Abstract
Introduction/Purpose
Body weight generally increases with aging in Western societies. Although training studies show that exercise produces acute weight loss, it is unclear whether the long-term maintenance of vigorous exercise attenuates the trajectory of age-related weight gain. Specifically, prior studies have not tested whether the maintenance of physical activity, in the absence of any change in activity, prevents weight gain.
Methods
Prospective study of 6119 male and 2221 female runners whose running distances changed < 5 km/wk between baseline and follow-up surveys 7 yr later.
Results
On average, men who maintained modest (0–23 km/wk), intermediate (24–47 km/wk), or prolonged running distances (≥ 48 km/wk) all gained weight through age 64; however, those who maintained ≥ 48 km/wk had one half the average annual weight gain of those who maintained < 24 km/wk. For example, between the ages of 35 and 44 in men and 30 and 39 yr in women, those who maintained < 24 km/wk gained, on average, 2.1 and 2.9 kg more per decade than those averaging > 48 km/wk. Age-related weight gain, and its attenuation by maintained exercise, were both greater in younger than in older men. Men’s gains in waist circumference with age, and its attenuation by maintaining running, were the same in older and younger men. Regardless of age, women increased their body weight, waist circumference, and hip circumference over time, and these measurements were attenuated in proportion to their maintained running distance. In both sexes, running disproportionately prevented more extreme increases in weight.
Conclusion
As they aged, men and women gained less weight in proportion to their levels of sustained vigorous activity. This long-term beneficial effect is in addition to the acute weight loss that occurs with increased activity.
INTRODUCTION
Clinical trials and prospective studies have shown that sedentary men and women who start to exercise (e.g., running) lose weight and body fat compared with those who remain sedentary, and those who stop exercising gain body weight relative to those who remain active [25,29,30]. Moreover, the amount of weight change is proportional to the change in exercise dose [25,29,30]. Exercise is often prescribed in conjunction with calorie restriction to achieve more effective weight loss [17].
In addition to producing acute weight loss, vigorous exercise may inhibit age-related weight gain [20,23,24]. In Western societies, men and women generally gain weight as they age [3,18]. The National Institute of Medicine (IOM) currently recommends 60 min of walking per day, or its energy equivalent, to maintain healthy weight [8]. Multiple studies have examined the effects of short-term exercise training on body weight and support the IOM’s recommendations [1,5,6,17,28]. Few studies, however, have examined the long-term effects of exercise on weight gain, and none, to our knowledge, have examined the effect of sustained vigorous exercise on annual weight gain per se.
This paper tests whether running attenuates long-term weight gain independently of any change in activity level. Specifically, we include only runners whose reported exercise levels remained constant between baseline and follow-up. Heretofore, the relationship of weight to exercise has been framed largely in terms of whether increased energy expenditure causes weight loss prospectively, or whether physical activity is associated with leanness cross-sectionally [1,5,8]. The hypothesis examined herein is largely untested because few prospective cohorts have the sample sizes required to extract a subset of consistent exercisers. By contrast, the National Runners’ Health Study includes 8340 runners whose reported weekly running distance changed by less than 5 km/wk between their baseline and 7-yr follow-up surveys.
METHODS
The survey instruments and baseline characteristics of the National Runners’ Health Survey are described elsewhere [20,21,23–26]. Between 1991 and 1995, a two-page questionnaire, distributed nationally at races and to subscribers of the nation’s largest running magazine (Runner’s World, Emmaus PA), solicited information on demographics (age, race, education), running history (age when respondent began running at least 12 miles per week, average weekly mileage, number of marathons during the preceding 5 yr, and best marathon and 10-km times), weight history (greatest and current weight, weight when respondent started running, least weight as a runner, body circumferences of the chest, waist, and hips), diet (vegetarianism, and the current weekly intakes of alcohol, red meat, fish, fruit, vitamin C, vitamin E, and aspirin), current and past cigarette use, prior history of heart attacks and cancer, and medications for blood pressure, thyroid, cholesterol, or diabetes. Eighty percent of the 54,956 participants of the National Runners’ Health Study provided follow-up information or were deceased (N = 180). Runners were excluded if they smoked (N = 634), followed strict vegetarian diets (N = 418), or used thyroid (N = 934) or diabetes medications (N = 182), because of the possible influences of these factors on adiposity. To address the hypothesis of whether the maintenance of a constant activity affects weight gain, we restrict our analyses to runners whose follow-up weekly running distances were within 5 km/wk of their reported baseline value. The study protocol was approved by the committee for the protection of human subjects, and all participants signed committee-approved informed consents.
Change in body mass index (BMI) was calculated as the change in weight (kg) between the first and second questionnaire, divided by the square of the average height (m) from the two questionnaires. Self-reported waist and hip circumferences were elicited by the question “Please provide, to the best of your ability, your body circumference in inches” without further instruction. Weekly running distance was provided in response to our request for the average miles run each week for a specified year. Although other leisure-time physical activities were not recorded for this cohort, data from runners recruited after 1998 (when the question was introduced) show that running represents (± SD) 91.5 ± 19.1 and 85.2 ± 24.0% of all vigorous-intensity activity in men and women, respectively, and 73.5 ± 23.7 and 69.4 ± 25.7% of total leisure-time physical activity. Elsewhere, we have reported the strong correlations between repeated questionnaires for self-reported running distance (r = 0.89) [21], between self-reported and clinically measured height (r = 0.96) and weight (r = 0.96), and significant associations between self-reported running distance versus self-reported BMI and waist circumference in cross-sectional analyses [20-24]. Self-reported and clinically measured waist circumferences were moderately correlated (r = 0.68) [21]. Training studies in initially sedentary men have demonstrated that self-reported running distance correlates significantly with improvements in VO2max and plasma concentrations of high-density lipoprotein cholesterol [29,30].
