Abstract
In chronic infectious diseases, such as schistosomiasis, pathogen growth and immunopathology are affected by the induction of a proper balanced Th1/Th2 response to the pathogen and by antigen-triggered activation-induced T cell death. Here, by using S. japonicum infection or schistosome antigens-immunized mouse model, or antigens in vitro stimulation, we report that during the early stage of S. japonicum infection, nonegg antigens trigger Th2 cell apoptosis via the granzyme B signal pathway, contributing to Th1 polarization, which is thought to be associated with worm clearance and severe schistosomiasis. Meanwhile, after the adult worms lay their eggs, the egg antigens trigger Th1 cell apoptosis via the caspase pathway, contributing to Th2 polarization, which is associated with mild pathology and enhanced survival of both worms and their hosts. Thus, our study suggests that S. japonicum antigen-induced Th1 and Th2 cell apoptosis involves the Th1/Th2 shift and favorites both hosts and parasites.
1. Introduction
The balance between Th1 and Th2 cell responses to an infectious agent can influence both pathogen growth and immunopathology. In helminth infections, the parasites have evolved the capacity to induce Th2 responses in order to protect themselves against potentially toxic Th1-dependent antiparasitic effector mechanisms [1–3]. Many factors influence the differentiation of Th1 and Th2 cells, including the antigen dose and form, the affinity between the peptide antigen and the T cell receptor (TCR) [4], the nature and degree of co-stimulation [5], the presence of antigen-presenting cells (APC) [6, 7], and the cytokine milieu surrounding the differentiating cells [8]. In addition, antigen-triggered activation-induced cell death (AICD), which is the primary form of apoptosis for clonally expanded T cells, can influence both pathogen growth and immunopathology. AICD is considered the primary mechanism for deleting mature CD4+ T cells in the periphery and it plays an important role during adaptive immune responses by ensuring that a defined number of specialized T cells remain in the organism [9, 10].
Approximately 200 million people worldwide currently suffer from schistosomiasis, one of the most important human parasitic diseases. The main lesions resulting from schistosome infections are not caused by the adult worms. Instead, the lesions are caused by eggs trapped in human tissues, which stimulate immunopathological reactions including granulomas and fibrosis. Schistosomiasis is immunologically characterized by an early Th1 response that switches to a Th2-dominated response after the onset of parasite egg production [11]. Schistosomiasis has provided excellent models to study the induction and regulation of Th cell subset responses to infection. In the course of a schistosomal infection, the immune response progresses through at least three phases. (1) During the first three weeks of the infection, when the host is exposed to migrating immature and mature parasites, the dominant response is Th1-like. The response is induced by nonegg antigens, such as the cercariae, schistosomula, and schistosome worm antigens (SWA) [12, 13]. (2) As the parasites begin to produce eggs around week four, the response alters. The Th1 component begins to decrease, and this is associated with the emergence of a stronger Th2 response, which is primarily induced by egg antigens [13, 14]. (3) During the chronic phase of infection, the Th2 response is predominant and modulated. The granulomas that form around the eggs are smaller than at earlier times during the infection [13]. This produces a situation that is optimal for parasite survival concurrent with a condition that imparts minimal self-damage to the host. In addition to the Th1/Th2 shift, it has been reported that soluble schistosome egg antigen-(SEA-)stimulated T helper (Th) cell apoptosis occurs after egg laying and continues throughout the florid and downmodulated stages of schistosome infection, suggesting that Th cell apoptosis may represent a second significant method for controlling CD4+ T cells that mediate the immunopathology in schistosomiasis [15, 16].
To date, there is very little data available showing that Th1 or Th2 cell apoptosis is sensitive to the stimulation of different pathogen antigens. There is also little evidence that Th cell apoptosis contributes to the Th1/Th2 polarizations observed during different stages of schistosome infection. Here, we investigated whether different schistosome antigens could primarily trigger the death of different types of activation-induced Th cells and contribute to Th1/Th2 polarization during the stages of S. japonicum infection.
2. Materials and Methods
2.1. Mice, Infection, and Chemotherapy
Eight-week-old C57BL/6 female mice were provided by the Center of Experimental Animals (Nanjing University, Nanjing, China). All animal experiments were performed in accordance with Chinese animal protection laws and with permission from the Institutional Review Board. Oncomelania hupensis harboring S. japonicum cercariae were purchased from the Jiangsu Institute of Parasitic Diseases (Wuxi, China).
Each mouse was percutaneously infected with S. japonicum by placing a glass slide carrying 12 cercariae on its abdomen for 20 minutes. Some infected mice were orally administered artesunate (a gift from professor Junfan Shi, Zhejiang Institute of Parasitic Diseases, Hangzhou, China) dissolved in ddH2O in single 300 mg/kg doses at 7, 12, 17, 22, 27, 32, 37, and 42 days after infection. At 23, 35, and 56 days, six mice from each group were sacrificed.
2.2. Antibodies, Regents, and Antigen Preparation
Anti-CD3 (clone 145-2C11), anti-CD28 (clone 37.51), Fluorescein isothiocyanate-(FITC-)conjugated anti-CD4 (clone GK1.5), phycoerythrin-(PE-)conjugated anti-interferon-(IFN-)γ (clone XMG1.2) PE-conjugated anti-interleukin-(IL-)4 (clone 11B11), and their isotype control rat IgG1 antibodies were from eBioscience (San Diego, CA). Rabbit antimouse cleaved caspase-3 (Asp175) antibody and rabbit IgG isotype control antibody were from Cell Signaling (Danvers, MA). The Alexa Fluor 647F(ab′)2 fragment of the goat antirabbit IgG(H+L) was from Invitrogen (Eugene, OR). Phorbol12-myristate13-acetate (PMA) and ionomycin were from Sigma-Aldrich (St. Louis, MO). The CD4+ T Cell Isolation Kit was from Miltenyi Biotec (Bergisch Gladbach, Germany). The BD Cytofix/Cytoperm Plus Fixation/Permeabilization kit and Apo-Direct kit were from BD Pharmingen (San Diego, CA). The granzyme B (GrB) inhibitor Z-AAD-CMK was from Calbiochem (San Diego, CA). The caspase inhibitor Z-VAD-FMK was from BIOMOL International (Plymouth Meeting, PA).
