Abstract
Background
Restoration of a three-dimensional shape with soft tissue augmentation is a challenge for surgical reconstruction and esthetic improvement of intraoral mucosa and perioral skin tissues. A connective tissue graft or free gingival graft, classically used for such indications, requires a donor site, which may lead to various clinical complications.
Methods
In this article, a new three-dimensional scaffold made of silk fibroin that could be of great interest for these indications was studied. Mechanical tests were conducted to characterize the physical properties of the materials. The biocompatibility of such scaffolds was positively assessed in vitro using a combination of immunostaining, 5-bromo-2′-deoxyuridine proliferation assays, and histologic staining. Finally, the shaped material was grafted subcutaneously in nude mice for a long-time implantation study.
Results
Human fibroblasts embedded in this material had a survival rate up to 68.4% and were able to proliferate and synthesize proteins. One month after subcutaneous implantation, the three-dimensional soft tissue augmentation was stable, and histologic analysis revealed revascularization of the area through the biomaterial. A mild inflammatory reaction disappeared after 12 weeks.
Conclusion
The results indicate that silk-gel material was able to create a lasting three-dimensional soft tissue augmentation and is a promising biomaterial for periodontal and maxillofacial therapies, either as a scaffold for cells or alone as a biomaterial.
Keywords: Biocompatible materials, fibroblasts, grafts, regeneration, silk
Soft tissue augmentation is frequently required for the reconstruction of perioral skin tissues in facial deformities or trauma or for intraoral ridge defects,1,2 causing functional troubles and/or esthetic complaints. Several techniques for managing such deformities have been proposed, either by soft or hard tissue augmentation.1,3,4 Alveolar ridge augmentation by regenerating the lost bone has been widely studied to enable the use of dental implants. This reconstruction of the alveolar bone can be achieved by a variety of procedures including membrane techniques, bone grafts, and bone splitting.5 However, when dental implants are not planned or possible for medical reasons, soft tissue augmentation has been used to restore the esthetic contour.
Current therapeutic procedures for such soft tissue augmentation are based on three main approaches: autologous graft/flap, synthetic polymer injection, or tissue engineering.6 The soft tissue gingival augmentation techniques used in periodontal therapy, such as subepithelial connective tissue grafts or free gingival grafts, are considered to be standard procedures to create a stable crestal mucosa. Autologous tissues are useful to avoid rejection or an inflammatory response from the host. They are easily obtained from surrounding tissues using either flap or graft procedures.7,8 However, this approach is significantly hampered by the limited availability of donor tissue volume, inadequate or excessive rigidity, donor site morbidity, and/or lack of epithelialization resulting in an eroded mucosal surface.6,9 The donor area will also contain variable amounts of adipose tissue, possibly resulting in a reduction in volume associated with poor revascularization and a low rate of survival of the adipocytes.10,11 Liquid synthetic polymers, such as silicone, or biomacromolecule-containing solutions, such as human/bovine collagen or hyaluronic acid, are also used12,13 for soft tissue augmentation but are limited by adverse reactions6,14 and fast volumetric loss.15 Recently, new scaffolds, combining adequate physicochemical properties, biocompatibility, and tissue integration16 were developed. These scaffolds can be colonized by host cells, thus creating a soft tissue augmentation durable enough to facilitate revascularization of the area. Among these new scaffolds, silk fibroin protein-based biomaterials can uniquely address the needs for specific tissue repairs17 and provide better control of functional tissue outcomes because of the ability to control their morphology, structure, chemistry, biocompatibility, and mechanical properties.18,19
The hypothesis of the present study was that silk fibroin gels could be an alternative to acellular dermal matrices that are widely used for oral and maxillofacial soft tissue augmentation. Mechanical characteristics of the scaffold and the survival rate, proliferation rate, and protein synthesis of fibroblasts were assessed in vitro. Finally, in vivo experiments were performed in mice to study the filling efficacy and biocompatibility of the material.
MATERIALS AND METHODS
Silk Solution
Silkworm (Bombyx mori) silk fibroin consists of about 60% glycine and alanine repeats arranged in a β-sheet structure.20 Silk fibroin aqueous stock solutions were prepared as previously described.21 Briefly, cocoons of Bombyx mori were boiled for 40 minutes in an aqueous solution of 0.02 M sodium carbonate and rinsed thoroughly with pure water to extract the glue-like sericin proteins and wax. After drying, the extracted silk fibroin was dissolved in 9.3 M lithium bromide solution at 60°C for 4 hours, yielding a 20% (weight/volume [w/v]) solution. This solution was dialyzed against distilled water using dialysis cassettes for 2 days to remove the salt. The final concentration of silk fibroin aqueous solution was ~8%(w/v). Silk solutions with lower concentrations were prepared by diluting the 8% solution with water.
