Summary
Streptococcus pneumoniae is an important bacterial cause of sepsis, meningitis, pneumonia and otitis media. Pneumococcal disease is generally preceded by mucosal colonization with the homologous strain; hence, resistance to colonization may be an important aspect of resistance to disease. In humans, complement deficiency is a risk factor for development of pneumococcal disease. Although many studies have shown protective effects of complement during pneumonia and meningitis, there have been no studies reported that evaluate the role of complement in containment of pneumococcal colonization. To this end, we studied the role of complement in preventing progression of pneumococcal mucosal colonization to sepsis in a mouse model. Sepsis developed in sixty percent of complement-depleted mice following intranasal pneumococcal challenge, but not in control or neutrophil-depleted mice. Colonization density in the nasopharynx and local mucosal tissue was similar between complement-depleted and control mice before onset of sepsis. Immunization of complement-depleted mice with an intranasally administered whole cell pneumococcal vaccine (WCV) reduced progression toward sepsis and protected surviving mice against colonization comparably to complement-sufficient mice. We therefore conclude that complement prevents sepsis following pneumococcal colonization in a neutrophil-independent fashion, but WCV-induced adaptive immunity is complement-independent.
Keywords: Streptococcus pneumoniae, colonization, complement, innate immunity, adaptive immunity
Introduction
Streptococcus pneumoniae is an important bacterial cause of sepsis, meningitis, pneumonia and otitis media. Risk groups for pneumococcal disease include infants, the elderly and immunocompromised persons, specifically people with B-cell defects, complement-deficiencies, asplenia, interleukin-1 receptor defects and HIV infection [1]. Pneumococcal disease is generally preceded by mucosal colonization with the homologous strain; hence, resistance to colonization may be an important aspect of resistance to disease.
While a prerequisite for the development of invasive disease, colonization is usually asymptomatic. After a period of colonization, the host clears the pneumococcal strain from the nasopharynx [2]. Innate as well as adaptive immunity are believed to be involved in this process. An important role for complement has been implicated in both innate and adaptive immunity to many bacterial pathogens. Major functions of complement in host defense against infections are opsonization, leukocyte recruitment and activation, and lysis of bacteria and cells [3]. It is therefore not surprising that complement deficiencies in humans are correlated with an increased risk of pneumococcal invasive disease [1]. Animal studies using direct inoculation of pneumococci into normally sterile sites have confirmed this as well. Nakajima et al showed the protective role of complement in the development of experimental pneumococcal pneumonia in mice [4]. Kerr et al. have shown that innate immune defenses against pneumococcal pneumonia require pulmonary complement component C3 [5]. Propst et al. have recently shown the role of complement in early (non-neutrophil-mediated) and late (neutrophil-mediated) protection against pneumococcal pneumonia [6]. In meningitis, complement components C1q and C3 seem to be critical for the innate immune response to pneumococci [7].
However, to our knowledge, there have been no studies that evaluate the role of complement in containment of pneumococcal colonization. Two colonization studies in mice have described the effect of complement depletion on pneumococcal colonization 48 hours after challenge, but not on invasion [8, 9]. In the work described here, we wished to test the hypothesis that complement may serve to prevent colonizing bacteria in the nasopharynx from causing sepsis.
To this end, we studied the effect of complement depletion on colonization and disease following nasopharyngeal exposure in naïve mice. Our data show that although complement plays little or no role in controlling the initial density of pneumococcal colonization, it seems to be crucial for preventing the progression of colonization to sepsis. Furthermore, we evaluated whether acquired immunity to pneumococcal colonization was dependent on complement. To do so, we immunized mice with killed whole pneumococci and cholera toxin. This preparation is of interest both as a candidate whole cell vaccine (WCV) [10-12], and because the immunity induced appears to be very similar to that induced by mucosal exposure to live bacteria [[10-12], and hence may mimic the acquired immune response to natural colonization. We found that mice immunized with WCV were protected against colonization even when complement-depleted at the time of challenge. This suggests that this form of acquired immunity can act in the absence of complement.