Statistical analyses
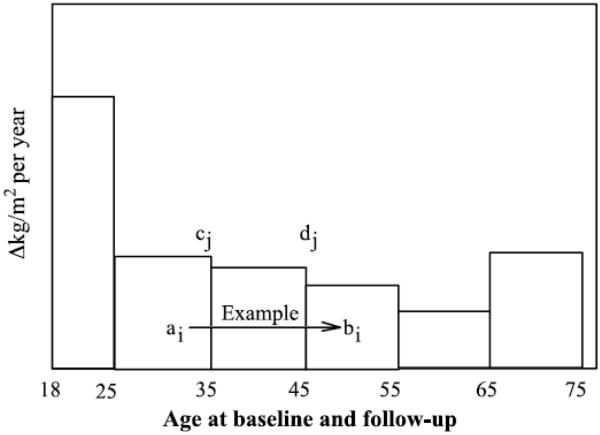
Results are given as means ± SE or as slopes ± SE, except where noted. We used multiple linear regression to estimate the annual mean changes in adiposity within six age intervals in men [18–24, 25–34, 35–44, 45–54, 55–64, and 65–74 yr old; Fig. 1) and four age intervals in women [18–29, 30–39, 40–49, and 50–74 yr old). Weight change was used as the dependent variable and age intervals as the independent variables in a zero-intercept multiple-regression model, where an individual’s total weight change was the sum of the time spent between the baseline and follow-up survey within each interval [26]. For example, Figure 1 shows that for a person who was 32 at baseline and 49 at follow-up, the total estimated weight gain would be 3/10 the estimated weight gain between 25 and 35 yr old, all (10/10) of the estimated weight gain between 35 and 45 yr old, and 4/10 the estimated weight gain between 45 and 55 yr old. Thus, ΔBMI is the value for the dependent variable, and 0, 3/10, 10/10, 4/10, 0, and 0 are the values for the independent variables—that is, the residence time within each of the six age classes. (Computationally, the contribution of the weight gain associated with the age class j, j = 1,...,6, to the total weight change of individual i, i = 1,...,N is zero if the individual was never in the age group j between surveys, and it is calculated as (minimum (bi - cj, dj - cj) - maximum (ai - cj,0))/(dj - cj) if they were, where cj and dj are the lower and upper limits of age class j, and ai and bi are participant’s i ages on their first and second survey; see Figure 1 and Williams and Wood [26].) Annual weight change was calculated by dividing the regression estimates of the average weight gain associated with the age interval by the number of years included in the interval.
FIGURE 1.
The additive contributions of weight gain within age intervals to an individual’s total weight change. Specifically, if an individual was 32 yr old at baseline and 49 at follow-up, the total estimated weight gain would be 3/10 the estimated weight gain between 25 and 35 yr old, all (10/10) of the estimated weight gain between 35 and 45 yr old, and 4/10 the estimated weight gain between 45 and 55 yr old. See Methods for computation.
We also tested whether the 10th, 25th, 50th, 75th, and 90th percentiles of annual weight gain diminished with weekly running distance. Bootstrap resampling was used to estimate standard error for the difference in percentile between distance categories, and their statistical significance [4]. This was done by computing the statistics on 10,000 sample datasets that were generated by sampling with replacement from the original data. The mean and standard deviation of the 10,000 statistics are the bootstrap estimate of the statistic and its standard error. Bootstrap resampling also was used to test whether the dose–response relationship between physical activity and annual changes in weight and regional adiposity were the same for all percentiles of weight change or whether it was disproportionately greater for higher or lower percentiles of weight gain [21,25]. The significance level for this test was twice the proportion of times that the bootstrap estimate of the difference was greater than zero, or less than zero— whichever was smaller (two-tailed test).
RESULTS
There were 6119 men and 2221 women who reported running within ± 5 km/wk of their baseline running distance (19% of the surviving cohort). Men and women, respectively, on the baseline surveys were, on average, middle aged (mean ± SD: 45.3 ± 10.1 and 39.6 ± 9.7 yr), college educated (16.7 ± 2.4 and 16.0 ± 2.3 yr of education), relatively lean (BMI of 24.1 ± 2.7 and 21.5 ± 2.7 kg/m2), narrow waisted (85.1 ± 6.3 and 69.3 ± 7.3 cm), and had run for 13.8 ± 8.0 and 10.4 ± 5.9 yr. Women’s baseline hip circumference averaged 91.9 ± 7.0 cm. The men gained 0.33 ± 0.64 kg and added 0.30 ± 0.71 cm around their waists annually during 7.5 ± 3.2 yr of follow-up. Women annually gained an average of 0.31 ± 0.69 kg in weight, 0.36 ± 1.07 cm in waist circumference, and 0.26 ± 1.08 cm in hip circumference during their 7.2 ± 2.3 yr of follow-up. The length of follow-up varies across individuals because of differences in when they were recruited and when their follow-up questionnaires were returned.
