Abstract
Objective
To determine whether graft survival over a 5-year follow-up period using corneal tissue from donors older than 65 years of age is similar to graft survival using corneas from younger donors.
Design
Multi-center prospective, double-masked, controlled clinical trial
Participants
1090 subjects undergoing corneal transplantation for a moderate risk condition (principally Fuchs’ dystrophy or pseudophakic corneal edema); 11 subjects with ineligible diagnoses were not included
Methods
43 participating eye banks provided corneas from donors in the age range of 12 to 75 with endothelial cell densities of 2300 to 3300 cells/mm2, using a random approach without respect to recipient factors. The 105 participating surgeons at 80 sites were masked to information about the donor cornea including donor age. Surgery and post-operative care were performed according to the surgeons’ usual routines. Subjects were followed for five years.
Main Outcome Measures
Graft failure, defined as a regraft or a cloudy cornea that was sufficiently opaque as to compromise vision for a minimum of three consecutive months.
Results
The 5-year cumulative probability of graft survival was 86% in both the <66.0 donor age group and the ≥66.0 donor age group (difference = 0%, upper limit of one-sided 95% confidence interval = 4%). In a statistical model with donor age as a continuous variable, there was not a significant relationship between donor age and outcome (P=0.11). Three graft failures were due to primary donor failure, 8 to uncorrectable refractive error, 48 to graft rejection, 46 to endothelial decompensation (23 of which had a prior, resolved episode of probable or definite graft rejection), and 30 to other causes. The distribution of the causes of graft failure did not differ between donor age groups.
Conclusions
Five-year graft survival for cornea transplants at moderate risk for failure is similar using corneas from donors ≥ 66.0 years and donors < 66.0 years. Surgeons and patients now have evidence that corneas comparable in quality to those used in this study from donors through age 75 years are suitable for transplantation.
INTRODUCTION
More than 33,000 cornea transplants are performed annually in the United States.1 Although the supply of donor corneas has been adequate for the last decade in the United States, this may not be the case in the future, particularly in light of recent changes in the Food and Drug Administration’s regulations regarding corneal transplantation2 which are expected to decrease the pool of eligible donors. In addition, there is a shortage of corneal tissue internationally.
Whether donor age should be used to determine suitability of a cornea for transplantation has been an area of considerable controversy among corneal surgeons in the United States. The debate will intensify if, as expected, the pool of tissue from younger donors shrinks and the availability of older donor tissue expands as the United States population over age 65 continues to increase. Already, approximately half of cornea donors are older than 60.1 Utilization of older donor tissue could potentially expand the donor pool perhaps by as much as 20–35%.3–7 However, many United States eye banks have arbitrarily set the upper age limit for donor eligibility at 65 years or less because some surgeons are reluctant to use corneas from older donors. This reluctance is not based on scientific evidence, as prior studies have not demonstrated that donor age is an important factor in determining transplant success if the quality of the donor cornea, including the endothelial cell density, is controlled for. These studies have often had inadequate experimental designs and/or small sample sizes which have hindered definitive conclusions as to the impact of donor age on graft survival. Thus, the question of whether graft survival is affected by donor age remains unanswered.
In order to evaluate the effect of donor age on cornea graft survival, the Cornea Donor Study (CDS) was designed to determine whether the graft-survival rate over a 5-year follow-up period is similar with corneal tissue from donors older than 65 years of age compared with that from younger donors.
METHODS
The protocol was approved by institutional review boards for all investigational sites. Each subject gave written informed consent to participate in the study. Study oversight was provided by an independent data and safety monitoring committee.
