SUMMARY
For two decades the glial sling has been hypothesized to act as a guidance substratum for developing callosal axons. However, neither the cellular nature of the sling nor its guidance properties have ever been clearly identified. Although originally thought to be glioblasts, we show here that the subventricular zone cells forming the sling are in fact neurons. Sling cells label with a number of neuronal markers and display electrophysiological properties characteristic of neurons and not glia. Furthermore, sling cells are continuously generated until early postnatal stages and do not appear to undergo widespread cell death. These data indicate that the sling may be a source of, or migratory pathway for, developing neurons in the rostral forebrain, suggesting additional functions for the sling independent of callosal axon guidance.
Keywords: Midline glia, Glial sling, Glial wedge, Corpus callosum, Axon guidance, Cell migration, Cortical development, Mouse
INTRODUCTION
In mouse, cortical axons forming the corpus callosum cross the midline at the boundary between the cingulate cortex and the septum (the corticoseptal boundary). The guidance of these axons across the midline has been thought to be provided by a group of cells, contiguous with the subventricular zone, called the glial sling (Silver et al., 1982). Sling cells migrate from the medial aspect of the lateral ventricles beginning on embryonic day (E) 15 of mouse development, forming the ‘sling’ structure by E17 at the cortical septal boundary (Silver et al., 1982; Hankin and Silver, 1988). Between the two cerebral hemispheres, the sling forms a continuous stream of cells with tight junctions linking the cells as they form the U-shaped sling structure (Silver et al., 1982). The axons of the corpus callosum cross the midline directly dorsal to the sling and the glial wedge (Silver et al., 1982; Shu and Richards, 2001), suggesting that the sling may be a guidance substratum for callosal axons.
Further evidence for the role of the sling in callosal axon pathfinding came from two types of experiments; ablation of the sling in utero which resulted in agenesis of the corpus callosum, and rescue of the sling in vivo in surgically induced acallosal animals (Silver et al., 1982; Silver and Ogawa, 1983; Katz et al., 1983; Smith et al., 1986). In the second series of experiments nitrocellulose membranes were inserted at the midline of acallosal mice and endogenous midline glia readily grew over the implant. Callosal axons were able to cross the midline only in the regions of the glial-covered implant (Silver and Ogawa, 1983). It was postulated that the cells that rescued the corpus callosum were derived from the sling.
However, in the early 1980s one of the limitations to studying the sling was the paucity of available markers for the sling. Based largely on their cellular morphology as determined by electron microscopic (EM) analysis, the sling was previously thought to be composed of glial cells (Silver et al., 1982). However since the sling did not label with GFAP and did not have the morphological characteristics of mature glia when viewed by EM (Silver et al., 1993), the cells were classified as glioblast cells rather than mature astrocytes. Given the lack of available markers for the sling, Silver and colleagues did not rule out the possibility that the sling may contain neurons or other cell types (Silver et al., 1982; Silver et al., 1993). In order to determine the putative axonal guidance molecules expressed by the sling we first sought to characterize the cellular makeup of the sling and identify molecular markers of these cells so that they could be easily studied and isolated.
Here we have identified a number of makers of the sling and characterized the electrophysiological properties of sling cells in whole-cell current clamp recordings. These data indicate that the sling is largely composed of neurons. We also find that the sling remains proliferative until early postnatal ages and that the disappearance of the sling by P10 is not due to widespread cell death. These findings suggest the hypothesis that the sling provides a pathway for developing neurons migrating from the subventricular zone (SVZ), possibly to populate adjacent regions of the brain.
MATERIALS AND METHODS
Animals
Embryonic and postnatal mice used in all experiments were derived from pregnant C57Bl/6J dams, where the day of the appearance of the vaginal plug was designated as E0. All procedures were approved by the University of Maryland School of Medicine and comply with NIH guidelines for the care and use of laboratory animals.
Immunohistochemistry
Embryos (collected as described below) were perfused with 4% paraformaldehyde and brains were sectioned with a vibratome into 50 μm thick sections. Immunohistochemistry was performed as previously described (Shu et al., 2000). Primary antibodies used were: (1) mouse anti-neuronal nuclei (NeuN; Chemicon, CA) 1: 3,000 for nickel-DAB reaction, 1:1000 for fluorescent Cy3 detection; (2) rabbit anti-cow glial fibrillary acidic protein (GFAP; DAKO, Denmark) 1:1000 for fluorescent Cy2 detection; (3) rabbit anti β-tubulin III (TUJ1; Babco, CA) 1:10,000 for Cy2 detection; (4) rabbit anti-calretinin (Swant) 1:500 for Cy2 detection; (5) mouse anti-bromodeoxyuridine (BrdU; DAKO) 1:1000 for Cy3 detection and (6) PCNA (DAKO) 1:1000 for Cy2 detection. Secondary antibodies used were: (1) biotinylated donkey anti-mouse (Jackson ImmunoResearch Laboratories) 1:600; (2) Cy2-conjugated donkey anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories) 1:400 and (3) Cy3-conjugated donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories) 1:400. Controls were performed for each antibody by eliminating the primary antibody to determine if there was non-specific staining from the secondary antibody alone.