Annual increases in weight by age, stratified by running distance
Average body weight, BMI, waist circumference, and hip circumference (women only) increased significantly over time for all age classes, regardless of distance run (Table 1, column 1). Young adults (18- to 24-yr-old men and 18- to 30-yr-old women) experienced the greatest annual increases in body weight, BMI, waist circumference, and hip circumference. In men, the rate of increase was diminished by over half after age 25 and by two-thirds after age 45, relative to the increases in young men. In women, annual increases in waist and hip circumference rates diminished by over half after age 29.
TABLE 1.
Annual average increases in body weight, BMI, and waist circumference (± SE) by age and average running distance in 6119 men and 2221 women
| Reported Average Weekly Running Distance at Baseline and Follow-up | ||||
|---|---|---|---|---|
| All | 0–23 km/wk | 24–47 km/wk | ≥ 48 km/wk | |
| Males | ||||
| Δbody weight (kg/yr) | ||||
| 18–24 | 1.37 ± 0.12§ | 1.56 ± 0.17§ | 0.86 ± 0.28† | 0.83 ± 0.22‡ |
| 25–34 | 0.46 ± 0.04§ | 0.63 ± 0.07§ | 0.37 ± 0.06§¥ | 0.26 ± 0.06§# |
| 35–44 | 0.44 ± 0.02§ | 0.52 ± 0.03§ | 0.42 ± 0.03§¥ | 0.31 ± 0.03§∞ |
| 45–54 | 0.32 ± 0.02§ | 0.41 ± 0.03§ | 0.26 ± 0.02§∞ | 0.23 ± 0.03§∞ |
| 55–64 | 0.17 ± 0.02§ | 0.14 ± 0.04§ | 0.21 ± 0.03§ | 0.13 ± 0.05† |
| 65–74 | 0.08 ± 0.04* | 0.10 ± 0.06 | 0.06 ± 0.06 | 0.08 ± 0.09 |
| ΔBMI (kg/m2 per yr) | ||||
| 18–24 | 0.42 ± 0.04§ | 0.48 ± 0.05§ | 0.28 ± 0.09† | 0.27 ± 0.07§ |
| 25–34 | 0.14 ± 0.01§ | 0.20 ± 0.02§ | 0.11 ± 0.02§¥ | 0.08 ± 0.02§# |
| 35–44 | 0.14 ± 0.01§ | 0.16 ± 0.01§ | 0.13 ± 0.01§¥ | 0.10 ± 0.01§∞ |
| 45–54 | 0.10 ± 0.00§ | 0.13 ± 0.01§ | 0.08 ± 0.01§∞ | 0.07 ± 0.01§∞ |
| 55–64 | 0.06 ± 0.01§ | 0.06 ± 0.01§ | 0.07 ± 0.01§ | 0.04 ± 0.01† |
| 65–74 | 0.03 ± 0.01* | 0.03 ± 0.02 | 0.02 ± 0.02 | 0.03 ± 0.03 |
| Δwaist circumference (cm/yr) | ||||
| 18–24 | 0.96 ± 0.16§ | 0.90 ± 0.23§ | 0.95 ± 0.33† | 1.00 ± 0.32† |
| 25–34 | 0.42 ± 0.05§ | 0.56 ± 0.08§ | 0.41 ± 0.07§ | 0.17 ± 0.08*# |
| 35–44 | 0.35 ± 0.02§ | 0.41 ± 0.04§ | 0.31 ± 0.03§¶ | 0.29 ± 0.04§ |
| 45–54 | 0.26 ± 0.02§ | 0.35 ± 0.03§ | 0.22 ± 0.03§# | 0.13 ± 0.04‡∞ |
| 55–64 | 0.27 ± 0.03§ | 0.29 ± 0.04§ | 0.28 ± 0.04§ | 0.15 ± 0.06† |
| 65–74 | 0.18 ± 0.05§ | 0.24 ± 0.07‡ | 0.18 ± 0.07† | 0.18 ± 0.12¥ |
| Females | ||||
| Δbody weight (kg/yr) | ||||
| 18–29 | 0.70 ± 0.12§ | 0.91 ± 0.20§ | 0.64 ± 0.17‡ | 0.39 ± 0.18* |
| 30–39 | 0.37 ± 0.03§ | 0.50 ± 0.05§ | 0.29 ± 0.04§¥ | 0.21 ± 0.06‡# |
| 40–49 | 0.31 ± 0.03§ | 0.42 ± 0.05§ | 0.23 ± 0.03§# | 0.23 ± 0.06§¶ |
| ≥50 | 0.21 ± 0.03§ | 0.31 ± 0.06§ | 0.11 ± 0.04*¥ | 0.15 ± 0.07* |
| ΔBMI (kg/m2 per yr) | ||||
| 18–29 | 0.24 ± 0.04§ | 0.31 ± 0.07§ | 0.24 ± 0.07‡ | 0.13 ± 0.07* |
| 30–39 | 0.13 ± 0.01§ | 0.17 ± 0.02§ | 0.11 ± 0.02§¥ | 0.08 ± 0.02§¥ |
| 40–49 | 0.12 ± 0.01§ | 0.16 ± 0.02§ | 0.08 ± 0.01§# | 0.08 ± 0.02§¶ |
| ≥50 | 0.08 ± 0.01§ | 0.12 ± 0.02§ | 0.04 ± 0.02†¥ | 0.06 ± 0.03* |
| Δwaist circumference (cm/yr) | ||||
| 18–29 | 0.91 ± 0.21§ | 1.01 ± 0.33† | 1.00 ± 0.38† | 0.64 ± 0.36 |
| 30–39 | 0.33 ± 0.05§ | 0.47 ± 0.09§ | 0.16 ± 0.08*¥ | 0.32 ± 0.11† |
| 40–49 | 0.40 ± 0.05§ | 0.48 ± 0.08§ | 0.36 ± 0.06§ | 0.28 ± 0.11† |
| ≥50 | 0.32 ± 0.06§ | 0.42 ± 0.09§ | 0.21 ± 0.08† | 0.31 ± 0.14* |
| Δhip circumference (cm/yr) | ||||
| 18–29 | 1.10 ± 0.22§ | 1.54 ± 0.33§ | 0.32 ± 0.41 | 1.10 ± 0.38† |