Eligible subjects were between 40 and 80 years old and had a corneal disease which placed them at moderate risk for graft failure (principally Fuchs’ dystrophy and pseudophakic corneal edema). An eye was not eligible if it was considered to be at high risk for failure (such as failed prior penetrating keratoplasty in the eye to be transplanted, chemical burns, significant cicatricial conjunctivitis, Herpes simplex/zoster, temporary keratoprosthesis, iridocorneal endothelial syndrome, any corneal condition in which there were two or more quadrants of stromal neovascularization, uncontrolled glaucoma or prior filtering surgery with placement of a shunt, or uncontrolled uveitis). Eyes at low risk for failure (such as keratoconus, stromal dystrophies, stromal scars without edema or post-refractive surgery with healthy endothelium) were also excluded. Only one eye per subject could be enrolled in the study. A complete listing of the subject eligibility criteria has been previously published.8
Donor corneas were procured and evaluated according to standard procedures of each eye bank, including assessment of endothelial cell density by specular microscopy. Eligible corneas were from donors 10 to 75 years old (though the youngest donor was 12 years old) with an eye bank measured endothelial cell density from 2300 to 3300 cells/mm2. The lower end was included to increase surgeon willingness to participate in the study without being provided with the actual cell count. The upper end was included to have sufficient balance in cell counts across the range of donor ages since higher cell counts are unusual in corneas from older donors. All donor tissue met Eye Bank Association of America (EBAA) standards for human corneal transplantation.9 Study-specific criteria were consistent with most eye banks’ tissue ratings of “good” to “excellent.” A complete listing of the donor cornea criteria has been published.10 Twenty-three of the eye banks participated in an ancillary study in which the donor specular images were sent to a central reading center for independent determination of endothelial cell density.11–12
On the day of tissue assignment for each study subject, a web-based computer program was used to select a cornea from those available at the eye bank that met the study eligibility criteria. The program randomly selected a cornea based on a two-level minimization procedure which attempted first to balance for each surgeon the number of corneas from donors ≥66 and <66 years old and then, when possible, to balance among age subgroups of 10–35, 36–50, 51–65, 66–70, and 71–75 years. The assignment was made without regard to recipient age or any other subject characteristics. If an eligible cornea was not available, one was imported from another eye bank.
Clinical investigators and subjects were masked to all characteristics of the donor cornea including age and endothelial cell density. Preoperative management, surgical technique, and postoperative care, including prescription of medications, were provided according to each investigator’s customary routine.
The follow-up visit schedule for the initial six months was left to each investigator’s discretion. Thereafter, the minimum follow-up visit schedule included a visit between six and 12 months and then one visit every 12 months through five years. The time window for the five-year visit extended from 58 months to 66 months. Additional visits were at the discretion of each investigator. Data collection at each visit was limited and included an assessment of graft clarity, signs of graft rejection, and intraocular pressure. A subject whose graft became cloudy and/or had signs of allograft rejection was treated according to the investigator’s usual routine.
Graft Failure
The primary study outcome was graft survival. The definition of graft failure, based on the definition used in Collaborative Corneal Transplantation Studies (CCTS)13, 14, was a regraft or, in the absence of regraft, a cloudy cornea in which there was loss of central graft clarity sufficient to compromise vision for a minimum of three consecutive months. Pachymetric measurement of corneal thickness was optional but was not formally a part of the definition of graft failure. When a cornea was cloudy at the last exam within the five-year examination window with less than three months of documented cloudiness, additional follow-up examination data were used to determine whether the cloudiness persisted for at least three months or if a regraft was performed. Cases were also considered to be graft failures when the cornea was cloudy at the last visit and there was no further follow up due to death, loss to follow up, or subject withdrawal. The date of graft failure was the date of the first examination at which the cornea was cloudy as part of the failure event. For cases in which the cornea was not documented to be cloudy prior to regraft, the date of regraft was considered to be the failure date. For subjects without graft failure who did not have an examination in the 5-year window (58 to 66 months) but had a later visit at which the cornea was clear, it was assumed for analysis that the cornea did not experience a graft failure by five years.
Eyes were not considered as graft failures if a serious operative complication such as an expulsive choroidal hemorrhage or major trauma that required surgical intervention occurred. The data for these eyes were censored at the time of surgery or the last completed visit prior to the trauma.
Graft rejection episodes were classified as definite when an endothelial rejection line was present in a previously clear graft and probable when there was inflammation (stromal infiltrate, keratic precipitates, cells in the anterior chamber, or ciliary injection) without an endothelial rejection line in a previously clear graft.
The primary cause of graft failure was classified as one of the following: (1) primary donor failure when the graft was cloudy on the first postoperative day and did not clear within two months, (2) graft rejection when an allograft reaction occurred that, in the investigator’s judgment, was the primary reason for graft failure, (3) refractive when a regraft was performed for this indication in a clear cornea, and (4) non-rejection when not classified in either of the other three categories. The primary reason for a non-rejection failure was classified as one of the following: endothelial decompensation, infection, epithelial defects, epithelial downgrowth, wound dehiscence, hypotony, corneal thinning, corneal edema, or glaucoma. Glaucoma was only considered to be the primary cause when a surgical procedure was performed for glaucoma.