Silk Gelation
Silk gelation was accomplished under sonication,22¶ which consisted of a power supply, converter, externally threaded disruptor horn, and 1/8-inch (3.175 mm)-diameter tapered microtip. For preparative tests, the silk concentration was 4% solution (w/v), and sonication time was varied from 5 to 30 seconds at the 20% amplitude setting. Solutions were incubated at 37°C after sonication, and the sol-gel transition was monitored visually. Samples were shaped like cylinders (Fig. 1A) with different thicknesses, depending on the experiment (see later), and scaffold-type ultrastructures (Fig. 1B).
Figure 1.
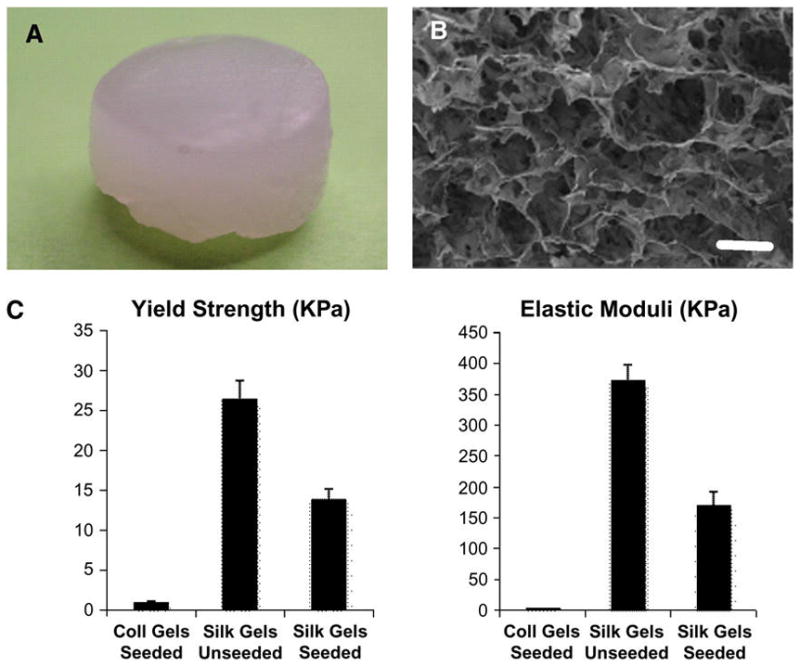
In vivo cylinder-shaped sample (8 × 6 mm) (A) and microstructural image of a liquid nitrogen frozen/freeze-dried gel observed under a field emission scanning electron microscope (B; bar = 1μm). Comparison of the mechanical properties of the silk and collagen gels using strain-to-failure and stress-relaxation tests. C) For each experiment, four samples were tested. Error bars represent the SD.
Cell Culture
For collagen gels, human dermal fibroblasts (HFFs) were derived from newborn foreskins using a collagenase and trypsin/EDTA mixture as previously described. 23 HFFs were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum,# penicillin–streptomycin, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid. Cells were usually seeded at a density 5 × 104 cells/ml (~10% confluent); cultures were replated when the cell density reached confluence. To construct the collagen matrix, HFF cells were added to neutralized type I collagen** to a final concentration of 5 × 105 cells/ml; 3 ml of this mixture was added to each 35-mmwell insert of a six-well plate and incubated for 7 days in media containing DMEM and 10% fetal calf serum until the collagen gel showed no further shrinkage.
For silk gels, a 4% (w/v) silk solution (1 ml) was sonicated in a laminar-flow hood at 50% amplitude for 30 seconds, and after 30 minutes incubation, the solution was sonicated again under the same conditions. After the second sonication, the solution was cooled to room temperature within 5 to 10 minutes, and 50 μl of the fibroblast suspension was added and mixed with the sonicated silk solution to reach a final concentration of 5 × 105 cells/ml. The control sample was sonicated in the same way, but 50 μl DMEM was added instead of the cell suspension after sonication. An aliquot of 1.5 ml of the mixtures was quickly transferred into 12-well cell-culture plates, with a total of three wells prepared for each sample group. All plates were incubated at 37°C and 5% CO2 for 7 days.
