Abstract
The tropical disease malaria, which results in more than one million deaths annually, is caused by protozoan parasites of the genus Plasmodium and transmitted by blood-feeding Anopheline mosquitoes. Parasite transition from the human host to the mosquito vector is mediated by gametocytes, sexual stages that are formed in human erythrocytes, which therefore play a crucial part in the spread of the tropical disease. The uptake by the blood-feeding mosquito triggers important molecular and cellular changes in the gametocytes, thus mediating the rapid adjustment of the parasite from the warm-blooded host to the insect host and subsequently initiating reproduction. The contact with midgut factors triggers gametocyte activation and results in their egress from the enveloping erythrocyte, which then leads to gamete formation and fertilization. This review summarizes recent findings on the role of gametocytes during transmission to the mosquito and particularly focuses on the molecular mechanisms underlying gametocyte activation and emergence from the host erythrocyte during gametogenesis.
1. Introduction
With an annual death toll of more than one million people, the tropical disease malaria is considered one of the most significant infectious diseases worldwide. Malaria is caused by protozoan parasites of the genus Plasmodium and transmitted by blood-feeding Anopheline mosquitoes. During their life cycle, plasmodia alternate between the human host and the insect vector, and thus the transmission stages of the parasite had to develop mechanisms for rapid adaptation to the new environment in order to coexist with the respective host.
Like most apicomplexan parasites, plasmodia further switch between tissue-specific multiplication cycles and a phase of sexual reproduction, which mediates the transition from the human to the mosquito and thus plays a crucial part in the spread of the disease. The malaria sexual phase begins with the differentiation of gametocytes in human erythrocytes, followed by their uptake during the blood meal of the mosquito and the formation of gametes within the insect midgut. The transformation of the fertilized zygote into the infective ookinete subsequently marks the end of the malaria sexual phase (reviewed in [1]).
Historically, scant research has been devoted to the malaria sexual stages, namely, gametocytes, gametes, and zygotes, since they neither contribute to the clinical picture of patients nor do they play a role for vector control. However, within the last two decades the dramatic increase of drug resistance in malaria parasites has forced researchers to broaden their consideration of tactics to combat the disease, including transmission blocking strategies aimed at the sexual stages. Such transmission blocking strategies, on the level of either drugs or vaccines, are designed to disrupt parasite reproduction and further development in the mosquito midgut, thus breaking the life cycle of the parasite. Research on the malaria transmission stages, however, was formerly hampered by cost- and time-consuming cultivation, as well as by the technically challenging infections of mosquitoes with parasites. This was particularly true for work on P. falciparum, the causative agent of malaria tropica.
In recent years knowledge on the malaria sexual phase has benefited from a dramatic resurgence provided by proteomic, microarray, and annotation projects that arose out of the genome sequence projects for multiple malaria species (e.g., [2–7]). As a result, a number of new sexual stage antigens have been identified, and progress has been made in the identification and functional characterization of enzymes and regulatory proteins that are involved in gametocyte differentiation and fertilization (reviewed in [1]).
Nowadays, three main questions regarding the malaria sexual phase are in the focus of interest. (1) Which are the mechanisms that cause a subset of erythrocytic parasites to enter the sexual stage pathway and to differentiate to gametocytes?. (2) How do gametocytes become activated within the mosquito midgut and how do they transform into gametes?. (3) In which way do the sexual stages interact with factors of the mosquito midgut? This review addresses the role of gametocytes during malaria transmission and particularly discusses the recent findings on gametocyte activation following entry of the mosquito midgut, as well as their egress from the host erythrocyte and transformation into gametes. Additional aspects of gametocytogenesis, sexual stage proteins, and malaria transmission can be found in other recent reviews [1, 8–11].
2. Gametocyte Differentiation in the Human
The gametocytes are the only stages within the life cycle of malaria parasites that are able to mediate the transition from the human host to the insect host. The development of asexual blood stage parasites to intraerythrocytic gametocytes, which is referred to as gametocytogenesis, starts approximately 7–15 days after the appearance of parasites in the human blood. It is not known to which degree gametocytes develop stochastically, with a small proportion of committed parasites leaving the asexual cycle and entering the sexual pathway, versus gametocytogenesis as a response to complex environmental signals during infection. Gametocytogenesis was previously shown to be influenced by different kinds of stress, including parasite density, anemia, host immune response or drug treatment (e.g., [12–20]; reviewed in [8, 10]). Up to date, little is known about parasite genes that regulate gametocytogenesis, but it was observed that reduced levels of gametocytes in parasite cultures are often associated with the loss of genetic information following subtelomeric deletion in the right arm of chromosome 9 [21].
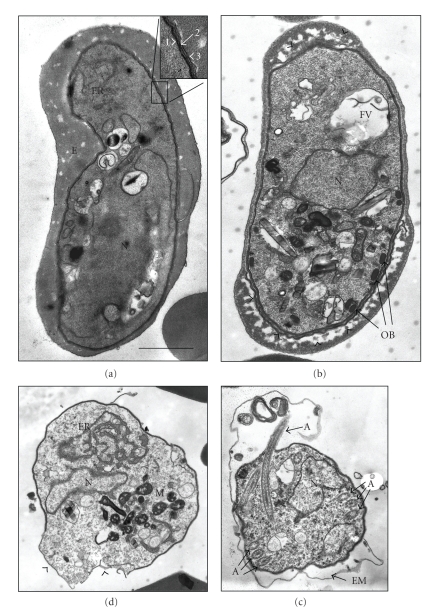
While in most Plasmodium species the sexual stages mature within less than two days, a time period of about 10 days is required for gametocyte development in the human malaria pathogen P. falciparum [22]. Gametocyte maturation can be classified into five morphological stages (stages I–V) [23], and mature P. falciparum gametocytes show an eponymous falcipare form. In these stages, the host erythrocyte has conformed to the crescent shape of the parasite and is reduced to a small cytoplasmic hem. The intraerythrocytic gametocyte lies within the parasitophorous vacuole (PV) and is shielded from the erythrocyte cytoplasm by the PV membrane (PVM), which is located adjacent to the parasite plasma membrane (PPM) (Figure 1(a)). Underneath the PPM is a typical gametocyte feature, the pellicular complex, which consists of a subpellicular membrane (SPM) vacuole subtended by an array of longitudinally oriented microtubules [24]. These structures probably give the gametocyte stability, and the electron-dense SPM disappears during gametogenesis (Figures 1(a), 1(b), 1(c), and 1(d)). Besides morphological changes, maturation of gametocytes also includes alterations on the molecular level in order to prepare the parasites for the rapid adaptation to the mosquito midgut. For instance, a large amount of mRNA is transcribed and stored in the cytoplasm of female gametocytes, as shown for the transcripts of the sexual stage surface proteins Pbs25 and Pbs28 in the rodent malaria model P. berghei [25], which will be transcribed only in the mosquito vector (see below).