Preparation of slices for electrophysiological recording
C57BL/6J mice between E17 and birth were obtained by anaesthetizing their mother with sodium phenobarbital (Nembutal; Abbott Laboratories, IL) at 0.07 mg/g body weight and then placing her on a warming pad to maintain body temperature. Once deeply anesthetized, the mother’s abdomen was opened to expose the uterus. Pups were removed sequentially and placed on ice, if older than E16, until deeply anesthetized. Pups were removed from the uterine horns and then decapitated before removing the brain. Postnatal mice were first anaesthetized on ice before decapitation and removal of the brain. Live brains were blocked in 3% low melting point agar (Sea plaque, FMC Bioproducts) in L-15 medium (Gibco BRL). Coronal sections were made on a vibratome (Leica) at 400 μm and the sections containing the sling were collected.
Slices were kept in a holding chamber that contained artificial cerebrospinal fluid (ACSF) at room temperature, aerated with 95% O2 and 5% CO2. ACSF was composed of (in mM): NaCl 124, NaHCO3 25, N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES) 5, KCl 3, MgSO4 1.3, CaCl2 2.0 and glucose 15. Recordings were made at room temperature in a submersion type chamber perfused with ACSF at 2 ml/minute. Electrode placement and cell selection were performed with near-infrared differential-interference-contrast microscopy (NIR-DIC), using 40× (0.8 NA) water immersion objective in a fixed-stage, upright microscope (BX50-WI; Olympus).
Whole-cell current-clamp recordings were obtained with an Axon 1D amplifier (Axon Instruments, Foster City, CA), from 36 sling cells from slices harvested from E17 (n=21), E18 (n=4), P0 (n=6), P1 (n=3) or P2 (n=2) mice and 4 glial wedge cells at E17. Recordings were digitized with an ITC-18 interface (Instrutech, Port Washington, NY) and acquired using PULSE software (HEKA Elektronik, Germany) on a Power PC Macintosh Computer (Apple, Inc., Cupertino, CA). The impedance of patch electrodes was 3 to 5 MΩ. The intracellular recording solution contained (in mM) potassium gluconate 120, KCl 10, Hepes 10, MgCl2 1, MgATP 2.5, Tris-GTP 0.2, BAPTA 0.1 and biocytin 2.6; pH was adjusted to 7.3. Chemicals were obtained from Sigma (St. Louis, MO).
Cells were filled with biocytin through the recording pipette. We recovered 10/36 of the recorded sling cells and 3/4 of the recorded glial wedge cells (due to fragility of the embryonic slices some cells were lost after recording probably as the patch pipette was removed). Slices were fixed overnight in a buffered solution containing 4% paraformaldehyde and then co-immunolabeled for either NeuN (sling cells) or GFAP (glial wedge cells) as described above. Visualization of the biocytin was performed as previously described (Gottlieb and Keller, 1997). In every case, biocytin-labeled cells stained with NeuN were localized to the sling, whereas biocytin-labeled, GFAP-positive cells were in the wedge, indicating that the anatomical location of these cells by infrared microscopy was adequate to identify the cells.
BrdU and TUNEL labeling
Bromodeoxyuridine was dissolved in sterile saline and injected intraperitoneally into pregnant dams at a concentration of 50 μg/g body weight on E15, E16, E17 and E18 respectively. Embryos from mothers injected on E15 and E16 were collected on E17 and those from mothers injected on E17 and E18 were collected on P0. Postnatally, P2 pups were injected with BrdU and collected on P3. The brains were processed for immunohistochemistry as described above. For time-lapse experiments BrdU was injected into pregnant dams at 9 am and embryos were collected at successive time points (separate litters were used for time points over 4 hours).
For TUNEL labeling, E17, E18, P0, P3, P5 and P10 brains were sectioned into 10 μm thick sections with a cryostat. An ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (S7111, Intergen) was used following the manufacturer’s protocol. The sections were further counterstained with ToTo-3 (Molecular Probes) to reveal brain morphology.
RESULTS
The ‘glial’ sling is largely composed of neurons
The sling is easily identified by its morphology and anatomical location by Nissl staining with Cresyl Violet (arrow in Fig. 1A). We have performed an extensive analysis of different markers to determine the cellular nature of the sling (Table 1). The nuclear neuronal marker NeuN (Mullen et al., 1992) labels sling cells as they migrate from the subventricular zone (SVZ) to the midline (Fig. 1B,C). Another marker for early differentiated neurons is TUJ1 (an antibody against β-tubulin), which labels the cell cytoplasm and processes (Moody et al., 1989). Fig. 1E,F shows neurons within the sling (arrows) double-labeled with NeuN (red in the nucleus) and TUJ1 (green in the cytoplasm). The calcium binding protein calretinin [a marker of the cortical subplate (Fonseca et al., 1995)] also labeled the sling (Fig. 1G,I), and double-labeled many of the cells that labeled with NeuN. However some neuronal markers such as neurofilament (Figlewicz et al., 1988), Hu (Okano and Darnell, 1997), GAP43 (Meiri et al., 1986; Goslin et al., 1988) and MAP2 (Ferreira et al., 1987; Niinobe et al., 1988) did not label the sling either pre- or postnatally (Table 1). We found that the same markers labeled the sling in both rostral and caudal regions of the sling. By contrast, the sling is not GFAP positive (Silver et al., 1993) as shown in figure 1D (green labeling), but other midline glial populations such as the glial wedge (Shu and Richards, 2001) (arrow in Fig. 1D) and glia within the indusium griseum are GFAP positive (Shu and Richards, 2001) (arrowhead in Fig. 1D).
Fig. 1.
