Abstract
In situ analysis of fetal semilunar valve leaflets has revealed cells co-expressing endothelial and mesenchymal markers along the endothelium, with diminished frequency seen in adult valves. To determine if such cells are progenitor cells, we isolated clonal populations from human pulmonary valves. The clones expressed endothelial markers, but showed potential to further differentiate into endothelium in response to vascular endothelial growth factor-A (VEGF). When exposed to transforming growth factor (TGF)-β2, individual clones adopted a mesenchymal phenotype to varying degrees and expressed markers of endothelial to mesenchymal transformation (EMT). Both VEGF and TGF-β2-induced phenotypic changes were partially reversible, indicating the plasticity of these cells. When challenged with VEGF or TGF-β2, a hierarchy of endothelial/mesenchymal potential could be seen among the clonal populations: cells initially closer to an endothelial phenotype showed a strong response to TGFβ2 that could be inhibited by VEGF, while cells closer to a mesenchymal phenotype responded to TGFβ2, but were resistant to endothelial-inducing effects of VEGF. These findings suggest the presence of bi-potential valve progenitor cells with ability to differentiate into either endothelial or interstitial cells of the valve leaflet. Understanding the differentiation potential and function of these cells may be important for understanding heart valve disease and may also be applied to current paradigms for creating tissue-engineered heart valves.
Introduction
Valvular heart disease is major cause of morbidity often resulting in the need to replace the valve with a mechanical or bioprosthetic valve. There is a pressing need for improved approaches, especially for children since calcification and destruction of the replacement valve occurs more rapidly in children and young adults 1. Possible strategies include stimulating endogenous repair pathways or using autologous progenitor cells to create tissue-engineered heart valves that will grow with the child. For both approaches, an increased understanding of mechanisms underlying the normal cellular turnover and repair throughout adult life will be essential.
During embryonic valve development, a subset of endocardial endothelial cells, driven by signals from the underlying myocardium, change their phenotype to mesenchymal cells and migrate into the cardiac jelly to form endocardial cushions, the primordial of valves and cardiac septa of the adult heart2. This transdifferentiation of endocardial cells to mesenchymal cells and migration away from the endothelial layer is termed endothelial-mesenchymal transformation (EMT). Studies from Markwald and co-workers show that during EMT, the activated endothelial cells lose cell-cell contacts, gain mesenchymal markers such as α-smooth muscle actin (α-SMA), reduce expression of endothelial markers and migrate into the cardiac jelly 3,4.
TGFβ superfamily members are well-studied signaling intermediates in endocardial cushion formation, with TGFβ1-3 isoforms expressed at or near the onset of EMT during chick and mouse cardiogenesis 4-6. VEGF is also expressed, by embryonic day 9.5, in the endothelial cells in the outflow tract and atrioventricular canal that undergo EMT7. Alterations in VEGF levels appear to have pronounced effects on the endocardial cushions depending on the timing, level and location during heart development 8-11. Following these embryonic events, Aikawa and colleagues demonstrated that human fetal valves possess a dynamic structure composed of proliferating cells, a nascent ECM and α-SMA-positive cells12. Hence, dynamic changes in cell phenotype, cell proliferation and apoptosis, and ECM remodeling occur after the endocardial cushions have remodeled into leaflets and continue throughout human fetal and post-natal development.
Vascular progenitor cells derived from murine embryonic stem cells can differentiate into either endothelial or smooth muscle cells when treated with VEGF or PDGF-BB, respectively 13. More recently, vascular progenitors have been isolated from arteries of adult mice 14. That valve progenitors exist and contribute to cell regeneration or repair in adult valves has been speculated upon15, however only recently has experimental evidence for valve progenitors been reported 16,17. Indeed, bone marrow transplantation experiments in mice have shown that clonal hematopoietic stem cells engrafted into the aortic, pulmonary and atrioventricular valves can differentiate into fibroblast-like cells 18. In the course of our studies on the endothelium of post-natal valves, we identified clonal populations from human pulmonary valve leaflets with progenitor-like properties, demonstrated by their ability to differentiate into endothelial cells in response to VEGF or into myofibroblast/SMCs reminiscent of valve interstitial cells in response to TGFβ2.
Methods
Immunohistochemistry of fetal and adult human semilunar valves
Fetal seminlunar valves at 14-19 weeks gestation, 20-39 weeks gestation, and from adults with a mean age 50.1±2.5 years were obtained at autopsy according to a protocol approved by the Human Research Committee at Brigham and Women's Hospital. No patient had documented cardiac disease or conditions known to predispose to heart valve disease. Valves were fixed in 10% buffered formalin, cut radially in the central cusp region through the adjacent arterial wall, and embedded in paraffin. Sections cut at 6 μm thickness were stained by the avidin-biotin-peroxidase method12,15. Images were analyzed using imaging software (IPLab version 3.9.3; Scanalytics, Inc., Fairfax, VA).
Isolation and culture of clonal population of human pulmonary valve endothelial/progenitor cells (HPVECs)
HPVECs were isolated from a pulmonary valve of a nine month old baby as described 19 and cultured on 1% gelatin-coated plates in EBM-2 supplemented with EGM-2 Single Quots (except the hydrocortisone aliquot was omitted), and 20% heat-inactivated FBS. This culture media will be referred to as EBM-2. Clonal populations were isolated from HPVECs at passage 2 by suspending in growth medium at 3.3 cells/mL and plating 100 μL /well of a 96-well plate. When the colonies covered two-thirds of the well, cells were passaged into 24-well dishes. Human dermal microvascular EC (HDMEC) were isolated as reported 20 but cultured in the same medium, EBM-2, as the HPVECs and HPVEC-derived clones.
On-line supplemental information describes materials and methods for indirect immunofluorescence, reverse transcriptase-polymerase chain reaction (RT-PCR), western blot analysis, flow cytometry, cellular migration and invasion assays and the HL-60 cell adhesion assay.