Mechanical Testing
Samples were tested for silk gels (with or without embedded cells) and fibroblast-contracted collagen on a machine†† equipped with unconfined compression platens and a 100-N load transducer, and sample data were exported using software.‡‡ Due to their irrelevance from the present study and difficulty in handling, uncontracted collagen gels were omitted from testing. Each sample was compressed at a controlled strain rate of 0.01 seconds−1. The compressive stress and strain were determined by normalizing raw load and extension data, respectively, against sample geometries (silk gel: 8 mm diameter × 3 mm height; collagen gel: 8 × 1 mm), and the elastic modulus was calculated as the slope of linear portion of each stress/strain plot. The compressive strength was determined by offsetting a line parallel to the best-fit line by a 0.5% strain, where the offset line that intersected the stress/strain response was defined as the value for yield strength.
Immunofluorescence and Proliferation Assay
For immunostaining, silk or collagen gels were frozen in embedding media§§ after incubating in 2 M sucrose for 2 hours at 4°C. Tissues were serial-sectioned at 8 μm. Tissue sections were blocked with 0.05% goat serum and 0.2% bovine serum albumin (v/v) in phosphate buffered saline (PBS) without fixation. Sections were first incubated with monoclonal antibody against procollagen|||| and later detected with fluorophore conjugated to goat immunoglobulin G.¶¶ Slides were mounted with a fluorescent mounting media## containing 1 mg/ml 4′-6-diamidino-2-phenylindole (DAPI) and visualized using a microscope.*** For apoptosis detection, cellular silk specimens were fixed in 10% neutral buffered formalin, and an in situ terminal deoxynucleotidyl transferase nick end labeling assay††† was performed in paraffin sections. Slides with no primary or secondary antibodies were used as immunostaining controls.
For 5-bromo-2′-deoxyuridine (BrdU) staining, prior to harvesting, silk or collagen gels were labeled with a 6-hour pulse of BrdU at a final concentration of 10 μM. Frozen tissues were sectioned at 8 μm, stained with monoclonal antibodies against BrdU,‡‡‡ and counterstained with DAPI.
Cell Viability Assays
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell viability. Briefly, 1 and 6 days in vitro (DIV) after seeding, scaffolds were washed three times with DMEM. The culture medium was replaced with DMEM and MTT (5 mg/ml in PBS), and the membranes were incubated for 2 hours at 37°C. After incubation, the reaction solution was removed from each well, and dimethyl sulfoxide was added. The membranes were gently agitated until the formazan precipitate was dissolved. Optical density values were measured using a spectrophotometer (550 nm) with a micro–enzyme-linked immunosorbent assay reader.§§§
Histologic Analyses
Biopsy specimens were fixed in 4% neutral buffered formalin, embedded in paraffin, and serially sectioned at 6 μm. Sections were stained with hematoxylin and eosin, visualized, and measured using a microscope|||||| equipped with a camera.¶¶¶
Grafting
All grafting experiments were performed under an animal protocol approved by Tufts Institutional Animal Care and Use Committee. Unseeded scaffolds made of silk or bovine collagen both shaped like a cylinder of 8 mm diameter × 6 mm height (Fig. 1A) were placed into dorsal subcutaneous pockets on the back of 6-week-old male Swiss nude mice.### Recipient mice were anesthetized with a mixture of ketamine 100 mg(75 mg/kg) and medetomidine hydrochloride 1 mg/ml (1 mg/kg) dissolved in saline. Animals were sacrificed at 7 and 14 days and 4 and 12 weeks after grafting.
RESULTS
Mechanical Properties
Unseeded silk gels, silk gels seeded with fibroblasts, and collagen gels seeded with fibroblasts exhibited an average compressive yield strength of 26.48 ± 2.30 KPa, 13.94 ± 1.20 KPa, and 0.87 ± 0.25 KPa, respectively, and an average elastic modulus of 373.65 ± 25.40 KPa, 171.09 ± 21.70 KPa, and 4.46 ± 0.3 KPa, respectively (Fig. 1C). Thus, unseeded and seeded silk gels demonstrated significantly higher compression strengths and elastic moduli than seeded collagen gels.