Neuronal markers label the subcallosal sling. (A–I) Coronal sections from E17 C57Bl/6J mouse brains stained with either Cresyl Fast Violet (A) or antibodies directed against the molecules indicated. In C–F NeuN labeling is red (nuclear labeling) and GFAP and TUJ1 are green. Scale bar in F: 400 μm (A,B); 120 μm (C); 200 μm(D,E); 35 μm (F). Scale bar in I: 100 μm.
Table 1.
Markers used to identify the cellular nature of the sling
| Marker | Sling labeling |
|---|---|
| BLBP | − |
| Calretinin | + |
| DCC | + |
| Emx1 | + |
| ErbB2 | − |
| ErbB4 | + |
| Gap43 | − |
| GFAP | − |
| GLAST | − |
| HuC/D | − |
| MAP2 | − |
| NeuN | + |
| Nfia | + |
| RC2 | − |
| Robo1 | + |
| TUJ1 | + |
positive for the label; −, negative for the label. BLBP, brain lipid binding protein; DCC, deleted in colorectal cancer; GFAP, glial fibrillary acidic protein.
We have also identified four other markers that label the sling: two transcription factors, Emx1 and NFIA (Table 1) (Preston et al., 2000; Shu et al., 2003), and two transmembrane receptors, DCC (deleted in colorectal cancer, which is a receptor for netrin 1) (Shu et al., 2000) (Table 1) and Robo1 (roundabout 1, a receptor for slit2; Table 1). How these axon guidance receptors play a role in the development of the sling is not yet known. However, each of these genes are expressed in neurons, providing further evidence that the sling is largely composed of neurons.
Cells within the sling express neuronal physiological properties
To determine if sling cells are capable of producing regenerative currents in response to membrane depolarization we obtained whole-cell, current-clamp recordings from sling cells in an in vitro slice preparation. Using near-infrared video microscopy (Stuart et al., 1993) to identify sling cells below the corpus callosum (Fig. 2D), we obtained recordings from 36 sling cells from slices harvested between E17 and P2. Biocytin was included in the pipette solution to intracellularly fill the recorded neurons (Fig. 3).
Fig. 2.
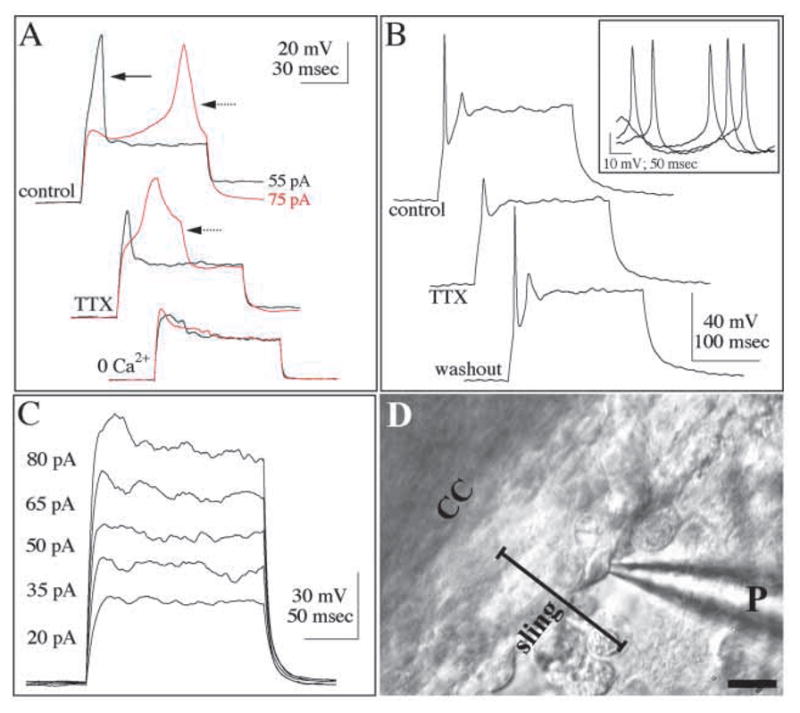
Patch-clamp recordings of sling neurons demonstrate that these cells fire action potentials. Current-clamp recordings in response to intracellular injection of depolarizing current pulses in (A,B) identified sling cells and (C) wedge cells. (A) Responses to 55 pA (black) and 75 pA (red), 100-msecond long current pulses of a sling cell at E18. The Na+-dependent spike (solid arrow) is suppressed after 8 minutes of TTX application, and the Ca2+-dependent spike (dotted arrow) is abolished in the absence of Ca2+ (0 Ca2+). At P2 (B) sling cells evoke TTX-sensitive Na+ spikes. Inset depicts spontaneous action potentials recorded, at rest, from the same cell; all spontaneous spikes were reversibly abolished by TTX. By contrast, identified glial wedge cells (C) respond to depolarizing currents with nearly linear increases in voltage responses, and evoke no regenerative spikes.
(D) Phase-interference contrast micrograph showing the sling lying directly underneath the corpus callosum (CC). A patch pipette (P) is in place to record from an identified sling cell. Sling cells were identified morphologically directly below the corpus callosum as they form a stream of tightly connected cells, with their leading processes pointing toward the midline (on the left). Only one focal plane is visible in this image, therefore surrounding sling cells are not in focus. Scale bar: 20 μm.
Fig. 3.

