Abstract
Purpose
To determine if particle shape can be engineered to inhibit phagocytosis of drug delivery particles by macrophages, which can be a significant barrier to successful therapeutic delivery.
Methods
Non-spherical polystyrene particles were fabricated by stretching spherical particles embedded in a polymer film. A rat alveolar macrophage cell line was used as model macrophages. Phagocytosis of particles was assessed using time-lapse video microscopy and fluorescence microscopy.
Results
We fabricated worm-like particles with very high aspect ratios (>20). This shape exhibits negligible phagocytosis compared to conventional spherical particles of equal volume. Reduced phagocytosis is a result of decreasing high curvature regions of the particle to two single points, the ends of the worm-like particles. Internalization is possible only at these points, while attachment anywhere along the length of the particles inhibits internalization due to the low curvature.
Conclusions
Shape-induced inhibition of phagocytosis of drug delivery particles is possible by minimizing the size-normalized curvature of particles. We have created a high aspect ratio shape that exhibits negligible uptake by macrophages.
Keywords: aspect ratio, curvature, drug delivery, macrophage, polystyrene
INTRODUCTION
Considerable advantages are gained over conventional delivery systems by encapsulating therapeutic drugs in polymer particles. These include controlled release, protection from metabolism and degradation, and targeting capabilities (1). However, the body’s immune response to foreign drug delivery particles can create a substantial barrier to successful drug delivery. Macrophage cells recognize and internalize foreign particles 1 μm and larger in a process known as phagocytosis (2). In some cases, infected macrophages are the intended target of drug delivery particles while in others, such as vaccines, macrophage internalization of particles is necessary for adequate protection (3). In many situations, however, macrophages are not the targeted cell type and phagocytosis prevents particles from releasing their therapeutic payload near or within the desired cell population. Regardless of whether particles are introduced in the blood, skin, muscle, or lungs, they come into contact with macrophages and are susceptible to phagocytosis (4,5). A great deal of research has focused on attempting to prevent phagocytosis through variation in particle surface chemistry, primarily using spherical particles. Chemical modification of drug delivery particles, using hydrophilic polymers such as polyethylene glycol or poloxamers, postpones uptake by temporarily preventing opsonization, the binding of proteins that increase recognition and receptor-specific attachment by macrophages (6).
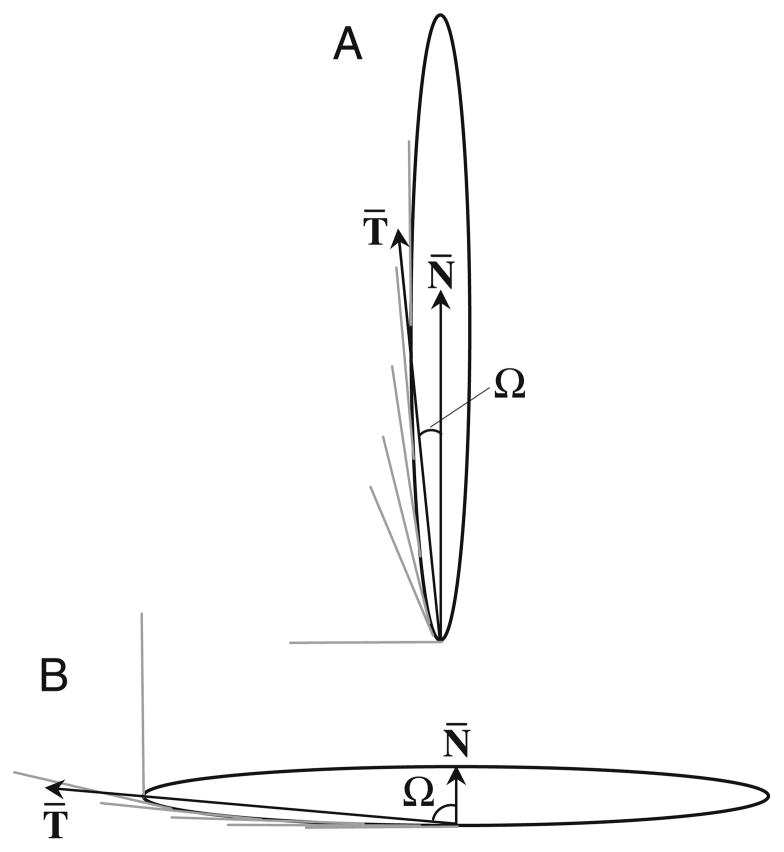
Recently, particle shape has been identified as having a significant effect on the ability of macrophages to internalize particles via actin-driven movement of the macrophage membrane (7). This phenomenon is independent of surface chemistry and depends only on the local particle shape where the cell attaches. Local particle shape, at the point of cell attachment, has been quantified as the angle between the membrane normal at the point of initial contact and tangent lines drawn to the particle contour near the point of initial contact (Fig. 1). This angle, Ω, is essentially a measure of the local curvature that has been normalized for size, the length over which that curvature exists. Length-normalized curvature, as defined by the parameter Ω, is the critical shape feature that dictates whether or not phagocytic internalization will occur. Local shapes that have low length-normalized curvature (Ω>45°, where 45° is the value for spherical