Abstract
Cardiomyocyte (CM) transplantation is one therapeutic option for cardiac repair. Studies suggest that fetal CMs display the best cell type for cardiac repair, which can finitely proliferate, integrate with injured host myocardium, and restore cardiac function. We have recently developed an engineered early embryonic cardiac tissue (EEECT) using embryonic cardiac cells and have shown that EEECT contractile properties and cellular proliferative response to cyclic mechanical stretch stimulation mimic developing fetal myocardium. However, it remains unknown whether cyclic mechanical stretch–mediated high cellular proliferation activity within EEECT reflects CM or non-CM population. Studies have shown that p38-mitogen-activated protein kinase (p38MAPK) plays an important role in both cyclic mechanical stretch stimulation and cellular proliferation. Therefore, in the present study, we tested the hypothesis that cyclic mechanical stretch (0.5 Hz, 5% strain for 48 h) specifically increases EEECT CM proliferation mediated by p38MAPK activity. Cyclic mechanical stretch increased CM, but not non-CM, proliferation and increased p38MAPK phosphorylation. Treatment of EEECT with the p38MAPK inhibitor, SB202190, reduced CM proliferation. The negative CM proliferation effects of SB202190 were not reversed by concurrent stretch stimulation. Results suggest that immature CM proliferation within EEECT can be positively regulated by mechanical stretch and negatively regulated by p38MAPK inhibition.
Introduction
The developing fetal myocardium has a high cell proliferation rate, which rapidly declines following birth.1,2 The loss of cardiomyocyte (CM) proliferation in the postnatal myocardium is the major barrier to myocardial regeneration following injury in higher vertebrates. Current treatment options for heart failure include medical management, cardiac transplantation, mechanical circulatory left ventricular assist devices, and other experimental surgical approaches, which all have both short- and long-term limitations.3 The limited efficacy and high morbidity of these current treatment approaches make the cardiovascular system an obvious target for tissue engineering.
Cellular cardiomyoplasty, utilizing a wide range of cell types, is now under intense investigation with the goal of regenerating functioning myocardium. Several approaches to increase the number of CMs within injured myocardium have been attempted, including (1) activation and stimulation of host myocardial regeneration by transplanted cells via angiogenic and/or paracrine effects,4,5 (2) direct transplantation of functional CMs or myogenic cells,6–8 and (3) implantation of tissue constructs containing CMs.9,10 In the latter two cases, the ideal donor CMs possess a high potential for division during cell preparation and potential for further proliferation following implantation to form a viable myocardial tissue within the surrounding injured recipient myocardium. Stem cell–derived CMs are considered as an alternative cell source for cardiac repair. However, the potential for CM proliferation must not include neoplastic or teratogenic potential, as has been noted for some multipotent stem cells.11,12
Studies suggest that fetal, finitely proliferating CMs display the best cell survival, functional integration, and sustained cardiac recovery and that three-dimensional (3D) in vitro culture conditions may be optimal for donor CM preparation.9,13–15 Tissue-engineered cardiac tissue constructs provide the necessary 3D environment for efficient cell survival, functional integration, and sustained cardiac recovery.9,10 We have recently developed a 3D engineered early embryonic cardiac tissue (EEECT) with similar cell proliferative activity and contractile properties to native developing fetal myocardium.16 Mechanical stretch stimulation of EEECT induces increased cellular proliferation, not cellular hypertrophy, and increases contractile function mimicking the adaptive fetal myocardium.16 However, it remained unclear which cellular subpopulation, CMs or non-CMs, responds to the cyclic mechanical stretch within this in vitro–engineered cardiac construct.
Numerous signaling pathways alone, or in combination with growth factors and/or environmental factors, have been investigated to identify the regulation of CM proliferation.17 p38-mitogen-activated protein kinase (p38MAPK) is a highly conserved signal transduction molecule that mediates extracellular signals to a variety of intracellular responses. p38MAPK has been extensively studied in postnatal CM growth (hypertrophy) and survival.7,18–20 Recent studies have also shown that p38MAPK plays a key role in CM proliferation in both developing fetal and postnatal myocardium.19,21–23 Therefore, the purpose of the present study was to elucidate the factors that regulate the proliferation activity of immature CMs within an in vitro–engineered cardiac tissue construct independent of the non-CM population proliferation activity. We tested the hypothesis that cyclic mechanical stretch increases specifically CM proliferation within EEECT and the inhibition of p38MAPK activity decreases CM proliferation.
Materials and Methods
EEECT construction
Fertile White Leghorn chicken eggs (Utah State University, Logan, Utah) were incubated in a forced-draft, constant-humidity incubator until Hamburger–Hamilton stage 31.24 Cells isolated from 10 to 15 embryonic ventricles were used to construct each individual EEECT.25 Our research protocols conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised 1985).