SWA and SEA were prepared and diluted with phosphate buffered saline (PBS) to a final concentration of 10 mg/mL, as previously described [17].
2.3. Antigen Immunization
Mice were injected subcutaneously in the back with 100 μl of a solution containing 50 μg of SEA, 100 μg of SWA, or PBS alone emulsified in incomplete Freund's adjuvant (IFA) [18]. Ten days after immunization, six mice from each group were sacrificed.
2.4. Cell Isolation
After infection, chemotherapy, or antigen immunization, the single cell suspension of splenocytes was prepared by teasing the spleen in PBS containing 1% fetal calf serum (FCS) (Gibco, Gaithersburg, MD) and 1% EDTA. Erythrocytes were lysed with ACK lysis buffer, and the remaining splenocytes were washed with PBS-1% FCS.
CD4+ T cells were purified from the splenocytes by negative selection with a CD4+ T Cell Isolation Kit and using a magnetic-activated cell sorter (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. The purity of the CD4+ population was >97%, as determined by fluorescence-activated cell sorting (FACS) analysis.
2.5. Cell Culture
For in vitro SWA or SEA restimulation of CD4+ T cells from infected mice with or without chemotherapy, 5 × 107 splenocytes from each mice were incubated in 10 mL of complete RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% FCS, 2 mM pyruvate, 0.05 mM 2-mercaptoethanol, 2 mM L-glutamine, 100 U of penicillin/mL, and 0.1 mg/mL streptomycin in a 10 cm plate (Costar, Cambridge, MA). The cells were incubated at 37°C in 5% CO2 in the presence of 20 μg/mL SWA or 20 μg/mL SEA. After 36 hours of incubation, CD4+ T cells were purified using MACS and prepared for staining with antibodies and FACS analysis.
To induce in vitro polarization and apoptosis of CD4+ T cells from normal mice with SWA and SEA, CD4+ T cells (6 × 106 cells/well) from normal mice were seeded in 12-well plates. The cells were preactivated overnight with anti-CD3 (2 μg/mL) and anti-CD28 (1 μg/mL) antibodies and then incubated in 3 mL of complete RPMI 1640 medium in the presence of specific antigens of 20 μg/mL SEA, 20 μg/mL SWA, or PBS alone for 36 hours at 37°C in 5% CO2.
For the caspase and GrB inhibition experiments, 5 × 107 splenocytes were incubated in 10 mL of complete RPMI 1640 medium in the presence of (1) SWA (20 μg/mL) and the GrB inhibitor Z-AAD-CMK (20 μM), (2) SEA (20 μg/mL) and the caspase inhibitor Z-VAD-FMK (20 μM), (3) PBS and Z-AAD-CMK (20 μM), or (4) PBS and Z-VAD-FMK (20 μM) in a 10 cm plate (Costar, Cambridge, MA) for 36 hours at 37°C in 5% CO2. After 36 hours of incubation, the CD4+ T cells in each group were purified and prepared for staining with antibodies and FACS analysis.
2.6. Intracellular Cytokines and Caspase-3 Staining
To detect intracellular cytokines and apoptotic molecules in CD4+ T cells, purified CD4+ T cells (2 × 106 cells/mL) were restimulated in the presence of PMA (25 ng/mL), ionomycin (1 μg/mL), and GolgiStopTM (0.66 μl/mL) in 24-well plates (2 mL/well) for 6 hours at 37°C in 5% CO2. Then the CD4+ T cells were washed in PBS containing 1% FCS and stained with 0.5 mg/mL FITC-conjugated anti-CD4 in the dark at 4°C for 30 minutes, followed by fixation and permeabilization with Cytofix/Cytoperm, according to the manufacturer's protocol. Next, the cells were stained intracellularly with 2 μl of rabbit antimouse caspase-3 monoclonal antibody or rabbit IgG isotype control antibody (mAb; 1 : 200) plus PE-conjugated anti-IFN-γ (0.2 mg/mL), PE-conjugated anti-IL-4 (0.2 mg/mL), or PE-conjugated rat IgG1 control antibodies for 1 hour at room temperature. Finally, the cells were washed in PBS containing 1% FCS and stained at room temperature for 30 minutes in the dark with 5 μl of the Alexa Fluor 647 F(ab′)2 fragment of the goat antirabbit IgG(H+L). FACS analysis was performed using the FACS Calibur (Becton Dickinson, San Jose, CA).
2.7. Terminal Transferase dUTP Nick End Labeling (TUNEL) Assay
In the caspase and GrB inhibition experiments, apoptotic cells were labeled with the Apo-Direct kit following the manufacturer's instruction. The cells were then detected by FACS.
2.8. Necropsy and Estimation of Worm and Egg Burdens in the Liver
At 23, 35, and 56 days postinfection in animals with or without orally administered artesunate, six mice from each group were sacrificed. The animals were perfused to measure the worm and liver egg burdens. The mouse livers were removed and weighed. A sample of each liver (0.5 g) was digested overnight at 37°C with 10 mL 5% KOH. In each sample, the total adult worm and liver egg burden was measured. The residues were dissected and immediately fixed in 10% buffered formalin prior to histopathological analysis.
2.9. Liver Histopathology
Liver sections were embedded in paraffin and stained with hematoxylin and eosin (HE) prior to microscopic examination. The number of granulomas was counted in 10 random microscopic fields in each mouse liver section using a microscope (magnification ×4; Olympus, Tokyo, Japan). The eggs and granulomas present in the livers were measured using a video micrometer (Olympus, Tokyo, Japan) in accordance with the manufacturer's instructions.
2.10. Statistical Analysis
For statistical evaluation of the data, two-tailed Student's t-tests were used, and P < .05 was considered statistically significant.
