Abstract
Breathing relies on a respiratory rhythm generator. A study characterizes an early emerging oscillatory group of Phox2b-expressing parafacial cells that entrain and couple with the preBötzinger Complex at the onset of fetal breathing.
Successful team performance requires practice; stepping onto the field with new players who have just met is not a recipe for success. Even if all the players have been working out individually, practicing together is essential to coordinate plays and to develop a successful team rhythm. At birth, mammals are thrust into a game of survival and to play this game must reliably breathe and suckle. To accomplish these goals, mammals practice breathing in utero. These episodic rhythmic fetal breathing movements (FBMs) are required for proper lung development and assure that respiratory muscles and the neural system that drives them are functional and coordinated at birth. In rodents1, the onset of FBMs involving the diaphragm is coincident with the onset of rhythmicity in the preBötzinger Complex (preBötC), a medullary area that is essential for respiration2. In this issue, Thoby-Brisson et al. have identified a second rhythmogenic area, the embryonic parafacial nucleus (e-pF), as being important in the neurogenesis of respiratory rhythms. They present elegant and definitive experiments showing that the e-pF is the source of the earliest behaviorally relevant rhythm for FBMs, starting at embryonic day 14.5 (E14.5) in mouse3. Furthermore, they show that it contributes substantially to the subsequent onset and development of rhythmicity at E15.5 in the preBötC, the presumptive onset and maintenance of FBMs, and reliable breathing at birth.
Since its identification in 19904, the preBötC has increasingly assumed the mantle of the principal rhythm generator for breathing. The preBötC drives inspiratory muscle activity and is the only known group of neurons that, when silenced, promptly results in a complete arrest of breathing, sufficient to asphyxiate conscious, unanesthetized adult rodents5. The coincident onset of rhythmicity in the preBötC and FBMs in rodents suggests a causal relationship. In 1996, however, it was shown that the preBötC is not sufficient to secure robust breathing during the perinatal period6. Mice with a deletion of the transcription factor Egr2, also known as Krox20, have alterations affecting rhombomeres 3 and 5 that remove an embryonic rhythmic source near the facial motor nucleus (nVII), resulting in markedly depressed breathing at birth. These results suggest that the e-pF, defined as the population of neurons flanking and partially capping the lateral aspect of nVII and extending approximately 200 μm caudal to nVII, is essential for driving breathing rhythm at birth when it acts as an ‘anti-apnea’ center7.
In this issue, Thoby-Brisson et al.3 examine the ontogeny of the e-pF and its relationship to the preBötC during prenatal development and reveal intriguing functional interactions in the respiratory rhythm generator. They treated blocks and slices of medulla from embryonic mice with a calcium indicator dye that fluoresces to reflect neuronal activity. Observing the ventral face of the embryonic brainstem, they found that the very first rhythmic neurons appeared at E14.5 (Fig. 1a). These bilateral neuronal populations each formed a cap over the ventrolateral and caudal part of nVII (that is, the e-pF). The e-pF oscillator on each side of the medulla is composed of about 260 Phox2b-positive glutamatergic neurons that are derived from Egr2-expressing progenitors. About 70% of these neurons express the neurokinin 1 receptor, which is also a marker for critical preBötC neurons2. Using pharmacology and knockout mice, Thoby-Brisson et al.3 found that rhythm generation in the e-pF network appears to be independent of glutamatergic synaptic transmission and opioid modulation, relying instead on a riluzole- and carbenoxolone-sensitive mechanism. This suggests the involvement of a persistent Na+ current and functional gap junction coupling. However, glutamatergic synaptic transmission is necessary for synchrony across the midline between bilateral e-pF areas (Fig. 1b).
Figure 1.