Embryonic ventricles were excised and enzymatically digested using 3 mg/mL collagenase type II in 1× PBS, with an average of 295 Collagen Digestion Units per mg, (Invitrogen, Carlsbad, CA) followed by 0.05% trypsin-EDTA solution, and then isolated cells were preplated for 1 h and floating cells were then cultured on a gyratory shaker (60 rpm) for 24 h to reaggregate viable CMs. Approximately 5.0 × 105 cells were mixed with acid-soluble rat-tail collagen type I (Sigma, St. Louis, MO) and matrix factors (Matrigel; BD Biosciences, Franklin Lakes, NJ) to generate a single EEECT.16 Briefly, isolated cells were suspended within a culture medium (modified Dulbecco's essential medium; Invitrogen) containing 20% FBS (Invitrogen). Acid-soluble collagen type I solution (pH 3) was neutralized with alkali buffer (0.2 M NaHCO3, 0.2 M HEPES, and 0.1 M NaOH) on ice. Matrigel (17% of total volume; BD Biosciences) was then added, and the cell suspension and matrix solution were mixed to reach a final collagen type I concentration of 0.67 mg/mL. Cylindrical-shaped EEECTs were constructed using collagen type I–coated silicone membrane culture plates (Tissue Train; Flexcell International, Hillsborough, NC) and FX-4000TT system (Flexcell International) as follows: (1) the center of the silicone membrane of a Tissue Train culture plate was deformed by vacuum pressure to form a 20-mm-length × 2-mm-width trough using a cylindrical loading post (Tissue Train and FX-4000TT); (2) approximately 200 μL of cell/matrix mixture was poured into the trough and then incubated for 120 min in a standard CO2 incubator (37°C, 5% CO2) to form a cylindrical-shaped construct. Both ends of the construct were held by anchors attached to the Tissue Train culture plate. When the tissue was formed, the culture plate was filled with a growth medium containing 10% FBS and 1% chick embryo extract (SLI, Horsted Keynes, UK). The vacuum pressure was then gradually released, allowing the construct to float within the culture plate. Growth medium was exchanged every 48 h.
Mechanical stretch stimulation and p38MAPK inhibition
To determine the effect of cyclic mechanical stretch and/or p38MAPK inhibition on EEECT cell proliferation, contractile force, and p38MAPK and Akt phosphorylation, we exposed culture day-10 EEECT to uniaxial cyclic mechanical stretch (0.5 Hz and 5% elongation); inhibition of p38MAPK with a selective inhibitor, SB202190 (10 μM); or concurrent p38MAPK inhibition (SB202190) with cyclic mechanical stretch. In the preliminary study, we found that 0.5 Hz and 5% strain of cyclic stretch increased both EEECT contractile force generation and cellular proliferation activity with minimal technical EEECT loss due to detachment of the tissue from anchors of the culture plate. We performed cyclic mechanical stretch stimulation or p38MAPK inhibition for 2 h or 48 h beginning on culture day 10. For 48-h p38MAPK inhibition, we treated EEECTs with SB202190-containing culture medium, and the culture medium was changed with fresh SB-containing culture medium at 24 h after initial treatment. For cyclic mechanical stretch stimulation, we also changed culture medium at 24 h after the beginning of cyclic mechanical stretch stimulation.
CM and non-CM proliferation assays
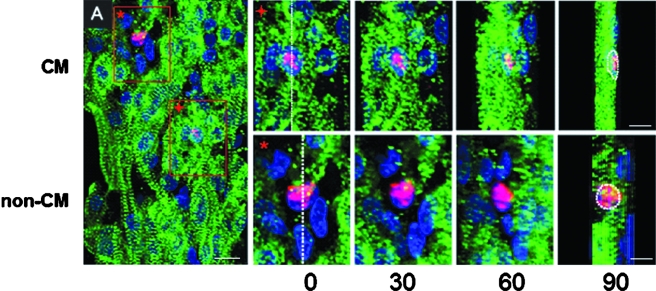
Each EEECT was fixed with 4% paraformaldehyde/PBS for 15 min. For bromodeoxyuridine (BrdU) staining, each tissue was incubated with 60 μg/mL BrdU (Sigma) for 16 h prior to fixation.16 Samples were then embedded in a 13% polyacrylamide gel oriented in the longitudinal direction, and 150-μm-thickness serial sections were made using a standard vibrating microtome (Vibratome-1000; Vibratome.com, St. Louis, MO).16 Sections were permeabilized with 0.1% Triton X-100 for 30 min and stained for BrdU using an Alexa Fluor 594–conjugated mouse-monoclonal anti-BrdU antibody (Invitrogen) and DAPI (Vector Laboratories, Burlingame, CA). To differentiate between proliferating CMs and non-CMs within EEECT, we used phospho-histone H3 (ser10; Upstate Cell Signaling Solutions, Temecula, CA) and Alexa Fluor 594 secondary antibody (Invitrogen) staining to detect cells undergoing mitosis while also counterstaining for CM phenotype with α-sarcomeric actinin (EA53; Sigma) and Alexa Flour 488 secondary antibody (Invitrogen). We reconstructed 3D projection images from stacks of z-axis optical scans (1-μm scanning interval, up to 50-μm thickness) using a standard laser confocal microscope system (FV1000; Olympus, Tokyo, Japan) and Scion Image software (Scion, Fredrick, MD).16 The reconstructed composite 3D projection images were visualized at different rotation angles to identify proliferating CMs by colocalization of phospho-histone H3–positive nuclei staining and α-sarcomeric actinin staining. Overlapping positive histone-H3 and α-sarcomeric actinin staining in all views confirmed CM phenotype (Fig. 1). To quantify total cellular and CM proliferation activities, 10–20 regions from 3D composite images from each EEECT were randomly chosen for analysis. Cellular proliferation and CM proliferation rates were calculated as: [BrdU (+) nuclei]/[DAPI (+) nuclei] (%) and [histone H3 (+) nuclei within α-sarcomeric actinin (+) cells]/[DAPI (+) nuclei] (%), respectively. We also calculated CM cell ratio (%) by [DAPI (+) nuclei with α-sarcomeric actinin (+) cells]/[DAPI (+) nuclei] (%).
FIG. 1.