Results
EMT in developing fetal heart valves
We showed previously that endothelial cells co-expressing α-SMA exist in aortic valve leaflets in juvenile sheep 15. To gain insights on the nature of these cells, we quantitated CD31/α-SMA positive cells in paraffin-embedded sections from human fetal and adult semilunar valve leaflets. Three subsets of cells were observed along the leaflet endothelium: cells expressing only CD31 (green), only α-SMA (red) and those co-expressing both CD31 and α-SMA (yellow) (Fig 1A). In fetal valves, some CD31-positive cells were seen in the interstitium of the leaflet. The CD31 expression might correspond to residual endothelial characteristics of the interstitial cells, similar to endothelial-specific GFP expression seen in embryonic valve leaflets 21. A-SMA-positive cells were observed predominantly along the sub-endothelial lining. In fetal valves, 5-10% of the CD31-positive cells were also α-SMA-positive, whereas only 1% double-positive cells were detected in adult valves (Fig 1B). The presence of dual CD31/α-SMA-positive cells suggests that a subset of valve endothelial cells have the potential to differentiate towards a mesenchymal phenotype, and perhaps migrate into the interstitial regions of the leaflet.
Figure 1.
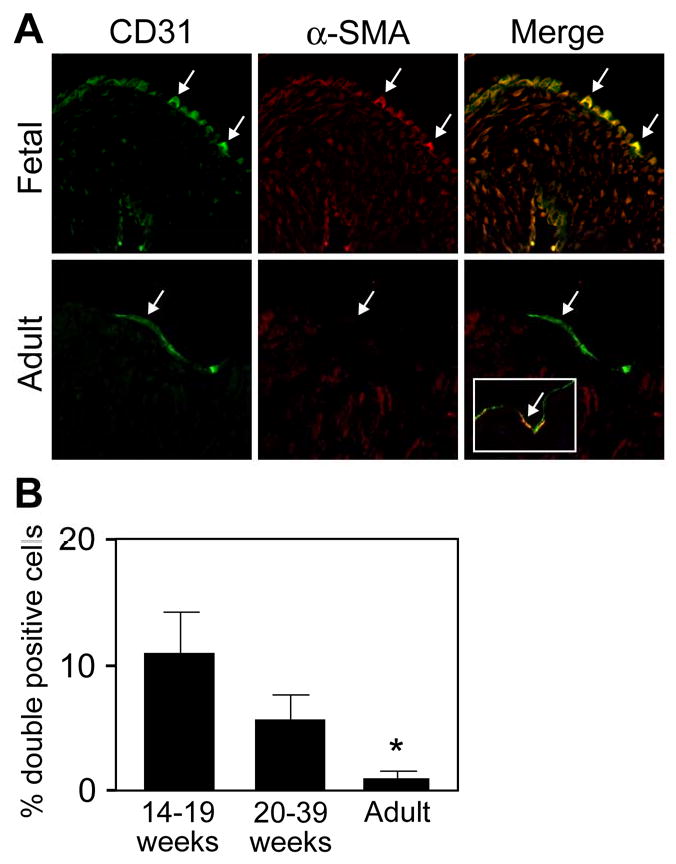
Cells co-expressing CD31 and α-SMA in fetal and adult semilunar valves (A) Sections were double-labeled with anti-CD31 (green) and anti-α-SMA (red). Arrows indicate double-positive cells along the arterial side of the leaflet. The inset in the lower right panel shows rare double-positive cells detected in adult valves. (B) Cells co-expressing both markers were quantitated: fetal valves from 14-19 weeks gestation, 10.8 ± 3.3% (n=11); 20-39 weeks gestation, 5.6 ± 2.0%, (n=10); and adult valves, 1.0 ± 0.5%, (n=10). Data are presented as mean ± SEM. P values: 14-19 weeks versus 20-39 weeks, not significant; 14-19 weeks versus adult, p<0.05; 20-39 week versus adult, p<0.05.
Isolation and characterization of human pulmonary valve endothelial cell (HPVEC) clones
To gain insight into the EMT potential of the CD31-positive and CD31/α-SMA-positive cells in human cardiac valves, we used a limiting dilution technique to isolate clonal populations. We isolated 5 clones, of which 4 exhibited features of a mesenchymal phenotype yet expressed endothelial markers. These clones showed slower growth kinetics and exhibited spindle-shaped morphology instead of the cobblestone morphology seen in parental HPVECs or HDMECs (Fig. 2A). Of the clones obtained, Clone 5 and Clone 8 were chosen for further characterization of markers specific to endothelial and mesenchymal lineages by three different techniques: RT-PCR (Fig 2B), indirect immunofluorescence (Fig 2C) and flow cytometry (Fig 2D). Both clones expressed the endothelial markers tested: CD31, VE-cadherin, vWF, VEGF-R2, eNOS, CD34, CD146 and CD105 as determined by PCR and by flow cytometry (Fig. 2D). HDMECs served as a positive control while parental HPVEC and human saphenous vein smooth muscle cells (HSVSMC) were analyzed for comparison. Clone 5 and 8 also expressed TGF-β-receptors I, II and III (Fig 2B). Immunofluorescence staining of Clone 8 and Clone 5 showed cell-cell border staining for CD31 and VE-cadherin, a punctate cytoplasmic staining for vWF, consistent with its localization in Weibel-Palade bodies, but little α-SMA (Fig. 2C). The flow cytometry analysis showed a similar pattern among HPVEC, Clone 8, Clone 5 and HDMECs and a clear distinction from HSVSMCs. The clones were negative for mesenchymal marker CD90 (Fig. 2D). Based on the expression patterns, Clone 8 and Clone 5 appeared to be endothelial cells despite lacking a typical cobblestone appearance.
Figure 2.