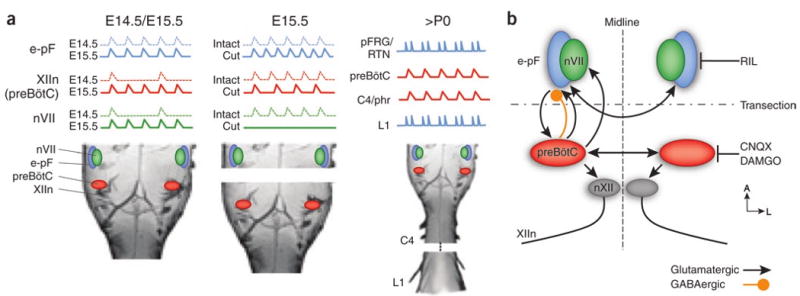
Development and properties of the respiratory rhythm generator, (a) Rhythmic activity appears earliest in the e-pF at E14.5 and only inconsistently drives FBMs in nVII and the hypoglossal nerve (XIIn). At E15.5, with the appearance of the preBötC, rhythmic FBMs are reliably generated. Transections do not eliminate rhythmic activity in either the e-pF or the preBötC, but the frequency is altered. After birth, the preBötC controls inspiratory motor activity, as recorded in the phrenic nerve (C4/phr), whereas a parafacial region, the pFRG/RTN, whose precursor is likely the e-pF, generates expiratory-modulated motor activity in abdominal muscles recorded in L1 and serves as a chemosensory area. (b) Schematic of the prenatal respiratory rhythm generator circuit. Arrows are schematic and may represent mono- or polysynaptic connections through intervening areas. Rhythmic activity in the e-pF is blocked by riluzole (RIL). Rhythmic activity in the preBötC can be silenced by DAMGO or CNQX.
PreBötC neurons begin to oscillate 1 d later (E15.5) in synchrony with the e-pF. These two regions can oscillate independently; when the en bloc brainstem is completely transected between the preBötC and the e-pF, both segments continue to oscillate endogenously. However, the frequency of oscillation is altered in both regions (Fig. 1a). These changes are probably the results of either the removal of interactions between the two oscillators and/or a modification in common, modulatory inputs, such as the raphe or locus coerelus. Although the e-pF contributes substantially to the establishment of a normal rhythmic activity in the preBötC, the e-pF does not appear to be essential for preBötC development. In mutants lacking the Egr2 gene, there is no rhythmic activity around nVII at E15.5, probably resulting from a loss of neurons that express Phox2b or neurokinin 1 receptor in the expected e-pF region. The respiratory rhythm measured in the hypoglossal nerve is still present, although it is slowed to half of the frequency of that observed in wild-type mice. Transection between the presumptive e-pF and the preBötC has no effect on this rhythm, suggesting that its origin is the preBötC, which presumably developed in the absence of the e-pF. At birth, breathing in these mutants is slow and variable, and most of the mice die shortly after birth. However, injection of an opioid receptor antagonist after birth can rescue the breathing defect and markedly improve mutant viability. The e-pF may therefore be essential for overcoming preBötC depression caused by the substantial opiate surge at birth2.
Although the e-pF is important during practice and in the earliest portion of the game, what happens after birth? Convergent data from many laboratories point to the parafacial region as a potential rhythmic source for breathing in postnatal rodents, and three main lines of supporting evidence are highlighted here. First, on the basis of its projections to the medullary respiratory network, we identified and named the retrotrapezoid nucleus (RTN)8, a small region that is ventral to nVII and is demarcated by neurons expressing the transcription factor Phox2b. We speculated that it was a site for central chemoreception8 and a potential respiratory oscillator4. Humans with mutations affecting Phox2b have congenital central hypoventilation syndrome, which is characterized by an inability to sustain robust breathing during sleep and a marked insensitivity to CO2 stimulation of breathing9. In mice, similar mutations severely disrupt breathing at birth and typically result in early postnatal death10. Second, another study found two sources of respiratory-phased rhythm in neonatal brainstem: the preBötC and a region ventral to nVII that was called the parafacial respiratory group (pFRG)11. Some RTN/pFRG neurons project caudally to brainstem premotoneurons, which drive spinal expiratory motoneurons, suggesting that these neurons are involved in the generation of expiratory movements12. Third, after depressing preBötC neurons with opioids, an unusual breathing pattern, called quantal slowing, can develop in both en bloc preparations and in young rats in vivo13. In this pattern, inspiratory motor activity skips beats, but expiratory motor activity is unaffected13,14. Transecting the brainstem rostral to the RTN/pFRG in juvenile rats does not substantially affect inspiratory and expiratory motor activity, but transection between RTN/pFRG and preBötC completely abolishes active expiratory motor activity with only a modest effect on inspiratory pattern14. These data underlie our hypothesis that in older rodents, and presumably other mammals, the preBötC drives the inspiratory-dominated respiratory pattern, whereas the RTN/pFRG produces a CO2/state-dependent rhythmic drive to expiratory muscles. The preBötC-driven inspiratory breathing pattern dominates at rest, during which the RTN/pFRG may only have tonic activity2,14. During exertion, however, when O2 consumption and CO2 production rise substantially, the activity of RTN/pFRG neurons may become increasingly rhythmic (Fig. 1a).
