Introduction
Dendritic cells (DC) have long been regarded as the most potent antigen presenting cells (APCs) within the immune system. Their ability to sample environmental antigens and stimulate T cell activity in a major histocompatibility complex (MHC)-restricted manner has attracted much attention given the poor antigen presenting ability and immunogenicity of tumor cells [1,2]. Although DCs constitute approximately 0.3% of all circulating blood leukocytes, they serve as the sentinels of the immune system and are found nearly ubiquitously throughout the body [3]. In their immature state, DCs are highly specialized antigen samplers capable of surveying their microenvironment through several mechanisms including engulfment, macropinocytosis, and receptor mediated endocytosis [3]. Upon encountering an antigen the DC processes it through MHC pathways and directs it to the cell surface to form an MHC-peptide complex (Figure 1). In line with traditional antigen presentation following uptake from the environment, many antigens are channeled through MHC-class II pathways with resultant MHC-peptide complexes being capable of stimulating CD4+ T cells. In addition, dendritic cells possess the unique ability to “cross-present” acquired antigens. In this process, DC endosomes release captured antigenic material into the cytosol where it is broken down by proteasomes [4]. The degraded peptides are then transported to the ER via a transporter-associated protein (TAP) and bound to MHC-class I molecules for presentation to CD8+ T cells [5,6]. These distinct mechanisms allow DCs to stimulate T cells in an MHC-class I and II manner, overcoming classical restrictions in antigen processing and presentation [7] and diversifying the resultant immune response.
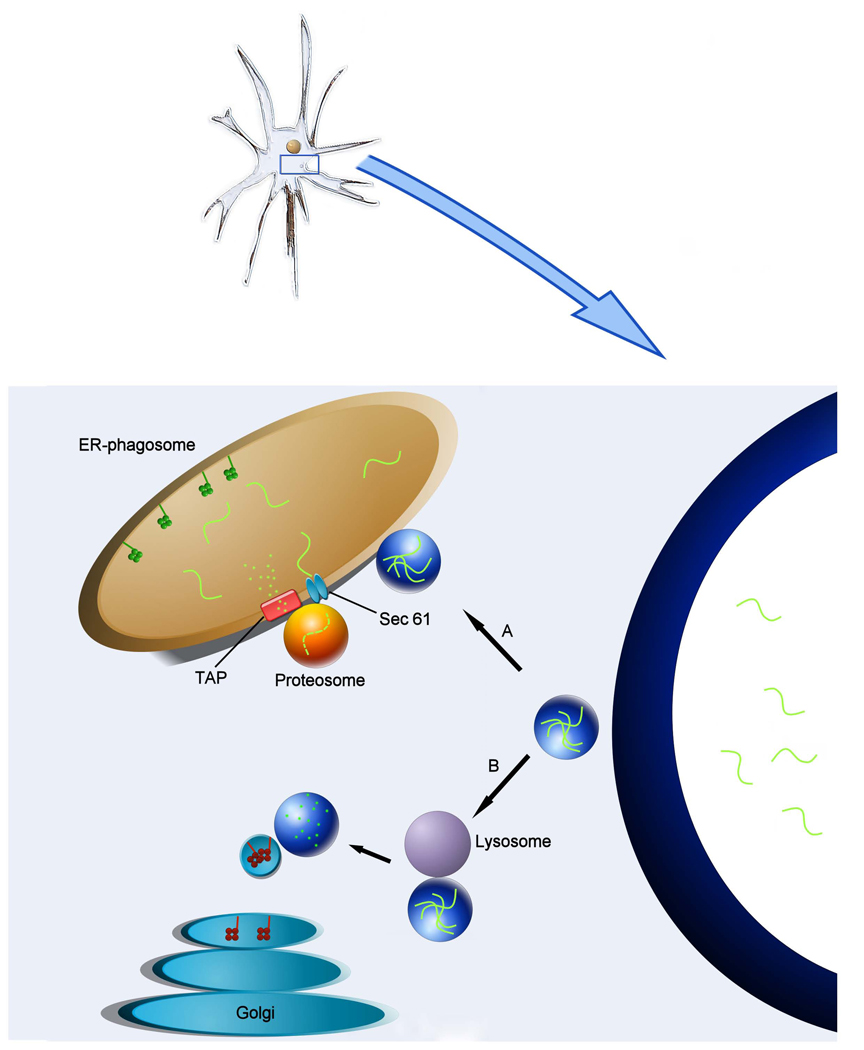
Figure 1.
Schematic of dendritic cell antigen processing and presentation via distinct MHC-I and MHC-II targeted pathways. Foreign antigens are sampled from the environment via dendritic cell phagocytosis or pinocytosis. Once vacuolized, antigen-containing vesicles are directed down one of two MHC pathways that result in cell surface presentation. A) Antigen containing vesicle encounters endoplastmic reticulum-phagosome. Antigen is retro-translocated into the cytoplasm via Sec 61 where proteosome complexes mediate peptide degradation. The resultant epitopes are translocated back into the ER-phagosome via the transporter-associated protein (TAP), where they are loaded onto MHC-I complexes and extruded for membrane integration and antigen presentation on the cell surface. B) Antigen-containing vesicle encounters lysosome which cleaves peptides using acid proteases. MHC-II complexes formed within the ER and subsequently processed and extruded from the Golgi apparatus are transported to peptide containing endosomes for antigen loading.
Dendritic cells are capable of handling a vast range of antigenic mediums. The sources of antigen that have been used in DC immunotherapy include exogenous MHC-restricted peptides, acid-eluted tumor peptides, tumor RNA and cDNA, viral vectors, apoptotic tumor cells, tumor cell lysate, and whole tumor cells. Many of these methods have been employed with varying degrees of success. However, a growing sentiment has emerged that argues for the use of a diverse range of antigens that cover both MHC classes rather than constructing specific MHC-matched peptides. The reasoning for this is multifold. First, stimulating T cells with a broad range of antigens reduces the likelihood of an escape phenomenon in which tumor cells lacking the specific antigens of interest avoid immune detection and continue to grow unhindered. Second, it is now well established that the stimulation of both CD4+ and CD8+ T cells is crucial in the activation and maintenance of anti-tumor immunity [7–10]. By allowing DCs to present and cross-present antigens on MHC-class II and I molecules, respectively, one avoids having to laboriously engineer peptides for each MHC class [9,11]. Finally, the methods employed to load the spectrum of antigens for a particular tumor obviate the need of characterizing each individual antigen used. Although the use of unfractionated tumor material containing unknown antigens has long raised the concern of inducing auto-immunity, particularly in the form of experimental allergic encephalomyelitis (EAE), no reports of this complication have been seen following DC vaccination in humans to date [3].
A dendritic cell vaccine is defined as DCs loaded with antigens, for example, those found on glioma, which are administered to patients in order to induce an antigen-specific T cell mediated anti-tumor response [12]. However, though immature dendritic cells are not functionally ideal for the loading of antigens, they are unable to activate lymphocytes until an inflammatory signal or pathogen induces their maturation [3,9,11]. Some groups argue that ex vivo maturation of DCs through CD40L or interferon (IFN)-γ [13] is thus necessary prior to vaccine administration to ensure proper antigen presentation and T cell activation [14–17]. Others maintain that maturation occurs naturally, and that no prior stimulus is required [18]. In the process of maturation, DCs lose their ability to uptake and process antigens. Moreover, they exchange their immature molecular signature for a mature (CD83+) phenotype, increasing expression of MHC-antigen complexes, lymphocyte costimulatory molecules (e.g. CD80/B7-1 & CD86/B7-2), TNF and TNF-receptor molecules (e.g. CD40), and many chemokines and chemokine receptors (e.g. IL-12, IL-15, IL-18) to aid in T-cell recruitment and DC navigation to lymphoid tissues (as reviewed by Steinman [11] and Soling [9]).