particles) inhibit internalization while spheres and local shapes that have high length-normalized curvature (Ω≤45°) permit internalization (7). Though the relationship between local particle shape and phagocytosis has been revealed, no global shape was seen to reduce uptake significantly compared to spherical particles. The challenge in drug delivery is to exploit this local shape internalization bias to create non-spherical drug delivery particles that exhibit significantly reduced phagocytosis. Global particle shapes with minimal regions of high length-normalized curvature and maximal low curvature regions are most likely to avoid uptake. We hypothesize that very high aspect ratio particles would meet these requirements and exhibit significantly decreased phagocytosis. In this report we demonstrate, using fluorescence and time-lapse video microscopy, that high aspect ratio worm-like particles successfully avoid phagocytic uptake by rat alveolar macrophages. This shape-induced inhibition of phagocytosis will have important ramifications in the successful use of polymer particles as drug delivery vehicles.
Fig. 1.
A schematic diagram illustrating how local shape is defined. T̄ represents the average of tangential angles near the point of cell contact. Ω is the angle between T̄ and the membrane normal at the site of attachment, N̄. A Ω=2.5° for cell attachment at end of worm. B Ω=87.5° for cell attachment on side of worm.
MATERIALS AND METHODS
Particle Preparation
Uncrosslinked polystyrene (PS) spherical particles (0.93± 0.1 and 2.98±0.083 μm in diameter) were purchased from Polysciences (Warrington, PA, USA). All chemicals used were of commercial grade. Fully hydrolyzed poly(vinyl alcohol) (PVA) was purchased from Sigma Chemicals (St. Louis, MO, USA). The protocol used for fabrication of non-spherical particles was adapted from Ho et al. (8). Specifically, 5% PVA was dissolved in 75°C water. After cooling to room temperature, 2% glycerol and 0.04% w/v spherical particles were added to the PVA mixture. The films were dried on a 19 cm×27 cm flat surface. The films were heated in a 150°C oil bath and stretched to nine times their original length using a custom-made apparatus. The stretching device consists of two opposing clamps connected on a screw. The clamps hold the ends of the film secure and rotation of the screw separates the clamps to stretch the film. Films were allowed to cool to room temperature and any residual oil was cleaned from the films with isopropanol. The films were soaked for 12 h in toluene containing excess coumarin to introduce fluorescence inside the particles. Toluene was removed from the particles without removing fluorescence by soaking the films in isopropanol containing coumarin for 12 h. The films were dissolved in 30% isopropanol/water at 65°C. The particles were washed by centrifugation with the same solution ten times to remove all PVA from the surface of the particles. To verify particle morphology, particles were coated with palladium (Hummer 6.2 Sputtering System, Anatech Ltd., Union City, CA, USA) and imaged with the Sirion 400 Scanning Electron Microscope (FEI Company, Hillsboro, OR, USA) at 3 eV. To promote internalization, particles were incubated with 0.25 mg/ml rabbit IgG (Sigma Chemicals) for 30 min at 37°C and washed twice with phosphate buffered saline (PBS) to remove unadsorbed IgG. Particle concentration was determined by mixing worms with red fluorescent spheres of known concentration (as measured by the supplier, Invitrogen, Carlsbad, CA, USA) and performing dual laser flow cytometry (FACS Aria, BD Biosciences, San Jose, CA, USA) to obtain the ratio of red spheres to green worms. With this ratio, worm particle concentration was calculated directly from the sphere standard concentration.
