Abstract
How the myocardium undergoes geometric, structural and molecular alterations that results in an end phenotype as might be seen in patients with dilated cardiomyopathy or following myocardial infarction (MI), is still poorly understood. Structural modification of the left ventricle which occurs during these pathological states results from chronic changes in loading conditions and is commonly referred to as “remodeling.” Remodeling may occur from increased wall stress in the face of hypertensive heart disease, valvular disease or perhaps most dramatically, following permanent coronary occlusion(Opie et al., 2006). A fundamental derangement of myocyte function is the most common perception for the basis of “remodeling” but the role of cells in the heart other than the muscle cell must of course be considered. While studies of the myocyte have been extensive, cardiac fibroblasts (CFs) have been studied less than myocytes. The fibroblast has a broad range of functions in the myocardium ranging from elaboration and remodeling of the extracellular matrix (ECM), to communication of a range of signals within the heart, including electrical, chemical and mechanical ones(Baudino et al., 2006). Integrins are cell surface receptors that are instrumental in mediating cell-matrix interactions in all cells of the organism, including all types within the myocardium. This review will focus on the role of integrins and related proteins in the remodeling process, with a particular emphasis on the cardiac fibroblast. We will illustrate this function by drawing upon two unique mouse models with perturbation of proteins linked to integrin function.
Integrin function: generalities and emphasis on the cardiac fibroblast
Integrins are heterodimeric transmembrane receptors composed of α and β subunits.(Hynes, 1987;Giancotti and Ruoslahti, 1999) The general arrangement of integrin subunits is that of a large extracellular domain (700–1100 amino acids), a single transmembrane segment and a short cytoplasmic tail, ranging from 20–60 amino acids.(Humphries, 2000). Ligand binding occurs to the extracellular domain of the integrin heterodimer, a process that is modified by range of amino acids spread throughout both the extracellular and transmembrane domains(Takada et al., 2007). The cytoplasmic domain of many of the β subunits is highly homologous, while the α subunits sequences are significantly more diverse. It is through the cytoplasmic tail that the integrins bind both a range of cytoskeletal linker molecules and also signal. Importantly, it has been recognized that binding of talin to integrin tails produces a change in integrin affinity for ligand, a process termed integrin activation(Calderwood, 2004;Calderwood et al., 1999). Integrin activation may not be solely restricted to talin binding but also may involve binding of other proteins to the integrin tail, such as kindlin-3(Moser et al., 2008). For additional data on detailed integrin structure the reader is referred to the excellent reviews such as(Green et al., 1998;Humphries, 2000;Hynes, 2002;Takada et al., 2007).
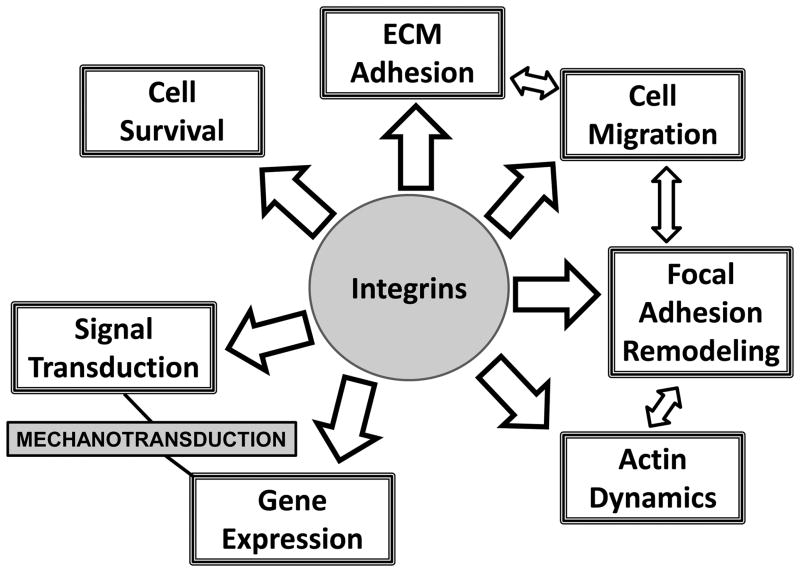
Initial studies determined that integrins were essential for adhesion of cells to the extracellular matrix (Hynes, 1981;Wylie et al., 1979;Ginsberg et al., 1983). Therefore, integrins are fundamental components in the interaction between the ECM and the cardiac myocytes or fibroblasts. Still, as shown in figure 1, it is now clear that integrins are multifunctional receptors with purposes that in addition to adhesion, include the regulation of cellular phenotype in the developing and post-natal myocardium, modulating such processes such as cell survival, cell migration, as well as remodeling of the cellular cytoskeleton and focal adhesion. A most intriguing role of integrins in the heart is their ability to serve as mechanotransducers during normal development and in response to physiological and pathophysiological signals. (Ingber, 1998;Simpson et al., 1999;Chen, 2008;Schwartz and DeSimone, 2008;Parker and Ingber, 2007);
Figure 1.