Upon localization to lymph organs rich in naïve T cells, mature DCs present their processed antigens in a MHC-restricted manner. Through various interactions they are able to mobilize many different arms of the immune system, including CD8+ cytotoxic T cells (CTLs), CD4+ helper T cells, natural killer (NK) and NK-like T cells [11]. Each of these cell types plays an essential role in the anti-tumor response (Figure 2). T cells expressing CD8 co-receptors recognize and lyse tumor cells in an MHC-class I restricted fashion, and have received much of the credit as the primary effector cell in immunotherapy. CD4+ T cells have traditionally been known for their part in the expansion and maintenance of CD8+ CTLs, secretion of stimulatory cytokines, and the induction of lasting immunity. Their critical role in immunotherapy has become increasingly appreciated over the past few years, as studies have demonstrated that their absence may result in deficient DC maturation as well as CTL tolerance [7,8]. Finally, NK and NK-like T cells have a unique niche in the leukocyte armament, being able to recognize and kill tumor cells that do not express surface markers such as MHC-class I. Although the exact mechanism of recognition and elimination of tumor cells in the absence of MHC-restriction is yet to be elucidated, they serve as an important complement in the killing of tumor cells that may possess diminished surface marker presentation and avoid CTL detection [3,11].
Figure 2.
Diagram depicting the multiple dendritic cell-lymphocyte interactions that take place in the immune cascade following antigen processing and presentation by DCs. Dendritic cell activation of NK T cells through self ligands (not shown) and IL-12 results in IFN-γ release and subsequent activation of CD4+ and CD8+ T cells. CD4 and CD8 T cell receptors (TCR) interact with peptide-MHC II and I complexes, respectively. CD40L-CD40 interactions between CD4+ T cells and a DC and CD28-B7 interactions between CD8+ T cells and a DC are critical costimulatory interactions that must occur for appropriate T-cell signaling and immune responsiveness.
Animal Models
Pre-clinical animal models explored many of the methodologies and safety concerns regarding dendritic cell vaccines. In the late 1990’s, we were among the first to describe the effectiveness of dendritic cell vaccines in the anti-tumor immunity of gliomas using a rat model [19]. Over the past decade, many groups have published similar studies with varying permutations in the choice of antigen, timing of vaccinations, and measures of therapeutic efficacy. The dissimilarities between study designs make it difficult to compare methodologies and their associated outcomes. However, their value lies within their ability to demonstrate the effectiveness of multiple different DC vaccine techniques in inducing antigen-specific cytotoxicity in vitro and in vivo. These studies have shown improved survival outcomes, as well as the safety of the strategy.
Many of the initial animal studies were key in evaluating the effectiveness of DC vaccination techniques for tumors located in the “immunologically privileged” central nervous system (CNS). Antigen sources used included synthetic peptides [20,21], acid-eluted tumor peptides [19], tumor lysate [21–26], DC-tumor fusion cells [27], and antigen containing vectors such as cDNA/RNA carrying viruses [28,29] and tumor extract carrying liposomes [30]. Many of these studies adopted strategies for antigen loading of DCs from previous experiments in peripheral neoplasms, and initial treatment schedules were similarly based on regimens that showed promise in non-CNS immunotherapy[19]. Nevertheless, the timing of DC administration variedly widely, with vaccinations being given before tumor inoculation [22,24,26–28], simultaneously with [21,23,25], or some time following tumor implantation [19,24,27,28,30]. The number of vaccinations ranged from two to five times across the various studies.
Overall, most groups concluded that vaccination with antigen-pulsed dendritic cells was able to produce a significant anti-tumor immune response. This was evidenced by increased overall survival in rats and mice, greater degrees of T-cell infiltration (primarily CD8+) on histological analysis of tumors, and more robust anti-tumor cytotoxicity assays when splenocytes were incubated with mouse glioma in vitro in immune responders. Although the studies conceded that pre-tumor vaccination resulted in greater survival in animal models, there are conflicting reports regarding the efficacy of DC vaccines when administered simultaneously or following tumor implantation [21–25]. Groups reporting no improvement in survival with DC vaccination after an established tumor suggest that it may be due to the immune system failing to generate an appropriate response quickly enough to counteract a rapidly growing tumor within a confined cranium[21,24]. However, studies in which T-cell mediated tumor killing was achieved showed that the animals that did respond to DC vaccination obtained lasting anti-tumor memory, with significantly improved survival following tumor rechallenge compared to unvaccinated controls [21,22,26].
These animal studies played an important role in alleviating some of the concerns regarding DC immunotherapy for CNS tumors. Experimental allergic encephalomyelitis (EAE) in particular was perhaps one of the most feared side effects, as previous studies have shown this lethal form of autoimmunity to occur following the injection of glioblastoma tissue into animals [31]. However, such signs of autoimmunity were not reported in the more recent studies conducted to date [21,22]. In addition to the information they provided regarding the applicability of different antigen sources and treatment schedules, the pre-clinical reports corroborated the idea that DC immunotherapy could be effectively used for intracranial neoplasms, and thus set the stage for further clinical studies.
Clinical Trials
In 2000, we published a case report on the first brain tumor patient to be treated with DC-based immunotherapy [32]. A patient with histologically confirmed GBM received three biweekly intradermal injections of DCs pulsed with acid-eluted, allogeneic MHC-I matched GBM peptides. Although we were able to appreciate an immune response as evidenced by an increased infiltration of CD3+ T cells in post-vaccination tumor, there was no objective clinical response from the treatment. The patient’s poor Karnofsky performance score (KPS) in addition to the possible lack of antigen homology between the allogeneic GBM and the patient’s tumor may have contributed to the lack of clinical response or prolonged survival.
In a Phase I dose-escalation clinical trial, we treated 12 GBM patients using DCs pulsed with autologous acid-eluted MHC tumor peptides in a dose-escalation study [33]. Patients were separated into three cohorts, each receiving 1, 5, or 10×106 DCs per injection. Subjects tolerated the procedure well with no signs of autoimmunity. There were only minimal Grade I toxicities related to the study vaccine, which were distributed similarly across all three dose groups. Although this study was not powered to measure efficacy, patients undergoing DC-based immunotherapy appeared to have an increased median time to progression (15.5 months) and overall survival (23.4 months) compared to historical controls. Of note, tumor burden and disease progression at the time of vaccination was a critical determinant of systemic CTL activity, tumor infiltration by T cells, as well as overall survival. All patients that generated a systemic CTL response showed no MRI evidence of progressive disease at the time of vaccination. Conversely, no patient with actively progressive disease developed statistically significant cytotoxicity. Moreover, only patients possessing minimal tumor burden at the time of vaccination were found to have tumor infiltrating lymphocytes (TILs) upon post-vaccination tissue examination. These findings suggest that active tumor progression or bulky residual burden can debilitate the initiation and propagation of an anti-tumor response. Interestingly, expression of the inhibitory cytokine TGF-β2 was found to be inversely proportional to the number of TILs found in tumor tissue following vaccination (IL-10 was not), implicating TGF-β2 as a possible mediator of immune evasion following vaccination. This study argues for the need for maximal resection and/or minimal residual disease to improve the efficacy of DC-mediated immunotherapy for glioma. Importantly, this clinical trial established the feasibility, safety, and immunological potential of DC vaccines for brain tumor patients.