Cellular morphology of sling and glial wedge cells filled with biocytin during current-clamp recordings. In cellular recordings of the type shown in Fig. 2, the recording pipette was used to fill cells with biocytin. (A,B) E17 filled cells (red) within the sling (A) and glial wedge (B, arrow). Slices were doubled labeled with GFAP to identify the glial wedge (green labeling in B). (C,D) Cells at P0 within the sling showed a more mature neuronal morphology with multiple dendrites (red). The cell in D is shown double labeled with NeuN (arrow indicates a yellow nucleus). Scale bar: 20 μm.
At all ages examined, sling cells responded to depolarizing current injections with regenerative spikes. Both the passive and voltage-dependent properties of sling cells, and the kinetics of the regenerative spikes, changed during development. The estimated input resistance gradually decreased from 7.7±2.6 GΩ at E17 to 4.1±1.9 at P2. The resting membrane potential, −38.8±14.2 mV, at E17 became gradually more hyperpolarized during development, reaching −62.0±2.8 mV at P2 (Fig. 2). At E17 and E18 depolarizing current injections (100–250 mseconds duration) typically evoked a single regenerative spike, which appeared at the onset of the current pulse, having an amplitude of 83.2±11.3 mV, and a half-width duration of 8.9±4.2 mseconds. Varying the current intensity typically evoked a different spike with an onset latency of >50 mseconds, and both a higher amplitude (187.6±76.4 mV) and a longer duration (26.1±17.5 mseconds at half-width). The early, short duration and the late, long-duration spikes resemble Na+ and Ca2+-mediated action potentials respectively, as described in other neuronal classes (Gutnick and Crill, 1995). Indeed, the presumed Na+ spike was reversibly suppressed by bath-applying the Na+ channel antagonist tetrodotoxin (TTX, 1 μM; 3 of 4 cells), whereas the presumed Ca2+ spike was reversibly suppressed by either removing Ca2+ from the extracellular solution (2 of 2 cells), or by adding the Ca2+ channel antagonist Cd2+ (1 mM) to the extracellular solution (2 of 2 cells) (Fig. 2A).
In more mature sling neurons (P0-P2), Na+ spikes dominated the response to current injections, whereas Ca2+ spikes occurred only in 4 of 12 cells (Fig. 2B). When Ca2+ spikes did occur, they had a shorter duration (12.7±4.9 mseconds at half width) and a smaller amplitude (82.8±83.4 mV), compared with those recorded from E17-E18 sling cells. The Na+ spikes at P0-P2 also had a shorter duration (4.4±2.3 mseconds at half-width), but an amplitude indistinguishable from that of E17-E18 neurons (79.8±21.3 mV). TTX reversibly suppressed Na+ spikes in 5 of 6 P0-P2 cells, and addition of Cd2+ reversibly suppressed Ca2+ spikes in 4 of 4 of these neurons. In response to hyperpolarizing current injections, 6 of 11 neurons recorded at P0-P2 produced a response consistent with an anomalous inward rectification, and a rebound spike immediately following the termination of the hyperpolarizing pulse. This response, consistent with the existence of a hyperpolarization-activated inward current (Solomon et al., 1993) (Ih), was never observed in E17-E18 sling cells. Finally, 5 of 11 P0-P2 sling cells produced spontaneous action potentials at rest, whose kinetics and suppression by TTX (5 of 5 cells), identified them as Na+-mediated spikes (Fig. 2B).
Following the recordings cells were filled with biocytin and then processed for NeuN labeling. At E17, biocytin-labeled sling cells possessed a long leading process oriented toward the midline (Fig. 3A) and at P0 had multiple processes resembling dendrites (Fig. 3C,D). These findings, and the fact that all biocytin-filled sling cells were immunoreactive for the neuronal marker NeuN (Fig. 3D), indicate that our recordings were obtained from neurons in the sling.
As a control we compared the electrophysiological properties of the identified sling cells with those of the glial wedge, a known population of GFAP-positive glia present at the ventrolateral edges of the sling (Shu and Richards, 2001). In response to intracellular injections of depolarizing currents, all glial wedge cells (E17, n=4) produced non-rectifying responses, and none produced Na+ or Ca2+ spikes (Fig. 2C). Three biocytin-filled glial cells were recovered and processed for GFAP immunohistochemistry. All labeled with GFAP (Fig. 3B) and had a typical radial glial morphology as previously described (Shu and Richards, 2001). Therefore sling cells possessed the electrophysiological characteristics of neurons and not glia, and together with the immunohistochemical data described above provide compelling evidence that the majority of cells within the sling are neurons.
Birthdating of the sling
It was originally reported that sling cells were generated between E15 and E17 of mouse development (Silver et al., 1982). We have performed a birth-dating analysis of the sling by injecting BrdU between E12 and P2 and sacrificing the animals at E17, P0 or P3. We found no BrdU-labeled cells within the sling when BrdU was injected at E12-E14, but labeled sling cells were present when BrdU was injected between E15-P2 (the oldest age examined; Fig. 4). This confirms previous results showing that sling cells are born on E15 (Silver et al., 1982), but extends these results to show that sling cells continue to be generated until at least P2.
Fig. 4.