3. Results
3.1. Effects of Artesunate on the Parasitology and Histopathology in Mice Infected with S. japonicum
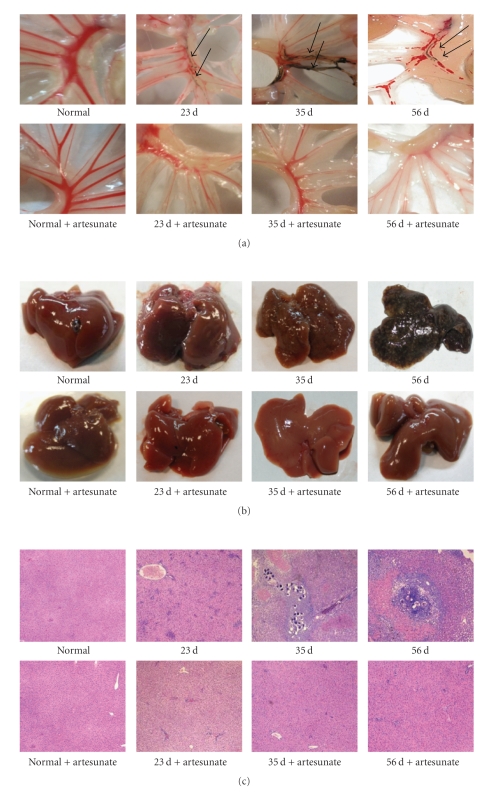
Adult worms were detected in the mesenteric veins of the mice 23 days post-infection (Figure 1(a)). At 35 and 56 days after infection, the adult worms had laid eggs. Egg nodes were observed in the surface of the mouse liver and tallied in the liver egg counts (Figure 1(b) and Table 1). Following administration of artesunate, the number of adult worms and fecundity were completely reduced in our study. No adult worms and eggs were detected in the artesunate-treated mice at the three time points after infection (Figures 1(a), 1(b), and Table 1).
Figure 1.
Parasitological and histopathological examination of infected mice treated with or without artesunate. In each experiment, twelve mice were percutaneously infected with S. japonicum and among these mice, six were orally administered artesunate dissolved in ddH2O as described in Materials and Methods. At 23, 35, and 56 days mice were sacrificed and perfused to measure the worm and liver egg burdens and for histopathological analysis. (a) Adult worms in the mesenteric veins of infected mice. (b) Egg nodes in the surface of the livers. (c) Microscopic examination of hepatic granulomatous inflammation in liver sections (Magnification: ×400). Measurements were made at 0 d and at three time points postinfection. Each micrograph is representative of three experiments.
Table 1.
Worm burdens and eggs counts in the livers of infected mice orally treated with or without artesunate.
| 0 d | 23 d | 23 d | 35 d | 35 d | 56 d | 56 d | |
|---|---|---|---|---|---|---|---|
| artesunate | artesunate | artesunate | |||||
| Adult worms | 0 | 9.75 ± 0.96*** | 0 | 10.5±1.29*** | 0 | 10 ± 1.63*** | 0 |
| Liver eggs (/g) | 0 | 0 | 0 | 26650.33 ± 1912.95*** | 0 | 51184.58 ± 1663.26*** | 0 |
Mice were percutaneously infected with S. japonicum; some were orally administered artesunate as described in Section 2. At 0 (before infection) and 23, 35, and 56 days postinfection, six mice from each group were sacrificed for worm burdens and eggs counts measurements. ***P < .001 compared to the artesunate groups at same time point.
We measured the magnitude of hepatic granulomatous inflammation, which is commonly used as an indicator of disease severity, and observed that there were significant differences in liver histology between mice that were or were not administered artesunate (Figure 1(c)). Severe hepatic granulomatous inflammation was observed around eggs 35 days after infection in mice that were not administered artesunate. However, in artesunate-treated, infected mice, no eggs were present, and there was only a mild hepatic inflammatory response observed. This mild inflammatory response may have been induced by schistosomula and early stage adult worms before they were killed by artesunate or by antigens released by destroyed parasites (Figure 1(c)).
3.2. Kinetic Analysis of Th1/Th2 Cell Polarization and Apoptosis during Different Stages of S. japonicum Infection
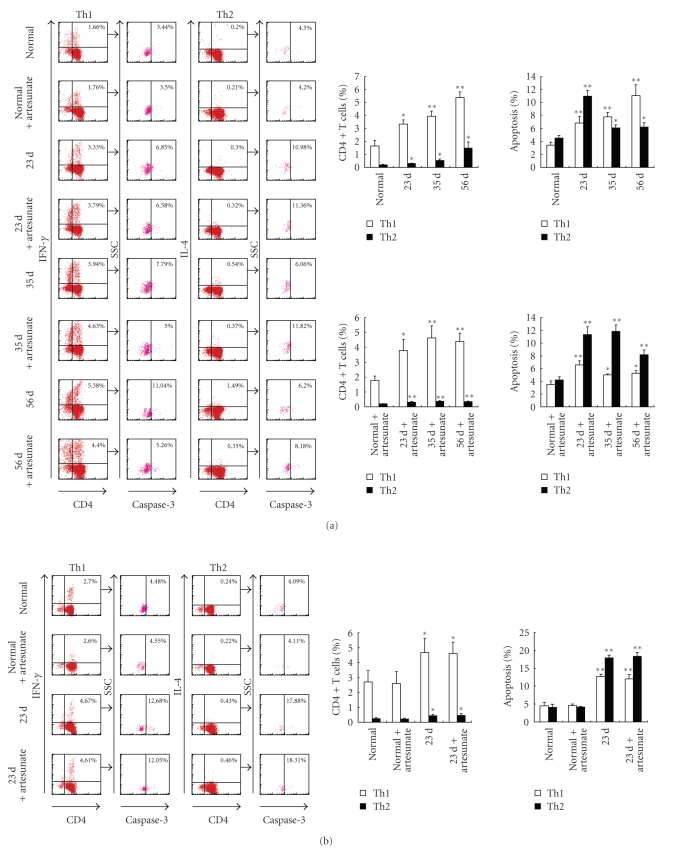
During schistosome infection of humans [19] and mice [11, 20], parasite-specific antigens (i.e., SWA or SEA) are believed to stimulate different types of responses. SWA has been shown to stimulate the type 1 response, especially during early infection, whereas SEA stimulates type 2 responses later in infection. Consistent with this, our results showed that the proportion of Th1 cells rose to 3.33% 23 days postinfection in comparison to the normal controls (1.66%). Meanwhile, the proportions of Th2 cells only slightly increased (Figure 2(a) left and upper-right panels). This demonstrates that there is a Th1-biased response due to nonegg antigens that occurs before the schistosomes lay eggs. In contrast, after eggs deposition, the number of Th2 cells rapidly increased (from 0.30% at 23 days to 0.54% at 35 days and 1.49% at 56 days). The increase in Th2 cells was more dramatic than the increase in Th1 cells (from 3.33% at 23 days to 3.94% at 35 days and 5.38% at 56 days), suggesting that the egg antigen induces a Th2-biased response.