Figure 1.
Ultrastructural changes in P. falciparum gametocytes during activation. (a) Transmission electron micrograph of a mature, non-activated gametocyte. The erythrocyte is reduced to an electron-light hem. The PVM is located adjacent to the PPM and the PV is therefore not discernable. No osmiophilic bodies are detectable, indicating that a male microgametocyte is pictured. Inset shows the pellicular membrane complex, depicting the SPM (1), the PPM (2) and the PVM (3). (b) A female macrogametocyte two-minute postactivation. The gametocyte is in the process of rounding up, thereby loosing its crescent shape. The osmiophilic bodies become closely associated to the parasite surface. At the poles, the PVM separates from the PPM and the erythrocyte is in the process of degrading (arrowheads). (c) Ultrastructure of an exflagellating microgametocyte. The axoneme of a forming microgamete is visible as longitudinal section, and several other cross sections of axonemes are detectable inside the microgametocyte. The PVM has disappeared and the EM ruptured. (d) A female macrogamete after emergence. This stage is marked by pronounced ER and the highly branched single mitochondrion. The SPM is in the process of disintegrating (arrowheads). A, axoneme; EM, erythrocyte membrane; ER, endoplasmic reticulum; FV, food vacuole; M, mitochondrion; N, nucleus; OB, osmiophilic body; PPM, parasite plasma membrane; PV, parasitophorous vacuole; PVM, PV membrane; SPM, subpellicular membrane. Bar, 1 μm.
The gender is predetermined in the developing gametocytes [26]. Gender specificity becomes established in the schizont committed to gametocytogenesis [27], and the gender ratio is typically female-biased with one male for about five female gametocytes, depending on the respective parasite clone [28]. This difference might be explained by the fact that one male gametocyte forms approximately eight microgametes, thus establishing a roughly 1 : 1 ratio of micro- and macrogametes in the mosquito midgut, thereby leading to most efficient fertilization in a monoclonal infection [8, 28]. Recently, Reece et al. demonstrated in the mouse model that parasites were able to adjust the sex ratio according to parasite density and the number of parasite clones coinfecting the mammalian host [29]. For instance, a less female-biased sex ratio would increase the probability of a successful fertilization of females of the respective clone, when competing with others [29–32]. In contrast, in vitro studies on P. falciparum did not show an adjustment of sex ratio to gametocyte density. However, an impact of the sex ratio on the infection rate, depending on gametocyte density, was observed [33].
The fact that single, haploid asexually replicating malaria parasites are able to develop into gametocytes of both sexes in the absence of sex chromosomes indicates that gametocyte gender determination is governed by differential gene expression [34]. Gametocyte stages I to IV were reported to sequester in the bone marrow and spleen, while terminally differentiated stage V gametocytes are then released in the peripheral blood system [35, 36] and only become infectious to mosquitoes after a further two or three days of circulation [37, 38]. Bloodstream gametocytes might not be distributed homogeneously, as evidenced by a significant aggregation pattern observed in midgut smears of P. falciparum-fed mosquitoes [39], and it is an intriguing hypothesis that parasites increase the likelihood of fertilization in the mosquito midgut by promoting uptake in preformed complexes of gametocytes.
3. Gametocyte Activation in the Mosquito Midgut
While feeding on an infected human, the female mosquito takes up malaria gametocytes together with the blood meal. By entering the midgut, the parasites receive environmental signals, which indicate the switch from warm-blooded host to insect vector and which initiate the development of gametes. Such signals include a drop of temperature by approximately 5°C [40], and the presence of the mosquito-derived molecule xanthurenic acid (XA), a byproduct of eye pigment synthesis [41, 42]. An additional signal reported to induce gametocyte activation is an increase of pH from 7.2 to about 8 [40, 43], but such a pH shift was later discussed to be an artificial inductor of exflagellation [9]. While XA appears to initiate a number of signaling events in the parasite (see below), the quest for a receptor that binds XA was hitherto unsuccessful.
Gametocyte activation is routinely measured by the formation of exflagellation centers, although a time period of almost 15 minutes lies between these two events [44]. This is due to the fact that exflagellation can easily be observed under the light microscope and quantified by counting of exflagellation centers. Exflagellation is the process when the activated male microgametocyte forms motile flagellar microgametes, which detach from the residual body by binding to erythrocytes (see below). Two previous studies showed that induction of exflagellation involves a fast increase in intracellular calcium and cGMP [45, 46]. An initial benchmark in elucidating sexual stage signaling was the identification of two guanylyl cyclases (GCα and GCβ) as integral membrane proteins in P. falciparum [47], which are activated by addition of XA (Figure 2) [48]. Noteworthy, the subsequent disruption of the GCβ ortholog in P. berghei resulted in normal exflagellation, but motility-impaired ookinetes, indicating that the role of GCβ is not essential for gametocyte activation [49]. The increase of cGMP triggers the activation of a cGMP-dependent protein kinase, PKG. Activation of PKG leads to rounding up of the gametocyte, a process that appears to be independent from calcium increase [50]. Gametocyte exflagellation further involves the presence of the second messengers diacylglycerol and inositol triphosphate (IP3), hydrolysis products of phospholipase C activity [51]. The latter eventually mediates the release of intracellular calcium from the endoplasmic reticulum (ER) (Figure 2).
Figure 2.
Schematic overview of signaling pathways identified to date that are involved in gametocyte activation (modified from [83]). Gametocyte activation is induced by a decrease in temperature and the presence of the mosquito-derived molecule XA. So far, a receptor involved in activation has not been identified. Activation effects PLC and GC, resulting in an increase of IP3 and cGMP. The latter activates a PKG. IP3 mediates release of intracellular calcium from the ER, which activates CDPK4 and consequently Map-2 in the male microgametocyte, eventually leading to exflagellation. A possible link between PKG and calcium release has not yet been confirmed. Signaling pathways in the activated macrogametocyte that are downstream of calcium release were not yet identified. Black lines indicate direct interactions and dashed lines indicate indirect interactions. Ca2+, calcium ion; CDPK, calcium-dependent protein kinase; cGMP, cyclic guanosine monophosphate; DAG, diacylglycerol; DNA, deoxyribonucleic acid; E, erythrocyte; ER, endoplasmic reticulum; GC, guanylyl cyclase; GTP, guanosine triphosphate; IP3, inositol triphosphate; Map-2, Mitogen-activated protein kinase 2; PDE, phosphodiesterase; PIP2, phosphatidylinositol-4,5-bisphosphate; PKG, cGMP-dependent protein kinase; PLC, phospholipase C; PV, parasitophorous vacuole; R, receptor; T, temperature; XA, xanthurenic acid.