Cells
Continuous alveolar rat macrophage cells NR8383 (American Type Culture Collection [ATCC], Manassas, VA, USA) were used as model macrophages. Cells were cultured in F-12K media (ATCC) supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/streptomycin (Sigma Chemicals) under standard culture conditions (37°C, 5% CO2, humidified).
Time-Lapse Video Microscopy
Cells (2×105 cells/ml) were allowed to attach in dishes lined with coverslip glass in F-12K media supplemented with 10% FBS and 25 mM HEPES (Sigma Chemicals). The dishes were placed on an Axiovert 25 microscope (Carl Zeiss Inc., Thornwood, NY, USA) at 100× with phase contrast filters and equipped with Bioptechs Delta T Controlled Culture Dish System® (Bioptechs Inc., Butler, PA, USA) to keep the cells at 37°C. Particles (one particle per cell) were added to the dishes and bright-field images were collected every 30 s for 2 h by a CoolSNAPHQ CCD camera (Roper Scientific, Tucson, AZ, USA) connected to the Metamorph® software. In some cases cells were observed for 12 h and cell behavior was the same as for 2 h. Observed cells were randomly chosen from the entire population thus discounting potential bias due to heterogeneity in macrophage size (radius 7.5±2.5 μm). Images were condensed into movies and analyzed manually for phagocytic events. Successful phagocytosis exhibited membrane ruffling at the site of attachment, blurring the crisp boundary of the membrane, and subsequent reforming of the membrane boundary after internalization. Phagocytosis was analyzed based on internalization of one attached particle by a single cell.
Fluorescence Microscopy
In response to limitations of confocal microscopy and flow cytometry, we designed a new method to distinguish between internalized and attached particles. This method involves fixing the macrophages after incubation and using solvent to remove the fluorescence of only externally bound particles. In this procedure cells (2×105 cells/ml) were allowed to attach to glass coverslips in 24-well plates containing F-12K media supplemented with 10% FBS. Fluorescent particles (30 particles per cell) were incubated with the cells at 37°C and 5% CO2 for 22 h. After particle-cell incubation, coverslips were rinsed in cold PBS to stop internalization and remove excess free particles. The cells were fixed with 2% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 1.5 h to protect both the cells and internalized particles from the solvent. After fixation the coverslips were washed twice with water to remove excess glutaraldehyde and air dried for 20–25 min depending on particle size. Without the drying step, water surrounding the external particles shields them from the solvent. The coverslips were then dipped in ethyl acetate for varying amounts of time (5 s to 1 min) depending on particle size, gently pressed on a tissue to remove excess solvent and submersed in water. The solvent exposure time increased as particle size decreased and as the air drying time decreased, likely due to the water shielding affect for smaller particles. After the process, cells were viewed with an Axiovert 25 microscope (Carl Zeiss) equipped with a mercury lamp and fluorescence filters at 40× or 100×. The number of fluorescent particles in each cell was counted manually and at least 50 cells were recorded in each sample.
Scanning Electron Microscopy (SEM)
SEM was used for high magnification examination of cell membrane interactions with the particles. After 60 min of incubation with particles at 37°C and 5% CO2, cells were fixed with 2% EM grade glutaraldehyde (Electron Microscopy Sciences). They were washed with serial dilutions of water and ethanol, dried under vacuum, and coated with palladium (Hummer 6.2 Sputtering System). Cells were imaged with the Sirion 400 SEM at 2 eV.