Yu et al. reported another Phase I clinical trial using DC pulsed with autologous, acid-eluted peptides for glioma patients [34]. In this study, nine patients with newly diagnosed malignant glioma received 3 biweekly subcutaenous injections of DCs loaded with acid-eluted tumor peptide. Systemic antitumor cytotoxicty was detected in 4 of the 7 patients assessed; intratumoral CD8+ CTL and CD45RO+ memory T-cell infiltration was found in 2 of the 4 patients who underwent a second resection due to tumor progression. Patients receiving DC vaccination were found to have an increased median survival (455 days) compared to that of those in the control group (257 days).
Kikuchi et al. used DC-glioma fusion cells (FCs) to vaccinate glioma patients in their Phase I clinical trial [35]. Eight patients with malignant gliomas received FCs intradermally every 3 weeks, with the total number of injections ranging from 1 to 9. An increased percentage of NK cells was found on FACS analysis in the peripheral blood of patients. In addition, an increase in IFN-γ release in PBMC-tumor co-incubation with both autologous as well as allogeneic glioma was seen following DC vaccination. Two patients experienced a minor response and no serious side effects were observed. These findings suggested that non-specific anti-tumor cytotoxicity may play a role in the DC-based immunotherapy of glioma.
Kobayashi et al. vaccinated five patients with autologous glioma RNA-pulsed DCs [36]. They were able to demonstrate the presence of a strong CD8+ CTL response against autologous glioma accompanied by a weaker NK cell-mediated cytotoxicity in their patients. This finding was significant in 3 of the 5 patients treated. Notably, in the two patients with minimal immune responses, a constitutively increased expression of the inhibitory cytokine IL-10 and decreased expression of IFN-γ by CD8+ T cells was found in vitro.
Yamanaka et al. compared different routes of DC injection in their Phase I/II clinical trial of 10 patients [37,38]. Dendritic cells were pulsed with autologous tumor lysate and administered to patients intradermally (n = 5) or both intradermally and intratumorally via an Ommaya (n = 5) reservoir every 3 weeks for a total number of injections ranging from 1 to 10. Immunologically, they observed an increased percentage of NK cells and increased T-cell mediated anti-tumor activity. In addition, there was an increased intratumoral infiltration of CD4+ and CD8+ T cells in the two patients who underwent reoperation following vaccination. Radiographically, the two minor responses seen were in patients included in the combined intradermal/intratumoral administration group, suggesting that the additional intratumorally injected DCs may stimulate a more efficient anti-tumor immune response.
Wheeler et al. published a report examining the correlation between thymic function, as manifest through CD8+ recent thymic emigrant production, age, and patient outcome in 17 GBM patients undergoing DC immunotherapy [39]. They found that thymic function, as reflected by its ability to produce CD8+ T cells, was directly proportional to good clinical outcomes in mice and human GBM patients and inversely proportional to age. Although patient age has long been a predictor of mortality and prognosis, their findings suggest that it is actually thymic function, which is inversely correlated with age, which may be the more telling factor. Thus, this non-specific immune parameter may later serve as an important prognosticator in glioma immunotherapy.
Caruso et al. conducted a Phase I study of 9 pediatric brain tumor patients undergoing immunotherapy via autologous tumor RNA pulsed DCs [40]. The cohort was comprised of a wide range of different tumor histologies (see Table 1). Although they detected a modest increase in anti-tumor antibodies in some patients, they did not appreciate any increase in T-cell mediated antitumor immunity. This may be explained by their findings that their patients had impaired immunocompetency prior to the start of the trial. Despite this, they reported clinical responses in three patients during the course of their study.
Table 1.
Summary of Phase I & II clinical trials of DC vaccination for CNS tumors.
| Series | Number of patients (type of trial) |
Tumor Characteristics |
Antigen Source |
Dendritic Cell Characteristics |
Immunological response | Clinical response | Toxicity |
|---|---|---|---|---|---|---|---|
| Liau et al. (2000)[32] | 1 (case report) |
Recurrent GBM (n=1) |
Acid-eluted allogeneic MHC-I matched GBM |
PBMCs differentiated with GM-CSF & IL-4; 3 biweekly i.d. injections |
In vitro T-cell proliferative response against allogenic tumor peptides |
None | None |
| Yu et al. (2001)[34] | 9 (Phase I) |
AA (n=2) and GBM (n=7) |
Autologous acid-eluted tumor peptides |
PBMCs differentiated with GM-CSF & IL-4; 3 biweekly s.c. injections |
JAM assay: systemic T cell mediated cytotoxicity against tumor (n=4 of 7 tested) IHC: increased infiltration of CD8+ and CD45RO+ T cells in tumor following vaccination (n=2 of 4) |
Increased median survival time compared to controls (455 days vs 257 days) |
Mild transient fever, nausea and vomiting (n=1), generalized lymphadenopathy (n=1) |
| Kikuchi et al. (2001)[35] | 8* (Phase I) |
AA (n=3) and GBM (n=5) |
Autologous DC-tumor fusion cells |
PBMCs differentiated with GM-CSF, IL-4 & TNF-α; 1–9 injections i.d. every 3 weeks |
FACS assay: increased percentage of NK cells (n=4 of 5 tested) ELISA: increased PBMC IFN-γ release with autologous as well as allogeneic tumor (n=6 of 6 tested) |
Minor response (n=2) A) resolution of intractable headache B) improved hemiparesis |
Erythema at injection site (n=1) |
| Kobayashi et al. (2003)[36] |
5 | GBM (n=5) | GFP transfected autologous tumor RNA with cationic lipid |
PBMCs differentiated with GM-CSF & IL-4 |
In vitro cytotoxicity against tumor by CD8+ (major) and NK-like T cells (minor) (significant in n=3; minimal in n=2) ELISA: increased IL-10 & decreased IFN-γ production by CD8+ T cells in patients with minimal CD8 cytotoxicity |
Not reported | None |
| Yamanaka et al. (2003)[37] |
10 (Phase I & II) |
AA (n=3) or GBM (n=7) |
Autologous tumor lysate (with KLH) |
PBMCs differentiated with GM-CSF & IL-4; 1–10 injections i.d. (n =5) or i.d & i.t. (n=5), once every 3 weeks |
FACS assay: increased percentage of NK cells (n= 5 of 5 tested) and CD8-, CD16-, & CD19-positive T cells (n=4 of 5 tested) DTH: positive reaction to tumor lysate (n=3 of 6 tested); ELISPOT: increased T cell mediated antitumor activity (n=2 of 5 tested) IHC: increased infiltration of CD4+ & CD8+ T cells in patients undergoing reoperation for tumor progression (n=2 of 2) |
Minor response (n=2) A) decreased contrast enhancing portion of lesion B) improvement in convulsions and decrease in contrast enhancing portion of lesion |
Mild headache (n=1); erythema at injection site (n=2) |
| Wheeler et al. (2003)[39] | 17 (Phase I & II) |
New or recurrent GBM |
Autologous tumor lysate |
PBMCs differentiated with GM-CSF & IL-4; 3 injections s.c. biweekly (a 4th 6 weeks after 3rd in Phase II patients) |
Not reported; trial conducted to study the relationship between CD8+ recent thymic emigrants (RTEs) and survival |
||
| Caruso et al. (2004)[40] | 9* (Phase I) |
PA (n=1), AA (n=1), GBM (n=2), medullo- blastomas (n=1), ependymomas (n=3), and pleomorphic xanthoastro- cytomas (n=1) |
Autologous tumor RNA |
PBMCs differentiated with GM-CSF & IL-4; 0–5 injections i.v. and i.d. biweekly |
ELISA: modest increase in specific antitumor antibodies (n=2 of 5 tested) IFN-γ-producing assays: no significant antitumor response (n=4 of 4 tested) T cell proliferation assay: no significant antitumor response (n=3 of 3 tested) |
Tumor free, stable disease at 21, 6 and 2 months follow-up (n=3 of 7 treated) |
None |
| de Vleeschouwer et al. (2004)[17] | 1* (case report) |
Post-radiation AA (n=1) |
Autologous tumor lysate |
PBMCs differentiated with GM-CSF & IL-4; DCs matured with TNF-α, IL- 1β, & PGE2; 6 injections i.d. first 2 every 2 weeks, then every month thereafter |