Survival, Proliferation, and Protein Synthesis of Fibroblasts In Vitro
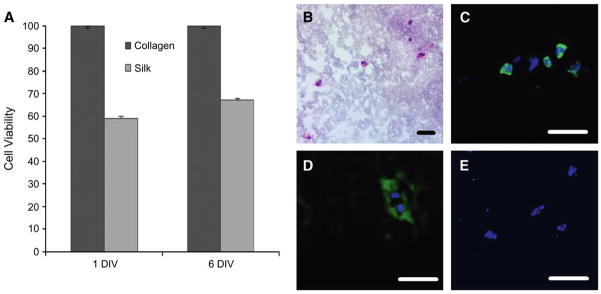
The cytotoxicity of the silk gels was assessed, and the viability of the encapsulated fibroblasts after 1 and 6 DIV was examined (Fig. 2A). The percentage of cells in silk scaffolds increased from 59.5% ± 2% after 1 DIV to 68.4% ± 1% after 6 DIV.
Figure 2.
In vitro evaluation of fibroblasts inside the silk gels. A) Survival rates after 1 and 6 DIV were measured using the MTT assay for cell viability. Each experiment was performed in triplicate. Error bars represent the SD. B) Inside the silk scaffold, fibroblasts presented a rounded morphology (hematoxylin and eosin stain). Cells were able to proliferate, as confirmed by BrdU staining (C), and synthesizing proteins, as shown by the presence of procollagen, a precursor of collagen, deposited around the fibroblasts (D) (procollagen immunostain). E) No apoptotic cells could be detected (TUNEL stain). Bar = 50 μm.
The localization of fibroblasts within silk gels (Fig. 2B) in vitro was similar to collagen gels (data not shown), with equivalent distribution in the three dimensions of the scaffold. The fibroblasts appeared elongated (spindle shaped) in collagen scaffolds, whereas they were more rounded with a lower degree of spreading in silk gels. BrdU staining was performed to determine the proliferation of fibroblasts in silk gels in the three-dimensional cultures (Fig. 2C). The rate of proliferation of the fibroblasts was high (52.4% ± 3%) compared to fibroblasts in collagen gels (14.8% ± 4%), especially after 7 DIV. Furthermore, immunohistochemical staining revealed that fibroblasts in the silk gels were able to produce type I procollagen (Fig. 2D), the precursor form of type I collagen synthesized in vivo by HFFs. In addition, apoptotic cells were not detected in the scaffold (Fig. 2E), indicating that the silk gels did not induce cell death.
Therefore, in the in vitro experiments, silk gels were non-cytotoxic and also allowed fibroblasts to proliferate and synthesize proteins.
Assessment of Silk Gels In Vivo
To assess the silk gels and determine the stability of the shape of the soft tissue augmentation in vivo, seeded silk gels were implanted subcutaneously in mice and observed up to 3 months after grafting. Visual macroscopic observations confirmed that the silk gels kept their shape, while progressively rounding at the periphery, after 7 days, 2 weeks, and 12 weeks (Figs. 3A, 3B, and 3C, respectively). After 1 month, at necropsy, histologic analysis of biopsy specimens showed healthy surrounding tissues with vasculature on the material (Fig. 3D). On days 7 and 14, silk gels and surrounding tissues showed signs of inflammation, with the presence of eosinophils, neutrophils, and macrophages present around, but not inside, the scaffold (Figs. 3G and 3H). After 1 month, the inflammation around the silk gels was greatly reduced with less acute inflammatory cells and the presence of histiocytes in interstitial infiltrates. No inflammatory cells could be detected after 3 months. The inflammation was more severe around the collagen gels after 1 week (Figs. 3E and 3F), with numerous neutrophils, eosinophils, and macrophages infiltrating the material. In terms of the durability of the biomaterial, in these experiments, the collagen implants could not be detected after 1 month. Histologic analyses of the silk-gel biopsy specimens (Fig. 4A) showed a progressive colonization of the interstitial spaces by stromal ingrowths associated with a vascularization of the area 12 weeks after grafting (Figs. 4B and 4C).
Figure 3.