It is not yet known how the signaling pathway involving IP3 and calcium release and the pathway involving cGMP and PKG activation are linked together, and whether PKG has an additional effect on calcium release from the ER (Figure 2). Current data suggest that at least three effector pathways exist (discussed in [50]): (1) a PKG-dependent, calcium-independent pathway that mediates rounding up of the activated gametocytes, (2) a calcium-dependent pathway that initiates microgamete formation, and (3) a calcium-dependent pathway that regulates emergence of activated gametocytes of both genders.
4. Gametocyte Egress from the Host Erythrocyte
Following uptake by the mosquito, both male and female gametocytes round up and then escape from the enveloping erythrocytes within about 10 minutes postactivation (Figures 1(b), 1(c), and 1(d)). In this period the microgametocyte replicates its genome three times in order to produce eight motile microgametes (reviewed in [1, 9]). Egress of the activated gametocyte from the host erythrocyte has been linked to the presence of osmiophilic bodies, gametocyte-specific secretory organelles that were first identified by electron microscopy due to their electron-dense features (Figure 1(b)) [24, 52]. They appear first in stage IV gametocytes and are particularly present in the female sexual stages. The osmiophilic bodies migrate to the PPM during activation and disappear within a few minutes post-activation, coevally with the rupture of the PVM (Figures 1(b), 1(c), and 1(d)) (G. Pradel, unpublished observations).
Osmiophilic bodies contain a gametocyte-specific and highly hydrophilic protein, Pfg377, which is considered a marker for these organelles [53, 54]. Only recently has the function of Pfg377 been investigated by reverse genetic methods. Gene-disruption studies showed that female P. falciparum gametocytes lacking this protein reveal a reduced number of osmiophilic bodies and fail to egress from the host erythrocyte, pointing to a pivotal role of this protein in gametocyte emergence [55].
Another protein, which was only recently identified, MDV-1/Peg3, has also been implicated with gametocyte egress. In P. falciparum, expression was reported to be initiated in stage I gametocytes in association with all membranous structures of the PVM and to persist until gametocyte maturation [56–58]. First gene disruption studies on P. falciparum described a reduced formation of particularly male gametocytes [57]. Two subsequent studies, however, indicated a role of the protein post-activation of gametocytes.Lal et al. [59] reported the presence of MDV-1/Peg3 in P. berghei gametocytes of both sexes and a subsequent focal localization at the anterior pole of the developing ookinete. Studies on parasites in which the respective gene was knocked out resulted in reduced ookinete formation. A study by Ponzi et al., on the other hand, showed that P. berghei MDV-1/Peg3 was associated with the gametocyte osmiophilic bodies [60]. Gametocytes lacking this protein failed to egress from the host erythrocyte, thus resulting in reduced fertilization and ookinete formation. Ponzi et al. [60] therefore suggested that MDV-1/Peg3 plays a major role in disrupting the PVM and the erythrocyte membrane (EM).
Independent from the life-cycle stage, host cell egress of malaria parasites involves rupture of two membranes, PVM and EM. The time line of rupture, however, was recently object to several brisk discussions. Particularly two models are currently under investigation, the inside-out model, in which the PVM ruptures prior to the EM [61], and the outside-in model, in which the EM is degraded first [62, 63] (reviewed in [64]). The timeline of parasite egress was hitherto mainly investigated in the asexual blood and liver stages, and no data are available for the egress of gametocytes. In the above mentioned study, Ponzi et al. [60] suggested that MDV-1/Peg3 is involved in PVM destabilization and that EM rupture depends on the absence of the PVM. In accord with this hypothesis, new studies from our laboratory indicated that the PVM disappears within a few minutes after gametocyte activation, and that the rupture of the EM follows several minutes later (G. Pradel, unpublished observations), thus supporting the inside-out model of egress.
The coming-out of malaria parasites from the host cell requires protease activity. A number of new studies engaged with the identification of proteases that mediate emergence of asexual blood stage merozoites. Data point to the involvement of the cytoskeleton-degrading malaria proteases falcipain-2 and plasmepsin II [64]. Particularly SERA (serine-rich antigen) proteins, which were identified in the PV of blood stage schizonts (e.g., [65, 66]), are supposed to mediate PVM rupture. It was shown for SERA-5 of P. falciparum that the protease is proteolytically activated by the serine-like subtilisin protease PfSUB1 [67]. While no detailed studies were yet performed on malaria gametocytes, it is worth mentioning that transcripts of select representatives of the above mentioned protease families are expressed in these stages, including falcipain-1, plasmepsin VI, SERA-6, SERA-7, and PfSUB3 [68, 69].
A popular strategy in investigating protease activity during rupture is the treatment of parasites with protease type-specific inhibitors. Again, these studies were mostly performed on blood stage parasites, particularly using cysteine protease inhibitors like E64. Treatment with this inhibitor, however, resulted to date in contradictory results, and it was reported that the inhibitor blocked either degradation of the PVM [62, 63] or rupture of the EM [70]. A similar egress study using protease inhibitors was recently performed on activated P. berghei gametocytes and showed that exflagellation can be blocked by the cysteine/serine protease inhibitors TPCK and TLCK [71]. Our laboratory subsequently confirmed these results in P. falciparum. Treatment of activated gametocytes with TPCK, TLCK, PMSF, or two novel falcipain-targeting cysteine protease inhibitors during activation reduced the formation of microgametes [72]. Furthermore, the aspartic protease inhibitor EPNP appeared to interfere with rounding up of gametocytes. The exact modes of action for these proteases during gametocyte egress from its host cell remain to be investigated.