| 30–39 | 0.33 ± 0.05§ | 0.40 ± 0.09§ | 0.32 ± 0.09‡ | 0.21 ± 0.10* |
| 40–49 | 0.18 ± 0.05§ | 0.27 ± 0.07‡ | 0.17 ± 0.07* | −0.04 ± 0.10¶ |
| ≥50 | 0.25 ± 0.06§ | 0.38 ± 0.09§ | 0.14 ± 0.09¶ | 0.13 ± 0.13 |
Significant weight increase:
P < 0.05
P < 0.01
P < 0.001
P < 0.0001.
Weight increase significantly different from the increase at 0–23 km/wk:
P < 0.05
P < 0.01
P < 0.001
P < 0.0001.
Individuals running modest (0–23 km/wk), intermediate (24–47 km/wk), or prolonged distances (≥ 48 km/wk) also demonstrated statistically significant annual increases in body weight, BMI, and waist circumference through age 64 in men and in all age groups in women (Table 1, columns 2–4). However, between ages 25 and 54 in men and 30 and 49 in women, the average annual gain in weight and BMI attenuated for those who ran intermediate and longer running distances. Those running ≥ 48 km/wk experienced only half the average annual increase in total weight and BMI as those who maintained < 24 km/wk. Waist circumference in men 25 yr and older also tended to increase significantly less for those who maintained their running distance ≥ 48 km/wk vs. < 24 km/wk.
Annual increases in weight by running distance, stratified by age
Table 2 presents the regression slopes for annual changes in body weight, BMI, and waist circumference versus maintained running distance (km/wk) within each age group. Annual increases in adiposity decreased significantly with running distance in young to middle-aged men (25–54 yr) but not in older men. Running produced progressively less attenuation of annual weight gain as the men got older. This was confirmed by the statistically significant interaction between the effects of age and running distance on annual changes in weight and BMI. In contrast, annual increases in waist circumference decreased with running distance in men 25 and older, regardless of age, and there was no significant interaction between age and distance. This suggests that the attenuation of the annual gains in waist circumference can be estimated by the single slope for pooled data adjusted for age (−0.004 ± 0.001 cm/yr per km/wk, P < 0.0001) rather than by separate coefficients for each age group.
Table 2.
Regression slope (± SE) of annual change in adiposity vs weekly running distance in men and women, stratified by age group
| Body mass (kg/yr per km/wk) |
BMI (kg/m2 per km/wk) |
Waist circumference (cm/yr per km/wk) |
|
|---|---|---|---|
| Males | |||
| 18-24 yr | −0.013 ± 0.006* | −0.004 ± 0.002 | −0.005 ± 0.006 |
| 25-34 yr | −0.009 ± 0.002§ | −0.003 ± 0.001§ | −0.007 ± 0.002‡ |
| 35-44 yr | −0.005 ± 0.001§ | −0.002 ± 0.000§ | −0.004 ± 0.001§ |
| 45-54 yr | −0.004 ± 0.001§ | −0.001 ± 0.000§ | −0.004 ± 0.001§ |
| 55-64 yr | 0.000 ± 0.001 | 0.000 ± 0.000 | −0.003 ± 0.001* |
| 65-74 yr | 0.000 ± 0.000 | 0.000 ± 0.001 | −0.007 ± 0.003† |
| Age x distance interaction | P < 0.0001 | P < 0.0001 | P = 0.20 |
| Females | |||
| 18-29 yr | −0.006 ± 0.002† | −0.002 ± 0.001* | 0.004 ± 0.006 |
| 30-39 yr | −0.007 ± 0.001§ | −0.002 ± 0.001§ | −0.003 ± 0.002 |
| 40-49 yr | −0.007 ± 0.001§ | −0.002 ± 0.000§ | −0.005 ± 0.002* |
| ≥50 yr | −0.003 ± 0.002 | −0.001 ± 0.001 | −0.003 ± 0.003 |
| Age x distance interaction | P = 0.35 | P = 0.54 | P = 0.42 |
Slope significantly different from zero:
P <0.05
P < 0.01
P < 0.001
P < 0.0001.
