Abstract
Calibrated functional magnetic resonance imaging (fMRI) provides a noninvasive technique to assess functional metabolic changes associated with normal aging. We simultaneously measured both the magnitude of the blood oxygenation level dependent (BOLD) and cerebral blood flow (CBF) responses in the visual cortex for separate conditions of mild hypercapnia (5% CO2) and a simple checkerboard stimulus in healthy younger (n = 10, mean: 28‐years‐old) and older (n = 10, mean: 53‐years‐old) adults. From these data we derived baseline CBF, the BOLD scaling parameter M, the fractional change in the cerebral metabolic rate of oxygen consumption (CMRO2) with activation, and the coupling ratio n of the fractional changes in CBF and CMRO2. For the functional activation paradigm, the magnitude of the BOLD response was significantly lower for the older group (0.57 ± 0.07%) compared to the younger group (0.95 ± 0.14%), despite the finding that the fractional CBF and CMRO2 changes were similar for both groups. The weaker BOLD response for the older group was due to a reduction in the parameter M, which was significantly lower for older (4.6 ± 0.4%) than younger subjects (6.5 ± 0.8%), most likely reflecting a reduction in baseline CBF for older (41.7 ± 4.8 mL/100 mL/min) compared to younger (59.6 ± 9.1 mL/100 mL/min) subjects. In addition to these primary responses, for both groups the BOLD response exhibited a post‐stimulus undershoot with no significant difference in this magnitude. However, the post‐undershoot period of the CBF response was significantly greater for older compared to younger subjects. We conclude that when comparing two populations, the BOLD response can provide misleading reflections of underlying physiological changes. A calibrated approach provides a more quantitative reflection of underlying metabolic changes than the BOLD response alone. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: aging, functional magnetic resonance imaging (fMRI), blood oxygen level dependent (BOLD) effect, cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), visual cortex
INTRODUCTION
The paramagnetic nature of deoxyhemoglobin produces a small local increase in blood oxygenation associated with brain activation that can be measured by functional magnetic resonance imaging (fMRI), called the blood oxygenation level dependent (BOLD) signal [Ogawa et al.,1992]. However the BOLD signal does not directly measure underlying neuronal activity but instead reflects a balance of changes in cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), and cerebral blood volume (CBV) initiated by a cascade of physiological events and mediators. Most fMRI studies using the BOLD signal have mapped brain activation in healthy young subjects for particular tasks. With increasing applications of fMRI to disease states [Matthews et al.,2006] a growing need exists to assess the possible effects of aging on brain function. Age‐related changes in brain anatomy, neuronal density, or cerebral metabolism could significantly influence the BOLD signal [Huettel et al.,2001].
Reported anatomical changes associated with aging of the human brain have included sulcal widening, increased ventricular size, and loss of synapses [Goldstein and Reivich,1991; Raz et al.,1992]. These structural changes are not concomitant with alterations in neuronal density as negligible neuronal loss has been observed in older healthy subjects at autopsy [Long et al.,1999]. Conflicting results have been observed concerning baseline blood flow and metabolic activity, as resting cerebral metabolism may either remain constant [Goldstein and Reivich,1991] or decline with age [Marchal et al.,1992; Yamaguchi et al.,1986]. Indeed, both anatomical variation and baseline metabolism may be regionally specific [Loessner et al.,1995] making generalizations quite problematic.
Several laboratories have recently investigated the effect of normal aging using BOLD fMRI [D'Esposito et al.,2003]. Direct comparisons of the magnitude of the BOLD hemodynamic response function (HRF) have typically been assessed between younger and older healthy subjects for either motor [Buckner et al.,2000; D'Esposito et al.,1999; Hesselmann et al.,2001; Mattay et al.,2002; Taoka et al.,1998] or visual tasks [Aizenstein et al.,2004; Buckner et al.,2000; Huettel et al.,2001; Raemaekers et al.,2006; Ross et al.,1997; Tekes et al.,2005]. In general, relatively simple tasks have been chosen with neuronal activity for the two aged populations assumed to be similar based on electrophysiology measures [Allison et al.,1984; Celesia and Daly,1977].
Conflicting results, though, have been observed within BOLD fMRI experiments. Some studies have observed a decrease in the magnitude of the BOLD signal with aging [Buckner et al.,2000; Raemaekers et al.,2006; Ross et al.,1997; Tekes et al.,2005] while others have failed to observe significant differences [Aizenstein et al.,2004; Huettel et al.,2001]. Observed group differences in the magnitude of the BOLD response may be sensitive to voxel selection [Aizenstein et al.,2004] or may be regionally specific [Buckner et al.,2000]. Interpretation of the effects of aging by simply studying the magnitude of the BOLD signal alone therefore remains problematic as it reflects a complex interaction between changes in CBF, CMRO2, and CBV.
By simultaneously combining measurements of both BOLD and CBF responses under separate conditions of mild hypercapnia (inhalation of a gas mixture of 5% CO2) and for a simple functional activation paradigm the BOLD signal can be “calibrated” to noninvasively determine the fractional changes in CMRO2 with functional activation [Davis et al.,1998]. This calibrated‐BOLD approach exploits the fact that the BOLD signal depends on changes in CBF and CMRO2, while the arterial spin labeling (ASL) signal [Williams et al.,1992] depends only on CBF changes. With the assumption that mild hypercapnia does not alter CMRO2 [Hafkenschiel et al.,1954; Jones et al.,2005; Kastrup et al.,1999; Kety and Schmidt,1948; Kim and Ugurbil,1997; Kliefoth et al.,1979; Novack et al.,1953; Sicard and Duong,2005], the hypercapnic response allows for the determination of the scaling parameter M that defines the maximal possible BOLD response for a brain region from that defined baseline state and for the particular image acquisition parameters. As has been recently demonstrated by Chiarelli and colleagues, accurate determination of M, rather than the use of assumed values, is critical for subsequently calculating fractional changes in CMRO2 [Chiarelli et al.,2007a,b] using the relatively simple mathematical model that has been proposed by Davis et al. [1998]. From these measurements, n, the fractional changes in CBF relative to CMRO2, [Leontiev and Buxton,2007] can be calculated for a particular functional activation paradigm.
While growing in popularity, calibrated BOLD studies have primarily been technical in nature, focusing on the feasibility of this technique in healthy younger volunteers that are less than 35‐years‐old [Brown et al.,2007; Chiarelli et al.,2007a,b; Davis et al.,1998; Hoge et al.,1999a,b; Leontiev and Buxton,2007; Stefanovic et al.,2004]. Extension of this method to healthy older populations has yet to be explored.