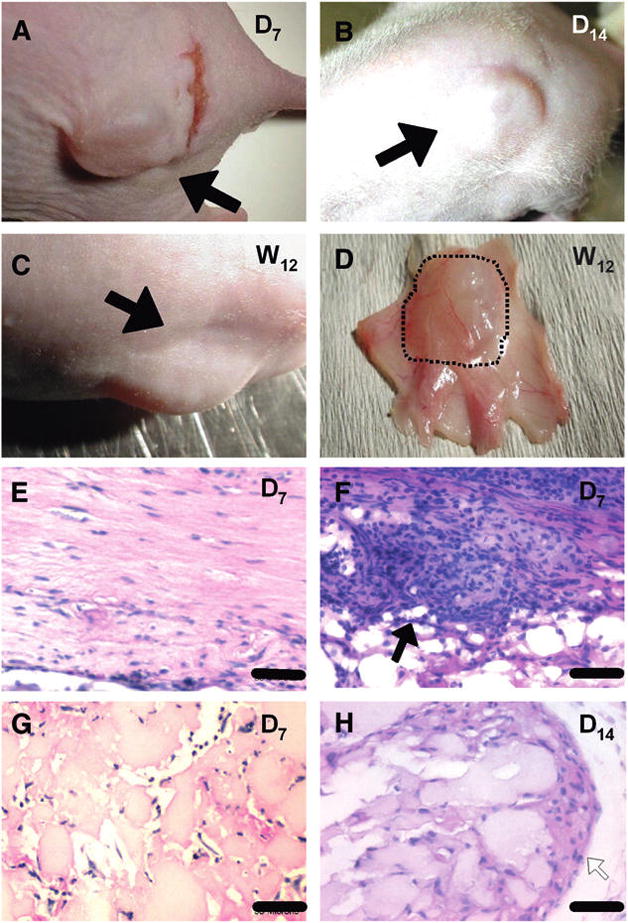
In vivo experiment. Macroscopic observations and short-term histologic analyses. Mice grafted with silk-gel cylinder-shaped samples on their back as observed on day 7 (A), day 14 (B), and week 12 (C). The augmentation created by the silk gels was clearly visible at each stage (black arrows). D) Visual inspection of a biopsy specimen showed healthy tissues surrounding the graft after 1 month. Histologic analysis with hematoxylin and eosin staining (E through H) showed infiltration by fibroblasts inside the two different gels on day 7 (E and G). A high-inflammation process (black arrow) was observed around collagen gels (F) with inflammatory cells, mainly neutrophils, eosinophils, and histiocytes. H) On day 14, a mild inflammation process with the presence of only histiocytes (white arrow) is observed at the periphery of silk gels. Bar = 50 μm (E and G) and 25 μm (F and H).
Figure 4.
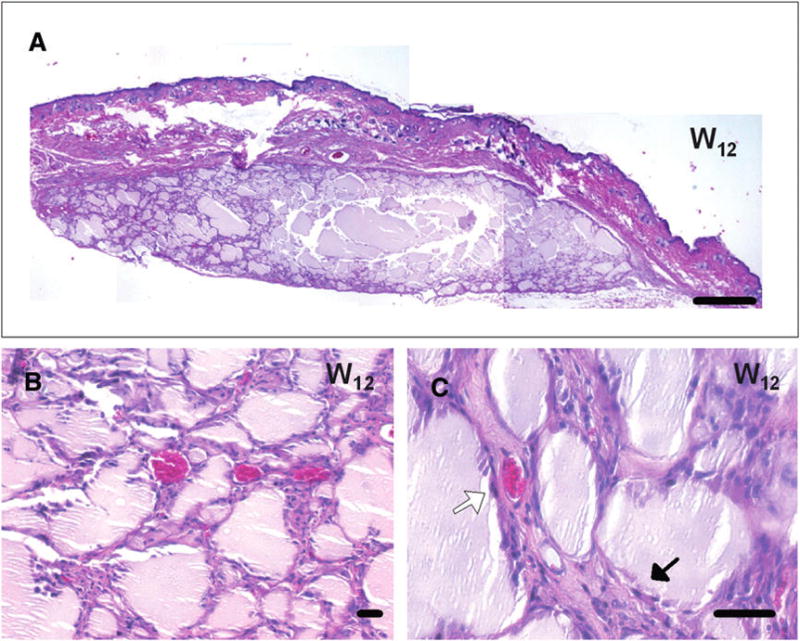
In vivo experiment: long-term histologic analyses. A through C) Histologic analyses with hematoxylin and eosin staining showed a complete disappearance of the inflammation process around silk gels at 12 weeks after grafting, with a partial fragmentation of the material. A) At this stage, a large piece of the silk material was detected. B and C) Fibroblasts and stromal tissues were found in the interstitial spaces between the silk-gel fragments (black arrow in C) associated with a complete vascularization (white arrow in C) of the area. Bar = 500 μm (A) and 50 μm (B and C).