RESULTS
We identified high aspect ratio worm-like particles as a shape with low phagocytosis potential, or maximum regions of low Ω (Fig. 2). Ω = cos−1 (N̄. T̄) and can be simplified for worm-shaped particles because they are essentially high aspect ratio (~22.5, measured from SEM images) ellipses (7). For an ellipse with a major axis a and minor axis b, Ω ~ arctan(b/a) for a particle attaching along the major axis (point) and Ω ~ arctan(b/a) for attachment along the minor axis (side). Though worms have very sharp ends with extremely high length-normalized curvature (Ω=2.5°), these Ω values exist only at the two discrete endpoints. The remainder of the shape is essentially flat (Ω=87.5° when approached from the side) and theoretically should not support phagocytosis. Worm-like particles were created from 1 and 3 μm diameter spherical polystyrene particles. The particles exhibited high aspect ratios and also some degree of flexibility. Particles in solution did not appear to bend or twist but some particles in SEM images appeared curved, likely due to capillary forces during the drying process.
Fig. 2.
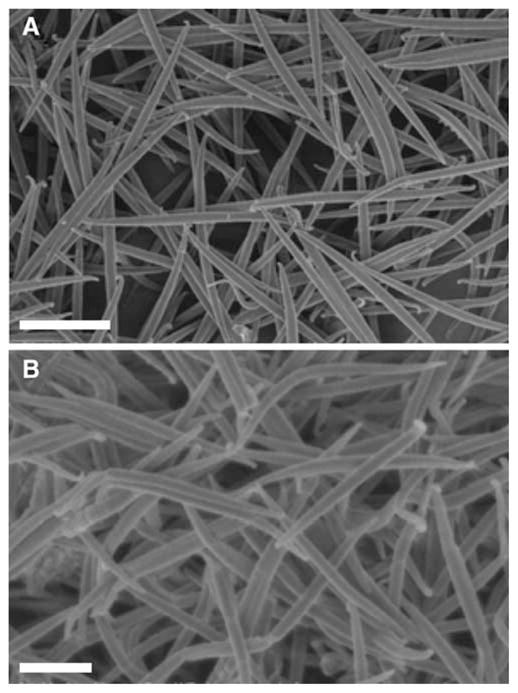
Scanning electron micrographs of polystyrene A worms stretched from 3 μm spheres and B worms stretched from 1 μm spheres. Scale bars = 10 μm (A) and 2 μm (B).
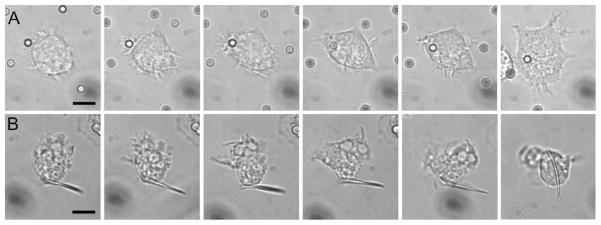
Individual phagocytic events involving worms and spheres of the same volume were compared. Volumes equivalent to 3 μm diameter spheres were chosen as an intermediate phagocytic size that attaches well to cells (data not shown). Time-lapse video microscopy was performed to observe interactions between alveolar macrophages and IgG-adsorbed shapes following particle attachment to cells. Time-lapse images clearly show spherical particles being internalized by the cells (Fig. 3A). On the contrary, no internalization of worms was seen following attachment to the sides of the particle (Fig. 3B). No attachment was observed to the pointed ends of the worms. Over all of the time-lapse events observed, about 55% of attached spheres were internalized while no worms were internalized (at least 27 interactions observed for each shape). This behavior has not been previously seen and is especially significant given that the particles were adsorbed with IgG to promote phagocytosis.
Fig. 3.
Time-lapse video microscopy clips at 0, 1, 2, 3, 4 and 32 min after attachment of IgG-adsorbed PS particles to alveolar macrophages. A Macrophage internalizes 3 μm sphere quickly following attachment. B Macrophage attaches to side of worm, but does not internalize it. Images have been cropped and magnified identically to show cell/particle detail. Scale bars = 5 μm.