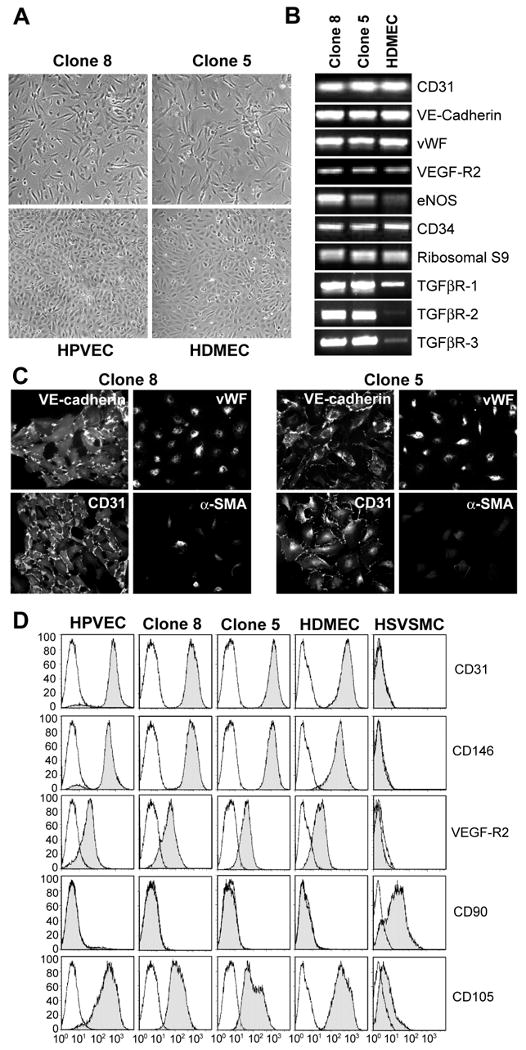
Clonal cell populations from human pulmonary valve express endothelial-specific markers. (A) Phase contrast micrographs of Clone 8 and Clone 5 (top panels) parental HPVECs and HDMECs (bottom panels) grown in EBM-2. (B) RT-PCR analysis of Clones 8 (lane 1), 5 (lane 2) and HDMECs (lane 3) for endothelial-specific transcripts and TGF-β-receptor transcripts. Ribosomal S9 served as a control. (C) Immunostaining of Clone 8 (left panels) and Clone 5 (right panels) with anti-VE-cadherin, anti-vWF, anti-CD31, and anti-α-SMA Abs. (D) Flow cytometric analysis of parental HPVEC, Clone 8, Clone 5, HDMEC and HSVSMC stained with FITC-conjugated CD31, CD146, VEFG-R2, CD90 and CD105 Abs.
TGFβ2 induces expression smooth muscle markers in HPVEC clones
TGFβ isoforms are well-documented in the differentiation of endothelial to mesenchymal cells in mouse and chick endocardial cushion formation4,6,15,19,22,23. However, whether TGFβ isoforms induce EMT in human valve endothelial cells is unknown. We tested whether the clonal populations from HPVECs can be induced to undergo EMT in response to TGFβ1, β2, or β3. Clonal cells are essential for this experiment in order to insure that effects are not due to preferential growth of mesenchymal cells that could contaminate a non-clonal endothelial cell culture. We treated the cells with TGFβ1-3 for 10 days and examined the changes in the expression of smooth muscle cell/mesenchymal markers α-SMA and calponin (Fig. 3A). TGFβ2, but not TGFβ1 or TGFβ2 isoforms, induced robust expression of α-SMA and calponin in Clone 8. Induction was also evident in Clone 5 and in the parental HPVECs, but to a lesser extent because the cells expressed α-SMA in the absence of TGFβ2. Increased expression of α-SMA and calponin was confirmed by RT-PCR (data not shown). HDMEC did not express α-SMA or calponin in either the absence or presence of TGFβ2. Consistently, TGFβ2 treatment attenuated CD31 expression in Clone 8 and Clone 5. In Fig. 3B, Clone 8 and Clone 5 treated without or with TGFβ2 for 10 days were analyzed for co-expression of CD31 and α-SMA by double-label immunofluorescence. The presence of both CD31 and α-SMA in individual cells supports the finding that the cells are undergoing EMT. The increase in α-SMA expressing (red) CD31-positive cells in response to TGFβ2, quantitated by counting fluorescent cells, was significant.
Figure 3.

TGFβ2-induced expression of α-SMA and calponin. (A) Clones 8 and 5, the parental HPVEC and HDMECs were grown for 10 days in absence (control, lane 1) or presence of 2ng/ml of TGFβ1 (lane 2), TGFβ2 (lane 3) or TGFβ3 (lane 4). Cell lysates were analyzed by western blot for the expression of CD31, α-SMA and calponin. (B) Clone 8 (left panels) and Clone 5 (right panels) were grown for 10 days in absence (control; top panels) or presence of 2ng/ml of TGFβ2 (TGFβ2; bottom panels). Cells were double-labeled with goat anti-human CD31/FITC-conjugated secondary Ab and mouse anti-α-SMA/Texas Red-conjugated secondary Ab. α-SMA positive cells, which also expressed CD31, were counted to determine number of positive cells in each clone. * P values<0.05.
TGFβ2 treatment results in up-regulation of EMT-related transcripts
Slug and its homolog Snail are zinc finger transcription factors that mediate both epithelial and endothelial transdifferentiation 24. Slug expression in developing valves is TGFβ2-dependent 25 and down-regulating Slug with anti-sense oligonucleotides inhibited EMT in chick endocardial cushion explants26. Therefore, we investigated if these markers were up-regulated when Clone 5 and Clone 8 cells were induced to undergo EMT (Fig 4A). TGFβ2 induced expression of Slug and caused a modest enhancement of Snail. The expression of other known EMT-related proteins, hHex, BMP-2 or Jagged-1 was unaltered by TGFβ2 (data not shown). Enhanced migration is another hallmark of EMT and crucial players involved in this process belong to the family of matrix metalloproteases (MMPs)6. TGFβ2 significantly enhanced the expression of MMP-1 and MMP-2 in HPVEC clones as assessed by RT-PCR (Fig 4A). To measure cellular migration directly, different factors were tested for ability to stimulate migration of Clone 8 (Fig 4B). Increased migration in response to serum, PDGF and bFGF was observed in cells treated with TGFβ2 compared to untreated control cells. We also measured the ability of Clone 8 to invade a collagen gel. TGFβ2-treated or untreated Clone 8 cells were compared to HDMEC treated with or without TGFβ2. In this assay, TGFβ2-treated cells were more invasive compared to the control cells, with TGFβ2-treated Clone 8 cells showing the most robust invasion (Fig 4D). In addition, Clone 5 and Clone 8 exhibited 4-5 fold higher levels of basal migration compared to the HDMECs (Fig 4C). As enhanced basal migration is a hallmark of endothelial progenitor cells27 the increased migratory activity of Clone 5 and Clone 8 is consistent with a progenitor phenotype.
Figure 4.
