Abstract
Significant data linking genetic causes to heart muscle diseases has only arisen in the last several decades, with hypertrophic cardiomyopathies being the subtype most extensively linked to single gene defects. Recent studies have shown that all major forms of cardiomyopathies can have a genetic basis. Lately enough scientific traction has arisen that clinical practice guidelines have been formulated by the American Heart Association and Heart Failure Association of America on genetic evaluation of cardiomyopathies. Mutations in genes from various classes have been identified as causal of these diseases including ones encoding sarcomeric proteins, those important for muscle metabolism or that lead to infiltrative diseases of the heart; ones critical for proper excitation-contraction coupling of the myocyte, or that are present in the myocyte cytoskeleton, and also proteins localized to the cardiac myocyte costamere. To illustrate these concepts, this review will focus on two costameric/membrane-associated proteins, vinculin and talin. We will discuss their general function, with emphasis on what is understood about their role in the cardiac myocyte and heart.
Keywords: vinculin, talin, costamere, intercalated disk, mechanotransduction
Evidence linking genetic causes to heart muscle diseases has only arisen in the last several decades, with hypertrophic cardiomyopathies being the subtype most extensively linked to single gene defects (1–4). In more recent time all major forms of cardiomyopathies have been shown to have a genetic basis, leading to enough scientific traction that clinical practice guidelines have been formulated by the American Heart Association and Heart Failure Association of America, on genetic evaluation of cardiomyopathies(5;6). Mutations in genes from various classes have been identified as causal of these diseases including one encoding sarcomeric proteins, those important for muscle metabolism or that can lead to infiltrative diseases of the heart; ones critical for proper excitation-contraction coupling of the myocyte, or that are present in myocyte cytoskeleton, and also in proteins localized to the cardiac myocyte costamere. Our lab has sought the basic role of costameric proteins in cardiomyocyte function and in this review focus on two of these proteins, vinculin and talin.
What is a costamere?
Costameres are sub-sarcolemmal structures in striated muscle that circumferentially align with the Z-disk of the myofibrils. They function to allow muscle adhesion to the extracellular matrix (ECM). These structures resemble focal adhesions (FAs) found in non-muscle cells, sharing with them many protein components such as integrins, α-actinin, focal adhesion kinase (FAK), integrin linked kinase (ILK), parvin, PINCH, vinculin, and talin (7).
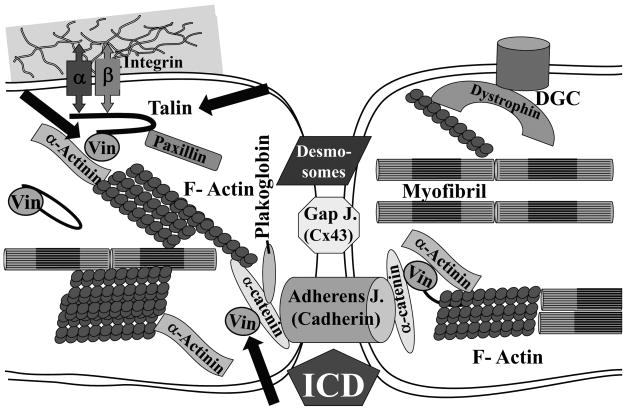
Beside their structural role in cell attachment costameres have been shown to be sites where contractile forces generated by cardiomyocytes are transmitted to the ECM (“inside-out”) (8), and where forces externally applied by the ECM are transmitted into the myocyte (“outside-in”) (9). Therefore costameres are sites where mechanical information is transduced bidirectionally across the cell membrane. Transmission using the “outside-in” mechanism may allow for the hypertrophic response of myocytes subjected to hemodynamic load. Still, it must be appreciated that myocyte mechanotransduction is not restricted to the costamere, but may also occur at Z-disks and intercalated disks (ICDs) within the cell (10). Within the costamere are two main protein complexes: the integrin complex and the dystrophin-glycoprotein complex (DGC)(11). (Figure 1) Defects or mutations in proteins of both structures lead to cardiomyopathies, indicating the importance of costameres in normal cardiac function and myocardial remodeling (12).
Figure 1. Costameric Proteins and the Intercalated Disk of two adjacent cardiac myocytes.
Shown are some of the key structural elements of the costameric protein complex of the cardiac myocyte, including ones linked to the integrin-talin-vinculin axis, as well as the dystrophic-glycoprotein complex (DGC). The intercalated disk (ICD) structure providing mechanical and electrical linkage of myocytes is also shown. The ICD mechanical linkages include the desmosomes and the adherens (cadherin-containing) junctions, while electrical coupling of cells occurs at gap junctions (gap j.) which contain connexins (Cx) such as Cx43. This diagram is provided to orient the reader to the location of vinculin and talin (arrows) within the cell, but is clearly a simplified version of costamere and ICD.