Figure 2.
Kinetic analysis of Th1/Th2 cell polarization and apoptosis in mice infected with S. japonicum. In each experiment, twelve mice were percutaneously infected with S. japonicum and among these mice, six were orally administered artesunate dissolved in ddH2O as described in Materials and Methods. At 23, 35, and 56 days mice were sacrificed and single cell suspension of splenocytes were prepared. (a) CD4+ T cells were purified from splenocytes using MACS, surface stained with anti-CD4-FITC and then intracellularly stained with rabbit antimouse caspase-3 antibody or rabbit IgG isotype control antibody plus anti-IFN-γ-PE, anti-IL-4-PE, or isotype IgG control mAbs for FACS analysis. The splenocytes were restimulated in vitro with SWA (b) or SEA (c) for 36 hours, and then the CD4+ T cells were purified by MACS and stained with above antibodies prior to FACS analysis. The percentage of apoptotic cells in the FACS data was derived from the CD4+ and IFN-γ+, or CD4+ and IL- 4+ cells and gated on the caspase-3+ population. Data are expressed as the mean ± SD of 18 mice from three independent experiments. *P < .05; **P < .01. Left panels: One representative experiment of flow cytometric analysis with the average percentage of Th or apoptotic cells shown in the FACS data. Right panels: The statistical analysis of 18 mice from three independent experiments.
To assess apoptosis in Th1 and Th2 cells during the different stages of schistosomal infection, CD4+ T cells were purified and labeled for FACS analysis without further stimulation in vitro. The results in Figure 2(a) (left and upper-right panels) showed that the level of apoptosis of both Th1 and Th2 cells increased in infected mice in comparison to normal mice. During the early stage of infection (23 days), the Th1-type polarized response induced by nonegg antigens increased. Th2 cell apoptosis increased (from 4.50% to 10.98%) slightly more quickly than apoptosis in Th1 cells (from 3.44% to 6.85%). By correlating the eggs deposition in the liver (at 35 and 56 days) and the SEA becoming the predominant stimulus, which directed a shift toward Th2 cell bias, the percentage of apoptotic Th1 cells remained higher (7.79% at 35 days and 11.04% at 56 days) than in Th2 cells. A lower level of Th2 cell apoptosis was observed (6.06% at 35 days and 6.20% at 56 days) postinfection in comparison to the level measured 23 days postinfection. These data suggest that SEA, rather than nonegg antigens, may be responsible for the changes observed after eggs deposition.
To clarify this issue, artesunate was administered immediately after S. japonicum infection to reduce fecundity and SEA-induced responses. We observed that artesunate administrated after infection resulted in strong antiparasitic activity, which stopped the infection before the adult worms laid eggs (Figure 1 and Table 1). Similar to the observations in infected mice, the nonegg antigens, whether released by live or drug-killed parasites, led to a Th1-biased response and more Th2 cell apoptosis 23 days after infection in artesunate-treated mice. At days 35 and 56, the predominant stimulus switched to SEA in the infected mice; however, in artesunate-treated mice, responses against nonegg antigens remained. In the artesunate-treated mice (at days 35 and 56), the percentages of Th1 cells (4.63%, 4.40%) remained higher, but the percentages of Th2 cells dropped to relatively lower levels (0.37%, 0.35%). Meanwhile, the level of Th1 cell apoptosis (5.00%, 5.26%) remained low, but there was significantly more Th2 cells apoptosis (11.82%, 8.18%) (Figure 2(a) left and lower-right panels). These results indicated that although both Th1 and Th2 cell apoptosis could be induced by either nonegg or egg antigens, these two subsets exhibited distinct susceptibilities to the two antigens. The nonegg antigens in S. japonicum triggered a Th1-biased response and more Th2 cell apoptosis. After the parasites produced eggs, the host responses developed against coexisting nonegg and egg antigens, but they were mainly induced by egg antigens, which shifted the response toward a Th2 bias. Meanwhile, more Th1 cells underwent apoptosis.
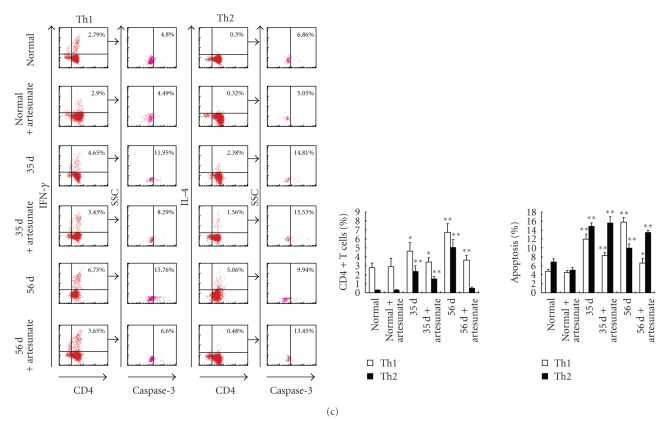
To rule out interference between nonegg and egg antigens after egg deposition, CD4+ T cells were isolated from mice at 23, 35, and 56 days after infection. Next, SWA and SEA were used in vitro to restimulate apoptosis in purified CD4+ T cells which were SWA and/or SEA antigen-primed and sensitized in vivo in infected animals [15]. SWA and SEA are well-characterized antigens that are involved in different types of Th response induction at different infection stages. When compared to the data from normal mice, restimulation with SWA resulted in an increase in both Th1 and Th2 cell apoptosis in CD4+ T cells derived from both artesunate-treated and untreated infected mice at 23 days; as shown in Figure 2(b), following SWA priming and sensitization, there was a greater level of apoptosis in Th2 cells (17.88%, 18.31%) than Th1 cells (12.68%, 12.05%). These results suggest that Th2 cells were more sensitive to SWA-triggered apoptosis. On the other hand, SEA was used to restimulate CD4+ T cells purified 35 and 56 days after infection from mice with or without artesunate administration, which were primed and sensitized by SWA alone or both SWA and SEA. As shown in Figure 2(c), after restimulation in vitro, Th1 cell apoptosis in infected mice at 35 and 56 days that were not treated with artesunate increased more markedly (from 4.80% in normal mice to 11.95% and 15.76%) than Th2 cells did (from 6.86% in normal mice to 14.81% and 9.94%). However, when compared at 35 and 56 days, much less Th1 cell apoptosis was detected in CD4+ T cells from the infected, artesunate-treated mice, which were not primed and sensitized with SEA in vivo (Figure 2(c)). These results further support the observation that Th1 cells are more sensitive to SEA-triggered apoptosis.