Identification of phospho-histone–positive CMs and non-CMs within EEECT. EEECTs were stained for α-sarcomeric actinin (green), phospho-histone H3 (pink), and DAPI (blue). 3D projection images were reconstructed using a laser confocal microscopy. Scale bar indicates 10 μm in panel (A). Red-boxed areas in panel (A) are shown at a higher magnification, and histone-positive nuclei (+; CM and *; non-CM) were visualized at several different rotation angles. At 0° rotation, the nuclei were sliced in half (white dotted line) and were visualized at 90° rotation angle. A proliferating CM was identified as a histone-positive nucleus surrounded by sarcomere (upper panel). Non-CMs were not surrounded by sarcomere staining in all views (lower panel). Scale bars indicate 5 μm in higher-magnification views.
SDS-PAGE western blot and ELISA assays
Whole cell lysates were prepared from EEECT and separated by SDS-PAGE (7.5% separating gel; Bio-Rad Laboratories). Immunoblotting was carried out using routine protocols. Each lane contained 20 μg of total protein. Mouse monoclonal β-actin antibody (Abcam, Cambridge, MA), mouse monoclonal anti-p38MAPK activated (diphosphorylated p38) antibody (Sigma), and mouse monoclonal anti-p38MAPK nonactivated antibody (Sigma), and mouse monoclonal α-sarcomeric actinin primary antibody (EA53; Sigma) were visualized with IR-Dye 800 donkey anti-mouse secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). Rabbit monoclonal phospho-Akt antibody (Millipore, Billerica, MA) was visualized with IR-680 donkey anti-rabbit secondary antibody (Rockland Immunochemicals). All proteins were visualized using an infrared western blot imaging system (Odyssey; LI-COR Biosciences, Lincoln, NE). Immunoblots were quantified using densitometry, and an expression ratio was calculated (Scion Image; Scion). Standard ELISA protocols from phospho-p38α-MAPK sandwich ELISA kit (Millipore) and Akt [pS473] ELISA kit (Invitrogen) were used to assay p38MAPK and Akt phosphorylation. We pooled three to four EEECTs for a single western blot or ELISA assay, and all western blot and ELISA assays were completed in triplicate (total 9–12 EEECTs were used per experimental group).
Contractile force measurement
EEECT passive and active forces were recorded as previously described.16 In brief, each EEECT was excised and transferred to a cold (25°C) calcium-free Ringer solution containing (in mM) 135.0 NaCl, 4.0 KCl, 10.0 Trizma-HCl, 8.3 Trizma-base, and 11.0 glucose and gassed with 95% O2/5% CO2 (pH 7.4). One end of the EEECT was attached using 10-0 monofilament nylon sutures to a force transducer (model 403A; Aurora Scientific, Ontario, Canada) and the other end to a rigid stainless bar mounted on a micromanipulator. The perfusion chamber containing the construct was then filled with warmed buffer (37°C, 2 cc total volume) and perfused at 1 mL/min with 2 mM Ca2+ Ringer solution. The construct was field stimulated (1 Hz, 4 ms, and 70–100 V) using a stimulator (Harvard Apparatus, Holliston, MA). EEECT length was increased stepwise in 5% increments up to a 15% elongation from original length or until total force reached maximum (Lmax). EEECT external diameters were recorded at each stretch increment using a video microscopy system (Model KPD-50; Hitachi, Tokyo, Japan, and PowerMac 8600 system; Apple Inc., Cupertino, CA), and cross-sectional area (mm2) was calculated assuming cylindrical geometry. Force (mg) was normalized by each specimen's adjusted cross-sectional area to yield active stress (mN/mm2).
Statistical analysis
Data are expressed as mean ± SE. One-factor analysis of variance (ANOVA) with Tukey post hoc test was performed to compare the p38MAPK and Akt activities after 2- and 48-h stimulation. Two-factor ANOVA with a Tukey test was performed to compare the BrdU-positive ratio, the phospho-histone H3–positive ratio, the p38-MAPK or Akt kinase phosphorylation ratios, and α-actinin/β-actin expression ratios. Two-factor repeated ANOVA with a Tukey test was performed to compare the active contractile force. Statistical significance was defined by a value of p < 0.05. All calculations were performed using SigmaStat (Systat Software, Point Richmond, CA).
Results
Effects of cyclic mechanical stretch and p38MAPK inhibition on EEECT CM and non-CM proliferation activity
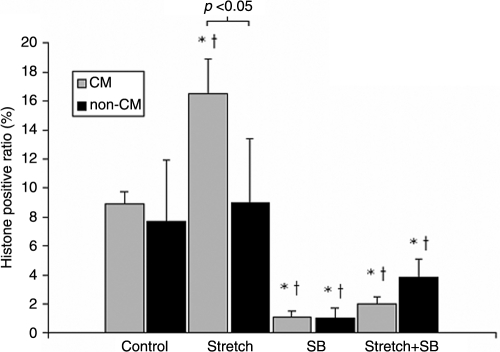
Culture day-12 EEECT, which developmentally corresponds to late fetal chick myocardium with the cumulative age of 19 incubation days of 21 incubation days (hatching), displayed a high BrdU-positive ratio comparable to native embryonic myocardium, and cyclic mechanical stretch further increased the BrdU-positive ratio (Table 1). EEECT CM cell ratio also increased in response to cyclic mechanical stretch [75.3 ± 3.4% (stretch, n = 3) vs. 63.9 ± 7.0% (control, n = 3), p < 0.05]. These results are consistent with our previous study.16 Both p38MAPK inhibition, SB202190 treatment, and concurrent p38MAPK inhibition and mechanical stretch stimulation decreased the EEECT BrdU-positive ratio (Table 1). Phospho-histone–positive ratios followed similar trends to the BrdU-positive ratios (Table 1 and Fig. 2). Notably, the increase in histone-positive cell ratio following cyclic mechanical stretch occurred only in the CM fraction but not in the non-CM fraction. In contrast, p38MAPK inhibition, SB202190 treatment, decreased both the CM and non-CM phospho-histone–positive ratios. Concurrent p38MAPK inhibition and mechanical stretch stimulation also decreased the CM and non-CM phospho-histone–positive ratios, similar to p38MAPK inhibition alone. Thus, the negative CM proliferation effect of SB202190 treatment was not reversed by concurrent cyclic mechanical stretch stimulation.
