co-delivered at high doses via adenoviral vectors.35
Although IGF-1 increased the amplitude of the response of the MSCs to TGF-β1 and BMP-2, increasing the cellularity and matrix synthesis, it did not appear to alter the morphology of the cells in the respective cultures or the endpoint composition of the aggregate matrix at 21 days. For example, we found COL X message in all aggregates stimulated with TGF-β1 or BMP-2. However, the aggregates receiving TGF-β1 showed only limited pericellular COL X protein according to immunohistochemistry, and morphologically, the cells in the matrix showed little evidence of hypertrophy (Fig. 2). The presence of IGF-1 did not affect this overall pattern. Chondrogenesis induced by BMP-2, with and without IGF-1, was more consistent with end-stage differentiation; a large percentage of the cells in the aggregates displayed a hypertrophic phenotype, and the extracellular matrix throughout showed robust immunostaining for COL X (Fig. 3). This suggests that co-expression of low-dose Ad.IGF-1 does not inhibit the progression or extent of chondrocytic differentiation induced by the respective growth factors. It has been argued that the detection of COL X mRNA early during MSC aggregate culture might limit its use as a marker for chondrogenic hypertrophy,45–47 and to a large degree our findings agree with this. From our studies, it appears that the best indicators of hypertrophy are cellular morphology and matrix composition; simple transcription of COL X is not a strong predictor of chondrocytic differentiation or hypertrophy.
Because IGF-1,36,37 BMP-2,36,48 and TGF-β138 have each been used successfully in animal models of tissue repair, the purpose of the present study was to explore methods to enhance chondrogenesis of primary MSCs using simultaneous delivery of these transgenes. Although the information gained in this study provided insight into the interactions between MSCs and adenoviral vectors and the chondrogenic effects of different transgenes alone and in combination in vitro, further in vivo work is required to determine its relevance to cartilage repair and regeneration. Gene-induced chondrogenesis of MSCs using multiple genes that act synergistically may enable the administration of lower viral doses in vivo and facilitate genetically expedited cartilage tissue-engineering approaches49 and could thus improve the outcome of gene- and cell-based therapies for cartilage repair.
Acknowledgments
The work was performed during a 2-year postdoctoral fellowship of A.F.S from 2002 to 2004 in the lab of C.H.E. supported by Deutsche Forschungsgemeinschaft (DFG) grant STE 1051/1-1. This work was supported by Grants AR48566 and AR50249 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to S.C.G. and Grant STE 1051/2-1 to A.F.S and U.N. from the DFG. We are grateful to Gary Gibson, Ph.D., Henry Ford Hospital, Detroit, Michigan, for kindly providing COL X antibodies.
References
- 1.Steinert A.F. Ghivizzani S.C. Rethwilm A. Tuan R.S. Evans C.H. Noth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nesic D. Whiteside R. Brittberg M. Wendt D. Martin I. Mainil-Varlet P. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev. 2006;58:300. doi: 10.1016/j.addr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Kuo C.K. Li W.J. Mauck R.L. Tuan R.S. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64. doi: 10.1097/01.bor.0000198005.88568.df. [DOI] [PubMed] [Google Scholar]
- 4.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Caplan A.I. Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 7.Noth U. Osyczka A.M. Tuli R. Hickok N.J. Danielson K.G. Tuan R.S. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060. doi: 10.1016/S0736-0266(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 8.Zuk P.A. Zhu M. Mizuno H. Huang J. Futrell J.W. Katz A.J. Benhaim P. Lorenz H.P. Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Chen F.H. Rousche K.T. Tuan R.S. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 10.Goldring M.B. Tsuchimochi K. Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33. doi: 10.1002/jcb.20652. [DOI] [PubMed] [Google Scholar]