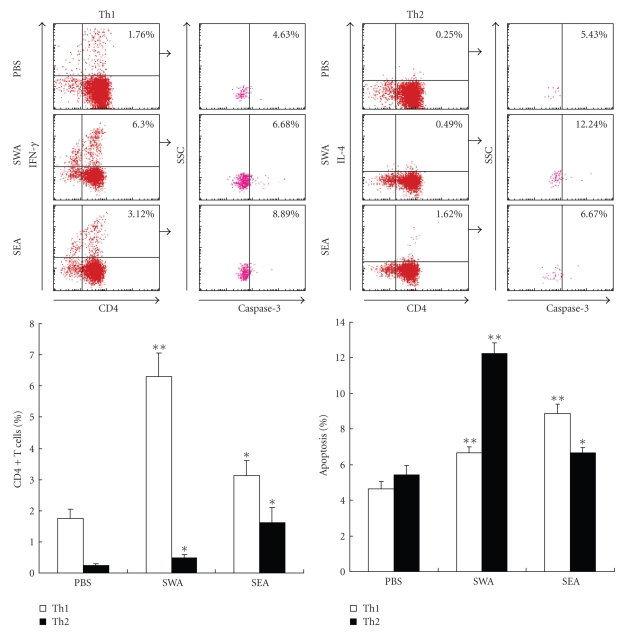
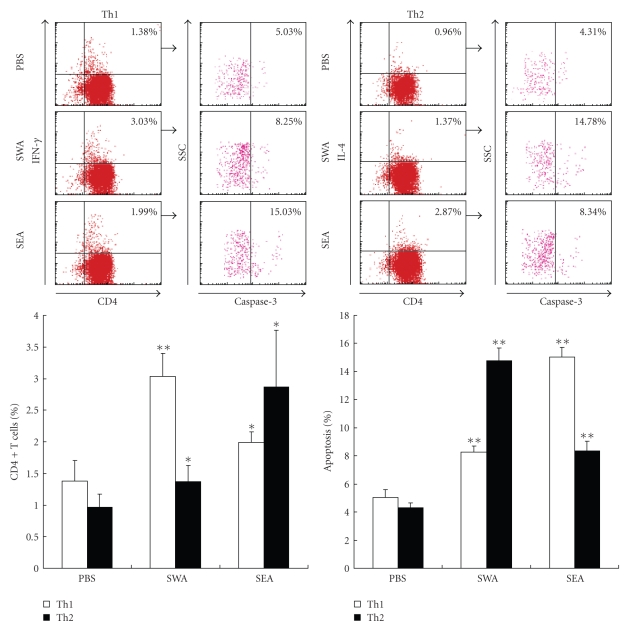
3.3. Polarization and Apoptosis of Th1/Th2 Cells in SWA and SEA Immunized Mice
To further investigate the Th cell apoptotic susceptibilities to the two different antigens, mice were immunized with SWA, SEA, or PBS as a control. After 10 days, the CD4+ T cells were purified from the immunized mice, labeled with antibodies, and analyzed with flow cytometry. As shown in Figure 3, compared with the control group, animals immunized with either SWA or SEA had increased percentages of Th1 and Th2 cells among the purified CD4+ T cells and showed both Th1 and Th2 cell apoptosis simultaneously. However, SWA immunization induced a quicker increase in Th1 cells (from 1.76% to 6.30%) than that Th2 cells (from 0.25% to 0.49%). There was also a significantly greater increase in Th2 cell apoptosis (from 5.43% to 12.24%) than Th1 cell apoptosis (from 4.63% to 6.68%). These results indicate that SWA immunization induced a Th1 polarization. Meanwhile, Th2 cell apoptosis was more susceptible to SWA than Th1 cell apoptosis (Figure 3). In contrast, SEA immunization predominantly induced a greater increase in Th2 cells (from 0.25% to 1.62%), compared to the increase of Th1 cells (from 1.76% to 3.12%). There was also a quicker increase in Th1 cell apoptosis (from 4.63% to 8.89%) than Th2 cell apoptosis (from 5.43% to 6.67%). These results indicate that SEA induced a Th2 cell polarization, and that Th1 cell apoptosis was more susceptible to SEA than Th2 cell apoptosis (Figure 3).
Figure 3.
Polarization and apoptosis of Th1/Th2 cells in SWA/SEA immunized mice. Mice were injected subcutaneously in the back with 100 μl of a solution containing 50 μg of SEA, 100 μg of SWA, or PBS alone emulsified in incomplete Freund's adjuvant (IFA). Ten days after immunization, six mice from each group were sacrificed. Splenocytes from SWA or SEA immunized mice were prepared, respectively, and then CD4+ T cells were purified from splenocytes with MACS. The cells were stained with anticaspase-3, anti-CD4-FITC, anti-IFN-γ-PE or anti-IL-4-PE mAb prior to FACS analysis as described in Materials and Methods. The percentage of apoptotic cells in the FACS data was derived from the number of cells that were CD4+ and IFN-γ+ or CD4+ and IL-4+ and gated on the caspase-3+ population. Data are expressed as the mean ± SD of 18 mice from three independent experiments. *P < .05; **P < .01. Upper panels: One representative experiment of flow cytometric analysis with the average percentage of Th or apoptotic cells shown in the FACS data. Lower panels: The statistical analysis of 18 mice from three independent experiments.