MATERIALS AND METHODS
Bacteria for animal challenge
S. pneumoniae strain 0603 is a clinical isolate of capsular serotype 6B strain described previously [11]. This strain was grown to mid-log phase in Todd-Hewitt broth with 0.5% yeast extract, and stored at −80°C, in THY with 10% glycerol. The bacteria were thawed just prior to challenge, centrifuged and resuspended in saline at a target concentration of 108 CFU/ml; the actual colony count was determined on blood agar.
Mouse model of colonization
Nasopharyngeal colonization (NP) was studied as described previously [11]. Briefly, 5-6 week old C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) were challenged intranasally with a dose of 106 CFU of live encapsulated pneumococci (strain 0603). We determined colonization at day 2-7 post-challenge. After euthanasia by CO2 inhalation, an upper respiratory culture was done by instilling sterile saline retrograde through the transected trachea, collecting the first 6 drops (about 0.1 ml) from the nostrils, and plating neat or diluted samples on blood agar plates containing gentamicin (2.5 μg/ml). At any time point during the experiment, any ill-appearing or moribund mouse was immediately sacrificed and nasal wash/blood cultures obtained. The lower limit of detection for bacteremia was 100 cfu/ml of blood. For graphical representation and statistical analysis, a sterile nasal wash sample was assigned half the lower limit of detection, or 0.8 CFU/nasal wash. In a separate experiment, after nasal washes were taken, nasal tissue was excised as described previously [10] and prepared for culturing. Briefly, the palate was cut carefully after which the strips of nasal-associated lymphoid tissue (NALT) were peeled off. These strips of cells were collected in PBS, homogenized and plated neat and diluted on blood agar plates containing gentamicin (2.5 μg/ml).
Complement depletion
Hypocomplementemia was induced by intraperitoneal (i.p.) injection of 15 U/animal of cobra venom factor (CoVF; Quidel, San Diego, California, United States) in PBS 18 h prior to bacterial challenge. One injection has been shown to deplete complement within 4 hrs after injection and to reduce levels of C3 to less than 3% of normal [13]. Hypocomplementemia was shown to persist for at least 48 h per injection [13]. For colonization experiments where hypocomplementemia had to last for 8 days, injections with CoVF were given at -1, +1 and +4 days around pneumococcal challenge.
Neutrophil-like cell depletion
Neutrophil-like cells were depleted as described previously [10]. Shortly, neutrophils were depleted by i.p. injection of 100 μg of monoclonal antibody RB6-8C5 (α-Ly6G antibody) in PBS on days −1, +1 and +4 relative to challenge. The concentration of neutrophils in peripheral blood was reduced 90-100% when measured 48 hrs after injection (data not shown).
Whole cell vaccine (WCV)
The WCV was prepared as described previously [11]. Briefly, strain Rx1AL- (a capsule- and autoysin-negative strain) was grown in THY to late-log phase, killed by the addition of 70% ethanol, and resuspended in Lactated Ringers (whole cell antigen (WCA)). The WCV contained 108 (killed) cfu equivalents of WCA plus 1 μg of cholera toxin (CT) as adjuvant (List Biological Laboratories, Campbell, CA) per 10 μl dose.
Immunization of mice
Adult C57BL/6 mice were immunized intranasally at age 5-6 weeks as described previously [11]. In summary, immunization was delivered by gently restraining the unanesthetized mice and applying 10 μl of WCV or CT alone atraumatically to the nostrils. A second dose was given one week after the primary dose. All mice were challenged 4-5 weeks after vaccination.
Statistical analysis
Proportions of colonized mice were compared by Fisher's exact test and colonization density was evaluated by the Mann-Whitney U (MWU) test. Comparison of survival curves was done by the Log-rank test using PRISM software (version 5.0; GraphPad Software, Inc.)