- 11.Steinert A. Weber M. Dimmler A. Julius C. Schutze N. Noth U. Cramer H. Eulert J. Zimmermann U. Hendrich C. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21:1090. doi: 10.1016/S0736-0266(03)00100-1. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 13.Nixon A.J. Brower-Toland B.D. Bent S.J. Saxer R.A. Wilke M.J. Robbins P.D. Evans C.H. Insulinlike growth factor-I gene therapy applications for cartilage repair. Clin Orthop Relat Res. 2000;379:S201. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 14.Worster A.A. Brower-Toland B.D. Fortier L.A. Bent S.J. Williams J. Nixon A.J. Chondrocytic differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-beta1 in monolayer and insulin-like growth factor-I in a three-dimensional matrix. J Orthop Res. 2001;19:738. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 15.Sekiya I. Colter D.C. Prockop D.J. BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun. 2001;284:411. doi: 10.1006/bbrc.2001.4898. [DOI] [PubMed] [Google Scholar]
- 16.Hanada K. Solchaga L.A. Caplan A.I. Hering T.M. Goldberg V.M. Yoo J.U. Johnstone B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81:284. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Barry F. Boynton R.E. Liu B. Murphy J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 18.Noth U. Rackwitz L. Heymer A. Weber M. Baumann B. Steinert A. Schutze N. Jakob F. Eulert J. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626. doi: 10.1002/jbm.a.31254. [DOI] [PubMed] [Google Scholar]
- 19.Mackay A.M. Beck S.C. Murphy J.M. Barry F.P. Chichester C.O. Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 20.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 21.Trippel S.B. Ghivizzani S.C. Nixon A.J. Gene-based approaches for the repair of articular cartilage. Gene Ther. 2004;11:351. doi: 10.1038/sj.gt.3302201. [DOI] [PubMed] [Google Scholar]
- 22.Evans C.H. Robbins P.D. Ghivizzani S.C. Wasko M.C. Tomaino M.M. Kang R. Muzzonigro T.A. Vogt M. Elder E.M. Whiteside T.L. Watkins S.C. Herndon J.H. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005;102:8698. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelse K. Schneider H. Ex vivo gene therapy approaches to cartilage repair. Adv Drug Deliv Rev. 2006;58:259. doi: 10.1016/j.addr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Cucchiarini M. Madry H. Gene therapy for cartilage defects. J Gene Med. 2005;7:1495. doi: 10.1002/jgm.824. [DOI] [PubMed] [Google Scholar]
- 25.Steinert A.F. Nöth U. Tuan R.S. Concepts in gene therapy for cartilage repair. Injury. 2008;39S1:97. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer G.D. Steinert A.F. Pascher A. Gouze E. Gouze J.N. Pilapil C. Betz O. Johnstone B. Evans C.H. Ghivizzani S.C. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Therapy. 2005;12:219. doi: 10.1016/j.ymthe.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Steinert A.F. Palmer G.D. Capito R. Hofstaetter J.G. Pilapil C. Ghivizzani S.C. Spector M. Evans C.H. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta1 complementary deoxyribonucleic acid. Tissue Eng. 2007;13:2227. doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 28.Longobardi L. O'Rear L. Aakula S. Johnstone B. Shimer K. Chytil A. Horton W.A. Moses H.L. Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21:626. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 29.Fukumoto T. Sperling J.W. Sanyal A. Fitzsimmons J.S. Reinholz G.G. Conover C.A. O'Driscoll S.W. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis Cartilage. 2003;11:55. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 30.Hardy S. Kitamura M. Harris-Stansil T. Dai Y. Phipps M.L. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71:1842. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer G.D. Gouze E. Gouze J.N. Betz O.B. Evans C.H. Ghivizzani S.C. Gene transfer to articular chondrocytes with recombinant adenovirus. Methods Mol Biol. 2003;215:235. doi: 10.1385/1-59259-345-3:235. [DOI] [PubMed] [Google Scholar]