A few recent studies have compared older and younger populations using variations of the calibrated BOLD technique. Restom et al. found that while the baseline CBF was reduced with aging, the fractional changes in CBF, but not BOLD changes, within the mesial temporal lobe for a visual encoding task were greater for older than younger healthy controls [Restom et al.,2007]. While hypercapnic calibration was not performed, using a number of assumptions these authors argued that the data was consistent with an increase in the fractional change in CMRO2 with aging. Handwerker and colleagues measured the BOLD but not the CBF responses for a visual saccade task and during breathholding normalization in healthy younger and older subjects [Handwerker et al.,2007]. They demonstrated that in V1 the change in the BOLD signal for the visual saccade, but not breathholding, was significantly lower in older compared to younger subjects.
Direct application of the calibrated BOLD technique in older populations will not only allow for further exploration of the effects of aging but also will allow for determination of baseline values that could serve as a biomarker for individuals at subsequent risk for disease [Prvulovic et al.,2005]. To our knowledge this is the first study to examine if differences in functionally measured variables of BOLD and CBF responses to hypercapnia and to a simple visual activation task, as well as calculated values of M, CMRO2, and n exist between a cohort of younger and older healthy volunteers. Our primary results are that: (1) the BOLD response was reduced in the older group, despite the finding that the changes in CBF, CMRO2 and the coupling ratio n were similar for the two groups; (2) The source of this effect was a reduction in the parameter M in the older group, associated with a reduced baseline CBF; and (3) The group averaged response showed similar BOLD poststimulus undershoots in the two groups, and yet only the older group showed a CBF poststimulus undershoot. These results highlight the difficulties involved in interpreting the BOLD response as a quantitative reflection of underlying physiological changes, and illustrate how a calibrated BOLD approach can begin to untangle the ambiguities of the BOLD response and provide a more quantitative assessment for comparisons between different subject populations.
METHODS
Subjects
A total of twenty healthy subjects performed identical tasks in a 3T GE Signa Excite whole body system with six subjects reported in a previous study [Ances et al.,2008]. The younger group consisted of 10 subjects who ranged in age from 21–35 years of age (mean 28‐years‐old; 6 males and 4 females) while the older group consisted of 10 subjects who ranged in age from 45 to 60 years of age (mean 53‐years‐old; 6 males and 4 females). All subjects were scanned according to guidelines approved by the Institutional Review Board (IRB) of the University of California San Diego (UCSD). Participants were selected without regard to ethnicity or race. Individuals with a previous reported significant history of substance abuse, neurological illness, or psychiatric disorders were excluded from the study.
Experimental Design
Each subject underwent two experiments within the same scanning session, one measuring the CBF and BOLD responses to mild hypercapnia, and one measuring the CBF and BOLD responses to a simple functional activation. In the first experiment, subjects breathed a gas mixture containing 5% CO2 through a mask that continuously sampled the end‐tidal CO2. In the second experiment, a black and white radial checkerboard flickering at 8 Hz was presented during the activation period while an isoluminant gray screen with a center fixation square was used during the rest portion. A block‐design sequence was utilized to maximize signal to noise ratio and consisted of 20 s of activation and 60 s of rest in one cycle. The full experimental run consisted of 60 s of baseline, followed by four cycles of task/rest, followed by an additional 30 s of rest, for a total run duration of 410 s. At least three functional activation runs were performed for each subject.
Hypercapnic Calibration
Hypercapnia was induced by breathing a gas mixture consisting of 5% CO2, 21% O2, and 74% N2 delivered through a nonrebreathing face mask (Hans Rudolph 2700 Series, St. Louis, MO). Two runs lasting ∼7 min in duration were performed and consisted of 2 min of breathing room air followed by 3 min of hypercapnia and then 2 min of room air. Throughout these runs subjects fixated on a white square centered on an isoluminent gray background, the same baseline condition as in the activation experiment. During all scans, including hypercapnia, cardiac pulse and respiratory effort data were monitored using a pulse oximeter (Invivo Millennia 3500; Invivo Research, Orlando, FL) while changes in end‐tidal CO2 (in mm Hg) were measured through a respiratory effort transducer (BIOPAC Systems, Goleta, CA). A pulse oximeter was placed on the subject's left index finger and a respiratory effort belt was placed around the subject's chest. Physiological data were sampled at 40 samples/s using a multichannel data acquisition board (National Instruments, Austin, TX).
MRI Parameters
Imaging data were acquired on a 3 Tesla whole body system (3‐T GE Excite, Milwaukee, WI) with an eight‐channel receive head coil. Quantitative ASL images were acquired with a single‐shot PICORE QUIPSS II [Wong et al.,1999] pulse sequence (TR = 2.5 s, TI1 = 700 ms, TI2 = 1500 ms, 20‐cm tag width, and a 1‐cm tag‐slice gap) with a dual‐echo gradient echo (GRE) readout and spiral acquisition of k‐space (TE1 = 9.4 ms, TE2 = 30 ms, flip angle = 90°, field of view (FOV) = 24 cm, 64 × 64 matrix). ASL acquisition alternates “tag” images, in which the magnetization of arterial blood is inverted before it flows into the selected slice, and “control” images in which the arterial magnetization is not inverted. The difference of the tag and control images from the first echo provides a CBF response time series, while the average of the tag and control images from the second echo yields a BOLD response time series. Four 7‐mm thick axial slices covering the entire visual cortex (VC) were acquired in a linear fashion from bottom to top. A high‐resolution structural scan was acquired with an inversion recovery prepared 3D fast spoiled GRASS (IR‐FSPGR) pulse sequence (TI = 450 ms, TR = 7.9 ms, TE = 3.1 ms, flip angle = 12°, FOV = 25 × 25 × 16 cm, matrix 256 × 256 × 124). The latter images were collected after the hypercapnia experiment and before the activation experiment, and thus provided additional delay between the two experiments to minimize any possible lingering effects of CO2 administration on the functional activation experiment.
In addition to these scans, a resting‐state CBF scan (200 s) was acquired at the scanning session for quantifying the baseline state. For conversion to absolute units, a cerebral spinal fluid (CSF) reference scan and a minimum contrast scan were also acquired. The CSF scan consisted of a single‐echo, single repetition scan acquired at full relaxation and echo time equal to 2.8 ms. This CSF scan was acquired using the same in‐plane parameters as the ASL scan, but the number of slices was increased to ensure coverage of the lateral ventricles. The minimum contrast scan was acquired with TR = 2 s, TE = 11 ms to ensure little contrast between gray matter, white matter and CSF. Two 8‐interleave repetitions were acquired using the same slice prescription as the CSF scan.
Data Analysis
Data for each subject were analyzed to generate average changes in the BOLD and CBF responses for both mild hypercapnia and for the functional activation paradigm within the VC. Dynamic ASL data were coregistered with AFNI software to correct for subject movement during the study [Cox,1996]. For each voxel, a CBF time‐series was computed by taking a running subtraction of the control and tag image series from the first echo data (TE = 9.4 ms). Each data point was calculated from the difference between that value and the average of the two nearest neighbors with adjustments made in the sign so that each point represents a subtraction of tag from control images. A BOLD‐weighted time series was computed from the running average of the second echo (TE = 30 ms), taking the average of each image with the mean of its two nearest neighbors [Liu and Wong,2005].