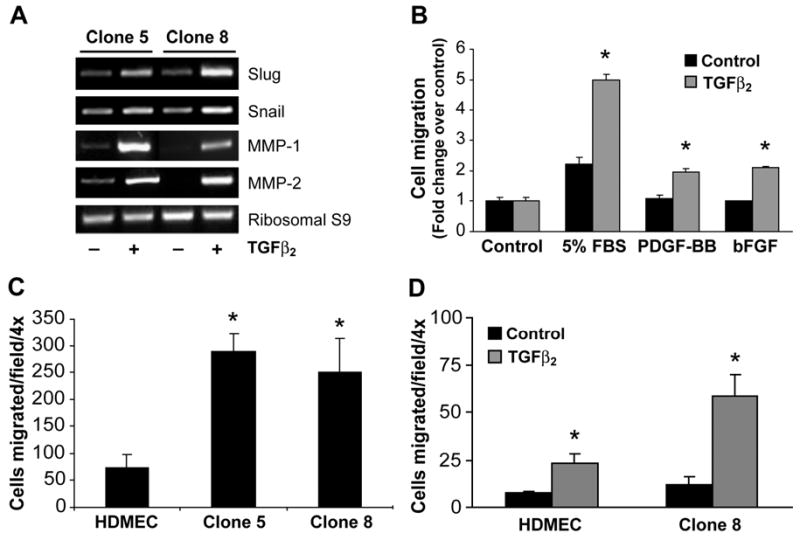
TGFβ2-induced expression of EMT markers, migration, and invasion. (A) Clone 5 (lanes1 and 2) and Clone 8 (lanes 3 and 4) were grown for 10 days in absence (lanes 1 and 3) or presence of 2 ng/ml TGFβ2 (lanes 2, 4). RNA was extracted and RT-PCR was performed with Slug, Snail, MMP-1 and MMP-2 primers. Ribosomal S9 served as a control. (B) Clone 8 cells cultured in absence (black bars) or presence (grey bars) of TGFβ2 for 10 days were tested for ability to migrate towards EBM (control), EBM with 5% FBS, 10 ng/ml PDGF-BB, and 10ng/ml bFGF. (C) HDMECs, Clone 5 and Clone 8 cells were cultured in EBM-2 medium and basal migration towards control medium was tested. (D) HDMECs and Clone 8 cells were cultured in absence (black bars) or presence (grey bars) of TGFβ2 in EBM-2 medium for 10 days and were tested for invasion into collagen gels for 72 h. Data from the migration and invasion assays are mean ± SD of two independent experiments performed in triplicates. In panels B-D, * represent p<0.05 compared control.
HPVEC clones exhibit endothelial/ mesenchymal plasticity
We also examined the effects of adding additional VEGF-A (10ng/ml) on differentiation of these clones. (EBM-2 supplemented with EGM-2 SingleQuots contains 2-5ng/ml VEGF-A.) The additional VEGF caused the cell clusters to form a confluent cobblestone-like monolayer (Fig. 5A), with CD31 localized at cell-cell borders (Fig 5B). To determined if this effect was specific to VEGF or a general response to growth factors, cells were treated with EBM-2 media without the added growth factors from Single Quots (-GF), with -GF media to which bFGF, EGF, PDGF, or VEGF had been added, and with full EBM-2 growth medium with Single Quots. In media without growth factors, both CD31 and α-SMA were expressed; the levels were not significantly altered by addition of bFGF or EGF. PDGF treatment led to reduction in CD31 and α-SMA expression. In contrast, cells treated with 10ng/ml VEGF or with complete EBM-2 medium showed enhanced CD31 expression and reduced α-SMA expression (Fig 5C) suggesting that VEGF drives these cells to stronger endothelial phenotype. Taken together, the results demonstrate that these cells have a dual potential to differentiate to an endothelial phenotype with VEGF or a mesenchymal phenotype with TGFβ2 (Fig 4 and Fig.5A). The fact that the clones were expanded from a single cell, coupled with the above findings, suggests that these clones are bi-potential progenitors that reside in the pulmonary valve leaflets.
Figure 5.
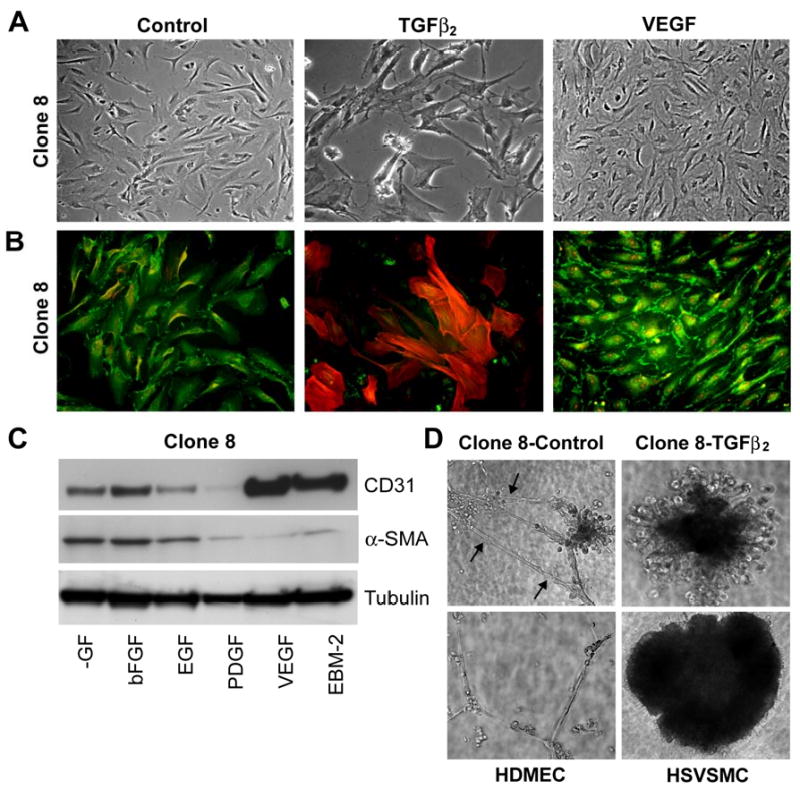
Phenotypic modulation of HPVEC clones. Clone 8 cells were cultured in absence (control) or presence of 2ng/ml TGFβ2 or 10ng/ml VEGF in EBM-2 medium for 10 days. (A) Phase contrast micrographs show cell morphology. (B) Double-label immunostaining of same cells with anti-CD31 (FITC) and anti-α-SMA (Texas Red). (C) Clone 8 cells were cultured in growth factor depleted medium (-GFl) or supplemented with bFGF (10ng/ml), EGF (10ng/ml), PDGF (10ng/ml), VEGF (10ng/ml), and EBM-2 medium for 10 days. Cell lysates were analyzed for the expression of CD31 and α-SMA, and tubulin to confirm equal loading. (D) Tube formation on Matrigel. Clone 8 cells were cultured in absence (control) or presence of TGFβ2 in EBM-2 medium for 10 days and then plated on Matrigel. Tube formation was assessed after 24 hours in comparison with HDMEC and HSVSMC that had not been treated with TGFβ2.