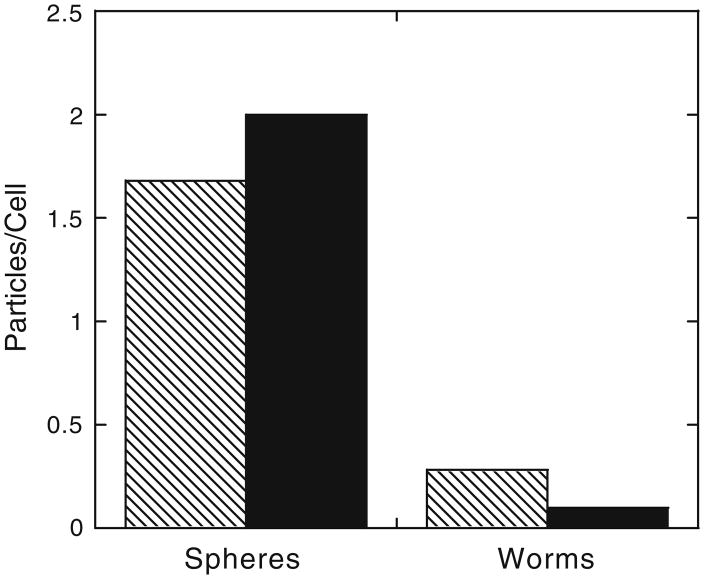
In order to verify that this shape bias holds over longer periods of interaction time with larger numbers of cells and particles, fluorescence microscopy was used to count internalized fluorescent particles distinguished from bleached externally bound particles. Figure 4 shows the number of phagocytosed 3 μm IgG-adsorbed spheres and worms after 22 h of incubation at a concentration of 30 particles per cell. Worms still exhibited very little internalization compared to spheres.
Fig. 4.
Average number of spherical and worm-like IgG-adsorbed particles internalized in 22 h as determined by fluorescence microscopy. Patterned bars indicate particle volumes equivalent to 3 μm spheres (p<0.0000031) and solid bars indicate particle volumes equivalent to 1 μm spheres (p<0.000024). At least 50 cells were counted for each sample.
Though shape clearly affects phagocytosis of intermediate sized micro-particles (3 μm), it is not known if shape retains this ability on smaller length scales. Non-phagocytic internalization processes, such as endocytosis, have been seen to be affected by the shape of nanoparticles (9). However, this result cannot be generalized to phagocytosis due to differences between the processes, particularly the strong actin-dependence of phagocytosis. To investigate the relationship between size and shape, phagocytosis was also measured for IgG-adsorbed spheres and worms with volumes equivalent to 1 μm diameter spheres, the smallest (spherical) size identified as purely actin-dependent phagocytosis (2). Figure 4 shows that the difference in phagocytic uptake between spheres and worms is retained, even enhanced, for 1 μm particles.
DISCUSSION
Previous work has revealed that particle shape plays an important role in the phagocytosis of polymer particles by macrophages (7). However, all of the shapes investigated exhibited significant uptake compared to spherical particles. The purpose of this study was to translate our understanding of the relationship between shape and phagocytosis to design and demonstrate a shape that exhibits negligible uptake by macrophages. Though it is impossible to create an entirely flat particle, by significantly increasing the aspect ratio we created a particle that is essentially flat except at the two discreet endpoints.
As hypothesized, decreasing the length-normalized curvature (Ω) over the majority of the particle significantly decreased uptake. Time-lapse video microscopy showed that no worm shaped particles were internalized while over half of all spheres that attached were internalized (3 μm volume). All worms that attached in the videos did so along the sides of the particles, not at the pointy, low Ω ends where phagocytosis should be possible. Though no internalization was observed in single cell time-lapse microscopy, low levels of worm internalization were seen with increased incubation time (2 to 22 h) and particle concentration (1 to 30 particles/cell). This is expected and is likely the result of increased cell-particle interaction as some worms attached to cells at the pointed (low Ω) end, which promotes internalization. These events, however, are still not common as internalization of worms is ~6 and ~20 times less than spheres of the same volume for 3 and 1 μm volumes respectively. These results demonstrate that it is possible to significantly decrease phagocytic uptake of particles through shape design.