DTH: positive reaction to tumor lysate (n =1) MRI: transient contrast enhancement after fifth vaccine MET-PET: transient increased metabolic uptake around resection cavity |
Tumor-free survival (60 mo following first vaccination) |
None |
| Yu et al. (2004)[43] | 14 (Phase I & II) |
Recurrent AA (n=3) or GBM (n-9) and new AA (n=1) or GBM (n=1) |
Autologous tumor lysate |
PBMCs differentiated with CM-CSF & IL-4; 3 injections s.c., biweekly |
qPCR: IFN-γ mRNA accumulation in PBMC (n=6 of 10 tested) JAM assay: systemic T cell mediated antitumor cytotoxicity (n=1 of 1 tested) Tetramer staining: increase in CD8+ antigen-specific T cell clones (n=4 of 9 tested) IHC: CD45RO+ memory T cells & CD8+ CTLs in resected progressive tumor (n=3 of 6) |
Increased median survival compared to controls (133 vs 30 weeks, respectively) |
Transient headache (n=3), erythema at the injection site (n=1), generalized seizures (n=2) |
| Rutkowski et al. (2004)[16] | 12* (Phase I) |
AA (n=4) and GBM (n=8) |
Autologous tumor lysate |
PBMCs differentiated with GM-CSF & IL-4; DCs matured with TNF-α, IL- 1β, & PGE2; 2–7 injections i.d., first two separated by 2 weeks, then monthly thereafter |
DTH: positive response to tumor lysate (n=6 of 8 tested) |
Following STR: stable disease (n=1 of 6), partial response (n=1 of 6) Following GTR: continuous complete remission 5 yrs after DC vaccine (n=2 of 6) |
Reversible Grade IV neurological deficits & lethargy (n=1), Grade II hematotoxicity (n=2), transiently increased morning stiffness (n=1), night sweats (n=1), meningismus (n=1) |
| Kikuchi et al. (2004)[44] | 15 (Phase I & II) |
AA (n=9) and GBM (n=6) |
Autologous DC-tumor fusion cells |
PBMCs differentiated with GM-CSF, IL-4, & TNF-α; 3–9 injections i.d., once every 2 weeks; systemic IL-12 given 2 and 5 days after each FC injection |
FACS analysis: percentage of cell types did not change significantly (n=7 of 7 tested) 51Cr release assay: increased cytotoxic activity (n=2 of 8) Intracellular ELISA (CD8+ T cells): increased IFN-γ (n=1 of 7) IHC: Robust infiltration CD8+ CTLs in patients undergoing reoperation for tumor progression (n=2) |
MRI/CT: Partial response (n=4 of 15); mixed response (n=1 of 15); stable disease (n=2 of 15) |
Transient grade I fever (n=4), generalized convulsions (n=1), erythema at injection site (n=13), transient liver dysfunction (n=6), leukocytopenia (n=7) |
| Yamanaka et al. (2005) [15] | 24 (Phase I & II) |
Recurrent AA (n=6) and GBM (n=18) |
Autologous tumor lysate (±KLH) |
PBMCs differentiated with GM-CSF & IL-4; ±OK- 432 for DC maturation (Phase II); 1–22 injections i.d. and/or i.t. (via Ommaya) every 3 weeks; immature DCs (Phase I) and both immature & mature DCs (Phase II) |
DTH: Positive response to tumor lysate (n=8 of 17 tested) ELISPOT assay: tumor specific CTLs increased (n=7 of 16) |
MRI/CT: Partial response (n=1); minor response (n=3); no change (n=10); significantly increased median survival (480 vs 400 days) |
Mild headache (n=1); mild erythema at cervical injection site (n=7) |
| Liau et al. (2005)[33] | 12 (Phase I) |
New (n=7) or recurrent (n=5) GBM |
Acid-eluted autologous tumor peptides |
PBMCs differentiated with GM-CSF & IL-4; 3 injections i.d., once every 2–4 weeks; dose escalation |
Alamar blue CTL assay: systemic tumor specific CTL (n=6 of 6 tested) IHC: CD8+/CD45RO+ memory T cell infiltration in tumors of patients undergoing reoperation for progression who subsequently had >30 mo survival (n=4 of 4); no increased TILs in tumors of patients undergoing reoperation who subsequently had <30 mo survival RT-PCR: lower expression of TGFβ2 in tumor of patients with detectable TILs |
Partial response (n=1); increased TTP (18.3 mo vs 8.2 mo in controls) and median survival (correlated with CTL response and minimal tumor burden) |
Fever and/or flu-like symptoms (n=4), nausea & vomiting (n=3), erythema at injection site (n=2), LAD (n=2), fatigue (n=5), seizures (n=1) |
| Walker et al. (2008)[45] | 13 (Phase I) |
AA (n=4) and GBM (n=9) |
Irradiated tumor cells |
PBMCs differentiated with GM-CSF & IL-4; 2–13 injections i.d., first 6 biweekly, thereafter every 6 weeks |
IHC: Increased CD8+ & CD45RO+ T cell infiltration in tumors of patients undergoing reoperation (n=3 of 3) |
Response to adjuvant chemotherapy: complete (n=1 of 8 treated), partial (n=4 of 8 treated), minimal/none (n=3 of 8 treated) |
None |
| de Vleeschouwer et al. (2008)[14] | 56* | Recurrent GBM | Autologous tumor cell lysate |
PBMCs differentiated with GM-CSF & IL-4; DCs matured with TNF-α, IL- 1β, & PGE2; 3–9 i.d. injections A) week 1 & 3 then every 4 weeks thereafter B) first 5 every 2 weeks, then every 4 weeks C) 4 weekly; boosts with autologous tumor lysate |
DTH: positive reaction to tumor lysate (n=9 of 21 tested) prior to vaccination, with no correlation with survival |
Significantly improved PFS in adults of cohort C; significantly improved OS in patients < 35 yo and PFS in patients with GTR |
Grade IV neurotoxicity (stupor) (n=1), Grade II hematotoxicity (n=2), transient increase in focal neurologic signs (n=6), headache (n=9), vomiting (n=2), flu-like symptoms (n=3), increased frequency of seizures (n=4), fatigue (n=7), myalgias (n=3), erythema around injection site (n=56) |
| Wheeler et al. (2008)[48] | 34 (Phase II) | New (n=11) or recurrent (n=23) GBM |
Autologous tumor lysate |
PBMCs differentiated with GM-CSF & IL-4; 4 injections (3 biweekly, then fourth 6 weeks after third) s.c. |
qPCR: progressively increased IFN-γ after vaccinations, peaking after 3 vaccinations (significantly in 17 of 31 tested) |
TTS and TTP significantly longer (but not in recurrent patients alone); TTS correlated logarithmically with postvaccine IFN-γ response magnitudes exclusively in responders; Significant increase in TTP during postvaccine chemotherapy interval compared to TTP following vaccine alone in both responders and non- responders (n=19) |
Cutaneous GBM with single lymph node involvement at site of DTH testing |
Study includes pediatric patients
Abbreviations used: GM-CSF = granulocyte macrophage colony stimulating factor; GBM = glioblastoma multiforme; PA = pilocytic astrocytoma; AA = anaplastic astrocytoma; IHC = immunohistochemistry; GFP = green fluorescent protein; KLH = keyhole limpet haemocyanin; GTR = gross total resection; PFS = progression free survival; TTS = time to survival; TTP = time to progression; DTH = delayed-type hypersensitivity; MRI = magnetic resonance imaging; CT = computerized tomography; PBMCs = peripheral blood mononuclear cells; CTLs = cytotoxic lymphocyte
De Vleeschouwer et al. explored the possibility of assessing immunotherapeutic progress through the use of magnetic resonance imaging (MRI) and methionine positron emission tomography (MET-PET) [17]. By monitoring contrast enhancement changes in relation to metabolic uptake ratios they could postulate at which point an immune response had occurred. This group published the findings from their Phase I clinical trial of 12 recurrent malignant glioma patients that were vaccinated with tumor lysate-pulsed dendritic cells [16]. Interestingly, they were the first to induce DC maturation ex vivo for glioma immunotherapy based on recent evidence arguing that the injection of mature DCs may mediate a more potent anti-tumor response [41,42]. The extent of resection was stressed in this study as prolonged disease free survival was only achieved in two patients who underwent gross total resection (GTR) prior to vaccination. Moreover, one patient who received only partial tumor resection suffered Grade IV neurotoxicity (National Cancer Institute common toxicity criteria) secondary to vaccination-induced peri-tumoral edema. As such, they argue that maximal resection may help avoid such dangerous complications during CNS immunotherapy. Akin to our conclusions [33], this study further champions the need for maximal resection to improve the potential efficacy of vaccination strategies for malignant gliomas.