DISCUSSION
Correction of alveolar ridge deformities has always been a challenge in therapy to periodontists, especially in the esthetic zone of the anterior maxilla. Based on Seibert’s classification,24 different surgical approaches have been proposed depending on the volume of the defect. Moreover, a series of staged surgical procedures is frequently necessary to augment the ridge to its former dimensions. Unfortunately, the final result of such periodontal grafts or flaps remains very technique sensitive and may not give constant and predictable esthetic results. In the study of Studer et al.,25 they suggested a prognostic evaluation depending on the Class of the defect: vertical and large defects, extending on 3 or 4 teeth, were not considered repairable by periodontal surgery. For such reasons, various xenografts and allografts have been introduced to the market and used for those specific indications. Correcting such voluminous defects may involve soft or hard tissue management. Most of the biomaterials indicated for hard tissue augmentation are used in association with rigid membranes (guided bone regeneration) that enable a predictable conformation of the site.5 These materials offer good results and are fully recommended when implants are secondary planned. However, these surgeries may present a couple of clinical complications that are not suitable when only an esthetic correction is indicated.26 Another way of achieving soft tissue augmentation is based on the progress of tissue engineering research. Thus, in periodontal therapy, dermal acellular scaffolds have been widely used for soft tissue augmentation27 but are limited by their processing and long-term evolution.13 Nevertheless, some countries do not authorize using such human-derived materials (e.g., France).28
In the present article, a new biomaterial formed from silk fibroin protein provided biocompatibility and long-term augmentation. The material degraded very slowly with minimal negative impact on the surrounding tissues. Furthermore, biomaterials using silk fibroin protein were proven to control the rate of degradation. In native fiber form, highly crystalline fibers (>50% β-sheet content) require months to years to fully degrade because the process is a surface enzymatic hydrolysis process.18,29 Upon reprocessing of the fibers into new material formats (e.g., gels, sponges, and films), the rates of degradation were modified. When three-dimensional porous sponges of silk were prepared,30 the mode of processing impacted these rates based on alterations in surface area, crystallinity, and bulk versus surface proteolytic digestion. A recent in vivo study31 in mice, demonstrated that three-dimensional porous silk materials implanted intramuscularly degraded in weeks (aqueous processing and low content of the β sheet) to >1 year (solvent processing and high content of the β sheet). In our experiments, collagen gels from bovine origin completely degraded after only 2 weeks. In the literature,32 when autologous collagen was used, the soft tissue augmentation obtained lasted up to 18 months if cross-linked with glutaraldehyde. However, collagen is a complex molecule and hard to harvest, and a large piece of tissue is needed to have enough material for the procedure. Therefore, silk gels, which are easy to produce, biocompatible, and long lasting after implantation, represent a significant improved alternative for soft tissue augmentation.
More than any other type of soft tissue augmentation, the one linked to the oral or maxillofacial sphere requires tunable mechanical properties from the material used. It needs to allow the structural changes of the tissues and the specific design shaping by the operator. Native silk fibers exhibit tensile properties and resistance to mechanical compression that exceed all other natural fibers and rival synthetic high-performance fibers.33 Our results for mechanical properties are comparable to previous publications34,35 where porous silk fibroin matrices demonstrated resistance to compression exceeding the commonly used skeletal tissue-engineering polymeric degradable scaffolds. In the present study, unseeded silk gels exhibited an elastic modulus of 0.37 MPa, close to the 0.66 MPa of the most flexible areas of the oral mucosa.36 These comparable values in compression strength may contribute to the ability of the silk gel to keep its shape over time.
Materials made of silk fibroin offer a wide flexibility in processing. Different morphologies can be formed depending on the need, such as conformal fill-ins (hydrogels), porous three-dimensional matrices, nanodiameter fibers, and films.19,30,37–39 Hydrogels, as used in this study, are the best type of morphology to realize soft tissue augmentation, but other morphologies may be useful for other oral applications. Moreover, the functionalization of the silks with specific growth factors was described21 and could be of great interest to offer tissue augmentation in combination with a biologic action in the reconstructed area. According to its mechanical properties, silk gel could be combined with membranes to ensure the required shape and accelerate bone formation.