RESULTS
Colonization leads to sepsis in complement-depleted, but not neutrophil-depleted or control mice
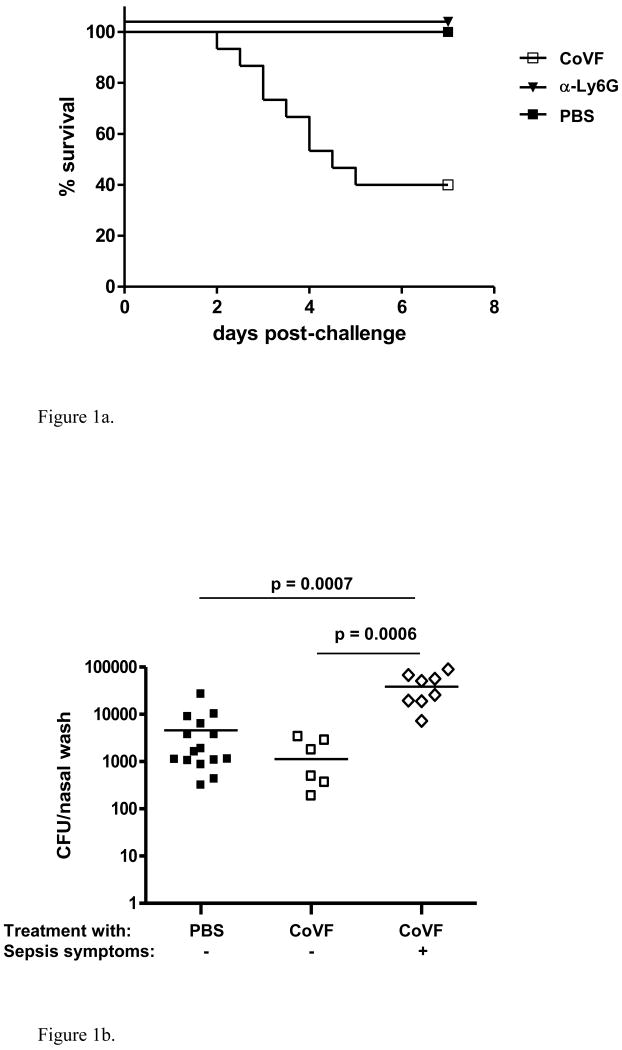
To study the role of complement in containment of pneumococcal colonization in mice, we depleted mice of complement for a period of 7 days with repeated doses of CoVF (C3 depletion), and challenged the mice intranasally with a serotype 6B pneumococcal strain. Between 2 and 7 days post-challenge 60% of the complement-depleted mice developed invasive pneumococcal disease (blood cultures positive for pneumococci with for most mice bacterial titers of more than 10ˆ4 CFU/ml) with rapid progression towards a moribund state, whereas none of the control mice showed any sign of illness nor detectable bacteremia at time of sacrifice (Log-rank p=0.0004, Figure 1a).
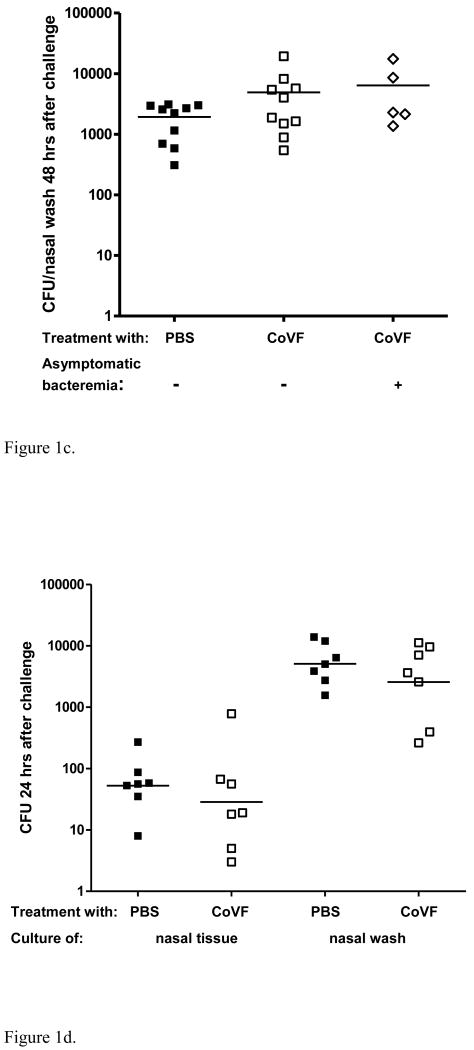
Figure 1.