The DGC is composed of several membrane-spanning and associated proteins. In muscle, the DGC includes dystrophin, sarcoglycans (α, β, γ, δ, ε, and ζ), dystroglycans (α and β), α-dystrobrevin, syntrophins (α1, β1 and β2), sarcospan, and NO synthase(11). Dystrophin is a 427-kDa protein that constitutes a core componentof the DGC. The DGC functions to anchor the sarcolemma to the ECM and to the sarcomere. It stabilizes the sarcolemma against physical forces during muscle contraction or stretch. Mutations in the many components of the DGC in man and in animal models have been shown to cause a variety of forms of skeletal muscular dystrophy as well as dilated cardiomyopathy (11;13;14). A comprehensive discussion of the DGC proteins is outside the scope of this paper and the reader is referred to some excellent recent reviews (11;15).
The other main complex in costameres is the integrin complex. (Figure 1) Integrins are heterodimeric surface receptors composed of α and β subunits that bridge the cytoskeleton and perhaps muscle sarcomere, with the ECM. They provide for cellular adhesion, and also act as mechanotransducers, converting mechanical stimuli to biochemical ones (16). Adult heart cardiac myocytes express α7β1 (a laminin receptor) as the main integrin, while α5β1 and α6β1 are expressed highly in myocytes during heart development. The function of integrins in cardiac myocytes has been analyzed by our own group and others, using both in vitro and in vivo studies. β1 has been linked to a hypertrophic phenotype using a neonatal rat cardiac myocytes model system(17). Transgenic and knockout (KO) mouse models have also shown how integrin function within the myocyte is essential for preservation of normal heart function (18–21). These data indicate that the connection of the ECM with cytoskeleton/sarcomere provided by myocyte integrins, is essential for accommodation to the transmission of the mechanical stress which occurs continuously in cardiac myocytes.
The connection of integrins with the actin filaments is indirect and occurs through several structural proteins such as talin, vinculin, α-actinin, ILK, filamin and tensin (22). Beside these structural connections, integrins also recruit signaling proteins that function in the transduction of mechanical stimuli. Important integrin mediated signaling events occur through focal adhesion kinase (FAK), melusin, paxillin, src, cas and PIPKI (23). For a discussion of integrin function and signaling, particularly as it relates to the heart the reader is referred to recent publications (24–26). This review will focus on two proteins that directly or indirectly connect to integrins: vinculin and talin.
Vinculin: general structure and function
Vinculin (Vcl) is ubiquitously expressed, membrane-associated protein that links the actin cytoskeleton to the sarcolemma. A splice-variant isoform of Vcl, termed metavinculin (MVcl), exists in muscle and platelets. MVcl arises from a 68-amino acid insertion at residue 915 in the 1066 Vcl sequence, thus producing this 1134 AA form(27). Some work indicates that Vcl and MVcl colocalize in cardiac myocytes(28). Their identical head regions interact with membrane associated proteins but the unique tail regions of each isoform provide for varied actin organization(28).
Because Vcl directly binds to the integrin associated protein talin and α-actinin, as well as to the cadherin associated proteins α/β-catenin, it is found in cell-matrix and cell-cell contact sites(29–33). In cardiac myocytes Vcl protein expression was first described in a transverse “rib-like” pattern. Since the Latin term for rib is costa, the location of this cell-matrix adhesion site of Vcl came to be defined as the costamere(34). Vcl binds indirectly to integrins through talin (see below) and thus it functions as a multi-protein linker connecting cell-matrix adhesions to the actin-based cytoskeleton(35;36). Cell-cell junctions in cardiac myocytes are called intercalated discs (ICD). (Figure 1) The ICD connects myocytes end-to-end in a staggered fashion and contains structures essential for both mechanical and electrical coupling. These include actin binding adherens junctions, intermediate filament interacting desmosomes as well as action potential conducting gap junctions(37). Vcl is highly expressed in ICDs as well as the costamere. Costameres and ICDs anchor contractile filaments to the sarcolemma and can be thought of as not only cellular junctions important for force transmission, but also as potential areas of force generation.
Vcl’s crystal structure has revealed a globular head followed by a hinge region and a flexible tail (38–40). Intramolecular head to tail association masks binding sites within Vcl, thereby keeping the protein in an inactive state (41). Molecules such as acidic phospholipids (42;43), talin (44) and α-actinin (45) were found to unmask Vcl’s binding sites, allowing for Vcl activation(46). Once activated, Vcl interacts with multiple proteins including talin, paxillin,α/β-catenin, Arp 2/3, VASP, vinexin, alpha-actinin, F-actin, alpha-synemin, PKCα, acidic phospholipids and Raver1(46). The interaction of Vcl and Raver1 is unique, since Raver1 harbors RNA recognition motifs and regulates mRNA processing(47;48).
Studies of Vcl deficient embryonic carcinoma cells found that they had poor cell-substrate adhesion and absent lamellipodia formation(49;50), suggesting that Vcl may stabilize matrix-adhesion sites by transferring mechanical stress, which drive cytoskeletal remodeling(51). More recently it was demonstrated that although Vcl-null embryonic fibroblasts still formed focal adhesions, these adhesions were smaller, less stable and turned over more rapidly than those in wild-type cells (52). Contractile arrest of neonatal cardiac myocytes led to depletion of Vcl from the costameres, and mechanical stress increased Vcl expression at these focal adhesion sites (53). Taken together, these data indicate that Vcl (and by extension MVcl) mechanically couples the actin-based cytoskeleton to the sarcolemma and regulates focal adhesion turnover.