5. Exflagellation and Gamete Formation
Exflagellation is the process in which the newly formed microgametes adhere to neighboring erythrocytes, thus forming rosettes called exflagellation centers, and then detach from the residual body of the activated male microgametocyte. The exflagellating microgamete adheres to sialic acids and glycophorin A of the erythrocyte surface [73] and this binding is probably mediated by Pfs230, an abundantly expressed adhesion protein that is associated with the gamete surface [74]. Interestingly, Pfs230 is proteolytically processed during gametocyte activation, and this processing can be inhibited by the metalloprotease inhibitor 1,10-phenanthroline [75, 76]. The same inhibitor blocks exflagellation by leaving the microgamete amotile [72], and it is tempting to speculate that processing of Pfs230 increases the adhesive properties of this protein, which are needed for the binding of the exflagellating microgamete to erythrocytes [74].
Astonishing advances have been made in unveiling the signaling cascades during gametogenesis of plasmodia. This might be explained by the fact that sexual stage proteins can easily be disrupted because of their nonessentiality for parasite proliferation, and thus a functional characterization can be obtained by phenotype analysis of parasites, in which the respective genes have been knocked out. Genome annotation has revealed an extensive catalog of parasite-encoded kinases, the malaria kinome, with at least 86 hypothetical kinases identified in P. falciparum [77, 78]. Several orthologs of these kinases were disrupted in P. berghei. An initial elegant study showed that the calcium-dependent protein kinase PbCDPK4 is involved in sexual stage signaling and regulation [79] (Figure 2). The kinase becomes activated by calcium increase following XA activation, resulting in genome replication in microgametocytes [79]. In P. falciparum, PfCDPK4 was reported to be gametocyte-specific and activated by phospholipase C [80]. In a subsequent step, the mitogen-activated protein kinase Pbmap-2 controls formation of male gametes at the stage of cytokinesis [81–83]. Downstream of these events, the protein kinases Pbnek-2 and Pbnek-4 trigger genome replication to the tetraploid level in the zygote stage [81, 84, 85]. Furthermore, PbCDPK3 is required for ookinete motility and engagement with the mosquito midgut epithelium [86, 87]. When highlighting these novel signaling pathways during gametogenesis, it has to be taken under consideration, however, that in some cases the results obtained for P. berghei and P. falciparum might differ. For example, a recent reverse genetics approach on the P. falciparum ortholog Pfmap-2 pointed to an essential function of this kinase for the parasite asexual blood cycle [88], contradictory to the abovementioned results on Pbmap-2. This indicates that insights gained by studying the rodent malaria model P. berghei cannot be as easily applied to human malaria pathogens as has so far been assumed.
Ingestion by the blood-feeding mosquito triggers molecular changes in the sexual stages of P. falciparum, with approximately 20% of stage-specific genes being activated during sexual stage development and parasite transmission [3, 5, 6]. This molecular switchover adjusts the gametocytes to the invertebrate host and on one hand initiates reproduction but on the other hand prepares the emerging gametes for the hostile environment of the mosquito midgut. Gametocyte development and gamete formation are particularly accompanied by the coordinated expression of numerous surface-associated proteins, including the EGF domain-containing proteins Pfs25 and Pfs28, the cysteine motif-rich proteins Pfs230 and Pfs48/45, as well as the multiadhesion domain PfCCp proteins. These proteins and their potential as transmission blocking targets were discussed previously [1] and will therefore not be focus of this review. Noteworthy is that the majority of these surface proteins have adhesive properties and can be divided in two classes. One class of sexual stage proteins, including Pfs230, Pfs48/45, and the six PfCCp proteins, is expressed within the PV of the developing gametocyte and subsequently present on the gamete surface, but expression of these proteins usually ceases during fertilization (Figure 3). The expression of the second class of surface proteins starts at the time point of fertilization, as was shown for Pfs25 and Pfs28, and expression often persists until the ookinete has formed [1]. The reason for this sudden onset of protein expression during fertilization is the translational repression of messenger RNA encoding for these proteins. This was interalia shown for the repression of Pbs25 and Pbs28 by the P. berghei RNA helicase DOZI (development of zygote inhibited) as part of a ribonucleoprotein complex [25]. Furthermore, transcript of the transcription factor AP2-O is present in female gametocytes. The factor, however, is only translated in the ookinete stage, where it then activates a set of genes encoding for adhesion proteins important for midgut invasion [89].
Figure 3.
Morphological changes of malaria parasites during transmission from the human host to the mosquito vector. The intraerythrocytic gametocyte stages mature in the human host and are taken up by the blood-feeding female mosquito. By entering the mosquito midgut, the gametocytes become activated and round up, before emerging from the enveloping host erythrocyte. Proteases (P) are involved in these processes. During gametogenesis, the female macrogametocyte transforms into a macrogamete, while the activated male microgametocyte forms eight microgametes. Within approximately twenty-minute post-activation, the motile microgamete fertilizes the macrogamete and the resulting zygote transforms within a day into the infective ookinete. Two classes of sexual stage proteins are expressed in association with the parasite surface. A first class of proteins (shown in green) is expressed in the parasitophorous vacuole of the developing gametocyte, where some of them assemble to form adhesive multiprotein complexes. The proteins are later exposed on the surface of the newly emerged gametes, but expression ceases during fertilization. Expression of a second class of surface-associated proteins (shown in pink) is repressed in the gametocyte stage, but repression is released during fertilization (R) and protein expression persists to the ookinete stage.
The reason for such a high number of adhesive proteins in the malaria parasite sexual stages remains elusive, but a new study from our laboratory might provide a first step towards answering this question. We showed that the six PfCCp proteins, which are characterized by a high number of adhesion modules, assemble to form multiprotein complexes during their expression in the PV, and these complexes are subsequently present on the surface of the newly emerged macrogametes [90]. Preliminary data point to an additional involvement of other surface-associated adhesion proteins in these complexes, like the transmembrane protein Pfs48/45, which might link the complex to the gamete surface [91] (S. Scholz, A. Kuehn, N. Simon, and G. Pradel, unpublished observations). We hypothesize that these protein complexes cover the macrogamete in the form of a sticky coat and that they are involved in important adhesive processes during malaria transmission to the mosquito. The complexes might play a role in promoting contact between the emerging gametes within the blood meal or in protecting the gametes from the aggressive environment of the mosquito midgut. Noteworthy, the gametes and zygotes are the only stages within the parasite's life cycle that, for more than one day, have to persevere outside a host cell. Here they are exposed to factors of the blood meal, including midgut bacteria and digestive enzymes, as well as components of the human immune system. This exposure results in an approximate 300-fold loss of parasite abundance during transmission to the mosquito, and the malaria transmission stages are therefore considered bottleneck stages of the parasite's life cycle [92].