In women under 50, maintaining longer running distances significantly reduced annual increases in total weight and BMI (Table 2). The attenuation of age-related weight gain was not significant in older women, but this may be partly attributable to limited statistical power, because there was no significant interaction between age and running distance. The pooled data adjusted for age suggest that each kilometer run per week attenuated the annual increase in body weight by −0.006 ± 0.001 kg, in BMI by −0.002 ± 0.000 kg/m2, and in waist circumference by −0.003 ± 0.001 cm. Maintaining longer running distances also attenuated annual increases in hip circumference (not displayed) in women 30–39 yr old (−0.006 ± 0.002 cm/yr per km/wk, P = 0.02) and 40– 49 yr old (−0.007 ± 0.002 cm/yr per km/wk, P = 0.002) but not in younger (−0.004 ± 0.006 cm/yr per km/wk, P = 0.50) or older women (−0.005 ± 0.003 cm/yr per km/wk, P = 0.07). However, there was, again, no significant interaction between age and distance, and the pooled slope for the annual attenuation in hip circumference per kilometer run per week, adjusted for age, was −0.006 ± 0.001 cm/yr per km/wk, P < 0.0001.
Percentiles of annual weight change by running distance
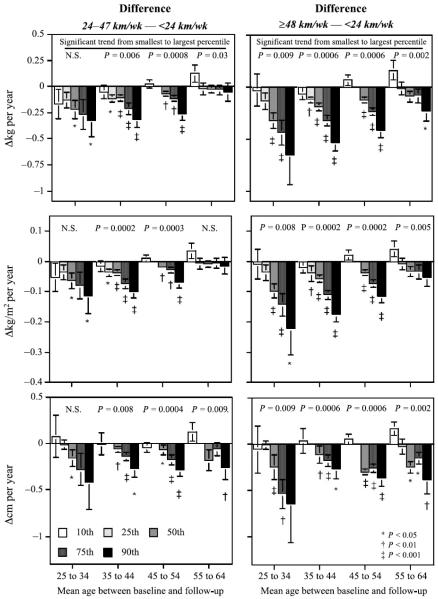
Figures 2 and 3 present the differences in the annual changes in body mass, BMI, and waist circumference between those who ran less than 24 km/wk versus 24–47 km/wk (left panels) and those who ran less than 24 km/wk versus ≥ 48 km/wk (right panels). Negative heights mean smaller annual increases in high-versus low-mileage runners. The differences are presented separately by age (x-axis) and by percentile of weight or circumference change (see legend). For example, the difference in the 90th percentile of BMI change is the 90th percentile of ΔBMI among runners who ran ≥ 48 km/wk minus the 90th percentile of ΔBMI among runners who ran < 24 km/wk. The bars for the 10th, 25th, 50th, 75th, and 90th percentiles would all have the same heights if running produced the same expected reduction in annual weight gain across all percentiles (i.e., if running more miles shifted the entire distribution of ΔBMI by a fixed amount). In contrast, the middle-right panel of Figure 2 shows that in 25- to 34-yr-old men, the 50th, 75th, and 90th percentiles of BMI change were significantly less for those who maintained ≥ 48 km/wk than for those who maintained running distances of less than 24 km/wk, whereas the 10th and 25th percentiles of BMI change did not differ significantly between the higher- and lower-mileage runners. The length of the bars increase progressively from the 10th through the 90th percentiles, and this trend is statistically significant (P = 0.008), suggesting further that running does not diminish weight gain uniformly but, rather, that the degree of attenuation increases with the percentile of ΔBMI.
FIGURE 2.
Difference in the annual change in body mass (top), BMI (middle), and waist circumference (bottom) between men who ran less than 24 km/wk, from those running 24–47 km/wk (left), and from those running ≥ 48 km/wk (right). Results are presented by age groups (x-axis) and percentile of weight change (legend). Significance levels for differences from zero are designated by symbols. Probabilities presented above the bars are for the significance of a linear trend going from the smallest to the highest percentile (percentile effect). SE are shown in brackets.
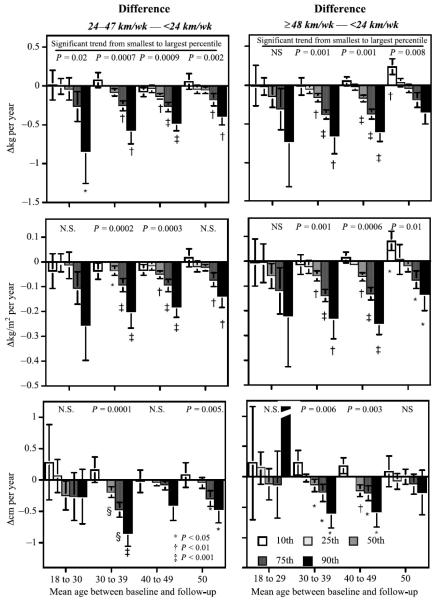
FIGURE 3.
Difference in the annual change in body mass (top), BMI (middle), and waist circumference (bottom) between women who ran less than 24 km/wk, from those running 24–47 km/wk (left), and from those running ≥ 48 km/wk (right). Results are presented by age groups (x-axis) and percentile of weight change (legend). Significance levels for differences greater than zero are designated by symbols. Probabilities presented above the bars are for the significance of a linear trend going from the smallest to the highest percentile (percentile effect). SE are shown in brackets.