In a Phase I/II study of tumor lysate-loaded DC vaccination for malignant glioma, subcutaneous injections of DCs loaded with tumor lysate were administered biweekly for a total of three injections [43]. Elevated IFN-γ mRNA levels in PBMCs, positive cytotoxicity assays, increased peripheral CD8+ CTLs, and increased infiltration of CD45RO+ memory and CD8+ T cells in progressive tumor corroborated a positive immune response. Additionally, this study reported an increased median survival in patients receiving vaccinations (133 weeks) compared to historical controls (30 weeks), further substantiating the viability of DC immunotherapy for glioma.
After their initial Phase I clinical trial, Kikuchi et al. [44] continued their work with human patients through a Phase I/II series modeled after their animal studies involving DC-glioma fusion cell injection with peri-vaccination IL-12 [27]. Fifteen patients were vaccinated intradermally with FCs on a biweekly basis for a total of three injects per course, with IL-12 administration on days 2 and 5 following each injection. Interleukin-12 was given as it had been shown to enhance the antitumor effects of FCs in mouse models. Similarly, they found that treatment efficacy using FC/IL-12 vaccination in human patients was better than FCs alone. Although, they were able to demonstrate cellular anti-tumor immunity in only a few of their patients, they observed much improved clinical outcomes, including four partial responses and one mixed response as determined by imaging. Patients tolerated the treatment regimen well and there were no reported signs of autoimmunity despite the use of systemic IL-12.
Walker et al. investigated the interaction between chemotherapy and dendritic cell vaccines in their Phase I clinical trial [45]. Thirteen patients with malignant glioma were treated with 6 biweekly injections (and every 6 weeks thereafter) of DCs pulsed with autologous irradiated tumor cells. Immunologically, they were able to appreciate an antitumor response by the presence of increased cytotoxic and memory T cells on post-vaccination resected tumor. Of the 8 patients that received adjuvant chemotherapy in addition to immunotherapy, 5 were reported to show objective radiological response to treatment, including one patient who had a complete response. This mirrors findings by Wheeler and colleagues [46] who through retrospective analysis determined that patients who received chemotherapy following DC immunotherapy did better in terms of overall survival and time to recurrence than patients who received either one alone. Although it was previously believed that chemotherapy and immunotherapy were antagonistic forms of treatment [47], this and other studies have added to the accumulating evidence that these two therapies may in fact be synergistic in nature.
In a recent paper, De Vleeschouwer et al. published an update of their work on DC immunotherapy for brain tumors, including 56 patients with recurrent glioblastoma [14]. Patients received intradermal injections of mature DCs pulsed with autologous tumor lysate according to three vaccination schedules that varied in regards to frequency of injections and the presence or absence of tumor lysate boosts (Table 1). In addition, DTH was assessed in 21 patients from which enough tumor material could be removed for appropriate testing. The treatment regimens were well-tolerated with the exception of one patient who developed vaccination-induced Grade IV neurotoxicity as was mentioned in their previous study [16] and two patients who experienced Grade II transient hematotoxicity. Analysis of patient survival and time to progression revealed that gross total resection prior to vaccination was the only independent predictor of progression free survival. Younger age (<35) and GTR were predictive of better overall survival, however, only in univariable analyses. Although it did not reach statistical significance, the regimen that included frequent vaccinations with tumor lysate boosting seemed to have improved PFS. Interestingly, DTH reactivity was not shown to have any correlation with clinical outcome.
Recently, Wheeler et al. reported on their Phase II trial in which they treated 34 patients with new or recurrent glioblastoma [48]. Patients received a total of 4 subcutaneous injections of autologous tumor lysate-pulsed DCs on weeks 0, 2, 4 and 10. Primary outcomes of interest were time to progression and time to survival (TTS). Immunological responses were quantified through measuring the differential expression of IFN-γ mRNA in lysate pulsed DCs expanded from PBMCs collected before and after vaccination. Using normalized IFN-γ production values as previously reported [49], 17 of the 31 patients tested showed a positive vaccine response (≥1.5 fold expression) after 3 vaccinations (responders). The magnitude of increased IFN-γ expression correlated logarithmically with TTS, however, only in vaccine responders. This finding was striking in that it was the first immunological predictor of immunotherapy outcome to achieve statistical significance, likely due to the large number of vaccine responders in this trial. Clinically, vaccine responders had significantly longer TTS (642 ± 61 days) compared to nonresponders (430 ± 50 days). Moreover, disease free progression in vaccine responders was improved by 4.5 months, with responders and non-responders having TTPs of 308 ± 55 days and 167 ± 22 days, respectively. It should be noted that these trends were not significant in patients with recurrent glioblastoma, only in those with newly diagnosed tumors. Finally, it was found that patients in this study experienced a 186-day to 190-day increase in TTP when the course of DC injections was followed by adjuvant chemotherapy, compared to DC therapy alone. This treatment effect was observed indiscriminately between responders and nonresponders, with differences only appreciable when comparing patients with 5-fold IFN-γ increase and all others. These findings supported recent data suggesting that chemotherapy may possibly potentiate the clinical effects of DC-based immunotherapy [46,47].
Current Status of DC Vaccines for Brain Tumors
Safety
Dendritic cell immunotherapy for brain tumors, throughout the 16 different clinical trials and over 200 patients treated to date, appears to be well tolerated across all variations in treatment protocols. A notable exception was one patient who experienced a Grade IV neurotoxicity following DC administration, which was felt to be due to peri-tumoral edema from the gross residual tumor [14]. Another patient, interestingly, developed a subcutaneous glioblastoma with single lymph node involvement following DTH testing [43]. Despite these outliers, most groups have predominantly reported Grade I and II toxicities in response to DC vaccine administration, with no treatment-associated deaths or permanent neurological defects. The most common reason for discontinuing DC-immunotherapy was tumor recurrence/progression, as would be the case with any other treatment modality for glioblastoma. Overall, the relative lack of serious adverse effects supports the safety of DC-based immunotherapies when used in the management of brain tumor patients.