Integrins are multifunctional cell surface receptors.
Most importantly, it is well accepted that integrins are important signal transducers(Schwartz and Ginsberg, 2002;Clark and Brugge, 1995;Hynes, 2002) The integrins function in a bi-directional manner across cell membranes. Ligand binding to the integrins results in intracellular signaling events, a cascade termed “outside-in” signaling. In this role, the integrins can influence a wide range of activities of the type mentioned above, including alterations in cell morphology, migration, proliferation, differentiation, survival; gene expression, suppression of tumorigenicity, changes in intracellular pH or concentration of cytosolic Ca++. Since the integrins do not themselves possess enzymatic activity, they signal through activation of downstream molecules such as kinases pp125 focal adhesion kinase (FAK) or integrin linked kinase (ILK), small GTPases such as Rho or Rac, and regulation of cytoskeletal components such as talin, paxillin or p130CAS, several of which may bind directly to the cytoplasmic domain of the integrin heterodimer. Ultimately, signaling from the integrins may influence pathways through which other cellular effectors (such as growth factors) may also signal, including those requiring Akt, Raf, phosphoinositide 3-kinase (PI3-K), or mitogen-activated protein kinases (MAPKs)/extracellular-signal regulated kinases (ERKs).
Integrin function can also be modified by agonists that bind to non-integrin cellular receptors and in turn modify integrin activation; a process termed “inside-out” signaling. Here, both increased binding of integrin to ligand as well as clustering of multiple integrins in close spacing within the cell membrane occurs. It is clear that integrin clustering in both myocytes and fibroblasts in the myocardium is associated with chemical and mechanical signaling.
The integrin family is large, with over 18 α and 8 β subunits identified in mammals. From these various subunits, over 24 pairs of integrins receptors can arise. While it is clear that all combinations of αβ heterodimers can form, how the preferential pairing of subunits occurs in a specific cell is not clear. The complex nature of this protein family is increased to an even greater extent by the numerous splice variant isoforms of individual subunits, including some expressed in the heart(de Melker and Sonnenberg, 1999;Song et al., 1993;Burkin and Kaufman, 1999). The integrins expressed on a particular cell type can be unique and can vary depending on developmental stage or pathological state. (Table I) Further complexity is evident since a single integrin receptor can bind to one or several ligands and in addition, a single ligand can be bound by several integrin heterodimers. For example cardiac fibroblasts express α5β1 (a fibronectin (FN)/osteopontin (OPN) binding integrin) as well as αvβ1, αvβ3, andαvβ5 (which bind FN, OPN as well as vitronectin) (Terracio et al., 1989;Terracio et al., 1991;Maitra et al., 2000;Ross and Borg, 2001). In addition, components of the ECM can bind to several different integrins, allowing potentially for even further functional redundancy and complexity.
TABLE 1.
Integrin Expression in CFs in the Basal and Diseased Myocardium
| αSubunit | Subunit(s) Partner | Ligand(s) | Basal Adult | Heart Failure (Relative to Basal Adult) | Hypertension (Relative to Basal Adult) |
|---|---|---|---|---|---|
| α1 | β1A, β3 | LN, Col | ++ | = | Decreased |
| α2 | β1A | LN, Col | + | = | Decreased |
| α3 | β1A | LN, Col, FN | + | Not known | Not known |
| α4 | β1A | FN, OPN | +++ | Not known | Not known |
| α5 | β1A | FN, OPN | +++ | Decreased | |
| α6 | β1A | LN | + | Not known | Not known |
| α8 | β1A | FN, VN, OPN | + | Not known | Not known |
| αV | β1A, β3, β5 | FN, VN, OPN | + | = | Not known |
| β1A | Not applicable | Not applicable | +++ | Decreased | Increased |
| β3 | Not applicable | Not applicable | ++ | Not known | Not known |
| β5 | Not applicable | Not applicable | + | Not known | Not known |
Col indicates collagen; FN, fibronectin; LN, laminin; VN, vitronectin.