- 32.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 33.Chomczynski P. Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 34.Park J. Gelse K. Frank S. von der Mark K. Aigner T. Schneider H. Transgene-activated mesenchymal cells for articular cartilage repair: a comparison of primary bone marrow-, perichondrium/periosteum- and fat-derived cells. J Gene Med. 2006;8:112. doi: 10.1002/jgm.826. [DOI] [PubMed] [Google Scholar]
- 35.Kawamura K. Chu C.R. Sobajima S. Robbins P.D. Fu F.H. Izzo N.J. Niyibizi C. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exp Hematol. 2005;33:865. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelse K. von der Mark K. Aigner T. Park J. Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- 37.Madry H. Kaul G. Cucchiarini M. Stein U. Zurakowski D. Remberger K. Menger M.D. Kohn D. Trippel S.B. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;15:1171. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 38.Pagnotto M.R. Wang Z. Karpie J.C. Ferretti M. Xiao X. Chu C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 39.Mizuta H. Sanyal A. Fukumoto T. Fitzsimmons J.S. Matsui N. Bolander M.E. Oursler M.J. O'Driscoll S.W. The spatiotemporal expression of TGF-beta1 and its receptors during periosteal chondrogenesis in vitro. J Orthop Res. 2002;20:562. doi: 10.1016/S0736-0266(01)00130-9. [DOI] [PubMed] [Google Scholar]
- 40.Nixon A.J. Saxer R.A. Brower-Toland B.D. Exogenous insulin-like growth factor-I stimulates an autoinductive IGF-I autocrine/paracrine response in chondrocytes. J Orthop Res. 2001;19:26. doi: 10.1016/S0736-0266(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 41.Smith P. Shuler F.D. Georgescu H.I. Ghivizzani S.C. Johnstone B. Niyibizi C. Robbins P.D. Evans C.H. Genetic enhancement of matrix synthesis by articular chondrocytes: comparison of different growth factor genes in the presence and absence of interleukin-1. Arthritis Rheum. 2000;43:1156. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Yaeger P.C. Masi T.L. de Ortiz J.L. Binette F. Tubo R. McPherson J.M. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 43.van Osch G.J. van den Berg W.B. Hunziker E.B. Hauselmann H.J. Differential effects of IGF-1 and TGF beta-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthritis Cartilage. 1998;6:187. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 44.Indrawattana N. Chen G. Tadokoro M. Shann L.H. Ohgushi H. Tateishi T. Tanaka J. Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320:914. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Loeser R.F. Pacione C.A. Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 46.DeLise A.M. Fischer L. Tuan R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 47.Mwale F. Stachura D. Roughley P. Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- 48.Zachos T. Diggs A. Weisbrode S. Bartlett J. Bertone A. Mesenchymal stem cell-mediated gene delivery of bone morphogenetic protein-2 in an articular fracture model. Mol Ther. 2007;15:1543. doi: 10.1038/sj.mt.6300192. [DOI] [PubMed] [Google Scholar]
- 49.Evans C.H. Palmer G.D. Pascher A. Porter R. Kwong F.N. Gouze E. Gouze J.N. Liu F. Steinert A. Betz O. Betz V. Vrahas M. Ghivizzani S.C. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 50.Hunter C.J. Imler S.M. Malaviya P. Nerem R.M. Levenston M.E. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials. 2002;23:1249. doi: 10.1016/s0142-9612(01)00245-9. [DOI] [PubMed] [Google Scholar]
- 51.Rees S.G. Flannery C.R. Little C.B. Hughes C.E. Caterson B. Dent C.M. Catabolism of aggrecan, decorin and biglycan in tendon. Biochem J. 2000;350:181. [PMC free article] [PubMed] [Google Scholar]
- 52.Gruber R. Mayer C. Bobacz K. Krauth M.T. Graninger W. Luyten F.P. Erlacher L. Effects of cartilage-derived morphogenetic proteins and osteogenic protein-1 on osteochondrogenic differentiation of periosteum-derived cells. Endocrinology. 2001;142:2087. doi: 10.1210/endo.142.5.8163. [DOI] [PubMed] [Google Scholar]
- 53.van Susante J.L.C. Pieper J. Buma P. van Kuppevelt T.H. van Beuningen H. van Der Kraan P.M. Veerkamp J.H. van den Berg W.B. Veth R.P.H. Linkage of chondroitin-sulfate to type I collagen scaffolds stimulates the bioactivity of seeded chondrocytes in vitro. Biomaterials. 2001;22:2359. doi: 10.1016/s0142-9612(00)00423-3. [DOI] [PubMed] [Google Scholar]