6. Mating of Malaria Parasites
During exflagellation, the microgamete detaches from the residual body and is freely motile, moving via sinusoidal or helical waves [93]. It is not known whether the microgamete meets the macrogamete by coincidence, whether it actively scans the blood meal, or whether it migrates along a gradient of an attractant that is released by the macrogamete. Interestingly, we recently identified filamentous protrusions of the P. falciparum gamete surface, which form immediately upon activation and which appear to establish long-distance contacts between parasites in the mosquito midgut (G. Pradel, unpublished observations). These filaments fit the typical characteristics of socalled nanotubes, novel organelles that were recently described for a number of animal cells (reviewed in [95, 96]). It has been proposed that nanotube-like filaments can be formed by almost all cells serving as a medium for exploring the extracellular environment [94] and therefore are likely to represent ancient features of unicellular eukaryotes. Nanotubes were reported to have a function in communication between cells, including calcium signaling and organelle transfer [95, 96]. We therefore hypothesize that the “nanotubes” of malaria gametes might be tools to facilitate association within the midgut in order to increase the chance of parasite mating.
Once the microgamete adheres to a macrogamete, fertilization begins by fusion of the plasma membranes. Two recent studies on P. berghei described the identification of the microgamete protein GCS1 (generative cell specific 1), also termed HAP2, which enables gamete fusion, and disruption of the respective gene results in male sterility and blocked fertilization [97, 98]. GCS1/HAP2 is a conserved protein of algae and plants, where it is involved in pollen tube guidance and seed formation [99, 100], and was also identified in protozoan parasites [97, 98, 101]. Importantly, GCS1/HAP2 does not mediate the initial binding between the two mating partners, which appears to involve other adhesion proteins [98].
Cell fusion is followed by nuclear fusion, and over the next 3 hours, meiosis occurs and the zygote becomes tetraploid [102]. During the following 24 hours, the zygote transforms into the infective ookinete stage, thus marking the end of the malaria sexual phase. The ookinete is motile and possesses an apical complex which enables it to disrupt and traverse the midgut epithelium, before settling down between epithelium and basal lamina. Parasite tetraploidy persists throughout the ookinete stage until sporozoite budding in the oocyst restores the haploid state [102].
7. Concluding Remarks
Despite intense work on the sexual stages of malaria parasites, they represent the least understood stages of the parasite's life cycle. Gametocyte differentiation and gametogenesis have mostly been studied in the human malaria pathogen P. falciparum, and for these sexual stages, a variety of proteins have been identified and characterized. On the other hand, the implication of malaria sexual stage proteins for malaria transmission was preferentially investigated in the murine P. berghei model, which is more easily accessible for genetic manipulations and transmission studies. Up to date it is challenging to combine information gained by both systems to receive the big picture on the malaria sexual phase. We expect that in the near future research on the sexual stages of malaria parasites will be dominated by two major tasks: (i) the big hunt for “the gene”, which enables the blood stage parasite to enter the sexual pathway and (ii) the analysis of the molecular mechanisms and signaling events of sexual stage parasites during fertilization in the mosquito midgut.
Acknowledgments
The authors thank Marc Kirschner for carefully reviewing the manuscript and Oliver Billker, Christian Doerig, and Tom Templeton for helpful discussions and support. The second author receives funding by an Emmy-Noether grant and the SFB479 of the Deutsche Forschungsgemeinschaft and by the 7th Framework Program of the European Union. The first author is associated member of the BioMedTec International Graduate School of Science “Lead Structures of Cell Function” of the Elite Network Bavaria.
References
- 1.Pradel G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology. 2007;134(14):1911–1929. doi: 10.1017/S0031182007003381. [DOI] [PubMed] [Google Scholar]
- 2.Carlton JM, Angiuoli SV, Suh BB, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419(6906):512–519. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- 3.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419(6906):520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 4.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419(6906):498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasonder E, Ishihama Y, Andersen JS, et al. Analysis of the Plasmodium falciparum proteome by high-accuracy mass spedrometry. Nature. 2002;419(6906):537–542. doi: 10.1038/nature01111. [DOI] [PubMed] [Google Scholar]
- 6.Le Roch KG, Zhou Y, Blair PL, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301(5639):1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 7.Hall NC, Karras M, Raine JD, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307(5731):82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 8.Talman AM, Domarle O, McKenzie FE, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malaria Journal. 2004;3, article 24 doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alano P, Billker O. Gametocytes and gametes. In: Sherman IW, editor. Molecular Approaches to Malaria. Washington, DC, USA: ASM Press; 2005. pp. 191–219. [Google Scholar]
- 10.Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Molecular Microbiology. 2007;66(2):291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 11.Babiker HA, Schneider P, Reece SE. Gametocytes: insights gained during a decade of molecular monitoring. Trends in Parasitology. 2008;24(11):525–530. doi: 10.1016/j.pt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smalley ME, Brown J. Plasmodium falciparum gametocytogenesis stimulated by lymphocytes and serum from infected Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75(2):316–317. doi: 10.1016/0035-9203(81)90348-5. [DOI] [PubMed] [Google Scholar]
- 13.Schneweis S, Maier WA, Seitz HM. Haemolysis of infected erythrocytes—a trigger for formation of Plasmodium falciparum gametocytes? Parasitology Research. 1991;77(5):458–460. doi: 10.1007/BF00931646. [DOI] [PubMed] [Google Scholar]
- 14.Lingnau A, Margos G, Maier WA, Seitz HM. The effects of hormones on the gametocytogenesis of Plasmodium falciparum in vitro. Applied Parasitology. 1993;34(3):153–160. [PubMed] [Google Scholar]