To extend these cellular studies to the whole organism, the Vcl gene was globally ablated in the mouse genome (54). With this, growth retardation of the embryo was evident from embryonic day (E)8 and embryonic death occurred by E10. The most prominent defects in these mice were lack of neural fold fusion, attenuation of cranial and spinal nerve development, and importantly from our perspective, abnormal cardiogenesis with severely reduced development of endocardial structures and an akinetic myocardium. Fibroblasts isolated from mutant embryos showed reduced matrix adhesion as well as a two-fold higher migration rate compared to wild-type cells, further amplifying the importance of Vcl in cell-matrix adhesion. This study clearly showed that Vcl was crucial for normal embryonic development, likely due to its role in the regulation of cell-matrix adhesion and cell locomotion.
Vinculin and the Heart: lessons from genetically manipulated mouse models
While homozygous global inactivation of the murine Vcl gene caused embryonic lethality (54) with embryos displaying a thin-walled myocardium, hemizygous KO mice (Vcl+/−) developed normally and were indistinguishable from wild type (Vcl+/+). We hypothesized that the reduced Vcl/MVcl expression in the Vcl+/− heart would lead to impaired cardiac function (55). With cardiac Vcl/MVcl protein levels reduced to less than 50% of wild-type levels in Vcl+/−, these Vcl+/− hearts were surprisingly histologically and functionally normal in the basal state. Electrocardiograms (ECGs) showed no difference in heart rate or PR intervals from WT controls, but displayed widened QRS complexes. Sub-cellular distribution of Vcl and its associated proteins talin, integrin and cadherin in the heterozygous KO hearts revealed only that the localization of cadherin was abnormal. Given these findings we evaluated the expression pattern of the gap junctional protein connexin 43 (Cx43) in Vcl+/− myocardium. Concordant with the abnormal cadherin expression we found widened and disturbed ICDs, visualized by anti-Cx43 immunostaining. Electron microscopic analyses of Vcl+/− hearts showed aberrant myofibril anchorage at the ICD and Z-lines. Since these findings were present in physiologically normal hearts we tested how Vcl+/− mice would tolerate pressure loading produced by transverse aortic constriction (TAC) (18;56). While all animals survived the surgery, 33% of the Vcl+/− TAC mice died spontaneously during the first 3–6 hours postoperatively. The surviving Vcl+/− mice showed appropriate hypertrophic responses, but beginning at 6-weeks post-TAC developed progressive left ventricular dysfunction. While 100% of control mice survived up to 12-weeks post-TAC, only 30% of Vcl+/− TAC mice survived. When Vcl+/− hearts were examined 4-weeks post TAC, when they were still physiologically normal, abnormalities in Z-line structure was detected. This mouse model gave further insight into the role of Vcl in maintaining function in the pressure loaded left ventricle.
To more specifically address if the cardiac phenotype in the global Vcl KO mice was caused by primary loss of Vcl from myocytes, we next generated mice where the Vcl/MVcl gene could be excised only in cardiac muscle cells (57). For this purpose we used a knock-in mouse line that expresses Cre recombinase as driven by the native myosin light chain-2 ventricular promoter (58). This Cre producing line leads to protein reduction only in the perinatal period (18;59;60). Homozygous floxed Vcl mice that also express the cardiac specific-Cre recombinase (termed cVclKO) were born with expected Mendelian distributions and developed 70% reduction of MVcl protein. By 6 weeks of age sudden death was evident in the cVclKO with less than 50% of these mice surviving till 12 weeks of age, despite preservation of cardiac function. Ventricular tachycardia was shown to be the cause of this early death, and the cVclKO hearts showed defective myocardial conduction. cVcl KO mice that survived through the vulnerable period of sudden death all developed dilated cardiomyopathy and died before 6 month of age. Reduced expression of cadherin and β1 integrin was seen in Vcl/MVcl deficient cardiac myocytes. Cx43 expression that was found mainly at the ICD in control mice was redistributed to the lateral cell membrane in the KO cells. Ultrastructural analysis of cVclKO samples obtained from animals that had preserved ventricular function, showed highly serrated ICD structures, with reduced electron-dense staining throughout the ICD, and mitochondria that were loosely arranged and disorganized. These results showed that loss of Vcl disturbs ICD structure, myofibrillar arrangement and mitochondria prior to the onset of myocardial dysfunction. In the adult heart Cx43 usually localizes to the ICD forming a low resistance pathway for propagation of the electrical impulse between cardiac myocytes. The altered Cx43 distribution we found in cVclKO mice likely provided for an arrhythmogenic substrate which predisposed to sudden death. The alteration of the ICD structure could also have predisposed cVclKo mice toward later myocardial dysfunction. Contrasting the approximate 2 year mouse lifespan with the 80-year life expectancy in humans, suggests the phenotype in our cVclKO mice might be akin to one that would arise in a human teenager. The findings in our mouse models emphasize the importance of maintaining normal Vcl expression in the heart.