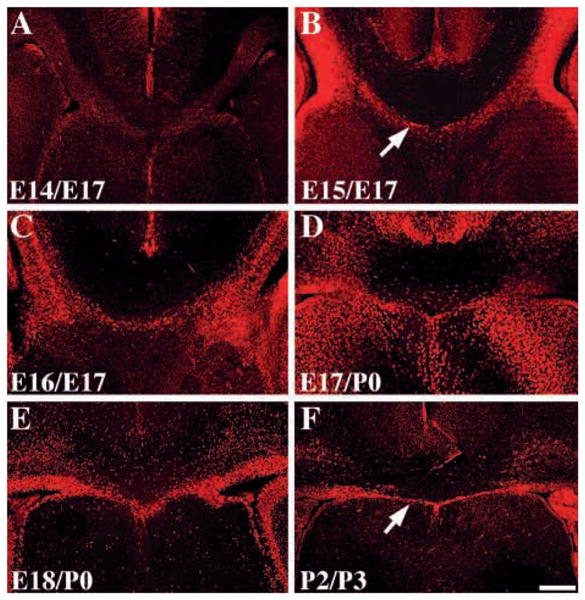
Birth dating of the sling. Coronal sections of brains from animals injected with BrdU on either E14 (A) E15 (B) or E16 (C) and sacrificed at E17. Animals were also injected at E17 (D) or E18 (E) and sacrificed at P0 or injected at P2 and sacrificed at P3 (F). All sections were processed for BrdU immunohistochemistry using a fluorescent (Cy3) secondary antibody. Arrows in B and F show the position of the sling. Scale bar: 250 μm in all panels.
Migration of the sling cells at the cortical midline
Based on the morphological development of the sling, it was previously hypothesized that the cells that make up the sling migrated from the SVZ to the midline (Silver et al., 1982). To investigate this directly we performed BrdU pulse-labeling experiments at both E16 and P3. Pregnant dams were injected with BrdU at 9 am on the day of the experiment and then sacrificed either 2, 4, 8, 16 or 24 hours later (Fig. 5A–E (E16) and Fig. 5G–J (P3)). The same litter was used for the 2 and 4 hour time points but all other data were obtained from separate litters. Sections were counterstained with the fluorescent marker Sytox green (Molecular Probes). BrdU has been reported to persist in the circulation for about half an hour (Packard et al., 1973; Nowakowski et al., 1989). Therefore a single BrdU injection should provide a snapshot of proliferating cells whose migratory distribution could then be followed in the short term. At both E16 and P3, BrdU labeling reached a maximum at 8 hours following the intraperitoneal injection into the pregnant dam (Fig. 5C,H). Animals sacrificed at successive time points revealed that BrdU labeling largely accumulated in the VZ/SVZ first, followed by the lateral edges of the sling and then more medial regions of the sling (arrows in Fig. 5A–D,H,I indicate the leading edge of the labeled migrating sling cells). This data indicated that sling cells were indeed migrating from the SVZ toward the midline.
Fig. 5.

Migration of the sling cells from the SVZ to the cortical midline. Individual litters were injected with BrdU at either E16 (A–F) or P3 (G–J) and then pups or whole litters were sacrificed at the times shown. Brains were sectioned coronally and processed for BrdU immunohistochemistry (red labeled cells in all panels) and counterstained with Sytox green (green cells in all panels except F). In F BrdU-labeled sections were double-labeled with PCNA which demonstrated the presence of proliferating cells within the sling (yellow cell in F). By using BrdU labeling sling cells could be seen migrating successively toward the midline (arrows in A–E and H and I). Scale bars: 200 μm in C for A–F and in J for G–J.
Some BrdU-positive cells were present at the midline as early as 8 hours after injection, indicating the possibility that some cells were generated within the sling itself rather than in the SVZ. To examine this we double-labeled BrdU-positive sections with the proliferative cell marker, proliferative cell nuclear antigen (PCNA; DAKO) 16 hours after BrdU injection. In this experiment some cells within the sling, particularly those present right at the midline, were undergoing proliferation. The rate of tangential cell migration within the cortex has been estimated at 15 μm/hour (O’Rourke et al., 1997) and in the rostral migratory stream (RMS) at 122 μm/hour while in the stream, and 24 μm/hour when invading the olfactory bulb (Wichterle et al., 1997). Therefore, based on these estimates sling neurons do have enough time to divide in the SVZ and migrate to the midline (a distance of ~400 μm in 24 hours), although clearly some cells within the sling are proliferating. Importantly, our successive analysis of BrdU-labeled cells demonstrates that the majority of sling cells migrate from the SVZ to the midline.
Postnatal development of the sling
Despite the continued proliferation of the sling cells, the structure itself does not continue to increase in size (other than to increase in proportion to the growth of the brain), and in fact it decreases slightly in size by P5 and disappears altogether by P10 (Fig. 6). Therefore the sling is a transient structure. In Nissl-stained, and NeuN-labeled sections, the sling forms a distinct structure at P0 (arrow in Fig. 6A,B) and P5 (Fig. 6C,D) but disappears by P10 (Fig. 6E,F).
Fig. 6.

Postnatal development of the sling. Coronal sections of P0 (A,B), P5 (C,D) or P10 (E,F) mouse brain were processed for NeuN immunohistochemistry (A,C,E) or for Cresyl Violet staining (B,D,F). The sling is present at P0 (arrows in A and B) and P5 (arrows in C,D), but absent by P10. Scale bar: 250 μm for all panels.
The disappearance of the sling has been attributed to a selective loss of the entire sling structure by an unknown mechanism of cell death (Hankin et al., 1988). In these experiments a number of picnotic nuclei were present in the sling at E17 and E18. Correlated with this was the formation of a small cavity called the cavum septum pellucidum (Hankin et al., 1988; Schneider and Silver, 1990). To investigate the extent of cell death within the sling we performed TUNEL labeling of the sling between E17 and P10. We found a few TUNEL-positive cells at E17 and E18 (arrows in Fig. 7A,B) but none at P0-P10 (Fig. 7C-F). This indicates that the disappearance of the sling may not be due to widespread cell death of the sling, but rather to migration of these cells away from the sling.