CONCLUSIONS
The results of this study showed that silk fibroin gels were an excellent alternative as an alloplastic biomaterial for soft tissue augmentation in the oral and perioral fields. Further experiments are currently in process to biofunctionalize this material and to study its properties after long-term implantation >1 year.
Acknowledgments
This study was supported by the Center for Integrated Tissue Engineering, School of Dental Medicine, Tufts University, by funds from the National Institute of Biomedical Imaging and BioEngineering (P41 Tissue Engineering Resource Center), National Institutes of Health, Bethesda, Maryland, and by the Foundation for Medical Research (to AS). The authors thank Dr. A. Braun, scientist at Charles River Laboratories, Shrewsbury, Massachusetts, for her comments and Dr. A. Allan, dermatopathologist at Pathology Services, Cambridge, Massachusetts, for her pathologic assessment of the inflammatory process. The authors report no conflicts of interest related to this study.
Footnotes
Branson 450, Ultrasonics, Danbury, CT.
HyClone Laboratories, South Logan, UT.
Organogenesis, Canton, MA.
3366 Instron, Instron, Norwood, MA.
Bluehill Software Version 2.0, Norwood, MA.
Tissue-Tek, Triangle Biomedical, Durham, NC.
Abcam, Cambridge, MA.
Alexa 488-conjugated goat IgG, Molecular Probes, Eugene, OR.
VECTASHIELD, Vector Laboratories, Burlingame, CA.
Nikon Eclipse, Nikon, NY.
TUNEL assay, Roche Diagnostics, Indianapolis, IN.
Boehringer-Mannheim, Indianapolis, IN.
Multiscan MK3, Thermo Labsystems, Franklin, MA.
Nikon Eclipse, Nikon.
SPOT RT Camera, Diagnostic Instruments, Sterling Heights, MI.
N:NIHS-nuf DF, Germantown, NY.
References
- 1.Evian CI, al-Maseeh J, Symeonides E. Soft tissue augmentation for implant dentistry. Compend Contin Educ Dent. 2003;24:195–198. 200–202, 204–206. quiz 208. [PubMed] [Google Scholar]
- 2.Frodel JL, Lee S. The use of high-density polyethylene implants in facial deformities. Arch Otolaryngol Head Neck Surg. 1998;124:1219–1223. doi: 10.1001/archotol.124.11.1219. [DOI] [PubMed] [Google Scholar]
- 3.Seibert JS, Salama H. Alveolar ridge preservation and reconstruction. Periodontol 2000. 1996;11:69–84. doi: 10.1111/j.1600-0757.1996.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Seibert JS. Treatment of moderate localized alveolar ridge defects. Preventive and reconstructive concepts in therapy. Dent Clin North Am. 1993;37(2):265–80. [PubMed] [Google Scholar]
- 5.Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(Suppl):49–70. [PubMed] [Google Scholar]
- 6.Ashinoff R. Overview: Soft tissue augmentation. Clin Plast Surg. 2000;27:479–487. [PubMed] [Google Scholar]
- 7.Cohen ES. Ridge augmentation utilizing the subepithelial connective tissue graft: Case reports. Pract Periodontics Aesthet Dent. 1994;6:47–53. quiz 55. [PubMed] [Google Scholar]
- 8.Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession. A systematic review. Ann Periodontol. 2003;8:303–320. doi: 10.1902/annals.2003.8.1.303. [DOI] [PubMed] [Google Scholar]
- 9.Griffin TJ, Cheung WS, Zavras AI, Damoulis PD. Post-operative complications following gingival augmentation procedures. J Periodontol. 2006;77:2070–2079. doi: 10.1902/jop.2006.050296. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen A, Pasyk KA, Bouvier TN, Hassett CA, Argenta LC. Comparative study of survival of autologous adipose tissue taken and transplanted by different techniques. Plast Reconstr Surg. 1990;85:378–386. [PubMed] [Google Scholar]
- 11.Perenack J, Wood RJ, Block MS, Gardiner D. Determination of subepithelial connective tissue graft thickness in the dog. J Oral Maxillofac Surg. 2002;60:415–421. doi: 10.1053/joms.2002.31229. [DOI] [PubMed] [Google Scholar]
- 12.Elson ML. Soft tissue augmentation. A review. Dermatol Surg. 1995;21:491–500. quiz 501–502. [PubMed] [Google Scholar]
- 13.Gapski R, Parks CA, Wang HL. Acellular dermal matrix for mucogingival surgery: A meta-analysis. J Periodontol. 2005;76:1814–1822. doi: 10.1902/jop.2005.76.11.1814. [DOI] [PubMed] [Google Scholar]