TGFβ2 abrogates the functional endothelial properties of the HPVEC clones
We then asked if these clones have the potential to function as normal endothelial and if so, what effect does TGFβ2 have. Tube formation in Matrigel is a characteristic feature of endothelial cells. Therefore, we investigated the tube-forming ability of these cells in presence or absence of TGFβ2 (Fig 5D). Untreated Clone 8 cells formed tube-like structures with transluscent slits along the length of the cords, indicating lumen formation. However, prior treatment with TGFβ2 inhibited tube formation of Clone 8 and instead caused the cells to contract into a large clump that appeared similar to untreated HSVSMCs. HDMECs treated with TGFβ2 were able to form tubes (data not shown) as were the un-treated HDMECs (Fig 5D).
A second functional property of endothelial cells is expression of adhesion molecules E-selectin, ICAM-1 and VCAM-1 in response to tumor necrosis factor (TNF)-α. Therefore, HPVEC clones cultured in either VEGF or TGFβ2 were exposed to TNF-α for 5 hours. Both clones as well as HDMECs showed TNF-α-induced expression of E-selectin, VCAM-1 and ICAM-1. Prior treatment with TGFβ2 greatly attenuated the TNFα-induced expression of the adhesion molecules in Clone 8 and Clone 5 but had no effect on the induction level in HDMECs (Fig 6A). Complementing these findings, a leukocyte adhesion assay revealed strong adhesion of HL-60 cells, a human promyelocytic leukemia line, to both Clone 8 and Clone 5 when stimulated with TNF-α for 5 hours. However, prior treatment with TGFβ2 inhibited binding of HL-60 cells to Clone 8 but not to HDMECs (Fig 6B). Treatment with VEGF prior to TNF-α induction led to modest increase in this adhesion response consistent with results in Fig 6A. These data demonstrate that TGFβ2 treatment abrogates endothelial functional properties specifically in HPVEC-derived clones but not in HDMECs,
Figure 6.
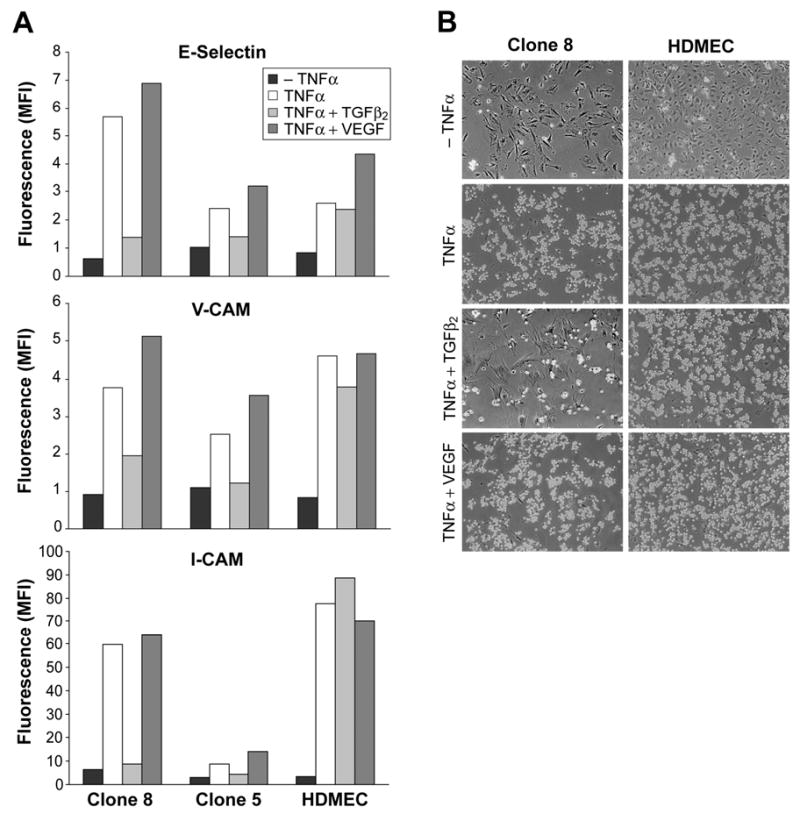
Cytokine-induced expression of leukocyte adhesion molecules and adherence of leukocytes. Clone 8 cells were cultured in absence or presence of 2ng/ml TGFβ2 or 10ng/ml VEGF in EBM-2 medium for 10 days. Cells were further treated without (-TNFα) or with TNF-α (TNF-α +) for 5 h and analyzed for the expression of leukocyte adhesion molecules by flow cytometry. B. HL-60 cell adhesion to Clone 8 and HDMECs with similar treatments as in A was determined by light microscope.