3.4. In Vitro Induction of CD4+ T Cell Polarization and Apoptosis in Naïve Mice Using SWA and SEA
After purifying CD4+ T cells from naïve mice, they were stimulated in vitro for 36 hours with PBS, SWA, or SEA. Flow cytometry analysis showed that in vitro stimulation of CD4+ T cells with either SWA or SEA increased the percentages of both the Th1 and Th2 cells in the purified CD4+ T cells from naïve mice as well as the number of apoptotic Th1 and Th2 cells. The data also showed that in vitro stimulation of CD4+ T cells with SWA preferentially increased the proportion of Th1 cells and the apoptosis of Th2 cells, but SEA preferentially increased the proportion of Th2 cells and increased the level of apoptosis in Th1 cells (Figure 4).
Figure 4.
In vitro induction of polarization and apoptosis in CD4+ T cells derived from normal mice with SWA and SEA. CD4+ T cells were purified from normal mice by MACS and preactivated overnight with anti-CD3 (2 μg/mL) and anti-CD28 (1 μg/mL) antibodies. Then the cells were stimulated with specific antigens of SEA, SWA or PBS alone for 36 hours at 37°C in 5% CO2, followed by staining with rabbit antimouse caspase-3 antibody or rabbit IgG isotype control antibody plus anti-CD4-FITC, anti-IFN-γ-PE anti-IL-4-PE mAbs, or isotype control antibodies prior to FACS analysis. The percentage of apoptotic cells in the FACS data was derived from the number of cells that were CD4+ and IFN-γ+ or CD4+ and IL-4+ and gated on the caspase-3+ population. Data are expressed as the mean ± SD of 18 mice from three independent experiments. *P < .05; **P < .01. Upper panels: One representative experiment of flow cytometric analysis with the average percentage of Th or apoptotic cells shown in the FACS data. Lower panels: The statistical analysis of 18 mice from three independent experiments.
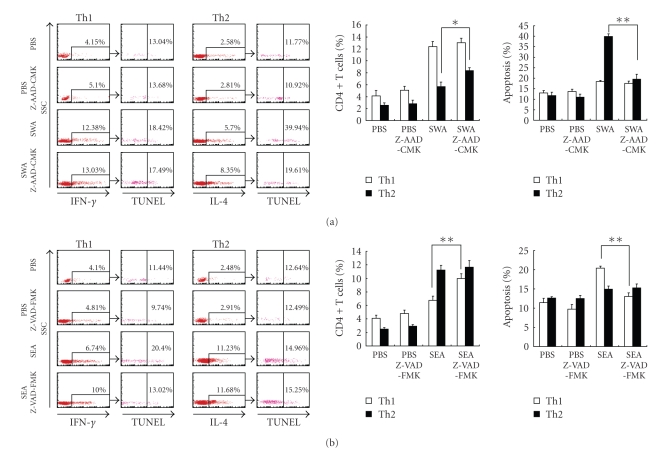
3.5. GrB and Caspase May Be Involved in Different Th Cells Apoptotic Mechanisms That Are Triggered by Different S. japonicum Antigens
Recent studies have provided strong evidence that Th1 and Th2 cells use different mechanisms to execute TCR-induced cell death (TCR-ICD) [21–25]. To investigate whether antigen AICD contributed to Th1/Th2 polarization in S. japonicum infection, we studied C57BL/6 mice immunized with SWA, SEA, or PBS. We also examined the effect of caspase inhibitor Z-VAD-FMK and the GrB inhibitor Z-AAD-CMK and measured cell apoptosis using the TUNEL assay considering that caspase inhibitor Z-VAD-FMK was used. As shown in Figure 5(a), SWA increased the percentages of Th1 and Th2 cells and increased the percentages of Th1 and Th2 cell apoptosis among the CD4+ T cells, in comparison to the controls. In the Z-AAD-CMK-treated groups, there was a marked decrease in SWA restimulation-related Th2 cell apoptosis and an increase in Th2 percentage; however, this was not observed for Th1 cell apoptosis and Th2 percentage. In contrast, Figure 5(b) shows that, compared to the PBS control, SEA increased both the percentages of Th1 and Th2 cells and apoptosis in Th1 and Th2 cells. However, in the Z-VAD-FMK-treated groups, the level of Th1 cell apoptosis induced by SEA significantly decreased and the proportion of Th1 cells significant increased, but the statistically significantly decrease in Th2 cells apoptosis and the increase in the proportion of Th2 cells were not observed in both the SEA and PBS groups when compared to their respective cocultures in the absence of Z-AAD-CMK.
Figure 5.
Caspase and GrB may be involved in different types of Th cell apoptotic mechanisms that are induced by different S. japonicum antigens. (a) Splenocytes from SWA-immunized mice were restimulated in vitro with SWA or PBS in the presence or absence of the GrB inhibitor Z-AAD-CMK. (b) Splenocytes from SEA immunized mice were restimulated in vitro with SEA or PBS in the presence or absence of the caspase inhibitor Z-VAD-FMK. After 36 hours, restimulated CD4+ T cells in (a) and (b) were purified by MACS and stained with anti-IFN-γ-PE or anti-IL-4-PE mAbs. Apoptotic cells were labeled with an Apo-Direct TUNEL assay kit and then detected by FACS. The percentage of apoptotic Th1 or Th2 cells among the purified CD4+ T cells was derived from the number of IFN-γ+ or IL-4+ cells and gated on the TUNEL positive population. Data are expressed as the mean ± SD of 18 mice from three independent experiments. *P < .05; **P < .01. Left panels: One representative experiment of flow cytometric analysis with the average percentage of Th or apoptotic cells shown in the FACS data. Right panels: The statistical analysis of 18 mice from three independent experiments.