Table 1.
Effects of Cyclic Mechanical Stretch and p38MAPK Inhibition on Engineered Early Embryonic Cardiac Tissue Cardiomyocyte and Non-Cardiomyocyte Proliferation
| Control | Stretch | SB | Stretch + SB | |
|---|---|---|---|---|
| BrdU-positive ratio (%) | 22.2 ± 2.4% (n = 4) | 40.7 ± 1.9% (n = 4)a | 14.8 ± 2.1% (n = 4)a | 16.2 ± 2.1% (n = 4)a,b |
| Histone-positive ratio (%) CM | 8.9 ± 0.9% (n = 4) | 16.5 ± 2.4% (n = 4)c,d,e | 1.1 ± 0.4% (n = 3)d,e | 2.0 ± 0.5% (n = 3)d,e |
| Non-CM | 7.7 ± 4.2% (n = 4) | 9.0 ± 4.4% (n = 4) | 1.0 ± 0.6% (n = 3)d,e | 3.8 ± 1.2% (n = 3)d,e |
Data are mean ± SE. n: number of samples.
p < 0.05 versus control EEECT within group.
p < 0.05 versus stretch EEECT.
p < 0.05 versus stretch non-CM fraction.
p < 0.05 versus control EEECT CM fraction.
p < 0.05 versus control EEECT non-CM fraction.
SB: p38MAPK inhibition, SB202190 treatment; Stretch: uniaxial cyclic mechanical stretch (0.5 Hz, 5% elongation); Stretch + SB: concurrent cyclic mechanical stretch and p38MAPK inhibition (SB202190).
FIG. 2.
Quantification of CM and non-CM histone H3–positive fractions in each group. *p < 0.05 versus control EEECT CM fraction. †p < 0.05 versus control EEECT non-CM fraction.
Effects of cyclic mechanical stretch and SB202190 treatment on p38MAPK and Akt activities
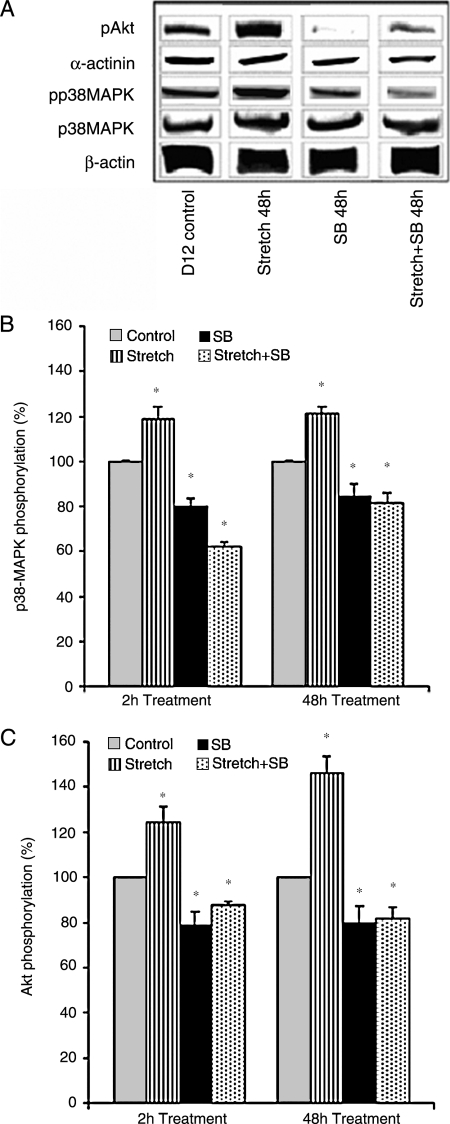
Western blots indicated that total p38MAPK and α-actinin (normalized by β-actin) expression did not change in response to 48-h cyclic mechanical stretch stimulation suggesting CM proliferation (Fig. 3A). ELISA analysis showed that both p38MAPK and Akt phosphorylation levels increased following 2-h cyclic mechanical stretch and decreased following 2-h SB202190 treatment or concomitant SB202190 treatment with cyclic mechanical stretch (Fig. 3B, C) at which EEECT contractile force and CM proliferation rate did not change from control EEECT (data not shown). Phosphorylated p38MAPK and Akt consistently increased following 48-h cyclic mechanical stretch and decreased following 48-h SB202190 treatment or concomitant SB202190 treatment with cyclic mechanical stretch (Fig. 3B, C).
FIG. 3.
Representative western blots and ELISA analysis of EEECT p38MAPK and Akt expression following mechanical stretch stimulation and/or p38MAPK inhibition. Stimulation or inhibition did not change β-actin, α-actinin, or p38MAPK nonphosphorylated expression. Stretch stimulation increased EEECT phosphorylated p38MAPK and phosphorylated Akt expression, while inhibition and concurrent stretch and inhibition decreased phosphorylated p38MAPK and phosphorylated Akt expression (A). Phosphorylated p38MAPK and Akt expression increased following 2 and 48 h of cyclic stretch, decreased following 2 and 48 h of p38MAPK inhibition, and decreased following 2 and 48 h of concurrent stretch and p38MAPK inhibition (B, C). *p < 0.05 versus normalized control EEECT expression.