- 15.Motard A, Landau I, Nussler A, et al. The role of reactive nitrogen intermediates in modulation of gametocyte infectivity of rodent malaria parasites. Parasite Immunology. 1993;15(1):21–26. doi: 10.1111/j.1365-3024.1993.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 16.Puta C, Manyando C. Enhanced gametocyte production in Fansidar-treated Plasmodium falciparum malaria patients: implications for malaria transmission control programmes. Tropical Medicine and International Health. 1997;2(3):227–229. doi: 10.1046/j.1365-3156.1997.d01-267.x. [DOI] [PubMed] [Google Scholar]
- 17.Hogh B, Gamage-Mendis A, Butcher GA, et al. The differing impact of chloroquine and pyrimethamine/sulfadoxine upon the infectivity of malaria species to the mosquito vector. American Journal of Tropical Medicine and Hygiene. 1998;58(2):176–182. doi: 10.4269/ajtmh.1998.58.176. [DOI] [PubMed] [Google Scholar]
- 18.Buckling A, Ranford-Cartwright LC, Miles A, Read AF. Chloroquine increases Plasmodium falciparum gametocytogenesis in vitro. Parasitology. 1999;118(4):339–346. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 19.Sokhna CS, Trape J-F, Robert V. Gametocytaemia in Senegalese children with uncomplicated falciparum malaria treated with chloroquine, amodiaquine or sulfadoxine + pyrimethamine. Parasite. 2001;8(3):243–250. doi: 10.1051/parasite/2001083243. [DOI] [PubMed] [Google Scholar]
- 20.Talman AM, Paul REL, Sokhna CS, et al. Influence of chemotherapy on the Plasmodium gametocyte sex ratio of mice and humans. American Journal of Tropical Medicine and Hygiene. 2004;71(6):739–744. [PubMed] [Google Scholar]
- 21.Day KP, Karamalis F, Thompson J, et al. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(17):8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinden RE. Gametocytes and sexual development. In: Sherman IW, editor. Malaria: Parasite Biology, Pathogenesis, and Protection. Washington, DC, USA: ASM Press; 1998. pp. 25–48. [Google Scholar]
- 23.Hawking F, Wilson ME, Gammage K. Evidence for cyclic development and short-lived maturity in the gametocytes of Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1971;65(5):549–559. doi: 10.1016/0035-9203(71)90036-8. [DOI] [PubMed] [Google Scholar]
- 24.Sinden RE. Gametocytogenesis of Plasmodium falciparum in vitro: an electron microscopic study. Parasitology. 1982;84(1):1–11. doi: 10.1017/s003118200005160x. [DOI] [PubMed] [Google Scholar]
- 25.Mair GR, Braks JAM, Garver LS, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313(5787):667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvestrini F, Alano P, Williams JL. Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology. 2000;121(5):465–471. doi: 10.1017/s0031182099006691. [DOI] [PubMed] [Google Scholar]
- 27.Smith TG, Lourenco P, Carter R, Walliker D, Ranford-Cartwright LC. Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology. 2000;121(2):127–133. doi: 10.1017/s0031182099006265. [DOI] [PubMed] [Google Scholar]
- 28.Paul REL, Brey PT, Robert V. Plasmodium sex determination and transmission to mosquitoes. Trends in Parasitology. 2002;18(1):32–38. doi: 10.1016/s1471-4922(01)02122-5. [DOI] [PubMed] [Google Scholar]
- 29.Reece SE, Drew DR, Gardner A. Sex ratio adjustment and kin discrimination in malaria parasites. Nature. 2008;453(7195):609–614. doi: 10.1038/nature06954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton WD. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreedinghas new implications in cytogenetics and entomology. Science. 1967;156(3774):477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- 31.Schall JJ. Evolutionary biology: sex ratios writ small. Nature. 2008;453(7195):605–606. doi: 10.1038/453605a. [DOI] [PubMed] [Google Scholar]
- 32.Schall JJ. Do malaria parasites follow the algebra of sex ratio theory? Trends in Parasitology. 2009;25(3):120–123. doi: 10.1016/j.pt.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Mitri C, Thiery I, Bourgouin C, Paul REL. Density-dependent impact of the human malaria parasite Plasmodium falciparum gametocyte sex ratio on mosquito infection rates. Proceedings of the Royal Society B. 2009;276(1673):3721–3726. doi: 10.1098/rspb.2009.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alano P, Carter R. Sexual differentiation in malaria parasites. Annual Review of Microbiology. 1990;44:429–449. doi: 10.1146/annurev.mi.44.100190.002241. [DOI] [PubMed] [Google Scholar]
- 35.Smalley ME, Abdalla S, Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75(1):103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- 36.Rogers NJ, Hall BS, Obiero J, Targett GAT, Sutherland CJ. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infection and Immunity. 2000;68(6):3455–3462. doi: 10.1128/iai.68.6.3455-3462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology. 1977;74(1):1–8. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- 38.Lensen A, Bril A, van de Vegte M, van Gemert GJ, Eling W, Sauerwein R. Plasmodium falciparum: infectivity of cultured, synchronized gametocytes to mosquitoes. Experimental Parasitology. 1999;91(1):101–103. doi: 10.1006/expr.1998.4354. [DOI] [PubMed] [Google Scholar]
- 39.Pichon G, Awono-Ambene HP, Robert V. High heterogeneity in the number of Plasmodium falciparum gametocytes in the bloodmeal of mosquitoes fed on the same host. Parasitology. 2000;121(2):115–120. doi: 10.1017/s0031182099006277. [DOI] [PubMed] [Google Scholar]
- 40.Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitology. 1997;115(1):1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- 41.Billker O, Lindo V, Panico M, et al. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392(6673):289–292. doi: 10.1038/32667. [DOI] [PubMed] [Google Scholar]
- 42.Garcia GE, Wirtz RA, Barr JR, Woolfitt A, Rosenbergt R. Xanthurenic acid induces gametogenesis in Plasmodium, the malaria parasite. Journal of Biological Chemistry. 1998;273(20):12003–12005. doi: 10.1074/jbc.273.20.12003. [DOI] [PubMed] [Google Scholar]
- 43.Kawamoto F, Alejo-Blanco R, Fleck SL, Sinden RE. Plasmodium berghei: ionic regulation and the induction of gametogenesis. Experimental Parasitology. 1991;72(1):33–42. doi: 10.1016/0014-4894(91)90118-g. [DOI] [PubMed] [Google Scholar]
- 44.Sinden RE, Canning EU, Bray RS, Smalley ME. Gametocyte and gamete development in Plasmodium falciparum. Proceedings of the Royal Society B. 1978;201(1145):375–399. doi: 10.1098/rspb.1978.0051. [DOI] [PubMed] [Google Scholar]
- 45.Kawamoto F, Alejo-Blanco R, Fleck SL, Kawamoto Y, Sinden RE. Possible roles of Ca2+ and cGMP as mediators of the exflagellation of Plasmodium berghei and Plasmodium falciparum. Molecular and Biochemical Parasitology. 1990;42(1):101–108. doi: 10.1016/0166-6851(90)90117-5. [DOI] [PubMed] [Google Scholar]