Vinculin and Heart: lessons from man
Mutations in a large number of genes have now been associated with the development of cardiomyopathies in man(5). Of this set of genes, MVcl was found essentially absent in one patient with dilated cardiomyopathy(61). A more comprehensive study found multiple mutations of MVcl associated with kindreds with both dilated and hypertrophic forms of human cardiomyopathy (62;63). Interestingly a mutation in Vcl was also associated with a predisposition towards only hypertrophic cardiomyopathy (64;65). These studies begin to show the genetic and functional evidence for Vcl as a cardiomyopathy gene.
Gene profiling of patients with non-ischemic cardiomyopathy that required left ventricular assist device (LVAD) support was performed and included analysis of changes in Vcl transcripts(66). Of the group that recovered sufficiently to allow explantation of the device and not go on to heart transplantation, Vcl transcripts decreased 1.7-fold from time of implantation to explantation, and Vcl protein decreased 4.1-fold. In contrast, the hearts from patients that could not be assisted with the LVAD and still required transplant showed 1.7-fold increased Vcl transcript levels. It was suggested that accumulation of membrane associated proteins, including Vcl, might be a compensatory mechanism typical for end stage heart failure independent of the underlying disease(67).
Chagas cardiomyopathy caused by Trypanosoma cruzi infection, leads to severe cardiac fibrosis, life threatening arrhythmias and cardiac dysfunction(68). In vivo and in vitro studies showed that Trypanosoma infection disrupts Vcl’s localization at the costamere(69). At all times of infection Vcl expression was reduced and Vcl was absent from the costameres of infected specimens that also showed irregular alignment of ICDs. Recently, de Melo et al (70) showed that in addition to Vcl downregulation in Chagas disease, a substantial reduction of pan-cadherin and beta-catenin expression were also seen at the ICD. Combined, these data suggest that the disruption of costameric Vcl, combined with abnormal cadherin based cell-to-cell contact sites, may predispose to the impaired cardiac function and rhythm disturbances found in Chagas’ disease.
Talin: Another Costameric Protein With Potential Importance in the Heart
Integrins are cell surface receptors that bind cells to ECM, signal and function as mechanotransducers. They have been shown by our group and others to have an important role in normal cardiac homeostasis as well as in pathological processes such as hypertrophy(24;26). Integrins can bind directly or indirectly to many structural and signaling proteins through their cytoplasmic domain. The function of some of these proteins has begun to be characterized in the heart, (e.g. FAK, ILK, Melusin, and Vinculin(55;57;60;71–73)) but others, such as Talin, have not. Talin is a large (270 KDa, 2540 amino acid) dimeric protein that connects integrins with the actin cytoskeleton. (Figure 1) It is essential for the structural integrity of FAs and therefore for the attachment of the cells to the ECM(74). In addition to its structural role, talin modulates the ligand binding activity of integrins and functions in signal transduction, recruiting signaling proteins like FAK and phosphatidylinositol(4) phosphate 5 kinase type I (PIPKIγ) to FAs(75). Talin contains a globular N-terminal head domain (~50kDa) and a large flexible C-terminal rod domain (> 200 kDa). The head contains a FERM domain ((F)4.1 protein, (E) ezrin, (R) radixin, and (M) moesin) with binding sites for β-integrin subunits, F-actin, Wech, H-Ras, layilin, PIPkinase and FAK. The rod domain contains an additional integrin binding site, 2 additional actin binding sites, multiple binding sites for Vcl and a binding site for the muscle-specific protein α-synemin(75).
There are two vertebrate talin genes (Tln1 and Tln2), which generate proteins that are 74% identical. To date, most studies of talin have not distinguished between the individual isoforms talin1 and talin2. Talin1 is ubiquitously expressed while talin2 expression is more restricted, having dominant expression in heart, brain and skeletal muscle. Many in vitro and in vivo studies, have analyzed the function of talin1. In vitro studies have shown that talin1 is required for the assembly of FAs(74) and the regulation of integrin affinity for ECM ligands. Talin1 null mice have been created and display an embryonic lethal phenotype at E8.5-9, due to gastrulation defects(76), indicating that talin1 is an essential gene and that talin2 cannot replace its function in the entire embryo.
Recent studies using genetically manipulated mouse models have begun to analyze the in vivo relevance of talin1 in specific organs and cell types. For example one study has demonstrated that talin1 is required for the activation of integrins in platelets(77) while another has shown that plasma membrane blebbing with loss of membrane association with the cytoskeleton, occurs in megakaryocytes devoid of talin1(78). Like its ubiquitous localization in FAs, talin is also found in muscle-specific integrin complexes such as costameres, ICDs and myotendinous junctions (79–81;81). Given the similarities between FAs and costameres, talin likely serves an essential function in muscles cells of all types. This has begun to be shown by another mouse-based study where talin1 was ablated specifically in skeletal myocytes(82). This work showed that within skeletal muscle, tln1 is mainly localized at myotendinous junctions (MTJs). The importance of talin at this site was then appreciated in that the tln1 muscle specific KO mice showed defects in MTJs, but not in the assembly or localization of costameric integrin complexes, containing β1 integrin, Vcl and α-actinin. These results combined with the relatively high expression of tln2 in other areas of muscle, suggest that tln2 may in part compensate for loss of skeletal muscle tln1 in these mice.