Reversibility of endothelial and mesenchymal phenotypes
The above results prompted us to investigate if the changes we observed with TGFβ2 and VEGF are transient or whether differentiation status is maintained after removal of the factors. We treated clonal cells with TGFβ2 or VEGF for 10 days, trypsinized and re-seeded the cells in presence of TGFβ2 and VEGF according to the scheme in Fig. 7A. Lanes 1-3 show that the cells differentiated as expected based on our previous results. When cells were first induced to a mesenchymal phenotype with TGFβ2, we found that removing TGFβ2 reversed the phenotype (compare lanes 4 and 5). The presence of additional VEGF did not result in any further decrease in α-SMA (compare lane 4 and lane 6). After TGFβ2 treatment, a 10 day treatment with VEGF did not fully restore the endothelial phenotype (compare lanes 3 and 6). When cells were first cultured in VEGF for 10 days, subsequent exposure to TGFβ2 resulted in a full reversal (compare lane 8 to lanes 2 or 5). These results suggest that 1) VEGF is required for the cells to maintain an endothelial phenotype; 2) TGFβ2-induced α-SMA can be partially reversed; and 3) prior treatment with TGFβ2 or VEGF does not diminish the plasticity of the cells (Fig 7B). Similar results were obtained with both Clone 5 and Clone 8. The reversibility may be influenced by the ECM such that removal of the cells from the ECM produced in response to TGFβ2 might exert control over the cellular phenotype. To address the role of ECM, we examined the effect of plating the cells on different ECM to determine if exogenous ECM might alter EMT. As seen in Fig 7C, cells plated on fibronectin showed markedly enhanced TGF-β2 induction of α-SMA, consistent with the potential role for fibronectin in EMT in endocardial cushions 6.
Figure 7.
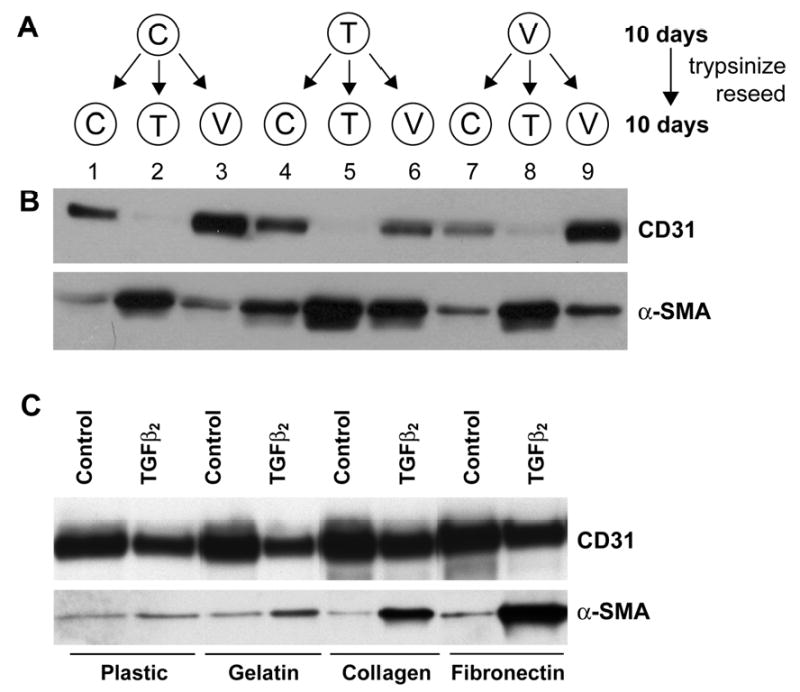
Changes induced by TGFβ2 and VEGF were reversible and effects of ECM on TGFβ2-induced EMT. Panel A: Schematic of culture conditions: Clone 8 cells were cultured in EBM-2 medium (C), EBM-2 medium with 2ng/ml TGFβ2 (T) or EMB-2 medium with 10ng/ml VEGF (V) for 10 days. Cells were then re-plated and cultured for 10 days in (C), (T) or (V). Panel B: Cell lysates were analyzed for the expression of CD31 and α-SMA. It is important to note that the EBM-2 medium (C) is supplemented with the commercially available SingleQuots, which contains 2-5 ng/ml VEGF-A. “V” medium is C supplemented with an additional 10ng/ml VEGF-A. Panel C: Clone 8 cells were plated on different ECM substratum in the absence or presence of TGFβ2 to assess the effects on induction of α-SMA.
A hierarchy of endothelial/mesenchymal plasticity among HPVEC clonal populations
To further understand the role of VEGF in the endothelial to mesenchymal differentiation, we analyzed three different clones, Clones 1, 8, 5, that varied in their potential to undergo EMT (Fig 8A). Clone 1 cells did not express α-SMA or calponin in response to TGFβ2 suggesting that this clone is unable to undergo EMT. However, addition of VEGF resulted in increased CD31 and VE-cadherin protein levels (Fig 8A); co-incubation with TGFβ2 had no effect on VEGF-enhanced expression of these endothelial markers. Clone 8 responded to both TGFβ2 and VEGF; TGFβ2 induced expression of α-SMA and calponin and a decrease in CD31 while VEGF increased CD31 and VE-cadherin. Strikingly, VEGF reduced the TGFβ2-induction of α-SMA and calponin in Clone 8. Clone 5, on the other hand, showed spontaneous transdifferentiation as seen by the co-expression of CD31 and α-SMA under control conditions (Fig. 8A, and 3B). TGFβ2 caused increased α-SMA and calponin and decreased CD31 as expected. However, VEGF was unable to block the effects of TGFβ2 on α-SMA and calponin. This suggests that Clone 5 is further differentiated towards a mesenchymal phenotype than Clone 8. The band intensities for CD31 and α-SMA for each clone in each condition from two separate experiments were quantitated by densitometry to substantiate the results (Fig 8B). In summary, these results support the concept that a hierarchy exists among these clonal cell populations in their propensity to transition from an endothelial to mesenchymal phenotype in response to VEGF or TGFβ2 (Fig 8C).
Figure 8.