- 46.Kawamoto F, Fujioka H, Murakami R-I, et al. The roles of Ca2+/calmodulin- and cGMP-dependent pathways in gametogenesis of a rodent malaria parasite, Plasmodium berghei. European Journal of Cell Biology. 1993;60(1):101–107. [PubMed] [Google Scholar]
- 47.Carucci DJ, Witney AA, Muhia DK, et al. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. Journal of Biological Chemistry. 2000;275(29):22147–22156. doi: 10.1074/jbc.M001021200. [DOI] [PubMed] [Google Scholar]
- 48.Muhia DK, Swales CA, Deng W, Kelly JM, Baker DA. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Molecular Microbiology. 2001;42(2):553–560. doi: 10.1046/j.1365-2958.2001.02665.x. [DOI] [PubMed] [Google Scholar]
- 49.Hirai M, Arai M, Kawai S, Matsuoka H. PbGCbeta is essential for Plasmodium ookinete motility to invade midgut cell and for successful completion of parasite life cycle in mosquitoes. Journal of Biochemistry. 2006;140(5):747–757. doi: 10.1093/jb/mvj205. [DOI] [PubMed] [Google Scholar]
- 50.McRobert L, Taylor CJ, Deng W, et al. Gametogenesis in malaria parasites is mediated by the cGMP-dependent protein kinase. PLoS biology. 2008;6(6, article e139) doi: 10.1371/journal.pbio.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin SK, Jett M, Schneider I. Correlation of phosphoinositide hydrolysis with exflagellation in the malaria microgametocyte. Journal of Parasitology. 1994;80(3):371–378. [PubMed] [Google Scholar]
- 52.Aikawa M, Carter R, Ito Y, Nijhout MM. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. Journal of Protozoology. 1984;31(3):403–413. doi: 10.1111/j.1550-7408.1984.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 53.Alano P, Read D, Bruce M, et al. COS cell expression cloning of Pfg377, a Plasmodium falciparum gametocyte antigen associated with osmiophilic bodies. Molecular and Biochemical Parasitology. 1995;74(2):143–156. doi: 10.1016/0166-6851(95)02491-3. [DOI] [PubMed] [Google Scholar]
- 54.Severini C, Silvestrini F, Sannella A, Barca S, Gradoni L, Alano P. The production of the osmiophilic body protein Pfg377 is associated with stage of maturation and sex in Plasmodium falciparum gametocytes. Molecular and Biochemical Parasitology. 1999;100(2):247–252. doi: 10.1016/s0166-6851(99)00050-x. [DOI] [PubMed] [Google Scholar]
- 55.de Koning-Ward TF, Olivieri A, Bertuccini L, et al. The role of osmiophilic bodies and Pfg377 expression in female gametocyte emergence and mosquito infectivity in the human malaria parasite Plasmodium falciparum. Molecular Microbiology. 2008;67(2):278–290. doi: 10.1111/j.1365-2958.2007.06039.x. [DOI] [PubMed] [Google Scholar]
- 56.Silvestrini F, Bozdech Z, Lanfrancotti A, et al. Genome-wide identification of genes upregulated at the onset of gametocytogenesis in Plasmodium falciparum. Molecular and Biochemical Parasitology. 2005;143(1):100–110. doi: 10.1016/j.molbiopara.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 57.Furuya T, Mu J, Hayton K, et al. Disruption of a Plasmodium falciparum gene linked to male sexual development causes early arrest in gametocytogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(46):16813–16818. doi: 10.1073/pnas.0501858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lanfrancotti A, Bertuccini L, Silvestrini F, Alano P. Plasmodium falciparum: mRNA co-expression and protein co-localisation of two gene products upregulated in early gametocytes. Experimental Parasitology. 2007;116(4):497–503. doi: 10.1016/j.exppara.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Lal K, Delves MJ, Bromley E, Wastling JM, Tomley FM, Sinden RE. Plasmodium male development gene-1 (mdv-1) is important for female sexual development and identifies a polarised plasma membrane during zygote development. International Journal for Parasitology. 2009;39(7):755–761. doi: 10.1016/j.ijpara.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Ponzi M, Sidén-Kiamos I, Bertuccini L, et al. Egress of Plasmodium berghei gametes from their host erythrocyte is mediated by the MDV-1/PEG3 protein. Cellular Microbiology. 2009;11(8):1272–1288. doi: 10.1111/j.1462-5822.2009.01331.x. [DOI] [PubMed] [Google Scholar]
- 61.Wickham ME, Culvenor JG, Cowman AF. Selective inhibition of a two-step egress of malaria parasites from the host erythrocyte. Journal of Biological Chemistry. 2003;278(39):37658–37663. doi: 10.1074/jbc.M305252200. [DOI] [PubMed] [Google Scholar]
- 62.Salmon BL, Oksman A, Goldberg DE. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):271–276. doi: 10.1073/pnas.011413198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soni S, Dhawan S, Rosen KM, Chafel M, Chishti AH, Hanspal M. Characterization of events preceding the release of malaria parasite from the host red blood cell. Blood Cells, Molecules, and Diseases. 2005;35(2):201–211. doi: 10.1016/j.bcmd.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Blackman MJ. Malarial proteases and host cell egress: an ‘emerging' cascade. Cellular Microbiology. 2008;10(10):1925–1934. doi: 10.1111/j.1462-5822.2008.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller SK, Good RT, Drew DR, et al. A subset of Plasmodium falciparum SERA genes are expressed and appear to play an important role in the erythrocytic cycle. Journal of Biological Chemistry. 2002;277(49):47524–47532. doi: 10.1074/jbc.M206974200. [DOI] [PubMed] [Google Scholar]
- 66.Aoki S, Li J, Itagaki S, et al. Serine repeat antigen (SERA5) is predominantly expressed among the SERA multigene family of Plasmodium falciparum, and the acquired antibody titers correlate with serum inhibition of the parasite growth. Journal of Biological Chemistry. 2002;277(49):47533–47540. doi: 10.1074/jbc.M207145200. [DOI] [PubMed] [Google Scholar]
- 67.Yeoh S, O'Donnell RA, Koussis K, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131(6):1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 68.Wu Y, Wang X, Liu X, Wang Y. Data-mining approaches reveal hidden families of proteases in the genome of malaria parasite. Genome Research. 2003;13(4):601–616. doi: 10.1101/gr.913403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenthal PJ. Cysteine proteases of malaria parasites. International Journal for Parasitology. 2004;34(13-14):1489–1499. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Glushakova S, Mazar J, Hohmann-Marriott MF, Hama E, Zimmerberg J. Irreversible effect of cysteine protease inhibitors on the release of malaria parasites from infected erythrocytes. Cellular Microbiology. 2009;11(1):95–105. doi: 10.1111/j.1462-5822.2008.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres JA, Rodriguez MH, Rodriguez MC, de la Cruz Hernandez-Hernandez F. Plasmodium berghei: effect of protease inhibitors during gametogenesis and early zygote development. Experimental Parasitology. 2005;111(4):255–259. doi: 10.1016/j.exppara.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Rupp I, Bosse R, Schirmeister T, Pradel G. Effect of protease inhibitors on exflagellation in Plasmodium falciparum. Molecular and Biochemical Parasitology. 2008;158(2):208–212. doi: 10.1016/j.molbiopara.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 73.Templeton TJ, Keister DB, Muratova O, Procter JL, Kaslow DC. Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. Journal of Experimental Medicine. 1998;187(10):1599–1609. doi: 10.1084/jem.187.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eksi S, Czesny B, van Gemert G-J, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Molecular Microbiology. 2006;61(4):991–998. doi: 10.1111/j.1365-2958.2006.05284.x. [DOI] [PubMed] [Google Scholar]