Effect of complement depletion in naïve mice. 1a. Effect of complement depletion on survival following intranasal challenge with a serotype 6B pneumococcal strain. Complement-depletion resulted in sepsis and death in 9/15 (60%) of the mice between 2-7 days after challenge whereas 15/15 (100%) of the control mice survived (Log-rank test: p = 0.0004). 1b. Effect of complement depletion on density of nasopharyngeal pneumococcal colonization 7 days post-challenge. Nasopharyngeal colonization density on day 7 was similar in complement-depleted but healthy mice (n=6) compared to control mice (n=15), whereas the complement-depleted mice that developed symptomatic invasive disease within the first 7 days (data available for 8/9 septic mice) were colonized with a density that was almost 100× higher at time of death compared to both the surviving complement-depleted (MWU: p = 0.0006) as well as the control mice (MWU: p = 0.0007). 1c. Effect of complement depletion on density of nasopharyngeal pneumococcal colonization 48 hrs post-challenge. Early nasopharyngeal colonization with a pneumococcal serotype 6B strain did not differ significantly between complement-depleted (n=15) and control mice (n=10). Moreover, there was no difference in colonization density between CoVF treated mice with asymptomatic bacteremia and mice without bacteremia (n=5 versus n=10, respectively). 1d. Effect of complement depletion on density of nasopharyngeal colonization and invasion of NALT 24 hrs post-challenge. Densities of colonization from nasal washes and cultures of NALT following nasal exposure to a pneumococcal serotype 6B strain did not differ between complement-depleted (n=7) and control mice (n=7).
We also studied the role of neutrophils in containment of pneumococcal colonization in mice. We depleted mice of neutrophils with repeated doses of α-Ly6G antibodies for a period of at least 7 days, and challenged the mice intranasally with serotype 6B pneumococci. All mice in the neutrophil-depleted group as well as the control group remained healthy and had no detectable bacteremia at time of sacrifice (Figure 1a).
Colonization density in complement-depleted mice is elevated after, but not before the onset of sepsis
We studied colonization density by obtaining nasopharyngeal washes from the surviving complement-depleted and control mice 7 days post-challenge or in case of sepsis symptoms at time of sacrifice (2-7 days post-challenge). The median colonization density for complement depleted mice was 1.1× 103 cfu/nasal wash, versus 1.6× 103 cfu/nasal wash in control mice (MWU p = 0.23) (Figure 1b). The median colonization density for complement-depleted mice with sepsis symptoms at time of sacrifice was significantly higher compared to both healthy complement-depleted mice (MWU p < 0.001) and healthy controls (MWU p < 0.001).
To evaluate the role of colonization-density in development of sepsis, we also determined colonization densities 48 hrs after challenge, just prior to the first sepsis symptoms. Nasopharyngeal colonization on day 2 did not differ significantly between complement-depleted mice and control mice (Figure 1c), Since one third of the mice had developed asymptomatic bacteremia at this time-point, we re-analyzed the data for mice with and without pneumococci in their bloodstream. Again, no significant differences were found in colonization density between non-bacteremic and bacteremic complement-depleted mice or between bacteremic complement-depleted and control mice (Figure 1c).
Pneumococcal tissue invasion is not elevated in complement-depleted mice
To evaluate the role of complement in local tissue invasion, we studied both surface colonization measured by nasal wash and nasal tissue invasion measured by homogenization and plating of NALT in both complement-depleted and control mice 24 hrs after challenge (Figure 1d). We found no difference between complement-depleted and control mice in either NP colonization (3.6×10*3 versus 5.0×10*3 cfu/nasal wash; MWU p =0.46) or the concentration of bacteria in NALT (19 versus 53 cfu; MWU p = 0.41).