Measures of outcome
One of the major criticisms of immunotherapy has been the lack of evidence supporting its objective clinical benefit (via MR imaging response criteria) despite the numerous studies that have validated its immunologic anti-tumor response [50]. However, this assertion was posed while evaluating clinical outcomes of immunotherapy using antiquated imaging criteria, which many now argue may not be an appropriate means of assessment in the presence of improved imaging technologies and greater emphasis on disease control/stability, quality of life, and overall survival [45,51,52]. Moreover, although systemic evidence of an anti-tumor responses following dendritic cell immunotherapy has been demonstrated on many occasions both in vitro and in vivo, its correlation with actual tumor lysis in human patients is inconsistent at best.
Several groups have tried to determine an immunologic correlate of clinical efficacy in their Phase I/II studies, including measures such as delayed type hypersensitivity (DTH) [14–16], the presence of tumor infiltrating lymphocytes (TILs) [33,34,43–45], and antitumor immunity in vitro from systemic CTLs [15,32–34,36,37,43]. Results have been mixed, particularly with DTH which was only shown to be predictive of improved survival in one study to date [15]. Tumor infiltrating lymphocytes (TILs) in relapsed tumors and systemic antigen specific CD8+ anti-tumor T cells following vaccination are ubiquitously found in patients that seem to respond to immunotherapy; however, they are not prognostically predictive as many non-responders present with such cells as well. It is thought that the microenvironment of the tumor itself, correlated with immunosuppressive cytokine release (e.g. TGF-β), may inhibit the exacting of actual tumor killing despite sufficient cellular immunity [33,36]. Questions regarding which biologic indices are predictive of clinical outcome will continue to be elucidated as larger cohorts are investigated in multi-center Phase II clinical trials for glioblastomas (e.g., DCVax™). Presently, time-to-progression (TTP) and overall survival (OS) remain the best measures of efficacy in dendritic cell immunotherapy.
Methods of Dendritic Cell Vaccine Development and Administration
Notwithstanding a decade of use, there still remains a great degree of variability in the development and administration of dendritic cell vaccines. Only a few studies have systematically examined these differences, resulting in a lack of data regarding the most effective means through which to carry out DC-based immunotherapy for CNS neoplasms. Some of these specifics have been resolved on account of information obtained from animal models or through empirical evidence gleaned from common practice. For example, although there are several different methods through which dendritic cells may be acquired, in all of the clinical trials involving DC immunotherapy for glioma to date, they were exclusively manufactured through the differentiation of peripheral blood mononuclear cells (PBMCs) ex vivo. There now exist many methods through which DCs can be produced efficiently and in large enough quantities for clinical trials [53–56].
Similarly, no studies exist comparing the efficacy of different sources of antigens in propagating anti-tumor immunity in human patients. The vast repertoire of antigen-loading strategies includes whole tumor cells [27,35,44], apoptotic tumor cells[45], acid-eluted tumor peptides [19,32–34], synthetic peptides [20,21], tumor lysate [14–17,21–26,38,39,43,48], and tumor cDNA/RNA [28,36,40,57]. The effectiveness of these methods in stimulating DC-mediated antitumor immunity has primarily been studied in animal models for proof-of-principle rather than comparative analyses. Although some animal studies have evaluated the efficacy of different sources of antigens in stimulating a DC-mediated anti-tumor response [21,28], the choice of antigenic stimuli in clinical trials seem largely based on previous work with pre-clinical models and theoretical considerations. Clearly, prior experience with a particular DC vaccination protocol allows for ready transition from bench to bedside. Theoretically, however, methods utilizing a wide range of autologous tumor antigens have been favored over peptide selection. This allows the vaccine to target all tumor associated antigens without requisite characterization, helping avoid clonal selection of antigen-loss variants and subsequent tumor-escape [58]. Choice of antigen must also be considered for pragmatic reasons, as poor availability of resected tumor tissue may favor the use of cDNA/RNA to pulse DCs as these antigens are readily amplified through molecular techniques [9]. Still, as most of the antigen sources available to immunotherapy have been shown to prime dendritic cells appropriately, their use may remain largely an empiric choice until future studies comparatively examining their functionality and practicality are conducted.
It is well accepted that antigen loading is most effective when pulsing phenotypically immature dendritic cells. However, the maturation state in which to administer DCs to patients following this step remains unclear. Numerous studies have shown that dendritic cell maturation is necessary for effective DC migration [59] and T-cell stimulation [42,60], thus making them more effective in generating an anti-tumor response [15]. Given the need for an inflammatory stimulus or cytokine to induce dendritic cell maturation, DCs have been matured ex vivo in order to theoretically ensure proper functioning once they reach the lymph nodes of the host [14–17]. This is supported by work by Yamanaka et al. who found that patients receiving mature DCs experienced a greater overall survival than patients receiving immature ones [15]. However, Barratt-Boyes et al. were able to demonstrate that immature antigen-pulsed dendritic cells undergo natural maturation when injected intradermally and are quite capable of stimulating appropriate anti-tumor T-cell pathways in vivo. Moreover, they argue that the administration of immature DCs may even be superior to that of mature DCs, as the latter have relatively decreased emigration rates from the injection site [18]. As clinical trials have demonstrated clinical benefit with DC regimens using both immature and mature dendritic cells, future studies comparing the two preparations will be needed to further evaluate the effect maturation status has on clinical efficacy and patient survival [61].
The frequency of DC injections in clinical trials was initially modeled after administration schedules found to be effective in immunotherapy for non-CNS tumors [19]. Since then, the majority of studies have roughly followed a biweekly injection regimen, with number of vaccinations varying from 1 to 22 times (Table 1). Given the many differences in other aspects of the vaccination protocol, it is difficult to compare the efficacy of DC administration frequency between published studies. It has been argued that vaccination should be given expediently following maximal surgical cytoreduction, chemotherapy, and/or radiotherapy in order to fully benefit from the rebound in immune function following gross total resection prior to tumor recurrence [52]. Although early initiation of DC immunotherapy is encouraged, data from animal [26] and patient [14] studies suggest early follow-up vaccinations are not as critical, and in fact may hinder the immune response by causing activation-induced death of recently activated T cells. Instead, these studies have demonstrated that booster injections with tumor lysate alone may be more beneficial in stimulating an anti-tumor response. Interestingly, many studies have employed the testing of delayed type hypersensitivity (DTH) using tumor lysate [14–17,37,48], which may have inadvertently served as a form of booster and improved anti-tumor immunity. Given the lack of controlled studies addressing these issues, the timing and frequency of DC vaccine administration remains largely based on empirical experience, and will require future studies to determine an optimal schedule.
The optimal dose of dendritic cells has similarly been questioned. Even from early pre-clinical studies, it was evident that low inoculations of DCs could stimulate an anti-tumor response [16]. Dose escalation protocols in clinical trials have substantiated the finding that DC-mediated immunity is an “on/off” rather than a dose-response phenomenon, as increasing numbers of these APCs do not affect the magnitude of the CTL response [14,33]. This is reassuring, as large quantities of autologous tumor lysate-pulsed dendritic cells were sometimes difficult to obtain during dose escalation protocols [33,40].