EEECT contractile force
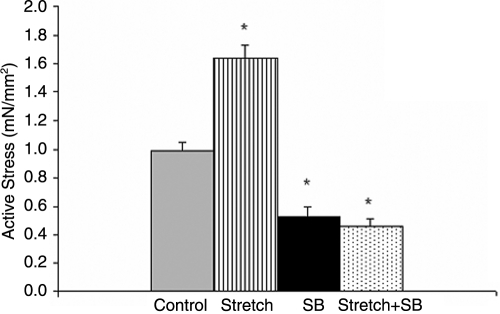
All EEECT specimens displayed a positive Frank-Starling response to increased construct length (p < 0.05). Cyclic mechanical stretch stimulation for 48 h increased EEECT active stress at Lmax [1.64 ± 0.09 mN/mm2 (n = 10), p = 0.017] while p38MAPK inhibition reduced active stress [0.53 ± 0.07 mN/mm2 (n = 8), p = 0.04], as did the combination of p38MAPK inhibition and mechanical stretch [0.46 ± 0.05 mN/mm2 (n = 8), p = 0.025] compared to control EEECTs [1.00 ± 0.06 mN/mm2 (n = 10)] (Fig. 4).
FIG. 4.
Active contractile force at Lmax of culture day-12 EEECT. Active force of culture day-12 EEECT increased in response to cyclic stretch stimulation for 48 h and decreased following either p38MAPK inhibition or concomitant stretch and p38MAPK inhibition. *p < 0.05 versus control EEECT.
Discussion
Increased mechanical loads trigger various molecular signaling pathways that can result in cardiac growth or pathologic hypertrophy in postnatal myocardium.26–28 In contrast, the immature embryonic/fetal myocardium responds to increased mechanical loads via cellular proliferation (myocardial hyperplasia).2,29,30 In our previous study, we showed that the engineered cardiac tissue from embryonic cardiac cells, termed EEECT, maintains a relatively high cellular proliferation activity, and cyclic mechanical stretch stimulation further increases cellular proliferation similar to the developing fetal myocardium.16 However, it was unclear which cellular subpopulation, CMs or non-CMs, responds to the mechanical stretch within this in vitro–engineered cardiac construct. In the present study, we found that both CMs and non-CMs proliferate within EEECT and that CMs, but not non-CMs, increase proliferation in response to cyclic mechanical stretch stimulation. In addition, mechanical stretch increased p38MAPK phosphorylation, which preceded increases in EEECT contractile force and CM proliferation. In contrast, the inhibition of p38MAPK significantly decreased CM proliferation activity, and the negative effects of p38MAPK inhibition overrode the positive cyclic mechanical stretch stimulation effects on EEECT contractile function and CM proliferation.
There are currently conflicting results regarding the role of p38MAPK in the regulation of CM growth (hypertrophy) and survival.18,26 Many of these studies investigate postnatal (neonatal or adult) CMs. Extrapolation of the results from the postnatal CMs to fetal CMs is difficult because of the marked phenotypic differences between the fetal and postnatal CMs, including age-dependent changes in protein kinase expression, β-adrenergic signaling,31 contractile apparatus maturity, and most importantly, by the hyperplastic32 versus hypertrophic33 responses to mechanical stress. Zimmermann et al. reported that culture day-14 engineered heart tissue (EHT) generated using neonatal rat CMs morphologically resembled adult myocardium, and the addition of mechanical stretch to the EHT induced cardiac hypertrophy.34 In our EEECT made from embryonic cardiac cells, we found that EEECT resembled developing immature fetal myocardium and that mechanical stretch induced CM proliferation. The disparate culture time and CM type is likely responsible for these differing results, underlining the phenotypic differences between postnatal and fetal CMs.16 Olson et al. recently reported that p38MAPK activity increased in response to increase in hemodynamic loads in a fetal ovine myocardium, suggesting a role for p38MAPK in regulating fetal CM proliferation.21,35 The stretch-induced increase in CM over non-CM phospho-histone H3 expression shown here may be dependent upon the activation of p38MAPK, since it was abolished by the selective inhibitor SB202190. These results suggest that p38MAPK is a mediator of stretch-induced fetal-type CM proliferation within in vitro–engineered cardiac tissue.
The observation that an increase in p38MAPK phosphorylation by cyclic mechanical stretch occurred coincident with increased Akt phosphorylation suggests that p38MAPK may regulate EEECT cell proliferation via Akt. Akt is a serine–threonine kinase and has been implicated in numerous intracellular processes including cell growth and survival.36,37 Akt has also been shown to be a downstream target of p38MAPK that can be directly regulated via gene transcription as well as by protein activation during myogenesis.38 Previous work has indicated that both Akt39 and p38MAPK40 are important for myogenesis. Gude et al. have recently shown that Akt acts as a facilitator of cellular proliferation for cardiac progenitor cells and young-committed CMs in the heart and that an increase in Akt correlated not only with replicating cells, but also with increased GATA-4 expression.41 Some myogenic-specific transcription factor downstream targets of p38MAPK, including GATA-4 and MEF2, have been shown to be activated directly by p38MAPK,42–44 and recent work has shown that the PI3 kinase pathway is involved in activating MEF2.38,39 Aouadi et al. reported that inhibition of p38MAPK activity reduced MEF2C expression and blocked CM lineage induction in embryonic stem cells, suggesting that the p38MAPK effect could be due to MEF2C regulation.45 We have shown that cyclic mechanical stretch stimulates p38MAPK and results in Akt activation and that inhibition of p38MAPK reduces Akt activation. These changes in p38MAPK and Akt content and activity within EEECT parallel changes in the CM proliferation. Therefore, it is likely that even though p38MAPK and Akt function via distinct, though parallel, signaling pathways, p38MAPK functions upstream of Akt in the response to mechanical stretch, suggesting that Akt function may be another important link in the regulation of CM proliferation.