TH17-mediated pneumococcal clearance following active immunization is effective in complement-depleted mice
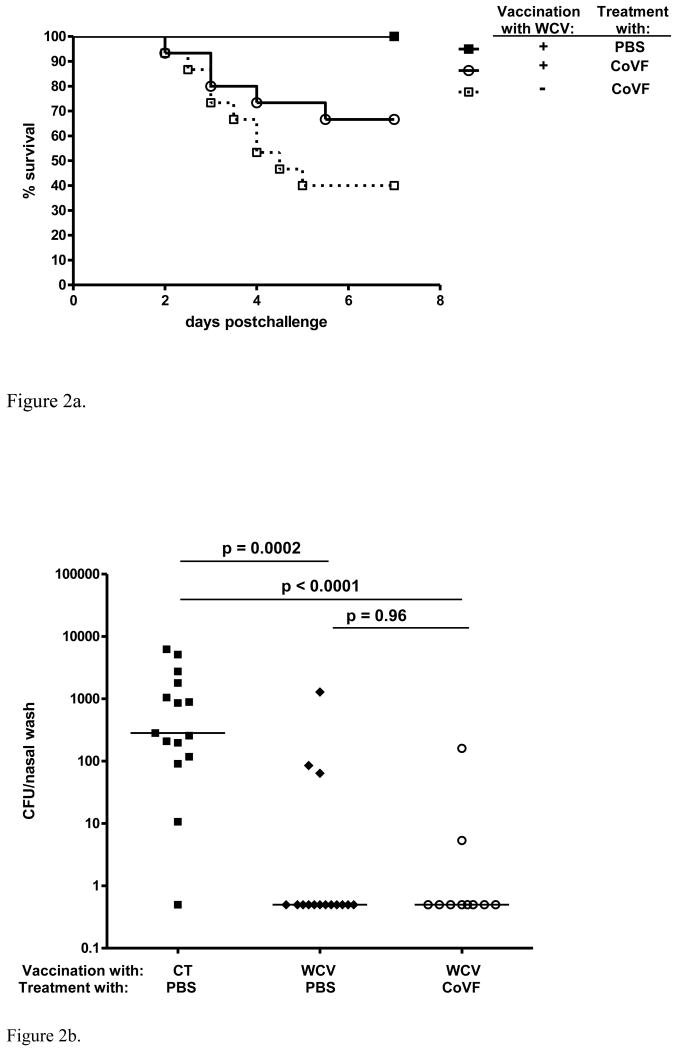
To evaluate whether complement is involved in the TH17-mediated clearance of pneumococci we immunized 6 week old mice twice with WCV. Four to six weeks after immunization we depleted these immunized mice of complement and challenged them with a serotype 6B pneumococcal strain. Groups of 15 mice were used. In WCV-immunized mice only 5 mice (33%) developed sepsis symptoms between 2-7 days post-challenge; this indicates an almost 50% reduction in sepsis incidence compared to non-immunized mice (9 of 15 mice, 60%), but this difference was not significantly different (Log-rank p = 0.13) (Figure 2a).
Figure 2.
Effect of complement depletion in WCV-immunized mice. 2a. Effect of WCV immunization on 7-day survival of complement-depleted mice challenged intranasally with a serotype 6B pneumococcal strain. Complement depletion resulted in sepsis and death in 5/15 (33%) of WCV immunized mice whereas all WCV-immunized mice (n=15) that were not complement-depleted survived without illness or bacteremia. 2b. Effect of complement depletion on WCV mediated protection against nasopharyngeal colonization with serotype 6B pneumococci 7 days post-challenge. As expected, in WCV-immunized mice that were not complement-depleted (n=15), density of colonization was reduced more than 100-fold compared to mice that received cholera toxin (CT) alone (n=15) and were not complement-depleted (MWU: p = 0.0002). Complement-depleted WCV-immunized mice (n=10) that survived 7 days post-challenge were equally protected against nasopharyngeal colonization as non-depleted WCV-immunized mice.