4. Discussion
Both Th1/Th2 shift and Th cell apoptosis are believed to benefit for both survival of parasites and reducing self-damage to the host during schistosome infection. Here, our results demonstrate that during the early stage of Schistosoma japonicum infection, nonegg antigens trigger Th2 cell apoptosis via the granzyme B signal pathway, contributing to Th1 polarization. Th1 polarization is believed to be associated with worm clearance and severe schistosomiasis. Following egg-laying, the egg antigens trigger Th1 cell apoptosis via the caspase pathway, contributing to Th2 polarization, which is associated with mild pathology and enhanced survival of both worms and their hosts. Thus, our study suggests that S. japonicum antigen-induced Th1 and Th2 cell apoptosis involves the deletion of mature Th cells in the periphery as well as a host Th response shift and polarization providing a mechanism to modulate the immune interaction between hosts and parasites.
Egg-laying is a critical event in adult schistosomes, which initiates both the formation of immunopathologic granulomas and the Th1/Th2 shift. Consistent with previous reports, we also observed that the initial oviposition occurred between 25 and 28 days after infection (unpublished data). Thus, we selected three time points (23, 35, and 56 days) postinfection as check points in our study that were representative of the response to nonegg antigen exposure, the initiation of granuloma formation in response to egg antigen exposure, and the initiation of modulation of the granuloma response.
To assess apoptosis in Th1 and Th2 cells during the different stages of schistosomal infection, CD4+ T cells were purified from mice at three time points mentioned above and labeled for FACS analysis without further restimulation in vitro. Our results showed that the level of apoptosis of both Th1 and Th2 cells increased in infected mice in comparison to normal mice. During the early stage of infection (23 days), the Th1-type-polarized response and Th2-biased apoptosis induced by non-egg antigens increased. At 35 and 56 days after infection, SEA became a predominant stimulus, and the Th2-type-polarized response and Th1-biased apoptosis induced by SEA increased. These in vivo data raise a question that SEA, rather than non-egg antigens, may be responsible for the changes observed after eggs deposition.
Thus, to illustrate this issue, the interference between non-egg and egg antigens after egg deposition has to be ruled out. Artesunate is a famous antimalarial drug [26, 27], which also displays activity against schistosomiasis [28, 29] through its vermicidal effect on 7- to 35-day-old schistosomes [30]. Following administration of artesunate in our study, the worm burdens and liver egg counts significantly decreased at 23, 35, and 56 days after S. japonicum infection, which indicates that oral administration of artesunate after infection successfully stops infection before the adult worms lay eggs. It provides us a chance to rule out interference to the host immune responses between non-egg and egg antigens after egg deposition. Results showed that, in artesunate-treated mice, without stimulation of SEA at 35 and 56 days after infection, Th1-biased responses and Th2-biased apoptosis remained.
Then, our experiments using in vitro stimulation of CD4+ T cells from S. japonicum infected mice with SWA resulted in a preferential increase in the proportion of Th1 cells and the apoptosis of Th2 cells. In contrast, SEA preferentially increased the proportion of Th2 cells and led to increased levels of apoptosis in Th1 cells. This was further supported by our in vivo data from mice immunized with SWA or SEA, respectively. Furthermore, we in vitro stimulated CD4+ T cells from naïve mice where SWA preferentially increased the proportion of Th1 cells and the apoptosis of Th2 cells, but SEA preferentially increased the proportion of Th2 cells and increased the level of apoptosis in Th1 cells. All these in vitro and in vivo findings provided further support for the notion that non-egg and egg antigens from the schistosome infection regulate the types of Th immune responses and are able to induce different biased types of Th cell apoptosis.
Recent studies have provided strong evidence that Th1 and Th2 cells use different mechanisms to execute TCR-induced cell death (TCR-ICD) [21–25]. Th1 cell death was shown to be triggered by the CD95-CD95L interaction and mediated via caspase 8, which initiated the activation cascade of caspases 3 and 9, which then leads to cell death [22–24]. Th2 cell apoptosis of Th2 cells was unrelated to the TNF-family proteins, since anti-CD95L, TR6, DR5, OPG, and anti-TNF did not affect TCR-ICD in Th2 cells. Instead, Th2 cells have been shown to undergo apoptosis by GrB dependent mechanisms [23, 25]. GrB was previously implicated in CD8+ T cell and NK cytotoxicity and was currently also shown to be crucial for Th2 cell death [25, 31]. On one hand, GrB triggers Th2 cell apoptosis by cleaving caspase-3 and some other caspases to initiate the caspase cascade [32]. On the other hand, GrB triggers Th2 cell apoptosis also by caspase independent pathways [23, 32–34]. Thus, in the presence of complete caspase inhibition, the caspase inhibitor Z-VAD-FMK, which does not inactivate GrB, could only block Th1 cell death, while the Th2 cells were little affected [23, 32]. In contrast, Th2 cell apoptosis could only be blocked by the GrB-specific inhibitor Z-AAD-CMK, whereas the caspase inhibitor Z-VAD-FMK had no effect [23, 25, 32] . Our results indicated that at least partially, the mechanism by which the non-egg antigens triggered Th2 cells apoptosis was GrB dependent and Th2 cell apoptosis might contribute to Th1 cell polarization. Meanwhile, caspases could be critical for the mechanism by which the egg antigens induced Th1 cell apoptosis and might contribute to Th2 cell polarization.
In conclusion, our study suggests that Th1/Th2 cell apoptosis is preferentially induced by different schistosome antigens during different stages of infection. This can partially explain the host Th cell immune response shift and polarization during schistosome infections. Additionally, Th1/Th2 cell apoptosis may contribute an additional mechanism that modulates the immune interaction between hosts and parasites. Th1/Th2 cell apoptosis may be used by hosts as an efficient way to restrict immunopathology and by parasites as a survival strategy to downregulate immune resistance and maintain infection in their hosts.
Acknowledgments
The authors thank professor Junfan Shi (Zhejiang Institute of Parasitic Diseases, Hangzhou, China) for providing artesunate. This work was supported by Grants from the National Basic Research Program of China (973 Program) (no. 2007CB513106), the National Natural Science Foundation of China (no. 30872206 and no. 30571629), and the grants of BK2007533 and 07KJA31023 from Jiangsu Province to Chuan Su.