To date, few studies have characterized the function of talin2. Studies in C2C12 skeletal muscle cells have shown that expression levels of talin2 increase during myoblast differentiation into myotubes, while talin1 expression remains constant (83). These authors also found that in rat cardiac myofibrils, talin2 was localized in costameres and ICDs while talin1 was not. This information suggests that talin1 and talin2 may have unique functions in skeletal and cardiac muscle.
Further studies on talin have capitalized on a gene trap mouse model which disrupts the talin2 gene(84). In this mouse, insertion of a beta-galactosidase transgene occurs in exon 28 of the talin2 gene, producing a chimeric talin2 protein which expresses AA 1–1295 of the 2540 total AA protein. With this, a large part of the talin2 rod domain is not produced but the mice are viable and fertile, suggesting that talin2 is not an essential protein for normal development or basal bodily functions, or that the talin2 chimeric protein contains the necessary domains or structures to be completely functional.
To emphasize the importance of tln2, recent work has studied tln2 deficient mice. Like the tln1 skeletal muscle specific KO mice, the tln2 deficient mice also show abnormal MTJ structure(82) although the defects were considerably more severe and at an earlier age with loss of tln2. This is consistent with the tenet that talin2 is the major talin isoform in skeletal muscle(85). In this same study, mice were also generated with loss of both tln1 and tln2 in skeletal muscle. These mice phenocopied the skeletal muscle specific beta-1 integrin KO mice(86) in that they showed disruption of skeletal muscle fiber cytoskeletal structure, had defects in both myoblast fusion and sarcomere assembly, and died shortly after birth. This data suggests that talin1 and 2 are both required for intact integrin function during muscle development and growth.
Given these findings in skeletal muscle, we hypothesize that both talin isoforms likely play essential roles in cardiac muscle development and remodeling and work is underway in our group to directly test this hypothesis. Indeed, work recently published on the mechanosensory protein cardiac ankyrin repeat protein (CARP), a Z-disc component known mostly for its interaction with titin and myopalladin, also suggested that some CARP mutants which lost binding to tln1 could be linked to familial cardiomyopathy in man (87).
Conclusion and Future Perspectives
Recent advances have clearly linked alterations in a large number of genes to all forms of cardiomyopathies(5;88;89). Amongst the range of causal genes are ones positioned within mechanical sensors of the cardiac myocyte: the Z-disk and the costamere(10;12). Our hope in this brief review was not to provide an exhaustive review of these proteins, but to specifically focus on two proteins under study in our laboratory which are examples of these types of proteins.
Mutations of Vcl and MVcl found in patients with cardiomyopathy; as well as the alteration of Vcl expression and localization in Chagas’ disease and in the advanced heart failure patient, all underscore the important role of Vcl in the human heart. Vcl KO mouse models as we have studied, further emphasize the critical role of Vcl and MVcl in maintenance of cardiac function. Heterozygous global Vcl KO mice showed no basal phenotype, but pressure overload of the left ventricle caused premature heart failure and increased their mortality compared to control animals. This model in mice has many parallels to the increased workload sensed by the heart in patients with hypertension. Since Vcl and MVcl mutations which have been linked to cardiomyopathy in man are heterozygous ones, we suggest that patients harboring these mutations could be further predisposed to developing premature heart failure when they also have other co-morbidities such as elevated blood pressure. The cardiac specific Vcl KO mouse model emphasizes the critical role of Vcl and MVcl in the maintenance of cell-to-cell and cell to matrix junctions in that they rapidly develop replacement fibrosis, arrhythmias and dilated cardiomyopathy. The profound phenotype in cVclKo mice clearly shows that Vcl is a fundamental structural protein in cardiac myocytes.
Still, despite these advances, there are many questions which remain to be answered about the role of Vcl in heart and heart disease. For example, though in vitro studies have demonstrated that the tails domain of Vcl and MVcl cause differential bundling of actin filaments(28), extending this biochemical work to the intact organ must be done. Further, it is clear that Vcl is highly expressed in the T-tubular system of the cardiac myocyte, but direct proof of Vcl’s role in maintaining physiological ion-channel function is currently unavailable. Similarly, our studies in the cVclKO myocardium suggest that Vcl/MVcl is instrumental in anchoring contractile filaments to costameres and ICDs, and may also be critical for normal gap junction function, perhaps through Vcl’s direct regulation of the ICD.