Fig. 7.
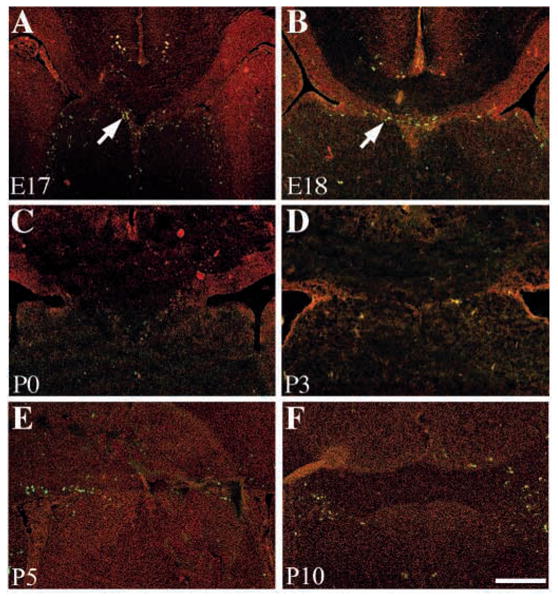
TUNEL labeling of the sling. Coronal cryostat sections at 10 μm were labeled using TUNEL and an FITC secondary antibody. TUNEL-positive cells were present within the sling at E17 and E18 (arrows in A and B respectively) but no TUNEL-positive cells were present at P0 (C) or P3 (D), despite the continuing generation of the sling cells at these ages (see Fig. 3). Even at P5 (E) and P10 (F) when the sling structure disappears there is no TUNEL labeling. Scale bar: 200 μm in all panels.
DISCUSSION
Our analysis of the sling reveals that these cells are differentiated neurons displaying typical neuronal physiological and immunohistochemical properties. In addition, sling cells do not label with glial markers such as RC2, BLBP (Kurtz et al., 1994; Feng et al., 1994), GLAST (Danbolt, 2001) or GFAP, and have distinct physiological properties from neighboring glial cells that do label with these markers.
The electrophysiological recordings we obtained from the sling cells changed during development. Early in development (E17-E18) sling neurons show long-duration Na+ and Ca2+ spikes, as do developing neurons elsewhere in the central nervous system (McCormick and Prince, 1987). Postnatally the Ca2+ spikes become smaller and less frequent, and the kinetics of Na+ spikes resemble those of more mature neurons (Gutnick and Modi, 1995). However, sling neurons did not label with markers of mature neurons such as neurofilament, GAP43 or MAP2 either pre- or postnatally, indicating they may represent an immature population of neurons. It is therefore unclear whether the same cells remain within the sling and become progressively more mature or whether new cells continue to migrate through the sling. The results of our BrdU time-lapse experiment suggest that the majority of neurons continue to migrate through the sling even at P3. It is therefore possible that sling cells migrating postnatally may have different cellular characteristics than those migrating at prenatal stages. In addition, given these results, more sling cells may be generated than previously thought. Further investigation is required to determine both the site of origin and the final destination of neurons migrating through the sling throughout development.
Originally the sling was described as only those cells that migrate from the medial aspect of the SVZ to the midline (Silver et al., 1982; Silver and Ogawa, 1983; Katz et al., 1983; Smith et al., 1986; Hankin and Sliver, 1988; Schneider and Silver, 1990). We have used this definition of the sling. In a later study Silver and colleagues (Silver et al., 1993) described a radial glial-like population of cells in cat that sit at the lateral edges of the sling. They postulated these may be the lateral extent of the sling and expanded the definition of the sling to include these cells. These radial glial-like cells are probably the equivalent of the glial wedge described in rodents (Shu and Richards, 2001). However, here we show that the glial wedge and the sling cells derived from the SVZ are clearly two different populations. Therefore here we have referred to the ‘sling’ as being only those cells originally described as the sling that are derived from the SVZ (Silver et al., 1982; Hankin and Silver, 1983; Silver and Ogawa, 1983; Katz et al., 1983; Smith et al., 1986; Schneider and Silver, 1990).
The prior classification of the sling as glioblast cells, the timing of sling formation and their location below the corpus callosum made it plausible that a function of the sling was to guide callosal axons across the midline. Furthermore, in vivo manipulations of the sling and rescue of the sling in surgically acallosal mice provided compelling evidence for their role in callosal axon guidance (Silver et al., 1982; Silver and Ogawa, 1983; Katz et al., 1983). Key to this hypothesis was the fact that the sling was formed prior to the time callosal axons cross the midline [which was previously thought to be at E18 (Floeter and Jones, 1985)]. However, recent experiments indicate that the pioneering axons of the corpus callosum (derived from the cingulate cortex) begin to cross the midline as early as E15.5 (Rash and Richards, 2001), before the sling structure has formed at E17 (Silver et al., 1982).