CFs can play critical roles in cardiac remodeling and may do so via an ECM/integrin interaction. The fibroblasts appear to play diverse functions. For example, Gullberg noted that PDGF-stimulated collagen gel contraction by CFs could be partly inhibited by β1 integrin antibodies(Gullberg et al., 1990). With stretch of cultured myocytes, release of angiotensin II (AngII) occurred(Sadoshima et al., 1993). Once produced, AngII stimulation of CFs modulated integrin localization and increased expression of both α8 and β1 integrins(Burgess et al., 1994;Thibault et al., 2001). Further, AngII increased adhesion of fibroblasts to collagen I via PKC epsilon(Stawowy et al., 2005). 14 day AngII infusion in rats caused increased α8β1 expression in myofibroblasts(Bouzeghrane et al., 2004). Carver et.al. have shown that α1β1 integrins play a role in collagen gel contraction by rodent CFs(Carver et al., 1995). Several studies have focused on various integrins, cytokines and signaling in the function of cardiac fibroblasts. For example stretch of rat cardiac fibroblasts resulted in both ERK2 and JNK1 activation, and activation varied dependent on the integrin subunits (e.g. α4 vs. α5) bound by ECM(MacKenna et al., 1998). Integrin overexpression can increase expression and activation of TGF-β, an important cytokine in the fibrotic response of the heart(Ma et al., 2003;Wang et al., 1999). Conversely, TGF-β stimulation of fibroblasts was found to increase expression ofα5β1 integrins(Dalton et al., 1999).
In the intact heart only a few studies on integrins and fibroblasts are available. Burgess, et. al. studied rats subjected to treadmill exercise or hypertension. β1 integrin was elevated with both hypertrophic stimuli; α1 and α2 levels both decreased, but α5 increased in the exercise group and decreased in the hypertensive one. Liu’s group studied the role of β integrins after myocardial infarction (MI) using rat and mouse as model systems. Both β1 and β3 integrins were low basally but increased at the infarct zone by day 3 post-MI, peaking at day 7 post-MI, then returned towards baseline. In line with the cell-type specific expression of integrins, β1A was found primarily on fibroblasts and inflammatory cells while β1D was expressed on myocytes and β3 was detected principally on endothelial and smooth muscle cells in the peri-infarct vessels. Thus while several studies of integrin function in the cardiac fibroblast have been performed, a full understanding of the role of integrins in these cells, particularly in the face of cardiac pathologies, is far from complete. For the remainder of this review, we would like to use two examples to further illustrate the function of integrins and related proteins in the myocardium and cardiac fibroblast.
Osteopontin is involved in hypertrophic and fibrotic responses of the heart
Osteopontin is a multifunction glycoprotein that is a member of the SIBLING (small integrin binding Ligand N-linked glycoproteins) family(Scatena et al., 2007). It is a soluble cytokine; a phosphoprotein that is secreted by many cells including cardiac myocytes and fibroblasts. As a matricellular molecule it does not serve a structural role in the ECM, but rather facilitates ECM responses. It can bind to numerous integrins includingαvβ3,αvβ1, αvβ5, α4β3, α4β1, α5β1, α8β1, α9β1, andα4β7, as well as the hyaluronic acid receptor CD44. Functioning through a series of downstream signaling pathways it can direct cellular adhesion, migration and survival. Despite its multiple functions, OPN homozygous knockout mice survive(Liaw et al., 1998) and therefore were used in our prior work by Collins, et.al. to study to function of OPN in the myocardium(Collins et al., 2004).