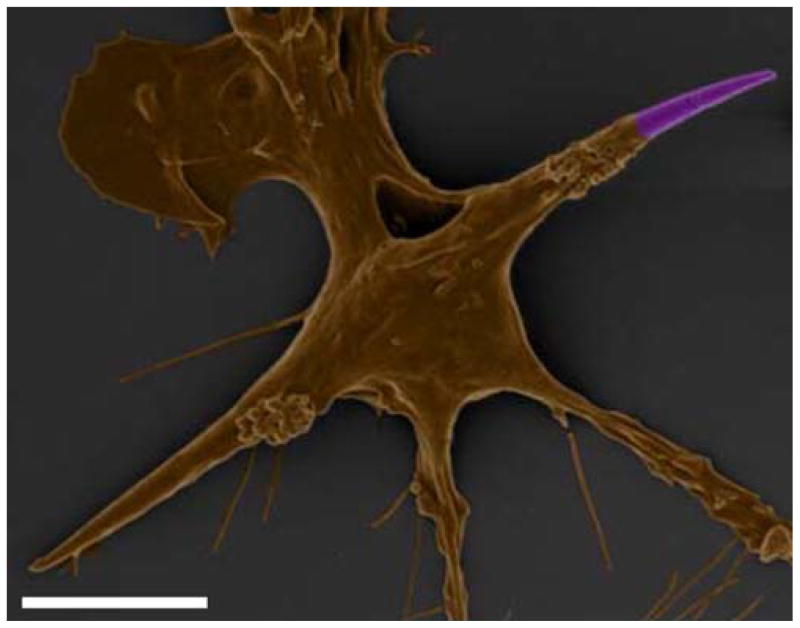
Although macrophages were usually unable to internalize worms, scanning electron microscopy of macrophages interacting with worms revealed unexpected macrophage behavior. As seen in Fig. 5, macrophages interact vigorously with the worms. The cells appear to manipulate and contort the particles. By attaching to multiple points on a worm, they are able to exert enough force to bend the particle. Despite the ability of the cells to organize their actin cytoskeleton and membrane to bend the particles, they are not capable of organizing the same structures for the purpose of internalization. This behavior has not been seen with other shapes. By creating very thin particles, we have altered not only the shape, but also the mechanical properties of a normally stiff, brittle particle. A flexible, non-phagocytosable particle could have exciting circulation properties in vivo (10).
Fig. 5.
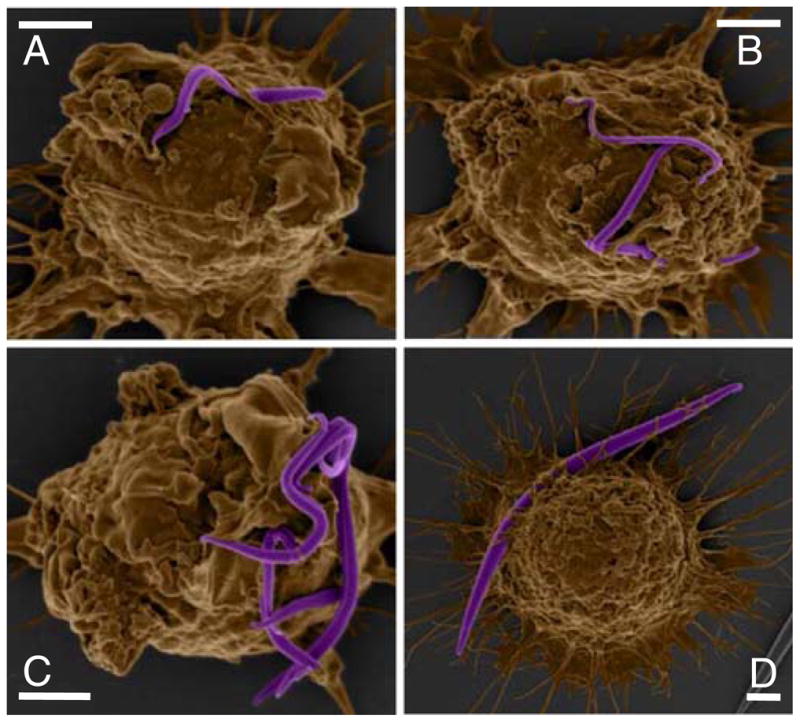
Colored scanning electron micrographs demonstrate the flexibility of IgG-adsorbed (A–C 1μm volume; D 3 μm volume) worm-like particles and ability of macrophages to bend worms by attachment at different points. Scale bars = 2 μm.