Signal averaging was performed within a manually defined region of interest (ROI) delineated on high‐resolution images. The VC mask was defined by the parietal‐occipital sulci, and contained not only the primary VC but also supplementary regions. These images were subsequently sampled to match the resolution of the functional images. A general linear model (GLM) analysis was performed to identify activated voxels within this region of interest (ROI) [Mumford et al.,2006; Restom et al.,2007]. A stimulus‐related regressor was obtained by convolving the block designed stimulus pattern with a gamma density function [Boynton et al.,1996]. Both cardiac and respiratory data were included in the GLM as regressors to model physiological modulation of the ASL signal, with constant and linear terms included as nuisance regressors. Prewhitening was performed using an autoregressive model [Burock and Dale,2000; Woolrich et al.,2004]. Functional runs were concatenated for the GLM analysis with separate physiological and nuisance regressors applied to each run [Restom et al.,2006].
Clusters of voxels exhibiting CBF activation within the VC mask were detected using an overall significance threshold of P = 0.05 applied to the first echo data. Correction for multiple comparisons was performed using AFNI AlphaSim program [Cox,1996]. In addition to this primary ROI based on CBF activation for the visual stimulus, we generated a ROI based on BOLD activated voxels for the visual stimulus with an overall significance threshold of P = 0.05.
For both ROIs, the CBF and BOLD time series for each subject were obtained by averaging voxel time courses within the VC after removal of the physiological noise components estimated from the GLM. For the functional paradigm, runs were averaged with both linear and quadratic drifts removed. For the mild hypercapnic paradigm, runs were averaged with only linear drifts eliminated in order to avoid removal of the single‐block response with a quadratic term.
For each subject, both average changes in the BOLD and CBF responses were normalized to their respective baseline values, calculated as the average of the first minute of the acquisition. The amplitude of the response to mild hypercapnia was calculated as the average over the last 2 min of CO2 inhalation, in order to reduce the influence of the transition regions. For the functional runs, the fractional changes in the CBF and BOLD responses were calculated as the average over a 15 s period starting at the midpoint of each stimulus presentation, approximating the plateau portion of the response. Group averages for all variables were determined by averaging the responses over subjects. For both hypercapnia and functional activation scans the first 10 s of each run were removed to reduce possible errors due to scanner drift.
The CBF time series from the baseline scan were corrected for inhomogeneities in the coil sensitivity profile using the smoothed minimum contrast images [Wang et al.,2005], and converted to physiological units (mL/100 mL/min) using the CSF image as a reference signal to determine the fully relaxed magnetization of blood [Chalela et al.,2000]. The mean resting CBF for each subject was calculated by averaging the CBF time series over all time points and over all voxels within the particular ROI. Correction was performed for partial volume effects. We also corrected the CBF measures for partial volume effects [Johnson et al.,2005]. This approach assumes that CSF has zero CBF.
Determination of CMRO2, n, CRC, and Baseline CBF
Calculation of CMRO2 changes with functional stimulation were determined by methods previously described by Davis et al. [1998]. In this model, the fractional BOLD signal change (ΔS/S 0) is related to the underlying fractional changes in CBF and CMRO2 from a defined baseline state. If we define f as the ratio of CBF to its baseline value, and r as the ratio of CMRO2 to its baseline value, the BOLD signal change is modeled as:
| (1) |
where the parameter value M represents a scaling factor specific to that brain region in the defined baseline state. That is, the parameter M reflects the deoxyhemoglobin content in the baseline state, and defines the maximum possible BOLD signal change or ceiling effect for a particular region. This parameter depends on the baseline CBV and oxygen extraction fraction (OEF), and also on experimental parameters such as field strength and echo time. The parameter α is the exponent of the empirical power law relationship between CBV and CBF, and is assumed to be equal to 0.38 [Grubb et al.,1974], while β is taken to be 1.5 based on numerical simulations [Boxerman et al.,1995b; Davis et al.,1998].
The mild hypercapnia experiment is required in order to accurately estimate M. With the assumption that mild hypercapnia does not alter CMRO2 [Hafkenschiel et al.,1954; Jones et al.,2005; Kastrup et al.,1999; Kety and Schmidt,1948; Kim and Ugurbil,1997; Kliefoth et al.,1979; Novack et al.,1953; Sicard and Duong,2005], the parameter r in Eq. (1) is assumed to be equal to one, and from the measured CBF and BOLD responses to hypercapnia M is calculated. This estimate of M is then combined with the measured CBF and BOLD responses to activation to calculate r for the activation experiment. From this estimate of the fractional changes in CMRO2 with activation the coupling ratio, n, can be determined.
Each of the functional runs was cropped into four block stimulus segments that were 80 s in length. Within this block the first 20 s consisted of baseline followed by 20 s of functional task presentation followed by an additional 40 s of baseline. This division of time period blocks was chosen after visual inspection of functional responses in order to ensure that a stable baseline had been achieved after stimulation. The signals within the first 20 s prior to the visual task were averaged and subtracted from the entire segment to normalize each block.
The hypercapnia experiment yielded a measure of vascular responsiveness. The cerebrovascular response to CO2 (CRC) was defined as the percentage change in CBF divided by the change in end‐tidal CO2 (in mm Hg).
Statistical Analysis
Simple paired t tests were performed between the two groups using a Sidak correction for multiple comparisons with a P‐value significant if P < 0.04.
RESULTS
The Resting Baseline CBF was Reduced for Older Subjects
We obtained baseline measures of CBF correcting for both degree of white matter involvement and partial volume within the VC of younger and older healthy subjects. As seen in Table I a significant reduction in baseline CBF was seen in the older group compared to the younger subjects.
Table I.
Measured values within visual cortex (VC) for younger (n = 10) and older subjects (n = 10) for a positive CBF activated region of interest (ROI) (mean ± standard error)
| Number of voxels | Fractional change in CBF with hypercapnia (%) | BOLD changes with hypercapnia (%) | Cerebral reactivity to CO2 (CRC) | Fractional change in CBF with activation (%) | BOLD changes with activation (%) | Baseline CBF (mL/100 mL/min) | |
|---|---|---|---|---|---|---|---|
| Younger (21‐ to 35‐years‐old) | 386 ± 52 | 47.4 ± 9.6 | 1.91 ± 0.22 | 8.3 ± 1.7 | 70.3 ± 9.4 | 0.95 ± 0.14 | 59.6 ± 9.1 |
| Older (45‐ to 60‐years‐old) | 297 ± 55 | *79.2 ± 14.9 | 1.72 ± 0.23 | 8.1 ± 1.5 | 66.1 ± 7.8 | *0.57 ± 0.07 | *41.7 ± 4.8 |
P < 0.04.