Continuous infusion of AngII via osmotic minipump was performed in wild-type mice at levels which evoked a 50 mmHg rise in systolic blood pressure. With this, increases in myocardial OPN transcript expression was detected by 4 days into the infusion, with subsequent normalization to baseline levels by 3 weeks. These results suggested that OPN is a component of the myocardial stress response invoked by AngII. Given this, the importance of OPN was investigated by use of the OPN KO mice mentioned above. With this infusion, hypertension, and related stress, the wild-type mice underwent the expected increases in heart weight/body weight ratios, indicative of ventricular hypertrophy, but the OPN KO mice showed a blunted morphometric response, despite similar increases in molecular markers of hypertrophy. With this, the amount of AngII induced fibrosis was assessed in the myocardium and it was seen that the absence of OPN leads to a reduced fibrotic response, even though ECM production (fibronectin, collagen I), β1 integrin and TGFβ transcripts, all increased similarly in the WT and KO mice. Given this, the effect of OPN on fibroblast function was evaluated using cardiac fibroblasts isolated from WT and OPN KO mice. The KO fibroblasts were seen to have reduced adhesion and proliferation, properties that could be partially restored towards normal by addition of exogenous OPN protein to the cultured KO cells. In support of these results were studies from Singh’s group(Trueblood et al., 2001;Xie et al., 2004) which showed that OPN KO hearts had a reduced hypertrophic response following aortic constriction, and also showed blunted collagen production and fibrosis following myocardial infarction. Finally, recent data from Lenga, et.al.(Lenga et al., 2008), has linked OPN to the formation of mature focal adhesions and transformation of fibroblasts to myofibroblasts, a process important for formation of scars during processes such as the post-myocardial infarction state. Thus it appears that OPN, as a protein produced by both cardiac myocytes and fibroblasts, is critically linked to both hypertrophic and fibrotic response pathways in the myocardium. It provides an important example of a substance involved in cell-matrix adhesion and modulation of integrin function, which could be potentially modulated in amount or function, to avoid adverse cardiac remodeling.
Focal adhesion kinase is linked to cardiac fibroblast proliferation, migration and transformation towards a myofibroblast phenotype
A second example of how integrin/focal adhesion function is involved in the cardiac stress response comes from our recent work on focal adhesion kinase (FAK)(Kang et al., 2006). As discussed above, integrins do not possess their own kinase activity and therefore to transduce signals, utilize a host of downstream kinases. Principal amongst them is FAK. We sought to assess the function of FAK in adult cardiac fibroblasts (CF) by culturing CF from homozygous FAK “floxed” mice originally produced by Beggs, et.al.(Beggs et al., 2003). With these cells in hand, we deleted FAK expression by infecting the cells with a recombinant adenovirus producing Cre recombinase. We thus produced cells devoid of FAK. First we assessed how these cells displayed focal adhesions (FAs) and expressed focal adhesion proteins. We found that the FAs were morphologically preserved and that the FA proteins vinculin, paxillin and talin were expressed equally in KO and control cells, but that in the FAK deficient CFs, expression of Pyk2, a cytoplasmic tyrosine kinase that shares approximately a 45% amino acid sequence identity with FAK, was upregulated. The FAK KO CF had normal adhesion, increased proliferation and reduced migration, when stimulated by platelet derived growth factor BB (PDGF-BB). Utilizing FAK-related non-kinase (FRNK) as an inhibitor of FAK function, we found that Pyk2 was not increased in the FRNK expressing, wild-type CF. FRNK caused reduction of CF migration, but did not alter cell proliferation, suggesting that Pyk2 was at least partly responsible for the proliferation defects in the FAK KO CF, but that it was not involved in the migratory defect seen in these cells.
Interesting work performed by Clemente and Franchini(Clemente et al., 2007) has begun to study the function FAK in vivo, and showed that FAK was present in both myocytes and fibroblasts of the murine heart, but increased dominantly in non-myocytes following aortic constriction. When mice were treated via single jugular vein injection with siRNA directed towards FAK, FAK expression was reduced over 70% and the mice were seen to have reduced fibrosis and collagen production following aortic constriction. Importantly, work by Insel and co-workers(Swaney et al., 2006), has linked FAK, like OPN, to the transformation of fibroblasts to myofibroblasts. Thus, FAK as an important mediator of integrin signaling appears also linked to CF proliferation, migration and conversion of CFs to myofibroblasts.