Figure 2 shows that men 35–54 yr old who consistently ran between 24 and 47 km/wk were less likely to have larger annual increases in weight and BMI than men who ran < 24 km/wk. Moreover, the attenuation of weight gain depended on the amount of exercise, the percentile of weight gain, and the age of the men. Specifically, the attenuation of weight gain increased progressively from the lowest to highest Δweight and ΔBMI percentile (percentile effect). These effects were more pronounced when men who maintained ≥ 48 km/wk are compared with those who maintained < 24 km/wk (Fig. 2, right panels) than when men who ran 24–47 km/wk are compared with those who ran < 24 km/wk (left panel, exercise dose effect). In men, there is a clear trend for age to reduce the attenuating effects of exercise on annual gains in the 50th, 75th, and 90th percentiles of Δweight and ΔBMI. Figure 2 also shows that annual increases in waist circumference were attenuated by running and that the degree of attenuation was greatest at the 90th percentile of Δwaist circumference, was intermediate at the 75th percentile, and was modest but significant at the 50th percentile of Δwaist circumference. The degree of attenuation was similar across age groups after age 35.
The effects of exercise maintenance on the percentiles of Δweight and ΔBMI in women are generally consistent with those observed in men (Fig. 3). Women 30–49 yr old who consistently ran between 24 and 47 km/wk or ≥ 48 km/wk had smaller annual increases in weight and BMI than those who ran < 24 km/wk. Also, the attenuation of weight gain increased progressively from the lowest to highest Δweight and ΔBMI percentile. The difference between maintaining 24–47 km/wk and maintaining ≥ 48 km/wk was less evident for women than for men. Age-related increases in the 50th, 75th, and 90th percentiles of waist circumferences were also diminished in women 30–39 yr old by running at least 24 km/wk and in women 40–49 yr old by running at least 48 km/wk. In men, years of education were associated with significantly smaller increases in BMI and waist circumference (P = 0.001). In women, parity was associated with significantly greater increases in BMI (P = 0.02) but not waist or hip circumference, whereas education was unrelated to changes in BMI and body circumference. Adjustment for education and parity did not affect the differences reported.
DISCUSSION
Our analyses confirm prospectively that middle-age weight gain occurs even among vigorously active men [20] and suggest that in Western society, weight gain is an intrinsic aspect of aging. Previously, in our initial cross-sectional analyses of 7059 men, we concluded that increases in BMI with age were constant regardless of activity level [20]. More recent cross-sectional analyses of over 60,000 men suggest that the rate of weight gain with age diminishes at higher activity levels [23]. Although suggestive, these cross-sectional studies could not distinguish aging and weight change (dynamic processes) from individual preferences to choose a level of vigorous activity on the basis of age and weight (self-selection).
The present results demonstrate an inverse dose–response relationship between the amount of exercise sustained for 7 yr and weight gain. The prospective design ensures that the effects were related to weight change per se. Unlike prospective analyses presented by others [2,7,11,13,27], our analyses are restricted to vigorously active men and women who maintained the same exercise levels at baseline and follow-up. This was done to eliminate the effects of change in physical activity, which is already known to produce weight loss [29,30]. Our analyses show that the benefits of vigorous exercise on weight extend beyond the acute weight loss after greater energy expenditure—it also reduces the rate of weight gain that occurs with aging.
In this paper, we estimate that between the ages of 35 and 44 yr in men and 30 and 39 yr in women, those who maintained less than 24 km/wk gained, on average, 2.1 and 2.9 kg more per decade than those averaging at least 48 km/wk (Table 1). The corresponding differences in the average increases in waist circumference were 1.2 and 1.5 cm. Willett et al. [19] have shown that even among relatively lean women whose BMI remains below 25 kg/m2, modest weight gain after age 18 is a significant predictor of coronary heart disease risk.
Individuals vary greatly in their tendency to gain weight with age; such variation may be attributable to behavioral differences, but it is also influenced by genetic and environmental variation [12]. The most important impact of maintaining vigorous exercise may be the prevention of more extreme weight gain. As shown in Figures 2 and 3, the differences in the 90th percentiles of weight gain between the high-mileage (≥ 48 km/wk) and low-mileage runners (< 24 km/wk) were 5.3 and 6.5 kg per decade in men and women, respectively. The corresponding differences for the 90th percentiles of the increases in waist circumference are projected to be 2.6 and 2.7 cm per decade in men and women, respectively.
Exercise had less impact on age-related increases in body weight and BMI in older men than in younger men, whereas the attenuation of age-related increases in waist circumference was similar in older and younger men. The degree of attenuation seems to be proportional to the expected changes in men’s body weight, BMI, and waist circumference with age—that is, the tendency for body weight to increase through age 50 and remain constant thereafter, and for waist circumference to continue to increase until later in life.