Less is even known about the function of talin in the heart and cardiac myocyte. Talin1 and talin2 share high similarity in sequence and structure, yet much data about their distinct roles in any cell, let alone ones in heart, is not currently available. Still, the few published studies on talin function in muscle(82;83), have led us to hypothesize that talin1 and talin2 function must clearly be distinct. Talin’s role as a mechanical linker and in force generation of non-muscle cells(74) together with its localization at costameres in skeletal and cardiac muscle, suggests that it could be an important mechanotransduction molecule in muscle As discussed above, this role is supported by the recent study where CARP/tln1 binding mutants were linked to dilated cardiomyopathy in human patients(87). Further studies will be necessary, and are ongoing in our own group, to analyze talin function in the basal and stressed heart, and to clarify the unique role of talin1 in contrast to talin2.
Clearly increased understanding of proteins like Vcl and talin will allow a greater understanding of the molecular basis for cardiomyopathies, increase our ability to screen and identify patients at high risk for profound heart failure or sudden death, and could potentially lead to novel new therapies.
Acknowledgments
This work was supported by grants to R.S.R. from the Veterans Administration (VA Merit) and the NIH (P01 HL066941 and RO1 HL088390).
References
- 1.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 2.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. doi: 10.1111/j.1540-8167.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 3.Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113:e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Seidman JG, Seidman CE. Cardiovascular genomics. Circulation. 2006;113:e450–e455. doi: 10.1161/CIRCULATIONAHA.105.560151. [DOI] [PubMed] [Google Scholar]
- 5.Hershberger RE, Lindenfeld J, Mestroni L, et al. Genetic evaluation of cardiomyopathy--a Heart Failure Society of America practice guideline. J Card Fail. 2009;15:83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 7.Ervasti JM. Costameres: the Achilles’ heel of Herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 8.Danowski BA, Imanaka-Yoshida K, Sanger JM, et al. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992;118:1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansour H, de Tombe PP, Samarel AM, et al. Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cepsilon and focal adhesion kinase. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- 10.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–H1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapidos KA, Kakkar R, McNally EM. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- 12.Cox L, Umans L, Cornelis F, et al. A broken heart: a stretch too far: an overview of mouse models with mutations in stretch-sensor components. Int J Cardiol. 2008;131:33–44. doi: 10.1016/j.ijcard.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 13.Quinlan JG, Hahn HS, Wong BL, et al. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 15.Ervasti JM, Sonnemann KJ. Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol. 2008;265:191–225. doi: 10.1016/S0074-7696(07)65005-0. [DOI] [PubMed] [Google Scholar]
- 16.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 17.Ross RS, Pham C, Shai SY, et al. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res. 1998;82:1160–1172. doi: 10.1161/01.res.82.11.1160. [DOI] [PubMed] [Google Scholar]
- 18.Shai SY, Harpf AE, Babbitt CJ, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 19.Keller RS, Shai SY, Babbitt CJ, et al. Disruption of Integrin Function in the Murine Myocardium Leads to Perinatal Lethality, Fibrosis, and Abnormal Cardiac Performance. Am J Pathol. 2001;158:1079–1090. doi: 10.1016/S0002-9440(10)64055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valencik ML, Keller RS, Loftus JC, et al. A lethal perinatal cardiac phenotype resulting from altered integrin function in cardiomyocytes. J Card Fail. 2002;8:262–272. doi: 10.1054/jcaf.2002.127335. [DOI] [PubMed] [Google Scholar]
- 21.Valencik ML, Zhang D, Punske B, et al. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ Res. 2006;99:1403–1410. doi: 10.1161/01.RES.0000252291.88540.ac. [DOI] [PubMed] [Google Scholar]
- 22.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 23.Wiesner S, Legate KR, Fassler R. Integrin-actin interactions. Cell Mol Life Sci. 2005;62:1081–1099. doi: 10.1007/s00018-005-4522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 25.Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–H2301. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- 26.Brancaccio M, Hirsch E, Notte A, et al. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–433. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Belkin AM, Ornatsky OI, Kabakov AE, et al. Diversity of vinculin/meta-vinculin in human tissues and cultivated cells. Expression of muscle specific variants of vinculin in human aorta smooth muscle cells. J Biol Chem. 1988;263:6631–6635. [PubMed] [Google Scholar]
- 28.Rudiger M, Korneeva N, Schwienbacher C, et al. Differential actin organization by vinculin isoforms: implications for cell type-specific microfilament anchorage. FEBS Lett. 1998;431:49–54. doi: 10.1016/s0014-5793(98)00723-6. [DOI] [PubMed] [Google Scholar]