SUMMARY
In this review we have begun to illustrate how integrins and their related ECM/matricellular and focal adhesion proteins, such as osteopontin and focal adhesion kinase, appear critically involved in the proliferative and fibrotic responses of the stressed or injured myocardium. Study into proteins of these types has just begun and a great deal of work is necessary to more fully understand their importance in cardiac disease processes. In this regard, caveats must be appreciated as we seek additional information about the function of these proteins in fibroblast function including the organ and species-specific nuances of fibroblasts, “not all fibroblasts are created equal;” that studies of cells from one developmental stage may yield unique results as compared to another (i.e. embryonic, neonatal and adult fibroblasts form the heart may have distinct properties); that culture studies may not always port-over to ones produced in the intact animal and similarly study in 2-dimensional culture may be different than those performed in 3D culture. Much work is still necessary but our progress to date surely illustrates how modulation of focal adhesion proteins may be an interesting future therapeutic target for cardiovascular diseases.
Acknowledgments
This work was supported by grants to R.S.R. from the Veterans Administration (VA Merit) and the NIH (P01 HL066941 and RO1 HL088390).
Reference List
- 1.Baudino TA, Carver W, Giles WR, Borg TK. Cardiac Fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 2.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borg TK, Goldsmith EC, Price R, Carver W, Terracio L, Samarel AM. Specialization at the Z line of cardiac myocytes. Cardiovasc Res. 2000;46:277–285. doi: 10.1016/s0008-6363(99)00433-2. [DOI] [PubMed] [Google Scholar]
- 4.Bouzeghrane F, Mercure C, Reudelhuber TL, Thibault G. Alpha8beta1 integrin is upregulated in myofibroblasts of fibrotic and scarring myocardium. J Mol Cell Cardiol. 2004;36:343–353. doi: 10.1016/j.yjmcc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Burgess ML, Carver WE, Terracio L, Wilson SP, Wilson MA, Borg TK. Integrin-mediated collagen gel contraction by cardiac fibroblasts. Effects of angiotensin II. Circ Res. 1994;74:291–298. doi: 10.1161/01.res.74.2.291. [DOI] [PubMed] [Google Scholar]
- 6.Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res. 1999;296:183–190. doi: 10.1007/s004410051279. [DOI] [PubMed] [Google Scholar]
- 7.Calderwood DA. Talin controls integrin activation. Biochem Soc Trans. 2004;32:434–437. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- 8.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 9.Carver W, Molano I, Reaves TA, Borg TK, Terracio L. Role of the alpha 1 beta 1 integrin complex in collagen gel contraction in vitro by fibroblasts. J Cell Physiol. 1995;165:425–437. doi: 10.1002/jcp.1041650224. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 11.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 12.Clemente CF, Tornatore TF, Theizen TH, Deckmann AC, Pereira TC, Lopes-Cendes I, Souza JR, Franchini KG. Targeting focal adhesion kinase with small interfering RNA prevents and reverses load-induced cardiac hypertrophy in mice. Circ Res. 2007;101:1339–1348. doi: 10.1161/CIRCRESAHA.107.160978. [DOI] [PubMed] [Google Scholar]
- 13.Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol. 2004;43:1698–1705. doi: 10.1016/j.jacc.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 14.Dalton SL, Scharf E, Davey G, Assoian RK. Transforming growth factor-beta overrides the adhesion requirement for surface expression of alpha(5)beta(1) integrin in normal rat kidney fibroblasts. A necessary effect for induction of anchorage-independent growth. J Biol Chem. 1999;274:30139–30145. doi: 10.1074/jbc.274.42.30139. [DOI] [PubMed] [Google Scholar]
- 15.de Melker AA, Sonnenberg A. Integrins: alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays. 1999;21:499–509. doi: 10.1002/(SICI)1521-1878(199906)21:6<499::AID-BIES6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg MH, Wencel JD, White JG, Plow EF. Binding of fibronectin to alpha-granule-deficient platelets. J Cell Biol. 1983;97:571–573. doi: 10.1083/jcb.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green LJ, Mould AP, Humphries MJ. The integrin beta subunit. Int J Biochem Cell Biol. 1998;30:179–184. doi: 10.1016/s1357-2725(97)00107-6. [DOI] [PubMed] [Google Scholar]