The Total Number of Functionally Activated Voxels Within the VC Did Not Differ with Age
Both groups had activation not only within the calcarine cortex but also fusiform areas. While the total number of active voxels tended to be greater for younger subjects than older subjects (Table I) this difference was not statistically different (P = 0.29).
For Mild Hypercapnia the Fractional Changes in CBF but Not the BOLD Response Magnitude Differed with Aging
Across all subjects, breathing CO2 enriched air increased end‐tidal CO2 by 6.5 ± 2.1 mm Hg (mean ± SD) for younger subjects and 8.1 ± 2.6 mm Hg for older subjects (P = 0.22). No significant changes in respiratory rate were observed within either of the groups during administration of 5% CO2. Robust changes in the BOLD and CBF responses to mild hypercapnia were seen in the VC of both groups (Fig. 1A,B). For mild hypercapnia, the fractional changes in CBF, but not the BOLD response magnitude, differed with aging (Fig. 1 and Table I). To determine if the larger fractional changes in CBF in the older group for mild hypercapnia were due to differences in the degree of CO2 change produced in the subjects, we determined the CRC, the CBF response per mm Hg change in end‐tidal CO2. The CRC was similar for the two groups (Table I). On the basis of the mismatch of the BOLD and CBF responses to hypercapnia, differences with aging were most likely due to M. The calculated values of M were significantly higher in the younger group (6.5 ± 0.8) than in the older group (4.6 ± 0.4) (Table I).
Figure 1.
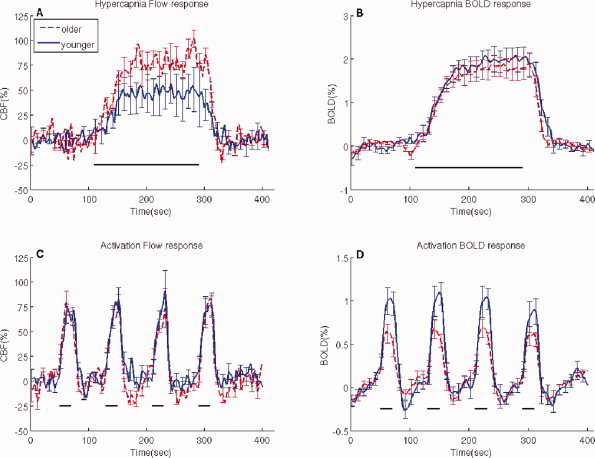
Functional changes in the blood oxygen level dependent (BOLD) and cerebral blood flow (CBF) response in the VC for mild hypercapnia and functional activation. The panels show average response curves and standard errors for the CBF activated voxels within healthy younger (n = 10) and older (n = 10) subjects. (A) Fractional changes in the CBF responses to hypercapnia, (B) Changes in the BOLD responses to hypercapnia, (C) Fractional changes in the CBF responses to activation, and (D) Changes in the BOLD responses to activation. A significant increase in the fractional changes in the CBF response to hypercapnia was seen in older subjects compared to younger subjects leading to a decrease in the M value for older subjects. For the functional activation task the BOLD response was greater for younger compared to older subjects. For all panels the solid bars indicate stimulus presentation. For all time series data the error bars are presented at every other time point that was sampled. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For Functional Activation, the Changes in BOLD, but Not CBF or CMRO2, were Reduced with Aging
Functionally modulated changes in the BOLD and CBF responses were obtained from the VC of both younger and older subjects during visual stimulation (Fig. 1C,D). A significant decrease in the BOLD signal was seen between the two groups. Older subjects had a significantly lower magnitude compared to younger subjects (P = 0.02) (see also Table IA). No significant differences were seen between older and younger subjects for fractional changes in the CBF response for the flashing checkerboard stimulus. Using M values determined for each subject from the hypercapnic experiments, we calculated the fractional changes in CMRO2 for the functional activation with no significant differences seen between the two groups (Table II). From these measures of fractional changes in CBF and CMRO2 we were able to determine the coupling ratio, n, for both groups. As highlighted by the scatter plot of fractional changes in CBF and CMRO2 for functional activation (Fig. 2A) the slope, n, was similar for both groups. Across subjects, no significant correlations existed between estimates of n and baseline CBF (Fig. 2B).
Table II.
Calculated values visual cortex (VC) for younger (n = 9) and older subjects (n = 8) for a region of interest based on positive CBF ROI (mean ± standard error)
| Fractional change in % CMRO2 with activation | n | M (%) | |
|---|---|---|---|
| Younger (21‐ to 35‐years‐old) | 32.3 ± 5.4 | 2.3 ± 0.2 | 6.5 ± 0.8 |
| Older (45‐ to 60‐years‐old) | 32.1 ± 4.6 | 2.2 ± 0.1 | *4.6 ± 0.4 |
P < 0.04.
Figure 2.
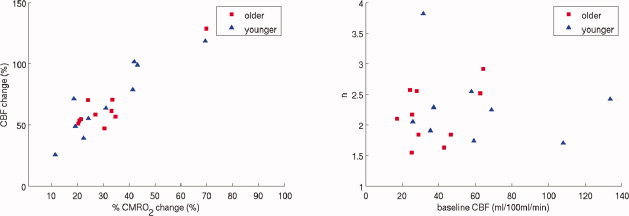
Covariance of calculated and measured parameters. (A) Fractional changes in CBF as a function of CMRO2 showing a similar slope, n, for both younger and older subjects. (B) Comparison of n with baseline CBF, showing no significant correlation. Younger subjects did have a significantly higher mean baseline CBF than older subjects. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Similar Trends were Seen When a BOLD Response ROI was Used
Data presented above were calculated from an ROI based solely on positive CBF activation to determine functional changes in the BOLD and CBF responses. To determine if observed values were affected by ROI selection criteria we performed an additional analysis that included an ROI consisting of positive magnitude BOLD activation maps (Tables III and IV) to determine functional changes in the BOLD and CBF responses. Selection of a ROI based on positive BOLD responses also allowed for comparison with previous studies that have investigated the effects of aging on the magnitude of the BOLD signal. Similar trends were seen with the BOLD ROI. In particular, older subjects had a significantly greater change in the CBF response for mild hypercapnia than younger subjects (Table III). Significant decreases in M and baseline CBF were seen in older subjects compared to younger subjects. For the functional activation paradigm both the fractional changes in the BOLD and CBF were similar for the two groups. A reduction in calculated fractional changes in CMRO2 was seen in older subjects compared to younger (Table IV) but this was not significantly different due to increased variability. Overall, values for fractional changes in CBF were reduced (∼50%) when compared to values obtained using a CBF based ROI, while the magnitude of the BOLD response was increased (∼20%).
Table III.