- 29.Lu MH, DiLullo C, Schultheiss T, et al. The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J Cell Biol. 1992;117:1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jockusch BM, Isenberg G. Interaction of alpha-actinin and vinculin with actin: opposite effects on filament network formation. Proc Natl Acad Sci U S A. 1981;78:3005–3009. doi: 10.1073/pnas.78.5.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- 32.Hazan RB, Kang L, Roe S, et al. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- 33.Weiss EE, Kroemker M, Rudiger AH, et al. Vinculin is part of the cadherin-catenin junctional complex: complex formation between alpha-catenin and vinculin. J Cell Biol. 1998;141:755–764. doi: 10.1083/jcb.141.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A. 1983;80:1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziegler WH, Gingras AR, Critchley DR, et al. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans. 2008;36:235–239. doi: 10.1042/BST0360235. [DOI] [PubMed] [Google Scholar]
- 36.Demali KA, Burridge K. Coupling membrane protrusion and cell adhesion. J Cell Sci. 2003;116:2389–2397. doi: 10.1242/jcs.00605. [DOI] [PubMed] [Google Scholar]
- 37.Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- 38.Bakolitsa C, de Pereda JM, Bagshaw CR, et al. Crystal structure of the vinculin tail suggests a pathway for activation. Cell. 1999;99:603–613. doi: 10.1016/s0092-8674(00)81549-4. [DOI] [PubMed] [Google Scholar]
- 39.Bakolitsa C, Cohen DM, Bankston LA, et al. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–586. doi: 10.1038/nature02610. [DOI] [PubMed] [Google Scholar]
- 40.Borgon RA, Vonrhein C, Bricogne G, et al. Crystal structure of human vinculin. Structure. 2004;12:1189–1197. doi: 10.1016/j.str.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Johnson RP, Craig SW. An intramolecular association between the head and tail domains of vinculin modulates talin binding. J Biol Chem. 1994;269:12611–12619. [PubMed] [Google Scholar]
- 42.Weekes J, Barry ST, Critchley DR. Acidic phospholipids inhibit the intramolecular association between the N- and C-terminal regions of vinculin, exposing actin-binding and protein kinase C phosphorylation sites. Biochem J. 1996;314 ( Pt 3):827–832. doi: 10.1042/bj3140827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 44.Izard T, Evans G, Borgon RA, et al. Vinculin activation by talin through helical bundle conversion. Nature. 2004;427:171–175. doi: 10.1038/nature02281. [DOI] [PubMed] [Google Scholar]
- 45.Bois PR, O’Hara BP, Nietlispach D, et al. The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin. J Biol Chem. 2006;281:7228–7236. doi: 10.1074/jbc.M510397200. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Huttelmaier S, Illenberger S, Grosheva I, et al. Raver1, a dual compartment protein, is a ligand for PTB/hnRNPI and microfilament attachment proteins. J Cell Biol. 2001;155:775–786. doi: 10.1083/jcb.200105044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huttelmaier S, Zenklusen D, Lederer M, et al. Spatial regulation of beta-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438:512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 49.Samuels M, Ezzell RM, Cardozo TJ, et al. Expression of chicken vinculin complements the adhesion-defective phenotype of a mutant mouse F9 embryonal carcinoma cell. J Cell Biol. 1993;121:909–921. doi: 10.1083/jcb.121.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldmann WH, Schindl M, Cardozo TJ, et al. Motility of vinculin-deficient F9 embryonic carcinoma cells analyzed by video, laser confocal, and reflection interference contrast microscopy. Exp Cell Res. 1995;221:311–319. doi: 10.1006/excr.1995.1380. [DOI] [PubMed] [Google Scholar]
- 51.Ezzell RM, Goldmann WH, Wang N, et al. Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp Cell Res. 1997;231:14–26. doi: 10.1006/excr.1996.3451. [DOI] [PubMed] [Google Scholar]
- 52.Saunders RM, Holt MR, Jennings L, et al. Role of vinculin in regulating focal adhesion turnover. Eur J Cell Biol. 2006;85:487–500. doi: 10.1016/j.ejcb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Sharp WW, Simpson DG, Borg TK, et al. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol. 1997;273:H546–H556. doi: 10.1152/ajpheart.1997.273.2.H546. [DOI] [PubMed] [Google Scholar]
- 54.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 55.Zemljic-Harpf AE, Ponrartana S, Avalos RT, et al. Heterozygous Inactivation of the Vinculin Gene Predisposes to Stress-Induced Cardiomyopathy. Am J Pathol. 2004;165:1033–1044. doi: 10.1016/S0002-9440(10)63364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rockman HA, Ross RS, Harris AN, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zemljic-Harpf AE, Miller JC, Henderson SA, et al. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol. 2007;27:7522–7537. doi: 10.1128/MCB.00728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Kubalak SW, Minamisawa S, et al. Selective requirement of myosin light chain 2v in embryonic heart function. J Biol Chem. 1998;273:1252–1256. doi: 10.1074/jbc.273.2.1252. [DOI] [PubMed] [Google Scholar]
- 59.Crone SA, Zhao YY, Fan L, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 60.Peng X, Kraus MS, Wei H, et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda M, Holder E, Lowes B, et al. Dilated cardiomyopathy associated with deficiency of the cytoskeletal protein metavinculin. Circulation. 1997;95:17–20. doi: 10.1161/01.cir.95.1.17. [DOI] [PubMed] [Google Scholar]