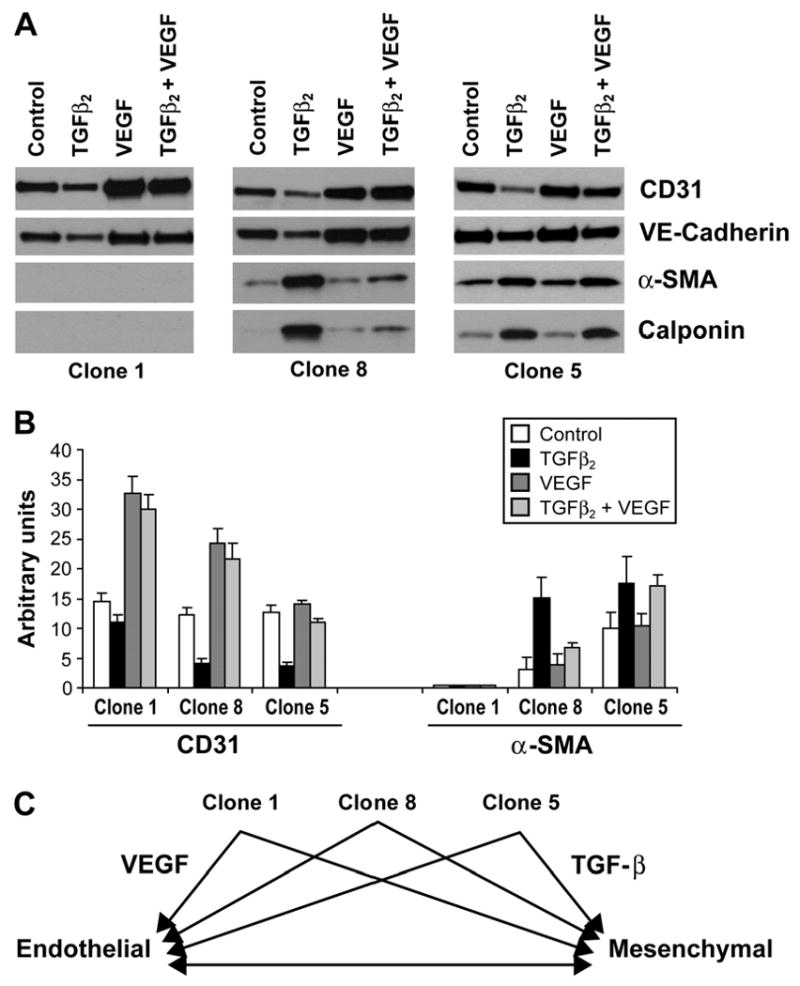
Endothelial/mesenchymal hierarchy in HPVEC clones. Clone 1 (left panel), Clone 8 (middle panel) and Clone 5 (right panel) were grown for 10 days in absence (control), 2ng/ml of TGFβ2 (TGFβ2), 10ng/ml VEGF (VEGF), or TGFβ2 and VEGF (TGFβ2+VEGF). Cell lysates were analyzed by western blot for the expression of CD31, VE-Cadherin, α-SMA and calponin. B. Quantitation of bands seen in A by densitometry. C. Schematic representation of hierarchy in plasticity of pulmonary valve progenitor cells.
Discussion
In the present study, we report for the first time that clonal endothelial-like cells isolated from human pulmonary valves transition to a mesenchymal phenotype, analogous to EMT, specifically in response to TGFβ2. TGFβ2 induced expression of EMT markers and increased cellular migration and invasion capacity. Endothelial functions such as ability to form tube-like structures and to adhere leukocytes in response to inflammatory signals were lost in TGFβ2-treated cells. The cells were not responsive to TGFβ1 consistent with the functional role for TGFβ2 in EMT in avian and murine endocardial cushions 28. In contrast, VEGF promoted formation of cell-cell contacts, a cobblestone morphology, and increased levels of CD31 and VE-cadherin. Our finding that VEGF can inhibit TGFβ2-induced EMT provides a potential mechanism to explain the negative role reported for VEGF in endocardial cushion formation 8,11. We postulate that the clonal populations are bi-potential valve progenitor cells based on their plasticity and their ability to grow in culture from a single cell. We further propose that these cells represent the CD31/α-SMA-positive cells seen by in situ immunostaining of human pulmonary valve leaflets. Whether the reversibility of the endothelial and mesenchymal phenotypes seen in vitro occurs in vivo will require further investigation. Finally, the differentiation potential seems to be restricted as clones failed to differentiate into adipocytes, chondrocytes osteocytes or hematopoietic progenitors in in vitro assays (data not shown).
We isolated valvular progenitor cells from discarded, surgically resected pulmonary valve tissue from three different patients. Two were infants, ages 5 months and 9 months, and the third was a fifteen year old. The number of single cells that grew into clonal populations was approximately 30%, but the number of bi-potential clones ranged from 1% from the 15 year old to 3-4% from the infant specimens. This corresponds well to our in vivo data showing that cells expressing both CD31 and α-SMA are found in higher numbers in fetal specimens compared to the adult valves (Fig 1). Though the valve progenitor cells are different from normal human microvascular ECs, such as HDMECs, they express endothelial markers and behave as functional endothelial cells in two in vitro assays. The endothelial properties we detected in the clones could be explained by that fact that they were expanded in a medium designed for growth of human endothelial cells, EBM-2, which contains 2-5 ng/ml VEGF. This level of VEGF was not sufficient to block the action of TGFβ2 in Clones 5 and 8 but may have been in Clone 1.
HDMECs were not responsive to TGFβ1–3 suggesting that post-natal EMT is specific to valve endothelium and perhaps to a subset of valve EC with progenitor properties. However, TGFβ-induced differentiation of large vessel-derived EC into SMC-like cells has been reported, suggesting that EMT may not be unique to the valve endothelium and that vascular progenitors may reside in the vessel wall. In 1992, bovine aortic EC were shown to express α-SMA, with a concomitant decrease in EC markers, in response to TGFβ129. DeRuiter and colleagues traced EC in the embryonic dorsal aorta that migrated into the subendothelial space and expressed α-SMA, indicating EMT in embryonic endothelium 30. A small percentage of cells in EC preparations from bovine arteries were also shown to undergo EMT 31. More recently, clonal populations of human umbilical vein ECs were shown to differentiate into SMC-like cells when FGF-1 was removed 32. Hence, although the valve endothelium appears to harbor endothelial cells that can be easily prompted to undergo EMT, endothelial cells with capacity to undergo EMT may reside throughout the vasculature and perhaps through embryonic, fetal, childhood and adult life.
How closely the EMT we observe in valve EC progenitors in vitro reflects the EMT that occurs in vivo, either during valve development or during post-natal life, is a critical question. Many of the hallmarks of EMT are seen in our in vitro system: loss of cell-cell contacts in the EC monolayer, increased expression of α-SMA, MMP-1, MMP-2 and Slug, increased cellular migration and invasion, and induction of Sox9 (unpublished results). The specificity of TGFβ2 for inducing these events is also consistent with in vivo studies of EMT in mouse endocardial cushions28. α-SMA is expressed transiently during EMT in developing valves, during steps of endothelial delamination and migration 4 but is found only in focal regions along the endothelium in healthy post-natal valves 12,15. Therefore, we postulate that the α-SMA-positive valve progenitors are recapitulating early events in EMT. Identification of specific markers of the interstitial cells of heart valves would provide important tools for studying the entire differentiation process.