In previous experiments in which the sling was severed, the corpus callosum could be rescued by implanting a piece of cellulose membrane at the midline over which GFAP-positive glial cells grew, followed by callosal axons (Silver and Ogawa, 1983; Smith et al., 1986). We show that the sling is largely composed of neurons indicating that the cells that rescued the corpus callosum in these experiments may not have been sling cells (at least not the SVZ-derived sling cells, which are not GFAP positive) but glial cells from other midline populations (possibly from the lateral edges of the sling). Our results do not rule out the possibility that the sling is involved in callosal axon guidance in some capacity, particularly at E17 when the sling is formed and the majority of callosal axons begin to cross the midline, or that it may be involved in the maintenance of the corpus callosum. It is still conceivable that a migratory population of neurons may be used by callosal axons to cross the midline after the pioneering axons have crossed. But our findings do indicate that the initial cortical axons that cross the midline (Rash and Richards, 2001) do not require the sling. Our data also suggest that the sling should not be grouped with other midline glial populations involved in guiding other midline commissures such as the optic chiasm and the anterior commissure (Marcus et al., 1995; Cummings et al., 1997; Pires-Neto et al., 1998) since the sling cells derived from the SVZ are not glia.
The postnatal disappearance of the sling has been attributed to a selective loss of the entire sling structure by an unknown mechanism of cell death (Hankin et al., 1988). However, our TUNEL labeling of the sling shows that very few of the cells die at E17 and E18 and that none die at postnatal ages. Therefore, unless cell death and clearance of the cells is occurring so rapidly that it cannot be detected with this technique, cell death alone cannot explain either the disappearance of the sling or the postnatal decrease in the size of the sling. Clearance of the sling has been proposed to occur by macrophage-mediated endocytosis, leaving the cavum septum pellucidum behind (Hankin et al., 1988). We have observed macrophage-like cells at the cortical midline (T.S. and L.J.R., unpublished observation) but have not determined which, if any, cells are removed by them. An alternative hypothesis for the disappearance of the sling is that the cells continue to migrate from the SVZ of the dorsal telencephalon to adjacent brain regions. Long distance migration of cells within the SVZ has been shown to occur from cells that originate from the medial ganglionic eminence (Anderson et al., 2001; Marin et al., 2001). In the rostral migratory stream, cells leave the SVZ and migrate to the olfactory bulbs where they give rise to granule neurons (Luskin, 1993; Lois and Alvaerz-Buylla, 1994). It is possible that sling cells do not cease to migrate when they reach the midline but continue to migrate and populate adjacent regions of the brain.
We therefore redefine the sling as a migratory population of neurons whose function may include the guidance of callosal axons across the midline. These new findings open the possibility for additional hypotheses about the developmental function of this distinct population of cells. Sling neurons label with many of the same markers as subplate neurons of the cortical plate, although sling neurons are born after those of the subplate. The subplate is involved in axonal guidance, and regionalization and patterning of the neocortex (McConnell et al., 1989; Ghosh et al., 1990; De Carlos and O’Leary, 1992; Ghosh and Shatz, 1992; Ghosh and Shatz, 1993; Ghosh and Shatz, 1994; McConnell et al., 1994; Molnar et al., 1998). The developmental significance of the sling is yet to be determined, but the possibility of novel functions for this population in forebrain development needs to be examined in order to determine their true potential.
Acknowledgments
We thank Ms Kimberley M. Valentino for excellent technical assistance, Dr N. Heintz (Rockefeller University, New York, USA), for the gift of the BLBP antibody and Dr N.C. Danbolt (University of Oslo, Norway) for the gift of the GLAST antibody. We are grateful for discussions and input from Dr Jerry Silver (Case Western Reserve University) on an earlier version of the manuscript. This work was supported by a Basil O’Connor Starter Research Award to L.J.R. from The March of Dimes Foundation for Birth Defects, grant 5-FY99-842 and NIH grants NS37792 (L.J.R.) and NS31078, NS35360 (A.K.).
References
- Anderson SA, Martin O, Horn C, Jennings K, Rubenstein JLR. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Malun D, Brunjes PC. Development of the anterior commissure in the opossum: midline extracellular space and glia coincide with early axon decussation. J Neurobiol. 1997;32:403–414. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- De Carols JA, O’Leary DD. Growth and targeting of subplate axons and establishment of major cortical pathways. J Neurosci. 1992;12:1194–1211. doi: 10.1523/JNEUROSCI.12-04-01194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Busciglio J, Caceres A. An immunocytochemcical analysis of the ontogeny of the microtubule-associated proteins MAP-2 and Tau in the nervous system of the rat. Brain Res. 1987;431:9–31. doi: 10.1016/0165-3806(87)90191-x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DA, Gremo F, Innocenti GM. Differential expression of neurofilament subunits in the developing corpus callosum. Brain Res. 1988;470:181–189. doi: 10.1016/0165-3806(88)90236-2. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Jones EG. The morphology and phased outgrowth of callosal axons in the fetal rat. Brain Res. 1985;354:7–18. doi: 10.1016/0165-3806(85)90064-1. [DOI] [PubMed] [Google Scholar]