Finally, the route of DC administration best for immunotherapy is still under investigation as well. Dendritic cells can be administered through a variety of ways, including subcutaneous, intradermal, intralymphatic, intranodal, and intratumoral injections. Several studies in mice and non-human primates have examined the differences in lymph node accumulation and T-cell stimulation with each route. Radioisotope tracing studies have shown that intravenous DC administration results in the accumulation of dendritic cells within the spleen and liver. However, interestingly this method results in the greatest humoral anti-tumor response as indicated by increased tumor antigen specific antibodies [62–65]. Conversely, intradermal [62,65], intralymphatic [62], intracranial [66], intranodal [59], and subcutaneous [63] injections of DCs have been shown to drain to lymph nodes and induce greater T-cell mediated immunity against tumor antigens compared to intravenous injections in pre-clinical models. Much attention has been given to the intranodal or perinodal administration of DCs, as lymph nodes are acknowledged as the processing centers responsible in mediating antigen presentation and T-cell activation [67]. Some investigators have questioned how the placement of these injections may alter the potency of the immune response. Recently Calzascia et al. were able to show that the distance from the cervical nodes was not as critical as the location of the tumor itself [68]. Although there was some improved tissue tropism for the CNS when DCs were administered into cervical lymph nodes, they found that the ultimate determinant of homing signals was the residence of the actual tumor, as was evidenced by CNS-tropic T cells following inguinal node DC injection in an intracranial tumor model. To date, only one clinical trial has investigated the differences in patient outcome between two injection routes. Yamanaka et al. found that patients who received both intratumoral and intradermal DCs had prolonged survival compared to those that received intradermal DCs alone [15]. Further studies comparing injection sites and modalities in inducing antitumor immunity are still needed.
Patient selection for Dendtic Cell Vaccines
The increasing volume of studies reporting on the clinical response to dendritic cell-based immunotherapy has allowed for the analysis of patient demographics to better determine who may benefit the most from this novel treatment modality. As with traditional therapies for malignant brain tumors, younger patients (<40 years old) receiving DC vaccines appeared to do better in terms of overall survival compared to older patients with similar tumor histologies [48,69]. Though this may in part be attributable to the general trend for younger glioma patients to have better prognoses, one particular study was able to demonstrate that this was primarily due to associated declines in thymus function with increasing age [39]. They maintained that CD8+ T-cell production from the thymus was a prognostic indicator of response to DC immunotherapy independent and superseding that of patient age.
Another critical patient characteristic that seems to have an effect on clinical outcome is surgical management. Those patients who underwent gross total resection of their brain tumor experienced significantly better progression free survival (PFS) compared to otherwise similar patients with appreciable residual tumor [14]. Moreover, bulky residual tumor or active tumor recurrence at the time of vaccination appears to debilitate the anti-tumor CTL response [33].
Finally, patients with newly diagnosed malignant glioma seem to achieve greater response rates than those with recurrent tumors [48]. Although these findings remain to be validated by future studies, it would appear that younger patients with newly diagnosed malignant glioma that are amenable to gross total resection would stand to benefit the most from DC based vaccines.
Synergy of Dendritic Cell Vaccines with other therapies
Although much progress has been made in DC-based immunotherapy for CNS tumors, objective clinical responses for vaccinated brain tumor patients remains inconsistent. Consequently, some groups have examined the use of adjuvant treatments to augment the effects of dendritic cell vaccination. These methods include adjuvant chemotherapy, cytokine administration, and toll-like receptor (TLR) agonists.
The use of cytokines to supplement DC-based immunotherapy in human patients is an extension of work done in pre-clinical animal studies [24,27,29]. Although systemic cytokine administration has only been used in one DC vaccine clinical trial to date [44], studies conducted in vitro as well as with human patients have shown that cytokines such as IL-10 [70], IL-18 [71], and IL-23 [72] may enhance the immune response of effector cells in DC immunotherapy. As the data regarding this adjuvant modality is scarce, further studies are needed before routine clinical use of systemic cytokines can be considered.
The use of standard treatments such as chemotherapy to aid immunotherapy has been considered as well. Chemotherapy has traditionally been regarded as an antagonist to the treatment effects of immunotherapy, because of its effects of bone marrow suppression causing lymphopenia. There has also been a belief that the dead apoptotic tumor cells would produce immune tolerance, exacerbating the lymphopenic state that results as well. However, mounting evidence argues that these apoptotic tumor cells may provide a rich antigen source for dendritic cells and that prompt DC vaccination following chemotherapy may actually provide greater benefit than delaying treatment [47]. Recently, studies have shown that when chemotherapy is used adjuvantly with DC-based immunotherapy, patients experience prolonged overall survival as well as increased time to disease progression [45,46,48]. As evidence suggests that chemotherapy in the setting of DC immunotherapy may actually be beneficial rather than obstructive, it may be prudent to further investigate multi-modality treatment strategies for the simultaneous treatment of brain tumor patients.
Conclusion
Over the past decade, dendritic cell-based immunotherapy for CNS tumors has progressed from pre-clinical rodent models and safety assessments to Phase I/II clinical trials in over 200 patients, which have produced measurable immunological responses and some prolonged survival rates. However, many questions regarding the methods and molecular mechanisms behind this new treatment option remain unanswered. Results from currently ongoing and future studies will help to elucidate which dendritic cell preparations, treatment protocols, and adjuvant therapeutic regimens will optimize the efficacy of DC vaccination. Additionally, it will be important to characterize the pathways underlying the immunosuppressive microenvironment of brain tumors that currently hinder anti-tumor responses. Combined with further advances in the manipulation of various lymphocyte subsets such as regulatory T cells and NK-like T cells, in addition to the usual armament of CD4+ & CD8+ T cells, understanding these immunologic intricacies will help maximize the cellular efficiency of immunotherapeutic techniques. As clinical studies continue to report results on DC-mediated immunotherapy, it will be critical to continue refining treatment methods and developing new ways to augment this promising form of glioma treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell DA, Fecci PE, Sampson JH. Immunotherapy of malignant brain tumors. Immunol Rev. 2008;222:70–100. doi: 10.1111/j.1600-065X.2008.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloisi F, Ria F, Adorini L. Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes. Immunol Today. 2000;21:141–147. doi: 10.1016/s0167-5699(99)01512-1. [DOI] [PubMed] [Google Scholar]
- 3.Parajuli P, Sloan AE. Dendritic cell-based immunotherapy of malignant gliomas. Cancer Invest. 2004;22:405–416. doi: 10.1081/cnv-200034909. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 5.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 6.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 7.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 8.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soling A, Rainov NG. Dendritic cell therapy of primary brain tumors. Mol Med. 2001;7:659–667. [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka R. Cell- and peptide-based immunotherapeutic approaches for glioma. Trends Mol Med. 2008;14:228–235. doi: 10.1016/j.molmed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–473. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 12.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 13.Tschoep K, Manning TC, Harlin H, et al. Disparate functions of immature and mature human myeloid dendritic cells: implications for dendritic cell-based vaccines. J Leukoc Biol. 2003;74:69–80. doi: 10.1189/jlb.0702352. [DOI] [PubMed] [Google Scholar]