Measured values within visual cortex (VC) for younger (n = 10) and older subjects (n = 10) for a positive BOLD activated ROI (mean ± standard error)
| Number of voxels | Fractional change in CBF with hypercapnia (%) | BOLD changes with hypercapnia (%) | Cerebral reactivity to CO2 (CRC) | Fractional change in CBF with activation (%) | BOLD changes with activation (%) | Baseline CBF (mL/100 mL/min) | |
|---|---|---|---|---|---|---|---|
| Younger (21‐ to 35‐years‐old) | 610 ± 62 | 40.9 ± 7.8 | 2.08 ± 0.33 | 7.9 ± 1.2 | 33.5 ± 3.9 | 1.12 ± 0.08 | 54.2 ± 10.0 |
| Older (45‐ to 60‐years‐old) | 554 ± 54 | *75.2 ± 14.8 | 2.47 ± 0.46 | 8.4 ± 1.3 | 35.6 ± 5.3 | 0.97 ± 0.07 | *37.3 ± 5.2 |
P < 0.04.
Table IV.
Calculated values visual cortex (VC) for younger (n = 10) and older subjects (n = 10) for a region of interest based on positive BOLD activated voxels (mean ± standard deviation)
| Fractional change in CMRO2 with activation | n | M (%) | |
|---|---|---|---|
| Younger (21‐ to 35‐years‐old) | 11.1 ± 3.2 | 1.8 ± 1.0 | 7.3 ± 0.5 |
| Older (45‐ to 60‐years‐old) | 7.2 ± 4.1 | 2.3 ± 1.7 | *5.7 ± 1.0 |
P < 0.04.
Despite Similar Poststimulus Undershoots in the BOLD Response, CBF Undershoots were Larger for the Older Group
In an attempt to further study the temporal dynamics of both measured changes in the BOLD and CBF responses we averaged the time series for each of these parameters across stimulus cycles to determine the average response to a single block stimulus. The mean measured changes in the BOLD and CBF responses to visual stimulation were obtained (see Fig. 3). Despite similar fractional changes in the CBF response (Fig. 3A), a significant decrease in the magnitude of the average BOLD response was seen in older compared to younger subjects (Fig. 3B). These results are consistent with the tabulated averages in Table I. The temporal dynamics of the average changes in the BOLD and CBF responses were similar for both groups. However, during the post‐stimulus period both groups showed an undershoot in the BOLD response, but an accompanying undershoot in the CBF response was only seen in the older group. Upon further quantification of the post stimulus period by determining the area under the curve there was a significant difference in the post stimulus undershoot for the fractional changes in the CBF (Fig. 3C) in older subjects compared to younger (P = 0.02) but not for the BOLD response (P = 0.97) (Fig. 3D).
Figure 3.
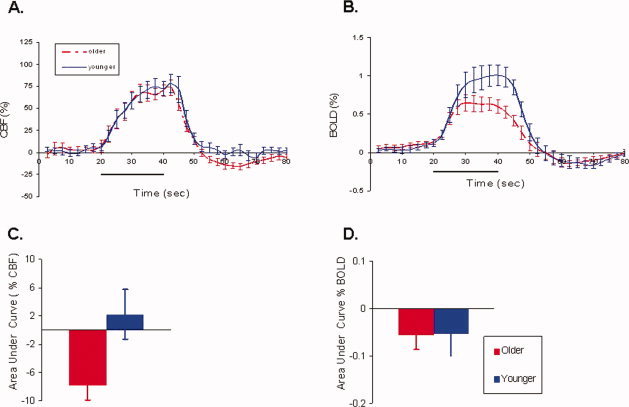
Single block averaged dynamics of the BOLD and CBF responses for older and younger subjects. A signal averaged block of 80 s (20 s of rest, 20 s of stimulus, and 40 s of rest) was assessed for both older and younger subjects. (A) The magnitude of the CBF response was similar for both groups. (B) A significant decrease in the magnitude of the BOLD response was seen in older compared to younger subjects. (C) In contrast, the undershoot period for the CBF response was significantly different. (D) However, a similar BOLD poststimulus undershoot was seen for both groups. For panels (A) and (B) the solid black bars indicate stimulus presentation. For each figure the standard errors are shown and are presented at every other time point sampled for time series data. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Brain imaging based on the BOLD signal alone provides a sensitive tool for mapping patterns of activation, but interpreting differences in the magnitude of the BOLD response with aging is more difficult due to the complexity of the BOLD effect. Additional approaches that provide quantitative measures are required to understand whether a change in the magnitude of the BOLD response reflects changes in neural activity, vascular responsiveness, coupling of CBF and CMRO2, or changes in the baseline state. To begin such explorations we performed calibrated BOLD studies within both younger and older healthy populations using a simple flashing checkerboard stimulus. For a ROI based on CBF activation, we found that the BOLD response to the visual stimulus was significantly reduced in the older group despite similar fractional changes in the CBF and CMRO2 responses. This reduction of the BOLD response for the older group was consistent with a reduction in M, the scaling parameter of the BOLD response that depends on baseline CBV and OEF. Along with the reduction in M, we found a significantly reduction in baseline CBF in the older group (Table II). In these studies we used a rather long off period between stimulus blocks (60 s) in order to be able to examine the dynamics of the response in the post‐stimulus period. Both groups showed nearly identical BOLD post‐stimulus undershoots, yet only the older group exhibited a CBF post‐stimulus undershoot (see Fig. 3).
Observed measurements of baseline CBF values of ∼ 60 mL/100 mL/min in younger subjects (Table I) are similar to values within the VC using other neuroimaging methods including MRI [Donahue et al.,2006] and PET [Mintun et al.,2002]. The observed age‐related decreases in resting‐state CBF are consistent with the ASL, PET, and single photon emission computed tomography (SPECT) studies that have shown regionally dependent decreases in CBF with advancing age [Bentourkia et al.,2000; Leenders et al.,1990; Marchal et al.,1992; Martin et al.,1991; Matsuda et al.,2003; Parkes et al.,2004]. While the measurements of baseline CBF are not novel, the introduction of these measurements while also performing functional studies provides a more complete picture of the effects of baseline CBF on the BOLD response.
In our study we found that with a simple visual stimulation the magnitude of the BOLD response was reduced in older subjects relative to younger adults (see Fig. 1). A number of previous studies [Buckner et al.,2000; Raemaekers et al.,2006; Ross et al.,1997; Tekes et al.,2005] also found a significant reduction in the BOLD response within the VC of older subjects compared to younger adults. However, a few studies [Aizenstein et al.,2004; Huettel et al.,2001] found no differences in the magnitude of the BOLD signal between older and younger groups when correcting for the number of negative voxels. When we used only voxels that had a positive change in the CBF response, we observed a significant reduction in the magnitude of the BOLD response in older subjects compared to younger adults.