In addition, we studied colonization density 7 days post-challenge in, non-immunized mice as well as immunized complement-depleted and non-depleted mice. In the complement-depleted immunized group 8/10 surviving mice and in the non-depleted immunized group 12/15 mice had cleared the pneumococci from their nasopharynx (MWU p = 0.96), whereas 14/15 non-immunized mice were colonized (Figure 2b).
Discussion
The natural route of pneumococcal infection in humans is nasopharyngeal colonization followed by invasion and disease. Humans with complement deficiency are known to be at increased risk of invasive disease [14]. We hypothesized that in mice exposed to pneumococci intranasally, complement depletion may result in invasive disease. To study this, we depleted mice of complement and challenged the mice intranasally with a serotype 6B pneumococcal strain that does not normally lead to bacteremia in mice. In contrast to control mice, which remained healthy, the majority of complement-depleted mice developed severe pneumococcal disease. These data suggest a clear role for complement in containment of pneumococci during natural colonization.
Following these results, we tested whether neutrophil depletion similarly increased the risk of sepsis in colonized mice. Interestingly, none of the mice depleted of neutrophil-like cells developed bacteremia or sepsis. These data thus imply a neutrophil-independent role for complement, specifically C3, in the prevention of pneumococcal invasion following colonization. Since this model is performed in naïve mice, an antibody-independent mechanism of protection by complement is suggested as well. This is supported by previous findings by our group as well as others showing that congenitally antibody-deficient mice challenged with strain 603 remain healthy for at least 7 days following exposure [9, 15].
When nasopharyngeal washes were obtained at the end of the experiment (7 days post-challenge), we found no difference in colonization density between the surviving complement-depleted and control mice. These data strongly suggest a role for complement, specifically C3, in prevention of pneumococcal invasion following colonization. However, the density of colonization in the septic complement-depleted mice at time of sacrifice was significantly higher compared to the non-septic (and surviving) complement-depleted and control mice, suggesting the alternative hypothesis that complement is involved in control of colonization density, and that higher colonization density in some mice therefore led to sepsis. Since the onset of symptoms of sepsis in this experiment began as early as two days post-challenge, this alternative hypothesis would imply that complement depletion results in increased colonization density by day 2 post-challenge, with a subsequent increased risk of invasive disease.
To test this hypothesis, we performed two additional colonization experiments in complement-depleted and control mice. First, to determine colonization densities just prior to onset of sepsis, we compared nasopharyngeal colonization density 48 hrs after challenge. In line with previous findings [8, 9], nasopharyngeal colonization on day 2 did not differ significantly between complement-depleted mice and control mice, although the median density was about 2-fold higher in the complement-depleted mice. Interestingly however, 48 hrs after challenge already one third of the mice had developed bacteremia, although this was not clinically evident. Also no difference in colonization density was observed between bacteremic and non-bacteremic complement-depleted mice.
Secondly, we considered the possibility that complement reduces local invasion of mucosal tissues [16], which might be an intermediate step between surface colonization (measured by nasal wash) and systemic invasion. We found no differences between complement-depleted and control mice in either NP colonization or the concentration of bacteria in homogenized NALT, arguing against the hypothesis that complement prevents local tissue invasion of pneumococcus and consequently bloodstream invasion. Taken together, the data from these experiments support the alternative hypothesis that complement's major role is to enable rapid killing of invading bacteria. In this light, the higher density of pneumococcal colonization in the septic mice after 7 days of colonization may represent the consequence of tissue invasion accompanying the overwhelming bacterial load rather then a consequence of complement depletion by itself. Alternatively, differences in duration of colonization (7 days in healthy mice versus 2-6 days in septic mice) might be (partially) responsible for the observed difference in colonization density between both groups.