- 75.Williamson KC, Fujioka H, Aikawa M, Kaslow DC. Stage-specific processing of Pfs230, a Plasmodium falciparum transmission-blocking vaccine candidate. Molecular and Biochemical Parasitology. 1996;78(1-2):161–169. doi: 10.1016/s0166-6851(96)02621-7. [DOI] [PubMed] [Google Scholar]
- 76.Brooks SR, Williamson KC. Proteolysis of Plasmodium falciparum surface antigen, Pfs230, during gametogenesis. Molecular and Biochemical Parasitology. 2000;106(1):77–82. doi: 10.1016/s0166-6851(99)00201-7. [DOI] [PubMed] [Google Scholar]
- 77.Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5, article 79 doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anamika, Srinivasan N, Krupa A. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins. 2005;58(1):180–189. doi: 10.1002/prot.20278. [DOI] [PubMed] [Google Scholar]
- 79.Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117(4):503–514. doi: 10.1016/s0092-8674(04)00449-0. [DOI] [PubMed] [Google Scholar]
- 80.Ranjan R, Ahmed A, Gourinath S, Sharma P. Dissection of mechanisms involved in the regulation of Plasmodium falciparum calcium-dependent protein kinase 4. Journal of Biological Chemistry. 2009;284(22):15267–15276. doi: 10.1074/jbc.M900656200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan SM, Franke-Fayard B, Mair GR, et al. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121(5):675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 82.Rangarajan R, Bei AK, Jethwaney D, et al. A mitogen-activated protein kinase regulates male gametogenesis and transmission of the malaria parasite Plasmodium berghei. EMBO Reports. 2005;6(5):464–469. doi: 10.1038/sj.embor.7400404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tewari R, Dorin D, Moon R, Doerig C, Billker O. An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Molecular Microbiology. 2005;58(5):1253–1263. doi: 10.1111/j.1365-2958.2005.04793.x. [DOI] [PubMed] [Google Scholar]
- 84.Reininger L, Billker O, Tewari R, et al. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. Journal of Biological Chemistry. 2005;280(36):31957–31964. doi: 10.1074/jbc.M504523200. [DOI] [PubMed] [Google Scholar]
- 85.Reininger L, Tewari R, Fennell C, et al. An essential role for the Plasmodium Nek-2 nima-related protein kinase in the sexual development of malaria parasites. Journal of Biological Chemistry. 2009;284(31):20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishino T, Orito Y, Chinzei Y, Yuda M. A calcium-dependent protein kinase regulates Plasmodium ookinete access to the midgut epithelial cell. Molecular Microbiology. 2006;59(4):1175–1184. doi: 10.1111/j.1365-2958.2005.05014.x. [DOI] [PubMed] [Google Scholar]
- 87.Siden-Kiamos I, Ecker A, Nyback S, Louis C, Sinden RE, Billker O. Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Molecular Microbiology. 2006;60(6):1355–1363. doi: 10.1111/j.1365-2958.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorin-Semblat D, Quashie N, Halbert J, et al. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Molecular Microbiology. 2007;65(5):1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- 89.Yuda M, Iwanaga S, Shigenobu S, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Molecular Microbiology. 2009;71(6):1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- 90.Simon N, Scholz SM, Moreira CK, et al. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. Journal of Biological Chemistry. 2009;284(21):14537–14546. doi: 10.1074/jbc.M808472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scholz SM, Simon N, Lavazec C, Dude M-A, Templeton TJ, Pradel G. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. International Journal for Parasitology. 2008;38(3-4):327–340. doi: 10.1016/j.ijpara.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 92.Vaughan JA, Noden BH, Beier JC. Sporogonic development of cultured Plasmodium falciparum in six species of laboratory-reared Anopheles mosquitoes. American Journal of Tropical Medicine and Hygiene. 1994;51(2):233–243. doi: 10.4269/ajtmh.1994.51.233. [DOI] [PubMed] [Google Scholar]
- 93.Sinden RE, Croll NA. Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology. 1975;70(1):53–65. doi: 10.1017/s0031182000048861. [DOI] [PubMed] [Google Scholar]
- 94.Ramirez-Weber F-A, Kornberg TB. Signaling reaches to new dimensions in Drosophila imaginal discs. Cell. 2000;103(2):189–192. doi: 10.1016/s0092-8674(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 95.Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nature Reviews Molecular Cell Biology. 2008;9(6):431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 96.Gerdes H-H, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Current Opinion in Cell Biology. 2008;20(4):470–475. doi: 10.1016/j.ceb.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 97.Hirai M, Arai M, Mori T, et al. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Current Biology. 2008;18(8):607–613. doi: 10.1016/j.cub.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y, Tewari R, Ning J, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes and Development. 2008;22(8):1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson MA, von Besser K, Zhou Q, et al. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168(2):971–982. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development. 2006;133(23):4761–4769. doi: 10.1242/dev.02683. [DOI] [PubMed] [Google Scholar]
- 101.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. Generative cell specific 1 is essential for angiosperm fertilization. Nature Cell Biology. 2006;8(1):64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- 102.Janse CJ, van der KLooster PFJ, van der Kaay HJ, van der Ploeg M, Overdulve JP. DNA synthesis in Plasmodium berghei during asexual and sexual development. Molecular and Biochemical Parasitology. 1986;20(2):173–182. doi: 10.1016/0166-6851(86)90029-0. [DOI] [PubMed] [Google Scholar]