Our data also suggest that p38MAPK activity within EEECT impacts contractile function in addition to cell proliferation. EEECT responded to mechanical stretch with increased active force and CM proliferation, which is similar to the developing fetal myocardium.25,46,47 Cyclic mechanical stretch has previously been shown to induce a variety of cellular processes, including activation of MAPKs,26,27,48,49 reprogramming of gene expressions,26,27 and an increase in protein synthesis,26,27 as well as trigger adaptive responses in muscle phenotype via the expression of contractile proteins.49,50 Recent studies in adult CMs have shown that p38MAPK activation upregulates Na+-Ca2+ exchanger expression similar to cardiac hypertrophy and failure in adult myocardium.51 In contrast, immature proliferative (not hypertrophic) embryonic/fetal CMs have different Na+-Ca2+ exchanger properties from adult CMs, and the changes in its properties depend on developmental stages.52,53 Some regulatory factors, PKA, PKC, and cyclic nucleotides, of the Na+-Ca2+ exchanger could also change expression, location, or compartmentalization with respect to developmental stages,53 which would also lead to altered Na+-Ca2+ exchanger function in embryonic/fetal CMs compared to adult CMs. There are no previous reports that investigated the relationship between p38MAPK (and other MAPKs) and Na+-Ca2+ exchanger function within developing immature CMs. We speculate that cyclic mechanical stretch–mediated p38MAPK activity within EEECT may not upregulate Na+-Ca2+ exchanger expression like what is seen in adult, mature CMs. Further investigation into the Na+-Ca2+ exchanger functions within EEECT and its role in regulating the active contractile force along with CM proliferation following stretch-induced p38MAPK activation is necessary.
Several limitations should be noted in the present study. First, cyclic mechanical stretch selectively increased CM proliferation and both p38MAPK and Akt phosphorylation, whereas p38MAPK inhibition reduced both CM and non-CM proliferation activities as well as reduced Akt activity. These results suggest that the positive cyclic mechanical stretch effects on CM proliferation may not be directly mediated by p38MAPK activity of CMs. The noted increase in p38MAPK activity and Akt activity induced by mechanical stretch may be independent events. It is also important to note that EEECT contains both CM population [65–75% in cell number, more than 90% in cellular volume ratio16] and non-CM population, such as fibroblasts, endothelial cells, vascular smooth muscle cells, and so on. CM-to-non-CM and CM-to-extracellular matrix interactions may greatly influence CM proliferation and p38MAPK/Akt activities. Second, the p38MAPK inhibition dose used in the current study (10 μM, SB202190) may also have toxic effects on both CM and non-CM populations other than its noted effects on cellular proliferation. In our preliminary study, we found that 1 and 5 μM SB202190 treatment for 48 h tended to decrease p38MAPK phosphorylation levels and CM proliferation rate (by histone H3–positive cell counting), while there was no change in the EEECT contractile force. Only 10 μM SB202190 treatment showed statistically significant decreases in p38MAPK phosphorylation, CM proliferation, and EEECT contractile force production. We note that EEECT architecture stained by α-sarcomeric actinin did not vary between treatment doses. Therefore, we chose the 10 μM SB202190 treatment dose for p38MAPK inhibition for the current study. However, other factors remain to be investigated, such as CM apoptosis, or altered CM metabolism, which could be triggered by p38MAPK inhibition.54 Third, we have not yet determined the roles of other MAPKs, such as JNK, or ERK that have also been closely associated with the regulation of CM growth, survival, and proliferation. These alternate kinases may directly or indirectly participate in the regulation of both CM and non-CM responses to cyclic mechanical stretch. Forth, while our current cyclic mechanical stretch protocol significantly increased EEECT force generation and CM proliferation, alternate stretch protocols may be optimal for immature CMs at different developmental stages. Finally, our finding of positive mechanical stretch effects on immature CM derived from avian and rat embryo (unpublished data) myocardium needs to be confirmed for immature CM derived from large mammals or in stem/progenitor cell–derived CMs. Nevertheless, our results suggest that immature CM proliferation can be positively regulated by cyclic mechanical stretch, and unwanted, excessive CM and non-CM proliferation can be negatively regulated by p38MAPK inhibition within this in vitro tissue culture environment, which is one of the key factors for the development of an optimal tissue culture method of functioning CM preparation for cardiac repair.
In summary, immature (fetal) CMs within a 3D engineered cardiac construct increased CM proliferation in response to cyclic mechanical stretch, and this proliferative response is blocked by p38MAPK inhibition. Our findings confirm a role for cyclic mechanical stretch and p38MAPK in the regulation of CM proliferation within tissue-engineered cardiac constructs that may be relevant in designing optimized donor CM preparation and culture approaches for cardiac repair paradigms. Further research is required to define the underlying mechanisms by which p38MAPK activation regulates the fetal CM proliferation, growth, and mechanical properties.