To help understand the distinct prenatal development of the preBötC and e-pF, we considered their evolutionary origin, a perspective that, although speculative, suggests a basis for their functional roles postnatally. The e-pF, which develops first, represents the phylogenetically ancient rhythm generator that drove breathing in aquatic vertebrates, whereas the preBötC represents the newer oscillator that emerged with the evolution of the lung and its complement of muscles. The evolutionary appearance of the diaphragm in mammals enabled a highly efficient inspiratory-driven pattern at rest that is sufficient to support endothermy. This would have led to the dominance of the preBötC at rest, with RTN/pFRG quiescent at rest, but becoming rhythmic to produce active expiration necessary for higher levels of ventilation, such as during exercise.
The most parsimonious interpretation, then, is that the e-pF becomes the pFRG. However, several issues warrant further investigation. First, in neonatal en bloc preparations, the activity pattern of pFRG neurons is markedly different from patterns at E14.5/E15.5. The inspiratory-modulated pattern in the e-pF is transformed into a peri-inspiratory pattern consisting of pre-inspiratory, and sometimes post-inspiratory, activity, but is silent during inspiration. The cause of this transformation may be changes in inspiratory-modulated Cl−-dependent inputs, presumably from the preBötC, which are depolarizing at E15.5, but are hyperpolarizing after birth (Fig. 1a). Second, it is not clear under what conditions the RTN/pFRG is rhythmic in the adult rat. Under resting conditions in anesthetized rats, there is little, if any, rhythmic activity in RTN15; there is also very little, if any, active expiration. Third, Egr2−/− mutants retain their responsiveness to CO2 after birth, whereas mutants lacking Phox2b-derived neurons do not. Perhaps there are two groups of Phox2b neurons, one of which is critical for chemoreception (RTN) and the other of which is rhythmogenic (e-pF/pFRG).
Thoby-Brisson et al.3 convincingly demonstrate that the e-pF is important in the ontogeny of rhythmic circuits for FBMs. They also provide tantalizing details regarding its function and its interactions with the preBötC. These findings contribute substantially to our understanding of how the neural network underlying the vital motor behavior of breathing prepares to perform for a lifetime.
References
- 1.Kobayashi K, Lemke RP, Greer JJ. J Appl Physiol. 2001;91:316–320. doi: 10.1152/jappl.2001.91.1.316. [DOI] [PubMed] [Google Scholar]
- 2.Feldman JL, Del Negro CA. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoby-Brisson M, et al. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- 4.Feldman JL, Connelly CA, Ellenberger HH, Smith JC. Eur J Neurosci. 1990;3 Suppl:171. [Google Scholar]
- 5.Tan W, et al. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacquin TD, et al. Neuron. 1996;17:747–758. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 7.Borday C, et al. Prog Biophys Mol Biol. 2004;84:89–106. doi: 10.1016/j.pbiomolbio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- 9.Gronli JO, Santucci BA, Leurgans SE, Berry-Kravis EM, Weese-Mayer DE. Pediatr Pulmonol. 2008;43:77–86. doi: 10.1002/ppul.20744. [DOI] [PubMed] [Google Scholar]
- 10.Dubreuil V, et al. Proc Natl Acad Sci USA. 2008;105:1067–1072. doi: 10.1073/pnas.0709115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onimaru H, Homma I. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janczewski WA, Onimaru H, Homma I, Feldman JL. J Physiol (Lond) 2002;545:1017–1026. doi: 10.1113/jphysiol.2002.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janczewski WA, Feldman JL. J Physiol (Lond) 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulkey DK, et al. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]