Statistical Methods
The study was designed as a non-inferiority study to evaluate whether the graft success rate was similar with tissue from older donors (66 to 75 years old) versus younger donors (10 to 65 years old). The requisite sample size originally was computed to be 858 subjects, each with one study eye, based on the following assumptions: 1:1 distribution of corneas between the two donor age groups, alpha = 0.05, power = 90%, 80% five-year success rate in each donor age group, and non-inferiority limit = 8% (10% of 80%). The non-inferiority limit represents the maximum width of the confidence interval on the difference in success proportions between donor age groups for which the success rate with older donor tissue would be considered similar to the success rate with younger donor tissue. The sample size was increased to 1000 to account for non-independent data when two subjects received corneas from the same donor and for incomplete follow up. In the middle of the enrollment period, the ratio of younger donor corneas to older donor corneas was about 2:1 (rather than the projected 1:1). To prevent the effect of this imbalance from reducing the intended statistical power, the sample size was increased to 1,100 to maintain statistical power at 90%.
Subjects who had an ineligible corneal diagnosis were not included in the analysis (N=11: 4 with corneal scar without edema, 2 with failure of a prior transplant, and 1 each with lattice dystrophy, keratoconus, prior endophthalmitis, prior retinal detachment repair with silicone oil, and Sjogren’s syndrome).
A comparison of the baseline endothelial cell density between donor age groups was performed using a Wilcoxon rank-sum test. When the baseline donor specular image was not available for endothelial cell density determination by the central reading center, the endothelial cell density determined by the eye bank was used. Cumulative probabilities of graft survival (subsequently referred to as “graft survival rates”) were calculated using the Kaplan-Meier method. Due to the varied timing of the 5-year visits, any failure or censoring within the visit window (58 to 66 months) was mapped to month 60 for analysis. Univariate comparisons of graft survival were based on the difference in the cumulative probabilities at 5 years using Greenwood’s formula to estimate the variance (not the log-rank test). The proportional hazards model was used to adjust for the baseline endothelial cell density and assess the relationship between graft survival and continuous donor age. No significant deviation from the proportional hazards assumption was detected for donor age or baseline cell density. The correlation of outcome between two corneas from the same donor (138 corneas from 69 donors) was evaluated using a generalized mixed model; no significant correlation was detected and results were similar with or without this adjustment (data not shown). The 95% confidence interval for evaluating non-inferiority is one-sided. All reported p-values are two-sided. Statistical analyses were conducted using SAS version 9.1 software (SAS Institute Inc., Cary, NC).
RESULTS
Baseline Characteristics and Surgical Procedure
Between January 2000 and August 2002, 1,090 eligible subjects were enrolled by 105 surgeons at 80 sites in the United States. Mean age was 70 ± 9 years; 697 (64%) were female, 1011 (93%) were Caucasian, 50 (5%) were African-American, 13 (1%) were Hispanic, and 16 (1%) were other race. Indications for corneal transplantation included Fuchs’ dystrophy in 676 (62%), pseudophakic/aphakic corneal edema in 369 (34%), and a variety of other causes in 45 (4%). Five hundred and thirty four (49%) eyes were pseudophakic and 66 (6%) aphakic prior to transplant. A history of glaucoma surgery was present for 71 (7%) eyes, and IOP-lowering medications were being used in 144 (13%) eyes at the time of surgery. Additional baseline characteristics have been previously reported.8 A cornea from a donor 12 to <66.0 years of age was assigned to 707 (65%) of the subjects, and a cornea from a donor 66.0 to <76.0 years was assigned to 383 (35%). Subject characteristics were similar in the older and younger donor age groups (Table 1). The correlation between subject age and donor age was 0.07 (95% confidence interval 0.02 to 0.13).
Table 1.