Funding Sources
This research was supported by the NIH R21HL79998 (B.B.K. and K.T.), NIH-NHLBI training grant T32-HL76124 (K.C.C.), and the Pennsylvania Department of Health (B.B.K. and K.T.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Li F. McNelis M.R. Lustig K. Gerdes A.M. Hyperplasia and hypertrophy of chicken cardiac myocytes during posthatching development. Am J Physiol. 1997;273:R518. doi: 10.1152/ajpregu.1997.273.2.R518. [DOI] [PubMed] [Google Scholar]
- 2.Sedmera D. Hu N. Weiss K.M. Keller B.B. Denslow S. Thompson R.P. Cellular changes in experimental left heart hypoplasia. Anat Rec. 2002;267:137. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- 3.Hagege A.A. Menasche P. Cellular cardiomyoplasty: a new hope in heart failure? Heart. 2000;84:465. doi: 10.1136/heart.84.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laflamme M.A. Zbinden S. Epstein S.E. Murry C.E. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annu Rev Pathol. 2007;2:307. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 5.Payne T.R. Oshima H. Okada M. Momoi N. Tobita K. Keller B.B. Peng H. Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 6.Laflamme M.A. Murry C.E. Regenerating the heart. Nat Biotechnol. 2005;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 7.Pasumarthi K.B. Field L.J. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 8.Roell W. Lewalter T. Sasse P. Tallini Y.N. Choi B.R. Breitbach M. Doran R. Becher U.M. Hwang S.M. Bostani T. von Maltzahn J. Hofmann A. Reining S. Eiberger B. Gabris B. Pfeifer A. Welz A. Willecke K. Salama G. Schrickel J.W. Kotlikoff M.I. Fleischmann B.K. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann W.H. Didie M. Doker S. Melnychenko I. Naito H. Rogge C. Tiburcy M. Eschenhagen T. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovasc Res. 2006;71:419. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann W.H. Melnychenko I. Wasmeier G. Didié M. Naito H. Nixdorff U. Hess A. Budinsky L. Brune K. Michaelis B. Dhein S. Schwoerer A. Ehmke H. Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaum J. Minami E. Laflamme M.A. Virag J.A. Ware C.B. Masino A. Muskheli V. Pabon L. Reinecke H. Murry C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 12.Bulić-Jakus F. Ulamec M. Vlahović M. Sincić N. Katusić A. Jurić-Lekć G. Serman L. Kruslin B. Belicza M. Of mice and men: teratomas and teratocarcinomas. Coll Antropol. 2006;30:921. [PubMed] [Google Scholar]
- 13.Chien K.R. Stem cells: lost in translation. Nature. 2004;428:607. doi: 10.1038/nature02500. [DOI] [PubMed] [Google Scholar]
- 14.Dowell J.D. Rubart M. Pasumarthi K.B. Soonpaa M.H. Field L.J. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 2003;58:336. doi: 10.1016/s0008-6363(03)00254-2. [DOI] [PubMed] [Google Scholar]
- 15.Roell W. Lu Z.J. Bloch W. Siedner S. Tiemann K. Xia Y. Stoecker E. Fleischmann M. Bohlen H. Stehle R. Kolossov E. Brem G. Addicks K. Pfitzer G. Welz A. Hescheler J. Fleischmann B.K. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation. 2002;105:2435. doi: 10.1161/01.cir.0000016063.66513.bb. [DOI] [PubMed] [Google Scholar]
- 16.Tobita K. Liu L. Janczewski A. Tinney J. Nonemaker J. Augustine S. Stolz D. Shroff S. Keller B.B. Engineered early embryonic cardiac tissue (EEECT) retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ahuja P. Sdek P. MacLellan W.R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clerk A. Sugden P.H. Inflame my heart (by p38-MAPK) Circ Res. 2006;99:455. doi: 10.1161/01.RES.0000241053.89089.c3. [DOI] [PubMed] [Google Scholar]
- 19.Engel F.B. Schebesta M. Duong M.T. Lu G. Ren S. Madwed J.B. Juang H. Wang Y. Keating M.T. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morissette M.R. Cook S.A. Foo S.Y. McKoy G. Ashida N. Novikov M. Scherrer-Crosbie M. Li L. Matsui T. Brooks G. Rosenzweig A. Signaling myostatin regulates cardiomyocyte growth through modulation of Akt. Circ Res. 2006;99:15. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson A.K. Protheroe K.N. Segar J.L. Scholz T. Mitogen-activated protein kinase activation and regulation in the pressure-loaded fetal ovine heart. Am J Physiol Heart Circ Physiol. 2006;290:H1587. doi: 10.1152/ajpheart.00984.2005. [DOI] [PubMed] [Google Scholar]
- 22.Tenhunen O. Rysä J. Ilves M. Soini Y. Ruskoaho H. Leskinen H. Identification of cell cycle regulatory and inflammatory genes as predominant targets of p38 mitogen-activated protein kinase in the heart. Circ Res. 2006;99:485. doi: 10.1161/01.RES.0000238387.85144.92. [DOI] [PubMed] [Google Scholar]
- 23.Tenhunen O. Soini Y. Ilves M. Rysä J. Tuukkanen J. Serpi R. Pennanen H. Ruskoaho H. Leskinen H. p38 Kinase rescues failing myocardium after myocardial infarction: evidence for angiogenic and antiapoptotic mechanisms. FASEB J. 2006;20:E1276. doi: 10.1096/fj.05-5618fje. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger V. Hamilton H.L. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49. [PubMed] [Google Scholar]
- 25.Tobita K. Garrison J.B. Liu L.J. Tinney J.P. Keller B.B. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. Anat Rec A Discov Mol Cell Evol Biol. 2005;283:193. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- 26.Komuro I. Katoh Y. Kaida T. Shibazaki Y. Kurabayashi M. Takaku F. Yazaki Y. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. J Biol Chem. 1991;266:1265. [PubMed] [Google Scholar]