- 14.Dadzie OE, Mahalingam M, Parada M, El Helou T, Philips T, Bhawan J. Adverse cutaneous reactions to soft tissue fillers–A review of the histological features. J Cutan Pathol. 2008;35:536–548. doi: 10.1111/j.1600-0560.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 15.Masuda T, Furue M, Matsuda T. Novel strategy for soft tissue augmentation based on transplantation of fragmented omentum and preadipocytes. Tissue Eng. 2004;10:1672–1683. doi: 10.1089/ten.2004.10.1672. [DOI] [PubMed] [Google Scholar]
- 16.Moharamzadeh K, Brook IM, Van Noort R, Scutt AM, Thornhill MH. Tissue-engineered oral mucosa: A review of the scientific literature. J Dent Res. 2007;86:115–124. doi: 10.1177/154405910708600203. [DOI] [PubMed] [Google Scholar]
- 17.Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends Biotechnol. 2008;26:244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 19.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 20.Bini E, Knight DP, Kaplan DL. Mapping domain structures in silks from insects and spiders related to protein assembly. J Mol Biol. 2004;335:27–40. doi: 10.1016/j.jmb.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter GD. Epidermal regeneration in the domestic pig. In: Rovee DT, Maibach HI, editors. The Epidermis in Wound Healing. Chicago: Yearbook Medical; 1972. pp. 71–112. [Google Scholar]
- 24.Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. Technique and wound healing. Compend Contin Educ Dent. 1983;4:437–453. [PubMed] [Google Scholar]
- 25.Studer S, Naef R, Scharer P. Adjustment of localized alveolar ridge defects by soft tissue transplantation to improve mucogingival esthetics: A proposal for clinical classification and an evaluation of procedures. Quintessence Int. 1997;28:785–805. [PubMed] [Google Scholar]
- 26.Strietzel FP. Risks and complications of membrane-guided bone regeneration. Retrospective analysis [in German] Oral and Maxillofacial Surgery. 2001;5:28–32. doi: 10.1007/s100060000248. [DOI] [PubMed] [Google Scholar]
- 27.Fowler EB, Breault LG. Ridge augmentation with a folded acellular dermal matrix allograft: A case report. J Contemp Dent Pract. 2001;2:31–40. [PubMed] [Google Scholar]
- 28.Pascal P, Damour O, Colpart JJ, Braye F. French legal framework relating to human tissues and cells. Med Biol Eng Comput. 2000;38:241–247. doi: 10.1007/BF02344783. [DOI] [PubMed] [Google Scholar]
- 29.Horan RL, Antle K, Collette AL, et al. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Rudym DD, Walsh A, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanke CW, Coleman WP. Dermal filler substances. In: Asken S, Alt TH, Hanke CW, Coleman WP, editors. Cosmetic Surgery of the Skin. St. Louis: Mosby Year Book; 1997. pp. 217–230. [Google Scholar]
- 33.Shao Z, Vollrath F. Surprising strength of silkworm silk. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- 34.Altman GH, Horan RL, Lu HH, et al. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. doi: 10.1016/s0142-9612(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Kim HS, Matsumoto A, Chin IJ, Jin HJ, Kaplan DL. Processing windows for forming silk fibroin biomaterials into a 3D porous matrix. Aust J Chem. 2005;58:716–720. [Google Scholar]
- 36.Inoue K, Arikawa H, Fujii K, Shinohara N, Kawahata N. Viscoelastic properties of oral soft tissue. 1. A method of determining elastic modulus of oral soft tissue. Dent Mater J. 1985;4:47–53. doi: 10.4012/dmj.4.47. [DOI] [PubMed] [Google Scholar]
- 37.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electro-spinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3:1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 38.Jin HJ, Park J, Valluzzi R, Cebe P, Kaplan DL. Biomaterial films of Bombyx mori silk fibroin with poly(ethylene oxide) Biomacromolecules. 2004;5:711–717. doi: 10.1021/bm0343287. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Kim UJ, Vunjak-Novakovic G, Min BH, Kaplan DL. Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials. 2005;26:4442–4452. doi: 10.1016/j.biomaterials.2004.11.013. [DOI] [PubMed] [Google Scholar]