Differences between our observations and others could result from inclusion of a relatively younger “older” group and/or ROI selection. Compared to previous studies that have included relatively older subjects many of our “older” subjects were younger when using these same standards. Overall our older subjects were ∼15 years younger than other aging studies. What was quite striking is that even though our “older” subjects were not as old, a significant reduction in the BOLD signal was still observed. This difference with aging was only observed when a ROI based on positive CBF responses was employed. When a ROI based on only positive BOLD responses was used a trend towards a reduction in the amplitude was seen (P = 0.12). We believe that selecting a ROI based on CBF activation rather than BOLD alone gives a closer approximation of neuronal changes, because a positive BOLD ROI is more likely to be dominated by draining venous artifacts. Our study demonstrates that derived estimates of CBF‐CMRO2 coupling can be affected by voxel selection in the ROI for averaging, as has been found in previous studies [Leontiev and Buxton,2007].
For a visual functional activation stimulus, aging was associated with a significant difference in the BOLD response but not fractional changes in the CBF and CMRO2 responses (Table II). The ratio of fractional changes in CBF to CMRO2, the coupling ratio n, was comparable between the two groups when either a CBF or BOLD ROI selection was used. An increase in the variance was present for a BOLD ROI. The observed reduction of the BOLD response is attributed to the reduction of M in the older group, so that the same fractional CBF response creates a weaker BOLD response. The difference in M was derived from the hypercapnic experiment, in which roughly similar BOLD responses were driven by a larger CBF response in the older group compared with the younger group. These results, with preserved CBF and CMRO2 responses, argue against the notion that the observed decrease in the BOLD response is due to a decrease in the neural activity response with aging. Our results are in good agreement with previous visual evoked potential studies that have demonstrated no differences with aging [Adachi‐Usami et al.,1988; Allison et al.,1984].
Our primary observation was that baseline CBF and the parameter M were both reduced in the older group. It is worthwhile to consider whether the reduction in M can be attributed to the reduction in baseline CBF. In a previous study [Brown et al.,2003] in which baseline CBF was raised by administration of acetazolamide, a ∼20% increase in baseline CBF produced a ∼35% reduction of the BOLD response to a finger tapping task. Broadly speaking, this result is counter to our current finding with aging, in the sense that with acetazolamide the higher baseline CBF was associated with a weaker BOLD response to activation, whereas in our current study the lower baseline CBF was associated with a weaker BOLD response. However, these results may be consistent, depending on what happens to baseline CMRO2 in each case. The parameter M reflects the amount of deoxyhemoglobin present in the baseline state, and in the context of the Davis model it is proportional to V 0 E 0 β, where V 0 is the baseline CBV, and E 0 is the baseline OEF and β is equal to 1.5 [Buxton et al.,2004]. With decreased CBF, we would expect V 0 to decrease as well, but the overall effect on M depends on whether the baseline OEF also changes. If E 0 remains constant, so that a reduction in CBF is also accompanied by a reduction in CMRO2, then M would track with a reduction in CBF, the pattern we observed. On the other hand, if CBF is increased with no change in CMRO2—the situation that is thought to apply in the acetazolamide experiment—then OEF will decrease significantly, overwhelming the effects of V 0 resulting in a decrease in M. The observed reduction of the BOLD response to a stimulus after administration of acetazolamide is consistent with this reduction of M [Buxton et al.,2004]. In short, the observed reduction in M in the older group is qualitatively consistent with a picture in which both baseline CBF and CMRO2 are decreased with aging, while fractional changes in CBF and CMRO2 to a functional stimulus are preserved. These considerations suggest that the relationship between baseline CBF and the BOLD response are complex. Further studies are needed to explore these questions, and in particular to assess other factors that may differ between our two groups.
In addition to examining the primary positive responses to activation, we also investigated the dynamics of the BOLD and CBF responses in the post‐stimulus period. An unexpected finding was the relative difference in the CBF post stimulus response between the two groups despite similar BOLD post‐stimulus undershoots. These results are quite comparable to a recent report by our colleagues [Restom et al.,2007], who also found a more pronounced CBF undershoot in the older group. The origin of the BOLD poststimulus undershoot in fMRI is not well understood. If there is a corresponding undershoot in the CBF response, then a BOLD undershoot could simply be a transient, but coupled, reduction of CBF and CMRO2 after the stimulus, consistent with a reduction in neural activity below baseline. Alternatively, a CBF reduction without a corresponding CMRO2 reduction, possibly related to the dynamics of vascular compliance [Behzadi and Liu,2005], would be expected to create an even larger BOLD undershoot. However, we observed a BOLD undershoot without an accompanying CBF undershoot suggesting a further uncoupling of physiological variables during the undershoot period. Two possibilities that explain these results could be: a slow return of CBV to baseline with a more rapid return of CBF and CMRO2 [Buxton et al.,1998; Mandeville et al.,1999], or a slow return of CMRO2 with a more rapid return of CBF and CBV [Liu and Wong,2005]. It is possible that each of these mechanisms may play a role either together or under different circumstances, and clearly further studies are needed to understand this unexpected change with aging.
The potential role of other physiological changes with aging on the magnitude of the BOLD response also requires additional studies. One observation from this study was that despite breathing the same inhaled gas mixture, the changes in end‐tidal CO2 tended to be higher in the older group. This may be due to better ventilatory compensation in the younger group, but we cannot support this with statistically significant differences in breathing between the groups. Nevertheless, these data do suggest the problems that may occur in using a standard CO2 inhalation to normalize the BOLD signal [Bandettini and Wong,1997; Handwerker et al.,2007]. In this normalization method, the BOLD response to activation is normalized to the BOLD response to hypercapnia. Ideally, this should reduce variability of the BOLD response due to regional variations in M. However, this approach may not work as well for normalizing responses between different aged groups. As seen in our study, the BOLD response to CO2 was similar in the two groups, while the activation responses were different. If we had only performed BOLD measurements, we could have argued that because the BOLD responses to CO2 were similar, the difference in the BOLD responses to activation was unlikely to be due to a difference in M. However, the underlying assumption of this normalization approach would be that CO2 elicits the same CBF response in both groups, and that was not the case. In short, a full calibrated‐BOLD approach with CBF as well as BOLD response measurements, rather than a CO2 normalization measuring only BOLD responses, is necessary to understand the origins of observed group differences in the BOLD response.
In summary, we have demonstrated that the calibrated BOLD approach is quite feasible within older healthy subjects. Our results demonstrate that interpretation of changes in the BOLD response alone in terms of underlying neural activity changes should be made with caution. For example, we found that different primary BOLD responses in the two groups were nevertheless associated with similar fractional changes in CBF and CMRO2, while similar poststimulus undershoots of the BOLD response were associated with different CBF undershoots. The additional measurements provided by a calibrated BOLD approach can begin to resolve the complexities involved in the interpretation of the BOLD response.
Acknowledgements
The authors are also deeply indebted to Thomas Liu and Kal Restom for their invaluable suggestions and assistance with code used for analyses.