- 27.Sadoshima J. Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Hoff M.J. Deprez R.H. Ruijter J.M. de Boer P.A. Tesink-Taekema S. Buffing A.A. Lamers W.H. Moorman A.F. Increased cardiac workload by closure of the ductus arteriosus leads to hypertrophy and apoptosis rather than to hyperplasia in the late fetal period. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:193. doi: 10.1007/s00210-004-0955-0. [DOI] [PubMed] [Google Scholar]
- 29.Barbera A. Giraud G.D. Reller M.D. Maylie J. Morton M.J. Thornburg K.L. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- 30.Saiki Y. Konig A. Waddell J. Rebeyka I.M. Hemodynamic alteration by fetal surgery accelerates myocyte proliferation in fetal guinea pig hearts. Surgery. 1997;122:412. doi: 10.1016/s0039-6060(97)90034-9. [DOI] [PubMed] [Google Scholar]
- 31.Schaffer W. Williams R.S. Age-dependent changes in expression of alpha 1-adrenergic receptors in rat myocardium. Biochem Biophys Res Commun. 1986;138:387. doi: 10.1016/0006-291x(86)90293-7. [DOI] [PubMed] [Google Scholar]
- 32.Rybin V.O. Steinberg S.F. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994;74:299. doi: 10.1161/01.res.74.2.299. [DOI] [PubMed] [Google Scholar]
- 33.Pandya N. Santani D. Jain S. Role of mitogen-activated protein (MAP) kinases in cardiovascular diseases. Cardiovasc Drug Rev. 2005;23:247. doi: 10.1111/j.1527-3466.2005.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann W.H. Schneiderbanger K. Schubert P. Didie M. Munzel J.F. Heubach J.F. Kostin S. Neuhuber W.L. Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 35.Olson A.K. Protheroe K.N. Scholz T.D. Segar J.L. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Investig. 2006;13:157. doi: 10.1016/j.jsgi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Kandel E.S. Hay N. Multiple regulators and multiple downstream effectors of the serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 37.Khwaja A. Akt is more than just a bad kinase. Nature. 1999;401:33. doi: 10.1038/43354. [DOI] [PubMed] [Google Scholar]
- 38.Cabane C. Coldefy A.S. Yeow K. De'rijard B. The p38 pathway regulates Akt both at the protein and transcriptional activation levels during myogenesis. Cell Signal. 2004;16:1405. doi: 10.1016/j.cellsig.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Tamir Y. Bengal E. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J Biol Chem. 2000;275:34424. doi: 10.1074/jbc.M005815200. [DOI] [PubMed] [Google Scholar]
- 40.Zetser A. Gredinger E. Bengal E. p38 Mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the MEF2C transcription factor. J Biol Chem. 1999;274:5193. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- 41.Gude N. Muraski J. Rubio M. Kajstura J. Schaefer E. Anversa P. Sussman M.A. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 42.Han J. Molkentin J.D. Regulation of MEF2 by p38 MAPK and its implication in cardiomyocyte biology. Trends Cardiovasc Med. 2000;10:19. doi: 10.1016/s1050-1738(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 43.Sedmera D. Thompson R.P. Kolar F. Effect of increased pressure loading on heart growth in neonatal rats. J Mol Cell Cardiol. 2003;35:301. doi: 10.1016/s0022-2828(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 44.Tenhunen O. Sarman B. Kerkela R. Szokodi I. Papp L. Toth M. Ruskoaho H. Mitogen-activated protein kinases p38 and ERK1/2 mediate the wall stress-induced activation of GATA-4 binding in adult heart. J Biol Chem. 2004;279:24852. doi: 10.1074/jbc.M314317200. [DOI] [PubMed] [Google Scholar]
- 45.Aouadi M. Bost F. Caron L. Laurent K. Le Y. Brustel M. Binetruy B. p38 Mitogen-activated protein kinase activity commits embryonic stem cells to either neurogenesis of cardiomyogenesis. Stem Cells. 2006;24:1399. doi: 10.1634/stemcells.2005-0398. [DOI] [PubMed] [Google Scholar]
- 46.Clark E.B. Hu N. Frommelt P. Vandekieft G.K. Dummett J.L. Tomanek R.J. Effect of increased pressure on growth in stage 21 chick embryos. Am J Physiol. 1989;257:H55. doi: 10.1152/ajpheart.1989.257.1.H55. [DOI] [PubMed] [Google Scholar]
- 47.Kyriakis J.M. Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 48.Komuro I. Kudo S. Yamazaki T. Zou Y. Shiojima I. Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. FASEB J. 1996;10:631. doi: 10.1096/fasebj.10.5.8621062. [DOI] [PubMed] [Google Scholar]
- 49.Rauch C. Loughna P.T. Static stretch promotes MEF2A nuclear translocation and expression of neonatal myosin heavy chain in C2C12 myocytes in a calcineurin- and p38-dependent manner. Am J Physiol Cell Physiol. 2005;288:593. doi: 10.1152/ajpcell.00346.2004. [DOI] [PubMed] [Google Scholar]
- 50.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 51.Xu L. Kappler C.S. Menick D.R. The role of p38 in the regulation of Na+ -Ca2+ exchanger expression in adult cardiomyocytes. J Mol Cell Cardiol. 2005;38:735. doi: 10.1016/j.yjmcc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Shepherd N. Graham V. Trevedi B. Creazzo T.L. Changes in regulation of sodium/calcium exchanger of avian ventricular heart cells during embryonic development. Am J Physiol Cell Physiol. 2007;292:C1942. doi: 10.1152/ajpcell.00564.2006. [DOI] [PubMed] [Google Scholar]
- 53.Reppel M. Fleischmann B.K. Reuter H. Sasse P. Schunkert H. Hescheler J. Regulation of the Na+/Ca2+ exchanger (NCX) in the murine embryonic heart. Cardiovasc Res. 2007;75:99. doi: 10.1016/j.cardiores.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Nagy N. Shiroto K. Malik G. Huang C.K. Gaestel M. Abdellatif M. Tosaki A. Maulik N. Das D.K. Ischemic preconditioning involves dual cardio-protective axes with p38MAPK as upstream target. J Mol Cell Cardiol. 2007;42:981. doi: 10.1016/j.yjmcc.2007.02.010. [DOI] [PubMed] [Google Scholar]