- Fonseca M, del Rio JA, Martinez A, Gomez S, Soriano E. Development of calretinin immunoreactivity in the neocortex of the rat. J Comp Neurol. 1995;361:177–192. doi: 10.1002/cne.903610114. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992;12:39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. A role for subplate neurons in the patterning of connections from thalamus to cortex. Development. 1993;117:1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. Segregation of geniculocortical afferents during the critical period: a role for subplate neurons. J Neurosci. 1994;14:3862–3880. doi: 10.1523/JNEUROSCI.14-06-03862.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JH, Banker G. Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature. 1988;336:672–674. doi: 10.1038/336672a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb JP, Keller A. Intrinsic circuitry and physiological properties of pyramidal neurons in rat barrel cortex. Exp Brain Res. 1997;115:47–60. doi: 10.1007/pl00005684. [DOI] [PubMed] [Google Scholar]
- Gutnick MJ, Crill WE. The cortical neuron as an electrophysiological unit. In: Gutnick MJ, Modi I, editors. The Cortical Neuron. New York: Oxford University Press; 1995. pp. 33–51. [Google Scholar]
- Gutnick MJ, Modi I. The Cortical Neuron. New York: Oxford University Press; 1995. [Google Scholar]
- Hankin MH, Silver J. Development of intersecting CNS fiber tracts: the corpus callosum and its perforating fiber pathway. J Comp Neurol. 1988;272:177–190. doi: 10.1002/cne.902720203. [DOI] [PubMed] [Google Scholar]
- Hankin MH, Schneider BF, Silver J. Death of the subcallosal glial sling is correlated with formation of the cavum septi pellucidi. J Comp Neurol. 1988;272:191–202. doi: 10.1002/cne.902720204. [DOI] [PubMed] [Google Scholar]
- Katz MJ, Lasek RJ, Silver J. Ontophyletics of the nervous system: development of the corpus callosum and evolution of axon tracts. Proc Natl Acad Sci USA. 1983;80:5936–5940. doi: 10.1073/pnas.80.19.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A, Zimmer A, Schnutgen F, Bruning G, Spener F, Mullen T. The expression pattern of a novel gene encoding brain-fatty acid binding protein correlates with neuronal and glial cell development. Development. 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Blazeski R, Godement P, Mason CA. Retinal axon divergences in the optic chiasm: uncrossed axons diverge from crossed axons within a midline glial specialization. J Neurosci. 1995;15:3716–3729. doi: 10.1523/JNEUROSCI.15-05-03716.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neruopilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol. 1987;393:743–762. doi: 10.1113/jphysiol.1987.sp016851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci USA. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody SA, Quigg MS, Frankfurter A. Development of the peripheral trigeminal system in the chick revealed by an isotope-specific anit-beta-tubulin monoclonal antibody. J Comp Neurol. 1989;279:567–580. doi: 10.1002/cne.902790406. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Niinobe M, Maeda N, Ino H, Mikoshiba K. Characterization of microtubule-associated protein 2 from mouse brain and its localization in the cerebellar cortex. J Neurochem. 1988;51:1132–1139. doi: 10.1111/j.1471-4159.1988.tb03078.x. [DOI] [PubMed] [Google Scholar]
- Nowakowski RA, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke NA, Chenn A, McConnell SK. Postmitotic neurons migrate tangentially in the cortical ventricular zone. Development. 1997;124:997–1005. doi: 10.1242/dev.124.5.997. [DOI] [PubMed] [Google Scholar]
- Packard DS, Menzies RA, Skalko RG. Incorporation of thymidine and its analog, bromodeoxyuridine, into embryos and maternal tissues of the mouse. Differentiation. 1973;1:397–405. doi: 10.1111/j.1432-0436.1973.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Pires-Neto M, Braga-De-Souza S, Lent R. Molecular tunnels and boundaries for growing axons in the anterior commissure of hamster embryos. J Comp Neurol. 1998;399:176–188. doi: 10.1002/(sici)1096-9861(19980921)399:2<176::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Preston SL, Shu T, Corte G, Rubenstein JLR, Richards LJ. Emx-1 is required for the development of the glial sling and formation of the corpus callosum. Soc Neurosci Abst. 2000;218(13):577. [Google Scholar]
- Rash BG, Richards LJ. A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol. 2001;434:147–157. doi: 10.1002/cne.1170. [DOI] [PubMed] [Google Scholar]
- Schneider BF, Silver J. Failure of the subcallosal sling to develop after embryonic X-irradiation is correlated with absence of the cavum septi. J Comp Neurol. 1990;299:462–469. doi: 10.1002/cne.902990406. [DOI] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glial during development of the corpus callousm. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Valentino KM, Seaman C, Cooper HM, Richards LJ. Expression of the netrin receptor, deleted in colorectal cancer (DCC), is largely confined to projecting neurons in the developing forebrain. J Comp Neurol. 2000;416:201–212. doi: 10.1002/(sici)1096-9861(20000110)416:2<201::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Shu T, Butz KG, Plachez C, Gronostajski RM, Richards LJ. Abnormal development of forebrain midline glia and commissural projections in Nfia knock-out mice. J Neurosci. 2003;23:203–212. doi: 10.1523/JNEUROSCI.23-01-00203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Edwards MA, Levitt P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- Silver J, Lorenz SE, Wahlsten D, Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- Silver J, Ogawa MY. Postnatally induced formation of the corpus callosum in acallosal mice on glia-coated cellulose bridges. Science. 1983;220:1067–1069. doi: 10.1126/science.6844928. [DOI] [PubMed] [Google Scholar]
- Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- Solomon JS, Doyle JF, Burkhalter A, Nerbonne JM. Differential expression of hyperpolarization-activated currents reveals distinct classes of visual cortical projection neurons. J Neurosci. 1993;13:5082–5091. doi: 10.1523/JNEUROSCI.13-12-05082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Eur J Physiol. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Alvarez-Buylla A. Direct evidence for homotypic, glia-independent neuronal migration. Neuron. 1997;19:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]