We have previously reported on 3973 men and 1444 women who quit running (detraining), 270 men and 146 women who started running (training), and 420 men and 153 women who remained sedentary during 7.4 yr of follow-up from this cohort [25]. Among those who quit running, the analyses demonstrated significant dose– response between decreases in the distance run per week and increases in weight and BMI in men (slope ± SE: −0.039 ± 0.005 kg per km/wk and −0.012 ± 0.002 kg/m2 per km/wk, respectively) and in older women (−0.060 ± 0.018 kg per km/wk and −0.022 ± 0.007 kg/m2 per km/wk). Among sedentary men and women who started running, the analyses again demonstrated significant dose–response between increases in the distance run per week and decreases in weight and BMI in men (−0.098 ± 0.017 kg per km/wk and −0.032 ± 0.005 kg/m2 per km/wk) and women (−0.062 ± 0.023 kg per km/wk and −0.021 ± 0.008 kg/m2 per km/wk). Waist circumference also changed in relation to the dose of running distance in men who quit (−0.026 ± 0.005 cm per km/wk) or started running (−0.078 ± 0.017 cm per km/wk).
Taken together, the current and previous studies suggest a two-component framework for describing the effects of physical activity on long-term changes in body weight: 1) an age–trajectory component that is attenuated by physical activity (demonstrated in this report); and 2) an acute component that is attributable to changes in the energy balance, as when energy expenditure is increased in relation to energy intake (demonstrated by us and others elsewhere [25,29,30]). The age–trajectory component may be of relatively minor significance in training or dieting studies, which usually span a year or less [14,29,30], during which the effects on weight of changing energy balance are likely to be large relative to aging. However, it may be of major significance to long-term prospective studies that relate weight change to physical activity.
Our proposed theoretical framework is useful for interpreting other prospective studies of exercise and weight, in which changes in weight between baseline and follow-up are compared with baseline [2,11,13], follow-up [2,27], or concurrent changes in physical activity [7,13,15]. Relationships between weight change and a single exercise measurement taken at either baseline or follow-up will reflect, in part, the effects of physical activity on age-related weight gain (the trajectory component) as well as any effects of change in activity that are related to either the baseline or follow-up activity (acute component). The third approach, which compares concurrent changes in weight and exercise, estimates the cumulative effects of changing energy expenditure (acute component) but not the effect of exercise on age-related weight gain (trajectory component), because it treats men and women who maintained a consistent level of exercise the same regardless of whether the activity level was high or low. In contrast, restricting the analyses to those who maintain a consistent exercise dose over time enables us to test whether exercise attenuates the trajectory component directly.
These observations have important clinical and public health implications. Excess body weight leads to a variety of metabolic disorders, including hypertension, diabetes, and hypercholesterolemia. It also contributes to increased cardiovascular disease risk. Preventing excess body weight may be more effectively addressed by preventing weight gain rather than by achieving weight loss in individuals who are already overweight. Physical activity should be promoted as sustained lifelong behavior rather than as a therapeutic lifestyle change, whose benefits are the weight not gained rather than the amount of weight lost. These benefits may be less apparent than acute weight loss by dieting and may only be perceived relative to the accumulation of excess body weight among others who are less active.
There are obvious limitations to our analyses. We lack data on the energy intake in these runners; this precludes our ability to claim directly that the inverse association between weight gain and exercise was independent of changes in energy intake. We also did not obtain usual running pace for this sample, and, therefore, we are unable to assess whether running intensity may have affected weight gain or specifically, whether the slower pace and reduced maximum aerobic power [9,10] of older runners may have contributed to the significant interaction between age and running distance. Education and parity did not account for the observed associations, but other measures of socioeconomic status were not obtained, and their affects are not known. The test– retest correlations for self-reported running distance suggest that the measure is highly reproducible; however, measurement error will bias the regression coefficients towards zero when distance is used as an independent variable. However, the runners included in these analyses were specifically chosen for the concordance of their baseline and follow-up running distances. Errors in recalling height, weight, and body circumferences should not bias the standard regression coefficients when used as dependent variables, but they will contribute to the error variance associated with the coefficients. The selection of this sample was based on reported differences in mileage between baseline and follow-up of less than ± 5 km/wk, but we do not know whether running distance deviated significantly between surveys, and this may have weakened the reported associations.
Another limitation is that we did not allocate participants at random to a prescribed running distance. Therefore, we cannot distinguish whether exercise caused the attenuation of age-related weight gain, or whether individuals who were genetically, behaviorally, or environmentally predisposed to a lower rate of weight gain over time were more successful at sustaining high levels of vigorous physical activity. Our analyses are based solely on runners, and running is a vigorous activity (> 6 METs); although we believe it likely that these results apply to other vigorous physical activities, this conjecture remains to be verified.
On the basis of the data presented here and by others, we conclude that maintaining a vigorously active lifestyle diminishes the apparently natural tendency to gain weight with age. Moreover, our analyses suggest that exercise prevents more extreme weight gain, and this may be especially beneficial given the curvilinear relationship between weight, morbidity, and mortality [16].