REFERENCES
- Adachi‐Usami E,Hosoda L,Toyonaga N ( 1988): Effects of aging on the temporal frequency characteristics determined by pattern visually evoked cortical potentials. Doc Ophthalmol 69: 139–144. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ,Clark KA,Butters MA,Cochran J,Stenger VA,Meltzer CC,Reynolds CF,Carter CS ( 2004): The BOLD hemodynamic response in healthy aging. J Cogn Neurosci 16: 786–793. [DOI] [PubMed] [Google Scholar]
- Allison T,Hume AL,Wood CC,Goff WR ( 1984): Developmental and aging changes in somatosensory, auditory and visual evoked potentials. Electroencephalogr Clin Neurophysiol 58: 14–24. [DOI] [PubMed] [Google Scholar]
- Ances BM,Leontiev O,Perthen JE,Liang CL,Lansing AE,Buxton RB ( 2008): Regional coupling differences in cerebral blood flow and oxygen metabolism: Implications for BOLD‐fMRI. Neuroimage 39: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA,Wong EC ( 1997): A hypercapnia‐based normalization method for improved spatial localization of the human brain activation with fMRI. NMR Biomed 10: 197–203. [DOI] [PubMed] [Google Scholar]
- Behzadi Y,Liu TT ( 2005): An arteriolar compliance model of the cerebral blood flow response to neural stimulus. Neuroimage 25: 1100–1111. [DOI] [PubMed] [Google Scholar]
- Bentourkia M,Bol A,Ivanoiu A,Labar D,Sibomana M,Coppens A,Michel C,Cosnard G,De Volder AG ( 2000): Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: Effect of aging. J Neurol Sci 181: 19–28. [DOI] [PubMed] [Google Scholar]
- Boxerman JL,Hamberg LM,Rosen BR,Weisskoff RM ( 1995b): MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 34: 555–566. [DOI] [PubMed] [Google Scholar]
- Boynton GM,Engel SA,Glover GH,Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human. VI. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG,Eyler Zorrilla LT,Georgy B,Kindermann SS,Wong EC,Buxton RB ( 2003): BOLD and perfusion response to finger‐thumb apposition after acetazolamide administration: Differential relationship to global perfusion. J Cereb Blood Flow Metab 23: 829–837. [DOI] [PubMed] [Google Scholar]
- Brown GG,Perthen JE,Liu TT,Buxton RB ( 2007): A primer on functional magnetic resonance imaging. Neuropsychol Rev 17: 107–125. [DOI] [PubMed] [Google Scholar]
- Buckner RL,Snyder AZ,Sanders AL,Raichle ME,Morris JC ( 2000): Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 12( Suppl 2): 24–34. [DOI] [PubMed] [Google Scholar]
- Burock MA,Dale AM ( 2000): Estimation and detection of event‐related fMRI signals with temporally correlated noise: A statistically efficient and unbiased approach. Hum Brain Mapp 11: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB,Uludag K,Dubowitz DJ,Liu TT ( 2004): Modeling the hemodynamic response to brain activation. Neuroimage 23 ( Suppl 1): S220‐S233. [DOI] [PubMed] [Google Scholar]
- Buxton RB,Wong EC,Frank LR ( 1998): Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn Reson Med 39: 855–864. [DOI] [PubMed] [Google Scholar]
- Celesia GG,Daly RF ( 1977): Effects of aging on visual evoked responses. Arch Neurol 34: 403–407. [DOI] [PubMed] [Google Scholar]
- Chalela JA,Alsop DC,Gonzalez‐Atavales JB,Maldjian JA,Kasner SE,Detre JA ( 2000): Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke 31: 680–687. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA,Bulte DP,Gallichan D,Piechnik SK,Wise R,Jezzard P ( 2007a): Flow‐metabolism coupling in human visual, motor, and supplementary motor areas assessed by magnetic resonance imaging. Magn Reson Med 57: 538–547. [DOI] [PubMed] [Google Scholar]
- Chiarelli PA,Bulte DP,Piechnik S,Jezzard P ( 2007b): Sources of systematic bias in hypercapnia‐calibrated functional MRI estimation of oxygen metabolism. Neuroimage 34: 35–43. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Deouell LY,Gazzaley A ( 2003): Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci 4: 863–872. [DOI] [PubMed] [Google Scholar]
- D'Esposito M,Zarahn E,Aguirre GK,Rypma B ( 1999): The effect of normal aging on the coupling of neural activity to the bold hemodynamic response. Neuroimage 10: 6–14. [DOI] [PubMed] [Google Scholar]
- Davis TL,Kwong KK,Weisskoff RM,Rosen BR ( 1998): Calibrated functional MRI: Mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ,Lu H,Jones CK,Pekar JJ,van Zijl PC ( 2006): An account of the discrepancy between MRI and PET cerebral blood flow measures. A high‐field MRI investigation. NMR Biomed 19: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Goldstein S,Reivich M ( 1991): Cerebral blood flow and metabolism in aging and dementia. Clin Neuropharmacol 14( Suppl 1): S34‐S44. [DOI] [PubMed] [Google Scholar]
- Grubb RL,Raichle ME,Eichling JO,Ter‐Pogossian MM ( 1974): The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke 5: 630–639. [DOI] [PubMed] [Google Scholar]
- Hafkenschiel JH,Friedland CK,Zintel HA ( 1954): The blood flow and oxygen consumption of the brain in patients with essential hypertension before and after adrenalectomy. J Clin Invest 33: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA,Gazzaley A,Inglis BA,D'Esposito M ( 2007): Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp 28: 846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V,Zaro Weber O,Wedekind C,Krings T,Schulte O,Kugel H,Krug B,Klug N,Lackner KJ ( 2001): Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett 308: 141–144. [DOI] [PubMed] [Google Scholar]
- Hoge RD,Atkinson J,Gill B,Crelier GR,Marrett S,Pike GB ( 1999a): Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: The deoxyhemoglobin dilution model. Magn Reson Med 42: 849–863. [DOI] [PubMed] [Google Scholar]
- Hoge RD,Atkinson J,Gill B,Crelier GR,Marrett S,Pike GB ( 1999b): Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci USA 96: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA,Singerman JD,McCarthy G ( 2001): The effects of aging upon the hemodynamic response measured by functional MRI. Neuroimage 13: 161–175. [DOI] [PubMed] [Google Scholar]
- Johnson NA,Jahng GH,Weiner MW,Miller BL,Chui HC,Jagust WJ,Gorno‐Tempini ML,Schuff N ( 2005): Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin‐labeling MR imaging: Initial experience. Radiology 234: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M,Berwick J,Hewson‐Stoate N,Gias C,Mayhew J ( 2005): The effect of hypercapnia on the neural and hemodynamic responses to somatosensory stimulation. Neuroimage 27: 609–623. [DOI] [PubMed] [Google Scholar]