In the unlikely case that a macrophage is able to internalize a worm-shaped particle, the effect on the cell is quite severe as seen in Fig. 6. Though worms stretched from 3 μm spheres have a volume ~125 fold smaller than the average alveolar macrophage, the length of the particles is approximately twice the diameter of the cells. Internalization of these particles completely distorts the cell. This image highlights the amazing ability of macrophages to orchestrate a seemingly impossible internalization feat that appears to contort the cell in an abnormal and possibly undesirable shape.
Fig. 6.
Colored scanning electron micrograph shows macrophage internalizing an IgG-adsorbed worm (3 μm volume) and the effect of internalization on cell shape. Scale bar = 5 μm.
Even in the cases where phagocytosis of particles is to be avoided, non-phagocytic uptake of these particles by desired cells can be critical to their performance as drug delivery carriers. Non-phagocytic cells will likely internalize these particles via endocytosis or pinocytosis pathways. In some cases, only cellular uptake of the released drug but not the entire particle is required. In either situation, understanding the dependence of shape on particle uptake by non-phagocytic cells is important (9). Shape dependence could be quite different given the varying roles of actin in phagocytosis, endocytosis and other internalization mechanisms.
CONCLUSIONS
The purpose of this work was to engineer a shape that exhibited negligible uptake by macrophages and demonstrate the feasibility of a shape-based approach to phagocytosis inhibition. The results presented here firmly establish that particle shape can be used to modulate phagocytosis of drug delivery particles and demonstrates significant reduction in particle uptake due solely to particle shape. Modification of surface chemistry and particle size are no longer the only viable options to reduce phagocytic uptake. Through manipulation of particle shape, we have identified worm-like particles that exhibit negligible phagocytosis when compared to traditional spherical particles. Shape inhibition of phagocytosis is successful by minimizing the regions of high length-normalized curvature on the particle. Worms, in particular, meet this criterion and have the potential to significantly affect drug delivery particle design for the avoidance of phagocytosis. In future design, all components of drug delivery systems including shape, size, and chemistry, must be combined and optimized for not only phagocytosis but also drug release, targeting and administration. Unexpectedly, this work also revealed the flexibility of both the worm particles and the macrophage membrane in dealing with such particles. Particle flexibility has been proposed to affect both phagocytosis and general drug delivery behavior (10,11). This feature and other shapes with similar characteristics could prove especially valuable in the design of future particulate drug delivery systems.
Supplementary Material
Acknowledgments
Authors acknowledge Alejandro Sanchez, Santosh Gupta, and Poornima Kolhar for assistance. JAC acknowledges a graduate fellowship from the National Science Foundation. This research was supported by the Program of Excellence in Nanotechnology by the National Institutes of Health.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11095-008-9626-z) contains supplementary material, which is available to authorized users.
References
- 1.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 2.Koval M, Preiter K, Adles C, Stahl PD, Steinberg TH. Size of IgG-opsonized particles determines macrophage response during internalization. Exp Cell Res. 1998;242:265–273. doi: 10.1006/excr.1998.4110. [DOI] [PubMed] [Google Scholar]
- 3.Xiang SD, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Plebanski M. Pathogen recognition and development of particulate vaccines: Does size matter. Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Illum L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood clearance and organ deposition of intravenously administered colloidal particles—the effects of particle size, nature and shape. Int J Pharm. 1982;12:135–146. [Google Scholar]
- 5.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharm Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 6.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Deliv Rev. 1995;17:31–48. [Google Scholar]
- 7.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho CC, Keller A, Odell JA, Ottewill RH. Preparation of monodisperse ellipsoidal polystyrene particles. Colloid Polym Sci. 1993;271:469–479. [Google Scholar]
- 9.Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Letters. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 10.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beningo KA, Wang YL. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci. 2002;115:849–856. doi: 10.1242/jcs.115.4.849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.