Abstract
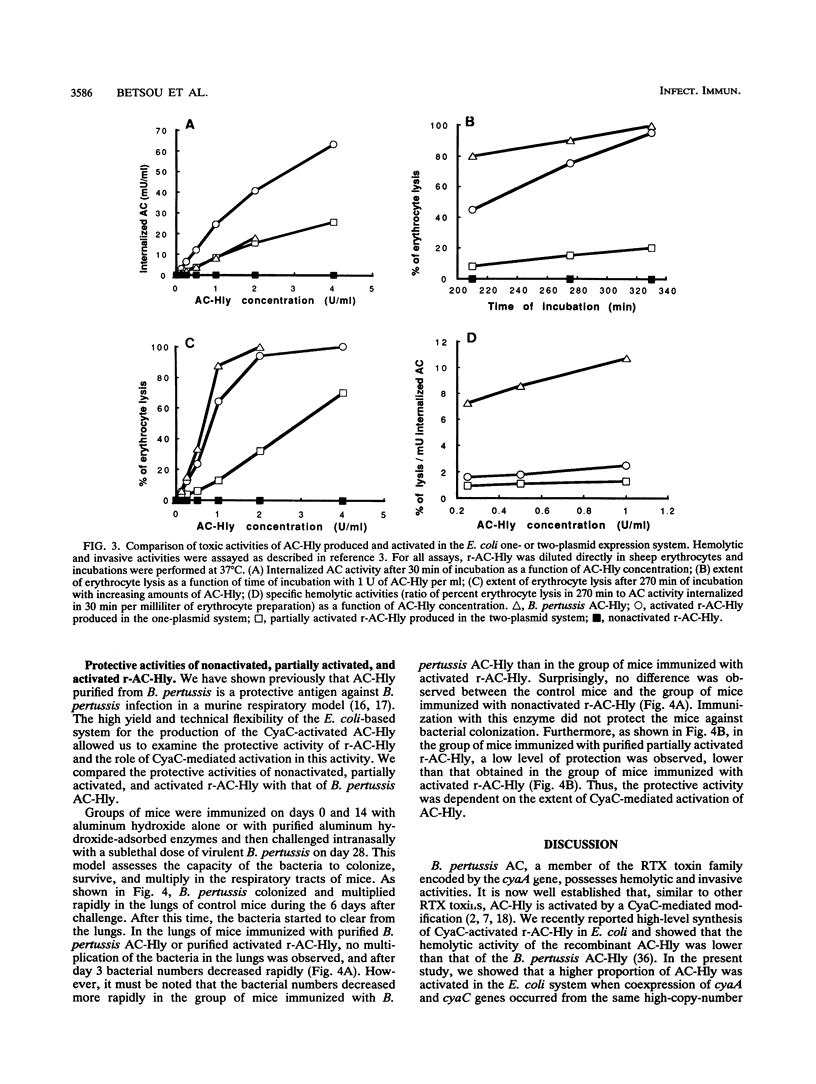
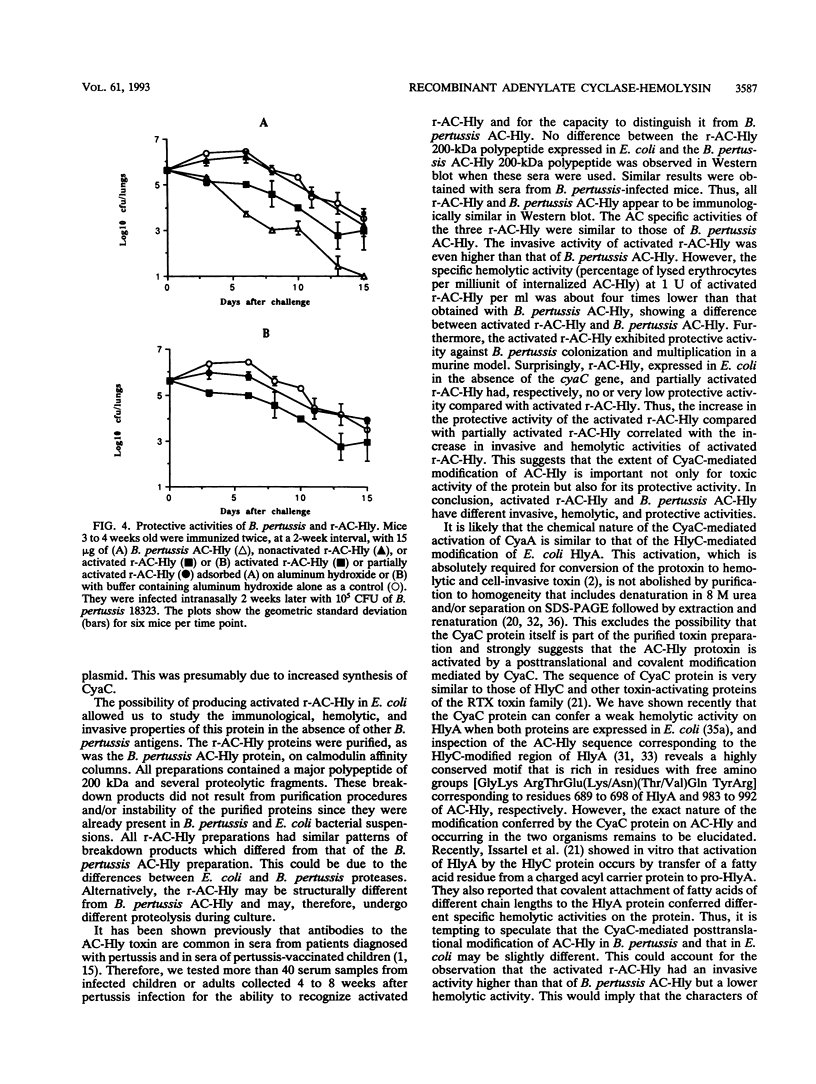
Bordetella pertussis adenylate cyclase-hemolysin (AC-Hly), encoded by the cyaA gene, belongs to the RTX family of toxins with extensive glycine-rich repeats in the carboxy-terminal portion. AC-Hly possesses both adenylate cyclase toxic and hemolytic activities that depend on a posttranslational modification mediated by the product of the cyaC gene. An improved system for AC-Hly synthesis and activation in Escherichia coli was developed. The results show that with purified AC-Hly (i) increased expression of the cyaC gene leads to a higher proportion of activated AC-Hly, (ii) the increase in protective activity of the activated recombinant AC-Hly correlates with the increase in its invasive and hemolytic activities, and (iii) the activated recombinant AC-Hly, but not the nonactivated recombinant AC-Hly, is a protective antigen against B. pertussis infection in a murine respiratory model. This suggests that possibly an immunodominant epitope required for protective activity is linked to the CyaC-mediated modification. Surprisingly, the protective and hemolytic activities of activated recombinant AC-Hly were lower than those of AC-Hly produced by B. pertussis, while its invasive activity was higher. This indicates that the modification of AC-Hly in B. pertussis and that in E. coli may differ.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arciniega J. L., Hewlett E. L., Johnson F. D., Deforest A., Wassilak S. G., Onorato I. M., Manclark C. R., Burns D. L. Human serologic response to envelope-associated proteins and adenylate cyclase toxin of Bordetella pertussis. J Infect Dis. 1991 Jan;163(1):135–142. doi: 10.1093/infdis/163.1.135. [DOI] [PubMed] [Google Scholar]
- Barry E. M., Weiss A. A., Ehrmann I. E., Gray M. C., Hewlett E. L., Goodwin M. S. Bordetella pertussis adenylate cyclase toxin and hemolytic activities require a second gene, cyaC, for activation. J Bacteriol. 