HPVEC clones also exhibit a hierarchy in which some clones are more endothelial-like while others are more mesenchymal-like in terms of response to exogenous factors. We hypothesize that these cells are valve progenitors that participate in cellular renewal of the valve endothelium and the interstitial cells of the leaflet. Differentiation towards one cell type or the other would depend on exposure to biochemical and mechanical signals and possibly even to pathological signals arising from disease or structural malformations. Alterations in valve progenitor cell proliferation or differentiation may occur in any number of deleterious conditions leading to calcification, leaflet thickening or even loss of integrity. Understanding the functional characteristics of these cells may lead to greater understanding of heart valve disease and eventually to strategies to manipulate these cells for therapeutic benefit.
Supplementary Material
Acknowledgments
We thank Dr. John E. Mayer, Jr, Cardiac Surgery, Children's Hospital Boston for providing human pulmonary valve leaflets for cell isolation. This work was supported by R01 HL 06490 to J.B. and a Bogue Research Fellowship, University College London to S.L.
References
- 1.Rabkin-Aikawa E, Mayer JE, Jr, Schoen FJ. Heart valve regeneration. Adv Biochem Eng Biotechnol. 2005;94:141–79. doi: 10.1007/b100003. [DOI] [PubMed] [Google Scholar]
- 2.Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. Am J Anat. 1977;148:85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- 3.Bernanke DH, Markwald RR. Migratory behavior of cardiac cushion tissue cells in a collagen-lattice culture system. Dev Biol. 1982;91:235–45. doi: 10.1016/0012-1606(82)90030-6. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima Y, Mironov V, Yamagishi T, Nakamura H, Markwald RR. Expression of smooth muscle alpha-actin in mesenchymal cells during formation of avian endocardial cushion tissue: a role for transforming growth factor beta3. Dev Dyn. 1997;209:296–309. doi: 10.1002/(SICI)1097-0177(199707)209:3<296::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–27. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 7.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–22. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 8.Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–8. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 9.Hallaq H, Pinter E, Enciso J, McGrath J, Zeiss C, Brueckner M, Madri J, Jacobs HC, Wilson CM, Vasavada H, Jiang X, Bogue CW. A null mutation of Hhex results in abnormal cardiac development, defective vasculogenesis and elevated Vegfa levels. Development. 2004;131:5197–209. doi: 10.1242/dev.01393. [DOI] [PubMed] [Google Scholar]
- 10.Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–6. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- 11.Chang CP, Neilson JR, Bayle JH, Gestwicki JE, Kuo A, Stankunas K, Graef IA, Crabtree GR. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell. 2004;118:649–63. doi: 10.1016/j.cell.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 14.Sainz J, Al Haj Zen A, Caligiuri G, Demerens C, Urbain D, Lemitre M, Lafont A. Isolation of “side population” progenitor cells from healthy arteries of adult mice. Arterioscler Thromb Vasc Biol. 2006;26:281–6. doi: 10.1161/01.ATV.0000197793.83391.91. [DOI] [PubMed] [Google Scholar]
- 15.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–43. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veinot JP, Prichett-Pejic W, Song J, Waghray G, Parks W, Mesana TG, Ruel M. CD117-positive cells and mast cells in adult human cardiac valves--observations and implications for the creation of bioengineered grafts. Cardiovasc Pathol. 2006;15:36–40. doi: 10.1016/j.carpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Deb A, Wang SH, Skelding K, Miller D, Simper D, Caplice N. Bone marrow-derived myofibroblasts are present in adult human heart valves. J Heart Valve Dis. 2005;14:674–8. [PubMed] [Google Scholar]
- 18.Visconti RP, Ebihara Y, Larue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An In Vivo Analysis of Hematopoietic Stem Cell Potential. Hematopoietic Origin of Cardiac Valve Interstitial Cells. Circ Res. 2006;98 doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 19.Johnson EN, Lee YM, Sander TL, Rabkin E, Schoen FJ, Kaushal S, Bischoff J. NFATc1 mediates vascular endothelial growth factor-induced proliferation of human pulmonary valve endothelial cells. J Biol Chem. 2003;278:1686–92. doi: 10.1074/jbc.M210250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest. 2001;107:745–52. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33:75–9. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima Y, Miyazono K, Kato M, Takase M, Yamagishi T, Nakamura H. Extracellular fibrillar structure of latent TGF beta binding protein-1: role in TGF beta-dependent endothelial-mesenchymal transformation during endocardial cushion tissue formation in mouse embryonic heart. J Cell Biol. 1997;136:193–204. doi: 10.1083/jcb.136.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima Y, Yamagishi T, Nakamura H, Markwald RR, Krug EL. An autocrine function for transforming growth factor beta 3 in the atrioventricular endocardial cushion tissue formation during chick heart development. Ann N Y Acad Sci. 1998;857:272–5. doi: 10.1111/j.1749-6632.1998.tb10130.x. [DOI] [PubMed] [Google Scholar]
- 24.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 25.Romano LA, Runyan RB. Slug is an essential target of TGFbeta2 signaling in the developing chicken heart. Dev Biol. 2000;223:91–102. doi: 10.1006/dbio.2000.9750. [DOI] [PubMed] [Google Scholar]
- 26.Romano LA, Runyan RB. Slug is a mediator of epithelial-mesenchymal cell transformation in the developing chicken heart. Dev Biol. 1999;212:243–54. doi: 10.1006/dbio.1999.9339. [DOI] [PubMed] [Google Scholar]
- 27.Khan ZA, MeleroMartin JM, Wu X, Paruchuri S, Boscolo E, Mulliken JB, Bischoff J. Endothelial Progenitor Cells from Infantile Hemangioma and from Umbilical Cord Blood Display Unique Cellular Responses to Endostatin. Blood. 2006;108:915–921. doi: 10.1182/blood-2006-03-006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–81. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- 29.Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–9. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 30.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–51. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 31.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–96. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 32.Ishisaki A, Hayashi H, Li AJ, Imamura T. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. J Biol Chem. 2003;278:1303–9. doi: 10.1074/jbc.M207329200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