Baseline Recipient Characteristics by Donor Age Group (N=1,090)
| Donor Age Group | |||
|---|---|---|---|
| Baseline Recipient Characteristic | Total N=1,090 |
< 66.0 N=707 (65) |
≥ 66.0 N=383 (35) |
| Age (years) | |||
| Mean (Standard Deviation) | 70 (9) | 69 (9) | 71 (8) |
| Median (Interquartile Range) | 72 (65, 76) | 72 (65, 76) | 73 (66, 77) |
| < 50.0 | 34 (3) | 24 (3) | 10 (3) |
| 50.0 – <60.0 | 128 (12) | 86 (12) | 42 (11) |
| 60.0 – <70.0 | 284 (26) | 192 (27) | 92 (24) |
| ≥ 70.0 | 644 (59) | 405 (57) | 239 (62) |
| Gender: Female | 697 (64%) | 445 (63%) | 252 (66%) |
| Race | |||
| White | 1,011 (93%) | 659 (93%) | 352 (92%) |
| African-American | 50 (5%) | 30 (4%) | 20 (5%) |
| Hispanic | 13 (1%) | 9 (1%) | 4 (1%) |
| Asian | 8 (<1%) | 4 (<1%) | 4 (1%) |
| Other | 8 (<1%) | 5 (<1%) | 3 (<1%) |
| Current Cigarette Smoker | 102 (9%) | 69 (10%) | 33 (9%) |
| Current Use of Glaucoma Medications | 144 (13%) | 96 (14%) | 48 (13%) |
| Prior Glaucoma Surgery | 71 (7%) | 40 (6%) | 31 (8%) |
| Diagnosis | |||
| Fuchs’ Dystrophy | 676 (62%) | 448 (63%) | 228 (60%) |
| Pseudophakic/Aphakic Corneal Edema | 369 (34%) | 232 (33%) | 137 (36%) |
| Other | 45 (4%) | 27 (4%) | 18 (5%) |
| Preoperative Lens Status | |||
| Phakic | 490 (45%) | 327 (46%) | 163 (43%) |
| Pseudophakic | 534 (49%) | 337 (48%) | 197 (51%) |
| Aphakic | 66 (6%) | 43 (6%) | 23 (6%) |
| Postoperative Lens Status | |||
| Phakic | 162 (15%) | 110 (16%) | 52 (14%) |
| Pseudophakic | 895 (82%) | 575 (81%) | 320 (84%) |
| Aphakic | 33 (3%) | 22 (3%) | 11 (3%) |
| Recipient Bed Size (mm)* | |||
| Mean (Standard Deviation) | 7.8 (0.3) | 7.9 (0.3) | 7.8 (0.3) |
N (%) unless otherwise specified
One subject with missing recipient bed size