Acknowledgments
Supported in part by grant HL-45652 and HL-72110 from the National Heart Lung and Blood Institute and DK066738 from the Institute of Diabetes and Digestive and Kidney Diseases. Conducted at the Lawrence Berkeley Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
REFERENCES
- 1.DiPietro L. Physical activity in the prevention of obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S542–S546. doi: 10.1097/00005768-199911001-00009. [DOI] [PubMed] [Google Scholar]
- 2.DiPietro L, Dziura J, Blair SN. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the Aerobics Center Longitudinal Study. Int J Obes. 2004;28:1541–1547. doi: 10.1038/sj.ijo.0802821. [DOI] [PubMed] [Google Scholar]
- 3.Drøyvold WB, Holmen J, Midthjell K, Lydersen S. BMI change and leisure time physical activity (LTPA): an 11-y follow-up study in apparently healthy men aged 20–69 y with normal weight at baseline. Int J Obes. 2004;28:410–417. doi: 10.1038/sj.ijo.0802569. [DOI] [PubMed] [Google Scholar]
- 4.Efron B. The Jackknife, the Bootstrap and Other Resampling Plans. Capital City Press; Montpelier, VT: 1982. pp. 1–92. [Google Scholar]
- 5.Erlichman J, Kerbey AL, James WP. Physical activity and its impact on health outcomes. Paper 2: prevention of unhealthy weight gain and obesity by physical activity: an analysis of the evidence. Obes Rev. 2002;3:273–287. doi: 10.1046/j.1467-789x.2002.00078.x. [DOI] [PubMed] [Google Scholar]
- 6.Fogelholm M, Kukkonen-Hajula K. Does physical activity prevent weight gain? A systematic review. Obes Rev. 2002;1:95–111. doi: 10.1046/j.1467-789x.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 7.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int J Obes. 1997;21:288–296. doi: 10.1038/sj.ijo.0800403. [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington, DC: 2005. [Google Scholar]
- 9.Jackson AS, Beard EF, Wier LT, Ross RM, Stuteville JE, Blair SN. Changes in aerobic power of men, ages 25–70 yr. Med Sci Sports Exerc. 1995;27:113–120. [PubMed] [Google Scholar]
- 10.Jackson AS, Wier LT, Ayers GW, Beard EF, Stuteville JE, Blair SN. Changes in aerobic power of women, ages 20–64 yr. Med Sci Sports Exerc. 1996;28:884–891. doi: 10.1097/00005768-199607000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes Relat Metab Disord. 2005;29:524–533. doi: 10.1038/sj.ijo.0802886. [DOI] [PubMed] [Google Scholar]
- 12.Maes HHM, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- 13.Owens JF, Matthews KA, Wing RR, Kuller LH. Can physical activity mitigate the effect of aging in middle-aged women? Circulation. 1992;85:1265–1270. doi: 10.1161/01.cir.85.4.1265. [DOI] [PubMed] [Google Scholar]
- 14.Ross R, Janssen I. Is abdominal fat preferentially reduced in response to exercise-induced weight loss? Med Sci Sports Exerc. 1999;31(Suppl 11):S568–S572. doi: 10.1097/00005768-199911001-00014. [DOI] [PubMed] [Google Scholar]
- 15.Taylor CB, Jatulis DE, Winkleby MA, Rockhill BJ, Kraemer HC. Effects of life-style on body mass index change. Epidemiology. 1994;5:599–603. doi: 10.1097/00001648-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Troiano RP, Frongillo EA, Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996;20:63–75. [PubMed] [Google Scholar]
- 17.Votruba SB, Horvitz MA, Schoeller DA. The role of exercise in the treatment of obesity. Nutrition. 2000;16:179–188. doi: 10.1016/s0899-9007(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 18.Wier LT, Ayers GW, Jackson AS, Rossum AC, Poston WS, Foreyt JP. Determining the amount of physical activity needed for long-term weight control. Int J Obes Relat Metab Disord. 2001;25:613–621. doi: 10.1038/sj.ijo.0801586. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk with the “normal” weight range. JAMA. 1995;273:461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 20.Williams PT. Evidence for the incompatibility of age-neutral overweight and age-neutral physical activity standards from runners. Am J Clin Nutr. 1997;65:1391–1396. doi: 10.1093/ajcn/65.5.1391. [DOI] [PubMed] [Google Scholar]
- 21.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obesity. 2004;28:120–128. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 22.Williams PT. Nonlinear relationships between weekly walking distance and adiposity in 27,596 women. Med Sci Sports Exerc. 2005;37:1893–1901. doi: 10.1249/01.mss.0000175860.51204.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med Sci Sports Exerc. 2005;37:1329–1337. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 24.Williams PT, Satariano WA. Relationships of age and weekly running distance to BMI and circumferences in 41,582 physically active women. Obes Res. 2005;13:1370–1380. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]
- 25.Williams PT, Thompson PD. Dose dependent effects of training and detraining on weight in 6406 runners during 7.4 years. Obesity (Silver Spring) 2006;14:1975–1984. doi: 10.1038/oby.2006.231. [DOI] [PubMed] [Google Scholar]
- 26.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes Relat Metab Disord. 2006;30:543–551. doi: 10.1038/sj.ijo.0803172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a US national cohort. Int J Obes. 1993;17:279–286. [PubMed] [Google Scholar]
- 28.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–S552. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 29.Wood PD, Stefanick ML, Dreon DM, et al. Changes in plasma lipids and lipoproteins in overweight men during weight loss through dieting as compared with exercise. N Engl J Med. 1988;319:1173–1179. doi: 10.1056/NEJM198811033191801. [DOI] [PubMed] [Google Scholar]
- 30.Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight-reducing diet, with or without exercise, in overweight men and women. N Engl J Med. 1991;325:461–466. doi: 10.1056/NEJM199108153250703. [DOI] [PubMed] [Google Scholar]