- 14.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 15.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski S, De Vleeschouwer S, Kaempgen E, et al. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91:1656–1662. doi: 10.1038/sj.bjc.6602195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vleeschouwer S, Van Calenbergh F, Demaerel P, et al. Transient local response and persistent tumor control in a child with recurrent malignant glioma: treatment with combination therapy including dendritic cell therapy. Case report. J Neurosurg. 2004;100:492–497. doi: 10.3171/ped.2004.100.5.0492. [DOI] [PubMed] [Google Scholar]
- 18.Barratt-Boyes SM, Zimmer MI, Harshyne LA, et al. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–2495. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- 19.Liau LM, Black KL, Prins RM, et al. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90:1115–1124. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 20.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 21.Grauer OM, Sutmuller RP, van Maren W, et al. Elimination of regulatory T cells is essential for an effective vaccination with tumor lysate-pulsed dendritic cells in a murine glioma model. Int J Cancer. 2008;122:1794–1802. doi: 10.1002/ijc.23284. [DOI] [PubMed] [Google Scholar]
- 22.Heimberger AB, Crotty LE, Archer GE, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103:16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 23.Ni HT, Spellman SR, Jean WC, Hall WA, Low WC. Immunization with dendritic cells pulsed with tumor extract increases survival of mice bearing intracranial gliomas. J Neurooncol. 2001;51:1–9. doi: 10.1023/a:1006452726391. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Choo YS, Koo T, et al. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol Lett. 2006;103:142–148. doi: 10.1016/j.imlet.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Pellegatta S, Poliani PL, Corno D, et al. Dendritic cells pulsed with glioma lysates induce immunity against syngeneic intracranial gliomas and increase survival of tumor-bearing mice. Neurol Res. 2006;28:527–531. doi: 10.1179/016164106X116809. [DOI] [PubMed] [Google Scholar]
- 26.Jouanneau E, Poujol D, Gulia S, et al. Dendritic cells are essential for priming but inefficient for boosting antitumour immune response in an orthotopic murine glioma model. Cancer Immunol Immunother. 2006;55:254–267. doi: 10.1007/s00262-005-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akasaki Y, Kikuchi T, Homma S, et al. Antitumor Effect of Immunizations With Fusions of Dendritic and Glioma Cells in a Mouse Brain Tumor Model. J Immunother (1991) 2001;24:106–113. [PubMed] [Google Scholar]
- 28.Yamanaka R, Zullo SA, Tanaka R, Blaese M, Xanthopoulos KG. Enhancement of antitumor immune response in glioma models in mice by genetically modified dendritic cells pulsed with Semliki forest virus-mediated complementary DNA. J Neurosurg. 2001;94:474–481. doi: 10.3171/jns.2001.94.3.0474. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka R, Yajima N, Tsuchiya N, et al. Administration of interleukin-12 and -18 enhancing the antitumor immunity of genetically modified dendritic cells that had been pulsed with Semliki forest virus-mediated tumor complementary DNA. J Neurosurg. 2002;97:1184–1190. doi: 10.3171/jns.2002.97.5.1184. [DOI] [PubMed] [Google Scholar]
- 30.Aoki H, Mizuno M, Natsume A, et al. Dendritic cells pulsed with tumor extract-cationic liposome complex increase the induction of cytotoxic T lymphocytes in mouse brain tumor. Cancer Immunol Immunother. 2001;50:463–468. doi: 10.1007/s002620100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigner DD, Pitts OM, Wikstrand CJ. Induction of lethal experimental allergic encephalomyelitis in nonhuman primates and guinea pigs with human glioblastoma multiforme tissue. J Neurosurg. 1981;55:32–42. doi: 10.3171/jns.1981.55.1.0032. [DOI] [PubMed] [Google Scholar]
- 32.Liau LM, Black KL, Martin NA, et al. Treatment of a patient by vaccination with autologous dendritic cells pulsed with allogeneic major histocompatibility complex class I-matched tumor peptides. Case Report. Neurosurg Focus. 2000;9:e8. doi: 10.3171/foc.2000.9.6.9. [DOI] [PubMed] [Google Scholar]
- 33.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 34.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 35.Kikuchi T, Akasaki Y, Irie M, et al. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi T, Yamanaka R, Homma J, et al. Tumor mRNA-loaded dendritic cells elicit tumor-specific CD8(+) cytotoxic T cells in patients with malignant glioma. Cancer Immunol Immunother. 2003;52:632–637. doi: 10.1007/s00262-003-0408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka R, Abe T, Yajima N, et al. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamanaka R, Tsuchiya N, Yajima N, et al. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J Neurosurg. 2003;99:746–753. doi: 10.3171/jns.2003.99.4.0746. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler CJ, Black KL, Liu G, et al. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- 40.Caruso DA, Orme LM, Neale AM, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol. 2004;6:236–246. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 42.Labeur MS, Roters B, Pers B, et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 43.Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 44.Kikuchi T, Akasaki Y, Abe T, et al. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15:114–121. doi: 10.1016/j.jocn.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 47.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 48.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 49.Kammula US, Marincola FM, Rosenberg SA. Real-time quantitative polymerase chain reaction assessment of immune reactivity in melanoma patients after tumor peptide vaccination. J Natl Cancer Inst. 2000;92:1336–1344. doi: 10.1093/jnci/92.16.1336. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 52.de Vleeschouwer S, Rapp M, Sorg RV, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery. 2006;59:988–999. doi: 10.1227/01.NEU.0000245595.38957.3E. discussioin 99–1000. [DOI] [PubMed] [Google Scholar]
- 53.Sorg RV, Ozcan Z, Brefort T, et al. Clinical-scale generation of dendritic cells in a closed system. J Immunother. 2003;26:374–383. doi: 10.1097/00002371-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Thurner B, Roder C, Dieckmann D, et al. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 55.Tuyaerts S, Noppe SM, Corthals J, et al. Generation of large numbers of dendritic cells in a closed system using Cell Factories. J Immunol Methods. 2002;264:135–151. doi: 10.1016/s0022-1759(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 56.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 57.Yamanaka R, Zullo SA, Ramsey J, et al. Marked enhancement of antitumor immune responses in mouse brain tumor models by genetically modified dendritic cells producing Semliki Forest virus-mediated interleukin-12. J Neurosurg. 2002;97:611–618. doi: 10.3171/jns.2002.97.3.0611. [DOI] [PubMed] [Google Scholar]
- 58.De Vleeschouwer S, Van Gool SW, Van Calenbergh F. Immunotherapy for malignant gliomas: emphasis on strategies of active specific immunotherapy using autologous dendritic cells. Childs Nerv Syst. 2005;21:7–18. doi: 10.1007/s00381-004-0994-3. [DOI] [PubMed] [Google Scholar]
- 59.De Vries IJ, Krooshoop DJ, Scharenborg NM, et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]
- 60.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engell-Noerregaard L, Hansen TH, Andersen MH, Thor Straten P, Svane IM. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58:1–14. doi: 10.1007/s00262-008-0568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 63.Eggert AA, Schreurs MW, Boerman OC, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 64.Mackensen A, Krause T, Blum U, et al. Homing of intravenously and intralymphatically injected human dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Cancer Immunol Immunother. 1999;48:118–122. doi: 10.1007/s002620050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morse MA, Coleman RE, Akabani G, et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 66.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173:2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 67.Barratt-Boyes SM, Figdor CG. Current issues in delivering DCs for immunotherapy. Cytotherapy. 2004;6:105–110. doi: 10.1080/14653240410005258. [DOI] [PubMed] [Google Scholar]
- 68.Calzascia T, Masson F, Di Berardino-Besson W, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 70.De Vleeschouwer S, Spencer Lopes I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol. 2007;84:131–140. doi: 10.1007/s11060-007-9362-y. [DOI] [PubMed] [Google Scholar]
- 71.Yamanaka R, Honma J, Tsuchiya N, et al. Tumor lysate and IL-18 loaded dendritic cells elicits Th1 response, tumor-specific CD8+ cytotoxic T cells in patients with malignant glioma. J Neurooncol. 2005;72:107–113. doi: 10.1007/s11060-004-3550-9. [DOI] [PubMed] [Google Scholar]
- 72.Hu J, Yuan X, Belladonna ML, et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]