1991 Jan;173(2):720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellalou J., Sakamoto H., Ladant D., Geoffroy C., Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990 Oct;58(10):3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Coote J. G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992 Feb;8(2):137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- Ehrmann I. E., Weiss A. A., Goodwin M. S., Gray M. C., Barry E., Hewlett E. L. Enzymatic activity of adenylate cyclase toxin from Bordetella pertussis is not required for hemolysis. FEBS Lett. 1992 Jun 8;304(1):51–56. doi: 10.1016/0014-5793(92)80587-7. [DOI] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M. S., Weiss A. A. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990 Oct;58(10):3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Young W. W., Jr, Lechler S. M., Gray M. C., Leppla S. H., Hewlett E. L. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem. 1989 Sep 5;264(25):14792–14796. [PubMed] [Google Scholar]
- Gray L., Baker K., Kenny B., Mackman N., Haigh R., Holland I. B. A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl. 1989;11:45–57. doi: 10.1242/jcs.1989.supplement_11.4. [DOI] [PubMed] [Google Scholar]
- Gross M. K., Au D. C., Smith A. L., Storm D. R. Targeted mutations that ablate either the adenylate cyclase or hemolysin function of the bifunctional cyaA toxin of Bordetella pertussis abolish virulence. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4898–4902. doi: 10.1073/pnas.89.11.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiso N., Rocancourt M., Szatanik M., Alonso J. M. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb Pathog. 1989 Nov;7(5):373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- Guiso N., Szatanik M., Rocancourt M. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb Pathog. 1991 Dec;11(6):423–431. doi: 10.1016/0882-4010(91)90038-c. [DOI] [PubMed] [Google Scholar]
- Hardie K. R., Issartel J. P., Koronakis E., Hughes C., Koronakis V. In vitro activation of Escherichia coli prohaemolysin to the mature membrane-targeted toxin requires HlyC and a low molecular-weight cytosolic polypeptide. Mol Microbiol. 1991 Jul;5(7):1669–1679. doi: 10.1111/j.1365-2958.1991.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Hewlett E. L., Gordon V. M., McCaffery J. D., Sutherland W. M., Gray M. C. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem. 1989 Nov 15;264(32):19379–19384. [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Khelef N., Danve B., Quentin-Millet M. J., Guiso N. Bordetella pertussis and Bordetella parapertussis: two immunologically distinct species. Infect Immun. 1993 Feb;61(2):486–490. doi: 10.1128/iai.61.2.486-490.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelef N., Sakamoto H., Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog. 1992 Mar;12(3):227–235. doi: 10.1016/0882-4010(92)90057-u. [DOI] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Ladant D., Michelson S., Sarfati R., Gilles A. M., Predeleanu R., Bârzu O. Characterization of the calmodulin-binding and of the catalytic domains of Bordetella pertussis adenylate cyclase. J Biol Chem. 1989 Mar 5;264(7):4015–4020. [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Vogel M., Goebel W. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol Gen Genet. 1987 Feb;206(2):238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Muller S., Briand J. P., Benz R., Schmid A., Goebel W. Haemolysin-derived synthetic peptides with pore-forming and haemolytic activity. Mol Microbiol. 1992 Jan;6(1):115–121. doi: 10.1111/j.1365-2958.1992.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Pellett S., Boehm D. F., Snyder I. S., Rowe G., Welch R. A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990 Mar;58(3):822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Schultz J. E., Brownlie R. M., Coote J. G., Parton R., Hanski E. Bordetella pertussis adenylate cyclase: purification and characterization of the toxic form of the enzyme. EMBO J. 1989 Sep;8(9):2755–2760. doi: 10.1002/j.1460-2075.1989.tb08417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Bellalou J., Sebo P., Ladant D. Bordetella pertussis adenylate cyclase toxin. Structural and functional independence of the catalytic and hemolytic activities. J Biol Chem. 1992 Jul 5;267(19):13598–13602. [PubMed] [Google Scholar]
- Sebo P., Glaser P., Sakamoto H., Ullmann A. High-level synthesis of active adenylate cyclase toxin of Bordetella pertussis in a reconstructed Escherichia coli system. Gene. 1991 Jul 31;104(1):19–24. doi: 10.1016/0378-1119(91)90459-o. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]