- Kastrup A,Kruger G,Glover GH,Moseley ME ( 1999): Assessment of cerebral oxidative metabolism with breath holding and fMRI. Magn Reson Med 42: 608–611. [DOI] [PubMed] [Google Scholar]
- Kety SS,Schmidt CF ( 1948): The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S‐G,Ugurbil K ( 1997): Comparison of blood oxygenation and cerebral blood flow effects in fMRI: Estimation of relative oxygen consumption change. Magn Reson Med 38: 59–65. [DOI] [PubMed] [Google Scholar]
- Kliefoth AB,Grubb RL Jr,Raichle ME ( 1979): Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem 32: 661–663. [DOI] [PubMed] [Google Scholar]
- Leenders KL,Perani D,Lammertsma AA,Heather JD,Buckingham P,Healy MJ,Gibbs JM,Wise RJ,Hatazawa J,Herold S,Beaney RP,Brooks DJ,Spinks T,Rhodes C,Frackowiak RSJ,Jones T( 1990): Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47. [DOI] [PubMed] [Google Scholar]
- Leontiev O,Buxton RB ( 2007): Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated‐BOLD fMRI. Neuroimage 35: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT,Wong EC ( 2005): A signal processing model for arterial spin labeling functional MRI. Neuroimage 24: 207–215. [DOI] [PubMed] [Google Scholar]
- Loessner A,Alavi A,Lewandrowski KU,Mozley D,Souder E,Gur RE ( 1995): Regional cerebral function determined by FDG‐PET in healthy volunteers: Normal patterns and changes with age. J Nucl Med 36: 1141–1149. [PubMed] [Google Scholar]
- Long JM,Mouton PR,Jucker M,Ingram DK ( 1999): What counts in brain aging? Design‐based stereological analysis of cell number. J Gerontol A Biol Sci Med Sci 54: B407–B417. [DOI] [PubMed] [Google Scholar]
- Mandeville JB,Marota JJ,Ayata C,Zaharchuk G,Moskowitz MA,Rosen BR,Weisskoff RM ( 1999): Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. J Cereb Blood Flow Metab 19: 679–689. [DOI] [PubMed] [Google Scholar]
- Marchal G,Rioux P,Petit‐Taboue MC,Sette G,Travere JM,Le Poec C,Courtheoux P,Derlon JM,Baron JC ( 1992): Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol 49: 1013–1020. [DOI] [PubMed] [Google Scholar]
- Martin AJ,Friston KJ,Colebatch JG,Frackowiak RS ( 1991): Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11: 684–689. [DOI] [PubMed] [Google Scholar]
- Matsuda H,Ohnishi T,Asada T,Li ZJ,Kanetaka H,Imabayashi E,Tanaka F,Nakano S ( 2003): Correction for partial‐volume effects on brain perfusion SPECT in healthy men. J Nucl Med 44: 1243–1252. [PubMed] [Google Scholar]
- Mattay VS,Fera F,Tessitore A,Hariri AR,Das S,Callicott JH,Weinberger DR ( 2002): Neurophysiological correlates of age‐related changes in human motor function. Neurology 58: 630–635. [DOI] [PubMed] [Google Scholar]
- Matthews PM,Honey GD,Bullmore ET ( 2006): Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci 7: 732–744. [DOI] [PubMed] [Google Scholar]
- Mintun MA,Vlassenko AG,Shulman GL,Snyder AZ ( 2002): Time‐related increase of oxygen utilization in continuously activated human visual cortex. Neuroimage 16: 531–537. [DOI] [PubMed] [Google Scholar]
- Mumford JA,Hernandez‐Garcia L,Lee GR,Nichols TE ( 2006): Estimation efficiency and statistical power in arterial spin labeling fMRI. Neuroimage 33: 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack P,Shenkin HA,Bortin L,Goluboff B,Soffe AM ( 1953): The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J Clin Invest 32: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S,Tank DW,Menon R,Ellermann JM,Kim SG,Merkle H,Ugurbil K ( 1992): Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 89: 5951–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes LM,Rashid W,Chard DT,Tofts PS ( 2004): Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn Reson Med 51: 736–743. [DOI] [PubMed] [Google Scholar]
- Prvulovic D,Van de Ven V,Sack AT,Maurer K,Linden DE ( 2005): Functional activation imaging in aging and dementia. Psychiatry Res 140: 97–113. [DOI] [PubMed] [Google Scholar]
- Raemaekers M,Vink M,van den Heuvel MP,Kahn RS,Ramsey NF ( 2006): Effects of aging on BOLD fMRI during prosaccades and antisaccades. J Cogn Neurosci 18: 594–603. [DOI] [PubMed] [Google Scholar]
- Raz N,Torres IJ,Spencer WD,White K,Acker JD ( 1992): Age‐related regional differences in cerebellar vermis observed in vivo. Arch Neurol 49: 412–416. [DOI] [PubMed] [Google Scholar]
- Restom K,Bangen KJ,Bondi MW,Perthen JE,Liu TT ( 2007): Cerebral blood flow and BOLD responses to a memory encoding task: A comparison between healthy young and elderly adults. Neuroimage 37: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K,Behzadi Y,Liu TT ( 2006): Physiological noise reduction for arterial spin labeling functional MRI. Neuroimage 31: 1104–1115. [DOI] [PubMed] [Google Scholar]
- Ross MH,Yurgelun‐Todd DA,Renshaw PF,Maas LC,Mendelson JH,Mello NK,Cohen BM,Levin JM ( 1997): Age‐related reduction in functional MRI response to photic stimulation. Neurology 48: 173–176. [DOI] [PubMed] [Google Scholar]
- Sicard KM,Duong TQ ( 2005): Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus‐evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 25: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic B,Warnking JM,Pike GB ( 2004): Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22: 771–778. [DOI] [PubMed] [Google Scholar]
- Taoka T,Iwasaki S,Uchida H,Fukusumi A,Nakagawa H,Kichikawa K,Takayama K,Yoshioka T,Takewa M,Ohishi H ( 1998): Age correlation of the time lag in signal change on EPI‐fMRI. J Comput Assist Tomogr 22: 514–517. [DOI] [PubMed] [Google Scholar]
- Tekes A,Mohamed MA,Browner NM,Calhoun VD,Yousem DM ( 2005): Effect of age on visuomotor functional MR imaging. Acad Radiol 12: 739–745. [DOI] [PubMed] [Google Scholar]
- Wang J,Qiu M,Constable RT ( 2005): In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med 53: 666–674. [DOI] [PubMed] [Google Scholar]
- Williams DS,Detre JA,Leigh JS,Koretsky AP ( 1992): Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA 89: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC,Buxton RB,Frank LR ( 1999): Quantitative perfusion imaging using arterial spin labeling. Neuroimaging Clin N Am 9: 333–342. [PubMed] [Google Scholar]
- Woolrich MW,Behrens TE,Beckmann CF,Jenkinson M,Smith SM ( 2004): Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21: 1732–1747. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T,Kanno I,Uemura K,Shishido F,Inugami A,Ogawa T,Murakami M,Suzuki K ( 1986): Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 17: 1220–1228. [DOI] [PubMed] [Google Scholar]