With respect to the adaptive immune response to pneumococci, much is known about the role of complement, especially C3b in the opsonophagocytic clearance of pneumococci by antibodies and neutrophils. Recently we have described a role for CD4+ TH17 cells and neutrophils, independent of antibody, in the prevention of pneumococcal colonization [10, 12]. The role of complement in this protection, however, has not been formally studied. To evaluate whether complement is involved in the TH17-mediated clearance of pneumococci we also performed complement-depletion in WCV-immunized mice. In WCV-immunized C3-depleted mice there was a trend towards improved survival with only 33% of the mice becoming systemically ill. Additionally, the surviving mice in the complement-depleted group were as well protected against nasopharyngeal colonization as control mice, suggesting that the protective effect of WCV against colonization is complement-independent [10-12, 15]. These data are potentially promising for the development of new preventive strategies for complement-deficient and other immunocompromised patients, although we have not rules out the possibility of an effect of complement-deficiency on the induction of TH17-type immunity by WCV.
In summary, we show here that complement plays an important role in prevention of invasive disease following mucosal exposure to pneumococcus. Given the large body of evidence about the role of complement in prevention of invasive pneumococcal disease, our data suggest that colonization may lead to frequent systemic pneumococcal invasion, which is readily controlled by complement. In contrast, when complement is absent, these challenges more often lead to invasive disease with measurable bacteremia.
Acknowledgments
We thank Dan Weinberger and Gili Regev-Yochay for helpful discussions. This work was supported by grants from the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences (D.B.) and by the National Institutes of Health grants R01 AI048935 (M.L.) and R01 AI066013 (R.M).
Footnotes
Presented in part at ISPPD-6, Reykjavik, Iceland, June 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Picard C, Puel A, Bustamante J, Ku CL, Casanova JL. Primary immunodeficiencies associated with pneumococcal disease. Current opinion in allergy and clinical immunology. 2003;3(6):451–9. doi: 10.1097/00130832-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Gray BM, Turner ME, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am J Epidemiol. 1982;116(4):692–703. doi: 10.1093/oxfordjournals.aje.a113452. [DOI] [PubMed] [Google Scholar]
- 3.Zwijnenburg PJ, van der Poll T, Florquin S, Polfliet MM, van den Berg TK, Dijkstra CD, et al. C1 inhibitor treatment improves host defense in pneumococcal meningitis in rats and mice. The Journal of infectious diseases. 2007;196(1):115–23. doi: 10.1086/518609. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima R, Namba K, Ishida Y, Une T, Osada Y. Protective role of complement in the development of experimental pneumococcal pneumonia in mice. Chemotherapy. 1990;36(4):287–93. doi: 10.1159/000238779. [DOI] [PubMed] [Google Scholar]
- 5.Kerr AR, Paterson GK, Riboldi-Tunnicliffe A, Mitchell TJ. Innate immune defense against pneumococcal pneumonia requires pulmonary complement component C3. Infection and immunity. 2005;73(7):4245–52. doi: 10.1128/IAI.73.7.4245-4252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Propst-Graham KL, Preheim LC, Vander Top EA, Snitily MU, Gentry-Nielsen MJ. Cirrhosis-induced defects in innate pulmonary defenses against Streptococcus pneumoniae. BMC microbiology. 2007;7:94. doi: 10.1186/1471-2180-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rupprecht TA, Angele B, Klein M, Heesemann J, Pfister HW, Botto M, et al. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J Immunol. 2007;178(3):1861–9. doi: 10.4049/jimmunol.178.3.1861. [DOI] [PubMed] [Google Scholar]
- 8.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS pathogens. 2005;1(1):e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infection and immunity. 2005;73(11):7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS pathogens. 2008;4(9):e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malley R, Lipsitch M, Stack A, Saladino R, Fleisher G, Pelton S, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infection and immunity. 2001;69(8):4870–3. doi: 10.1128/IAI.69.8.4870-4873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szalai AJ, Digerness SB, Agrawal A, Kearney JF, Bucy RP, Niwas S, et al. The Arthus reaction in rodents: species-specific requirement of complement. J Immunol. 2000;164(1):463–8. doi: 10.4049/jimmunol.164.1.463. [DOI] [PubMed] [Google Scholar]
- 14.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4(3):359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infection and immunity. 2006;74(4):2187–95. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]