- 62.Vasile VC, Will ML, Ommen SR, et al. Identification of a metavinculin missense mutation, R975W, associated with both hypertrophic and dilated cardiomyopathy. Mol Genet Metab. 2006;87:169–174. doi: 10.1016/j.ymgme.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Olson TM, Illenberger S, Kishimoto NY, et al. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105:431–437. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- 64.Vasile VC, Edwards WD, Ommen SR, et al. Obstructive hypertrophic cardiomyopathy is associated with reduced expression of vinculin in the intercalated disc. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.08.106. [DOI] [PubMed] [Google Scholar]
- 65.Vasile VC, Ommen SR, Edwards WD, et al. A missense mutation in a ubiquitously expressed protein, vinculin, confers susceptibility to hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2006;345:998–1003. doi: 10.1016/j.bbrc.2006.04.151. [DOI] [PubMed] [Google Scholar]
- 66.Birks EJ, Hall JL, Barton PJ, et al. Gene profiling changes in cytoskeletal proteins during clinical recovery after left ventricular-assist device support. Circulation. 2005;112:I57–I64. doi: 10.1161/CIRCULATIONAHA.104.526137. [DOI] [PubMed] [Google Scholar]
- 67.Hein S, Kostin S, Heling A, et al. The role of the cytoskeleton in heart failure. Cardiovasc Res. 2000;45:273–278. doi: 10.1016/s0008-6363(99)00268-0. [DOI] [PubMed] [Google Scholar]
- 68.Marin-Neto JA, Cunha-Neto E, Maciel BC, et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 69.Melo TG, Almeida DS, de Meirelles MN, et al. Trypanosoma cruzi infection disrupts vinculin costameres in cardiomyocytes. Eur J Cell Biol. 2004;83:531–540. doi: 10.1078/0171-9335-00419. [DOI] [PubMed] [Google Scholar]
- 70.de Melo TG, Meirelles MN, Pereira MC. Trypanosoma cruzi alters adherens junctions in cardiomyocytes. Microbes Infect. 2008;10:1405–1410. doi: 10.1016/j.micinf.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 71.DiMichele LA, Doherty JT, Rojas M, et al. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99:636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brancaccio M, Fratta L, Notte A, et al. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med. 2003;9:68–75. doi: 10.1038/nm805. [DOI] [PubMed] [Google Scholar]
- 73.White DE, Coutu P, Shi YF, et al. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Jiang G, Cai Y, et al. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Critchley DR. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys. 2009;38:235–254. doi: 10.1146/annurev.biophys.050708.133744. [DOI] [PubMed] [Google Scholar]
- 76.Monkley SJ, Zhou XH, Kinston SJ, et al. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 77.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang Y, Litvinov RI, Chen X, et al. Loss of PIP5KIgamma, unlike other PIP5KI isoforms, impairs the integrity of the membrane cytoskeleton in murine megakaryocytes. J Clin Invest. 2008;118:812–819. doi: 10.1172/JCI34239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Law DJ, Allen DL, Tidball JG. Talin, vinculin and DRP (utrophin) concentrations are increased at mdx myotendinous junctions following onset of necrosis. J Cell Sci. 1994;107 ( Pt 6):1477–1483. doi: 10.1242/jcs.107.6.1477. [DOI] [PubMed] [Google Scholar]
- 80.Zhang JQ, Elzey B, Williams G, et al. Ultrastructural and Biochemical Localization of N-RAP at the Interface between Myofibrils and Intercalated Disks in the Mouse Heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- 81.Anastasi G, Cutroneo G, Gaeta R, et al. Dystrophin-glycoprotein complex and vinculin-talin-integrin system in human adult cardiac muscle. Int J Mol Med. 2009;23:149–159. [PubMed] [Google Scholar]
- 82.Conti FJ, Felder A, Monkley S, et al. Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle. Development. 2008;135:2043–2053. doi: 10.1242/dev.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Senetar MA, Moncman CL, McCann RO. Talin2 is induced during striated muscle differentiation and is targeted to stable adhesion complexes in mature muscle. Cell Motil Cytoskeleton. 2007;64:157–173. doi: 10.1002/cm.20173. [DOI] [PubMed] [Google Scholar]
- 84.Chen NT, Lo SH. The N-terminal half of talin2 is sufficient for mouse development and survival. Biochem Biophys Res Commun. 2005;337:670–676. doi: 10.1016/j.bbrc.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 85.Conti FJ, Monkley SJ, Wood MR, et al. Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions. Development. 2009 doi: 10.1242/dev.035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwander M, Leu M, Stumm M, et al. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 87.Moulik M, Vatta M, Witt SH, et al. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54:325–333. doi: 10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paul M, Zumhagen S, Stallmeyer B, et al. Genes causing inherited forms of cardiomyopathies. A current compendium. Herz. 2009;34:98–109. doi: 10.1007/s00059-009-3215-8. [DOI] [PubMed] [Google Scholar]
- 89.Margulies KB, Bednarik DP, Dries DL. Genomics, transcriptional profiling, and heart failure. J Am Coll Cardiol. 2009;53:1752–1759. doi: 10.1016/j.jacc.2008.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]