Abbreviations
- AICD:
Activation-induced cell death
- HE:
Hematoxylin and eosin
- SEA:
Soluble schistosome egg antigen
- S. japonicum:
Schistosome japonicum
- SWA:
Soluble schistosome worm antigen
- Z-AAD-CMK:
Z-Ala-Ala-Asp-CH2Cl
- Z-VAD-FMK:
Z-Val-Ala-Asp(OCH3)-Fluoromethylketone.
References
- 1.Infante-Duarte C. Th1/Th2 balance in infection. Springer Seminars in Immunopathology. 1999;21(3):317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 2.Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature Reviews Immunology. 2007;7(12):975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald AS, Araujo MI, Pearce EJ. Immunology of parasitic helminth infections. Infection and Immunity. 2002;70(2):427–433. doi: 10.1128/iai.70.2.427-433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. Journal of Immunology. 1999;163(3):1205–1213. [PubMed] [Google Scholar]
- 5.Balkhi MY, Latchumanan VK, Singh B, Sharma P, Natarajan K. Cross-regulation of CD86 by CD80 differentially regulates T helper responses from Mycobacterium tuberculosis secretory antigen-activated dendritic cell subsets. Journal of Leukocyte Biology. 2004;75(5):874–883. doi: 10.1189/jlb.1003476. [DOI] [PubMed] [Google Scholar]
- 6.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. Journal of Clinical Investigation. 2007;117(5):1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiethe C, Debus A, Mohrs M, Steinkasserer A, Lutz M, Gessner A. Dendritic cell differentiation state and their interaction with NKT cells determine Th1/Th2 differentiation in the murine model of Leishmania major infection. Journal of Immunology. 2008;180(7):4371–4381. doi: 10.4049/jimmunol.180.7.4371. [DOI] [PubMed] [Google Scholar]
- 8.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann S, Krueger A, Kirchhoff S, Krammer PH. Regulation of T cell apoptosis during the immune response. Current Molecular Medicine. 2002;2(3):257–272. doi: 10.2174/1566524024605671. [DOI] [PubMed] [Google Scholar]
- 10.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nature Reviews Immunology. 2007;7(7):532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunological Reviews. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 12.Gobert GN, Chai M, McManus DP. Biology of the schistosome lung-stage schistosomulum. Parasitology. 2007;134(4):453–460. doi: 10.1017/S0031182006001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nature Reviews Immunology. 2002;2(7):499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunology. 2005;27(7-8):265–270. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundy SK, Lerman SP, Boros DL. Soluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL+ T and B cells during murine Schistosoma mansoni infection. Infection and Immunity. 2001;69(1):271–280. doi: 10.1128/IAI.69.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundy SK, Boros DL. Fas ligand-expressing B-1a lymphocytes mediate CD4+-T-cell apoptosis during schistosomal infection: induction by interleukin 4 (IL-4) and IL-10. Infection and Immunity. 2002;70(2):812–819. doi: 10.1128/iai.70.2.812-819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fallon PG, Smith P, Dunne DW. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. European Journal of Immunology. 1998;28(4):1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Boros DL. Identification of the immunodominant T cell epitope of p38, a major egg antigen, and characterization of the epitope-specific th responsiveness during murine schistosomiasis mansoni. Journal of Immunology. 1998;160(11):5420–5427. [PubMed] [Google Scholar]
- 19.Caldas IR, Campi-Azevedo AC, Oliveira LFA, Silveira AMS, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Tropica. 2008;108(2-3):109–117. doi: 10.1016/j.actatropica.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Wilson MS, Mentink-Kane MM, Pesce JT, Ramalingam TR, Thompson R, Wynn TA. Immunopathology of schistosomiasis. Immunology and Cell Biology. 2007;85(2):148–154. doi: 10.1038/sj.icb.7100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varadhachary AS, Perdow SN, Hu C, Ramanarayanan M, Salgame P. Differential ability of T cell subsets to undergo activation-induced cell death. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(11):5778–5783. doi: 10.1073/pnas.94.11.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XR, Zhang LY, Devadas S, Li L, Keegan AD, Shi YF. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: role of apoptosis in T helper subset differentiation. Cell Death and Differentiation. 2003;10(2):203–210. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- 23.Zheng L, Lenardo M. T helper 2 cells' preferred way to die. Immunity. 2006;25(2):187–188. doi: 10.1016/j.immuni.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Brunner T, Carter L, et al. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. Journal of Experimental Medicine. 1997;185(10):1837–1849. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devadas S, Das J, Liu C, et al. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25(2):237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Kashyap AS, Anand KP, Kashyap S. Artesunate for malaria. The New England Journal of Medicine. 2008;359(3):314–315. [PubMed] [Google Scholar]
- 27.Rosenthal PJ. Artesunate for the treatment of severe falciparum malaria. The New England Journal of Medicine. 2008;358(17):1829–1836. doi: 10.1056/NEJMct0709050. [DOI] [PubMed] [Google Scholar]
- 28.Shaohong L, Kumagai T, Qinghua A, et al. Evaluation of the anthelmintic effects of artesunate against experimental Schistosoma mansoni infection in mice using different treatment protocols. Parasitology International. 2006;55(1):63–68. doi: 10.1016/j.parint.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Jiraungkoorskul W, Sahaphong S, Sobhon P, Riengrojpitak S, Kangwanrangsan N. Schistosoma mekongi: the in vitro effect of praziquantel and artesunate on the adult fluke. Experimental Parasitology. 2006;113(1):16–23. doi: 10.1016/j.exppara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Ru W, Li Y, Dai J, et al. Studies on effect of artesunate against Schistosoma japonicum—I: susceptibility of different developed stages of worms in mice to artesunate . Chinese Journal of Schistosomiasis Control. 2006;18:161–164. [Google Scholar]
- 31.Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunological Reviews. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nature Reviews Immunology. 2003;3(5):361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 33.Thomas DA, Du C, Xu M, Wang X, Ley TJ. DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity. 2000;12(6):621–632. doi: 10.1016/s1074-7613(00)80213-7. [DOI] [PubMed] [Google Scholar]
- 34.Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. Journal of Biological Chemistry. 1998;273(43):27934–27938. doi: 10.1074/jbc.273.43.27934. [DOI] [PubMed] [Google Scholar]