- 19.Gullberg D, Tingstrom A, Thuresson AC, Olsson L, Terracio L, Borg TK, Rubin K. Beta 1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- 20.Humphries MJ. Integrin Structure. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 21.Hynes RO. Relationships between fibronectin and the cytoskeleton. In: Poste G, Nicolson GL, editors. Cytoskeletal elements and plasma membrane organization. North-Holland Pub. Co; Amsterdam: 1981. pp. 97–139. [Google Scholar]
- 22.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 23.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 24.Ingber DE. Cellular basis of mechanotransduction. Biol Bull. 1998;194:323–325. doi: 10.2307/1543102. [DOI] [PubMed] [Google Scholar]
- 25.Kang SM, Manso AM, Ross RS. Adenoviral-mediated knock-out of focal adhesion kinase in adult mouse cardiac fibroblasts promotes cell proleiferation and inhibits cell migration. Circulation. 2006;114:83. [Google Scholar]
- 26.Lenga Y, Koh A, Perera AS, McCulloch CA, Sodek J, Zohar R. Osteopontin expression is required for myofibroblast differentiation. Circ Res. 2008;102:319–327. doi: 10.1161/CIRCRESAHA.107.160408. [DOI] [PubMed] [Google Scholar]
- 27.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenna DA, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maitra N, Flink IL, Bahl JJ, Morkin E. Expression of alpha and beta integrins during terminal differentiation of cardiomyocytes. Cardiovasc Res. 2000;47:715–725. doi: 10.1016/s0008-6363(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 31.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 32.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 33.Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 35.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 36.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MA, DeSimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol. 2008;20:551–556. doi: 10.1016/j.ceb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 39.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res. 1999;85:e59–e69. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- 40.Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of alpha 7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106:1139–1152. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- 41.Stawowy P, Margeta C, Blaschke F, Lindschau C, Spencer-Hansch C, Leitges M, Biagini G, Fleck E, Graf K. Protein kinase C epsilon mediates angiotensin II-induced activation of beta1-integrins in cardiac fibroblasts. Cardiovasc Res. 2005;67:50–59. doi: 10.1016/j.cardiores.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Swaney JS, Patel HH, Yokoyama U, Head BP, Roth DM, Insel PA. Focal adhesions in (MYO)fibroblasts scaffold adenylyl cyclase with phosphorylated caveolin. J Biol Chem. 2006 doi: 10.1074/jbc.M513097200. [DOI] [PubMed] [Google Scholar]
- 43.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terracio L, Gullberg D, Rubin K, Craig S, Borg TK. Expression of collagen adhesion proteins and their association with the cytoskeleton in cardiac myocytes. Anat Rec. 1989;223:62–71. doi: 10.1002/ar.1092230110. [DOI] [PubMed] [Google Scholar]
- 45.Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res. 1991;68:734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- 46.Thibault G, Lacombe MJ, Schnapp LM, Lacasse A, Bouzeghrane F, Lapalme G. Upregulation of alpha(8)beta(1)-integrin in cardiac fibroblast by angiotensin II and transforming growth factor-beta1. Am J Physiol Cell Physiol. 2001;281:C1457–C1467. doi: 10.1152/ajpcell.2001.281.5.C1457. [DOI] [PubMed] [Google Scholar]
- 47.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Sun L, Zborowska E, Willson JK, Gong J, Verraraghavan J, Brattain MG. Control of type II transforming growth factor-beta receptor expression by integrin ligation. J Biol Chem. 1999;274:12840–12847. doi: 10.1074/jbc.274.18.12840. [DOI] [PubMed] [Google Scholar]
- 49.Wylie DE, Damsky CH, Buck CA. Studies on the function of cell surface glycoproteins. I. Use of antisera to surface membranes in the identification of membrane components relevant to cell-substrate adhesion. J Cell Biol. 1979;80:385–402. doi: 10.1083/jcb.80.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension. 2004;44:826–831. doi: 10.1161/01.HYP.0000148458.03202.48. [DOI] [PubMed] [Google Scholar]