A donor cornea was provided to each subject by one of 43 participating eye banks. Mean (SD) donor age was 58 (14) years. Median (interquartile range) endothelial cell density was 2666 (2462 to 2872) cells/mm2: 2680 (2466 to 2894) cells/mm2 for donors <66.0 years of age and 2624 (2448 to 2826) cells/mm2 for donors ≥66.0 years of age (P=0.02). There were no clinically important differences in the slit lamp characteristics or procurement factors of the donor corneas according to donor age. Additional information on the characteristics of the donor corneas has been previously reported.10
For 320 (29%) of the subjects, a lens extraction with intraocular lens placement was performed at the same time as the cornea transplant was performed. Post-transplant, 895 (82%) study eyes were pseudophakic, 162 (15%) were phakic and 33 (3%) were aphakic. Among the 895 subjects with an intraocular lens, 798 (89%) had a posterior chamber lens, 95 (11%) an anterior chamber lens, and 2 (<1%) an iris-fixated lens. Information on operative and immediate post-operative complications has been previously reported.8
Follow Up
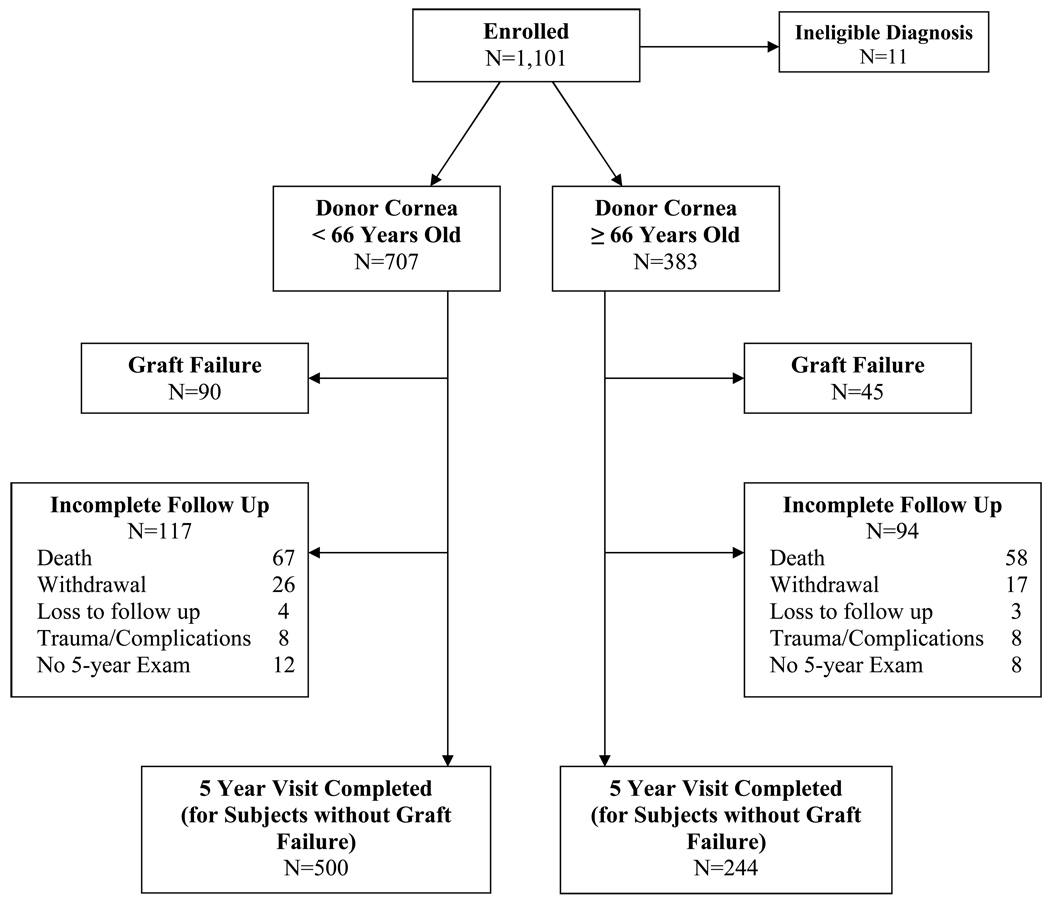
Overall, 125 (11%) subjects died and 50 (5%) withdrew or were lost to follow up prior to the 5-year post-operative examination. The data of 16 other subjects were censored prior to 5 years (i.e., not considered to be a graft failure) due to either choroidal hemorrhage at the time of the transplant (3 in the older and 2 in the younger donor age group) or major trauma during follow up (5 in the older and 6 in the younger donor age group). Figure 1 (available at http://aaojournal.org) provides data on the completeness of follow up for the older and younger donor age groups. No investigators or subjects were unmasked to donor age.
Figure 1 (on line).
Flow chart of follow up in the older and younger donor age groups
Graft Outcome
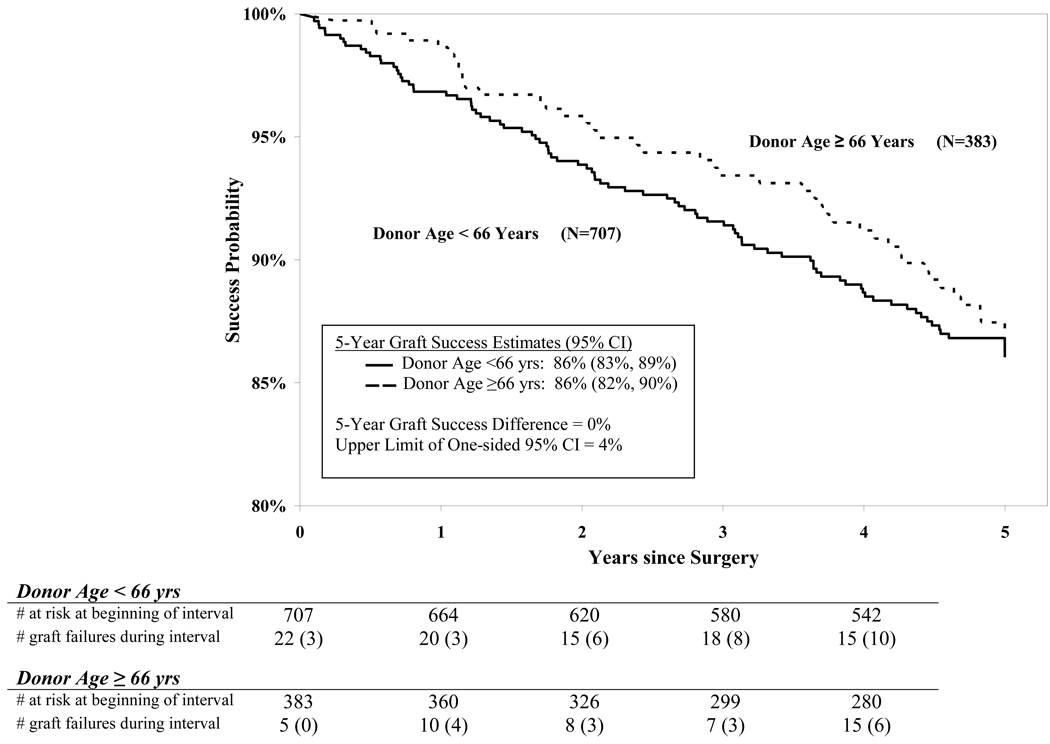
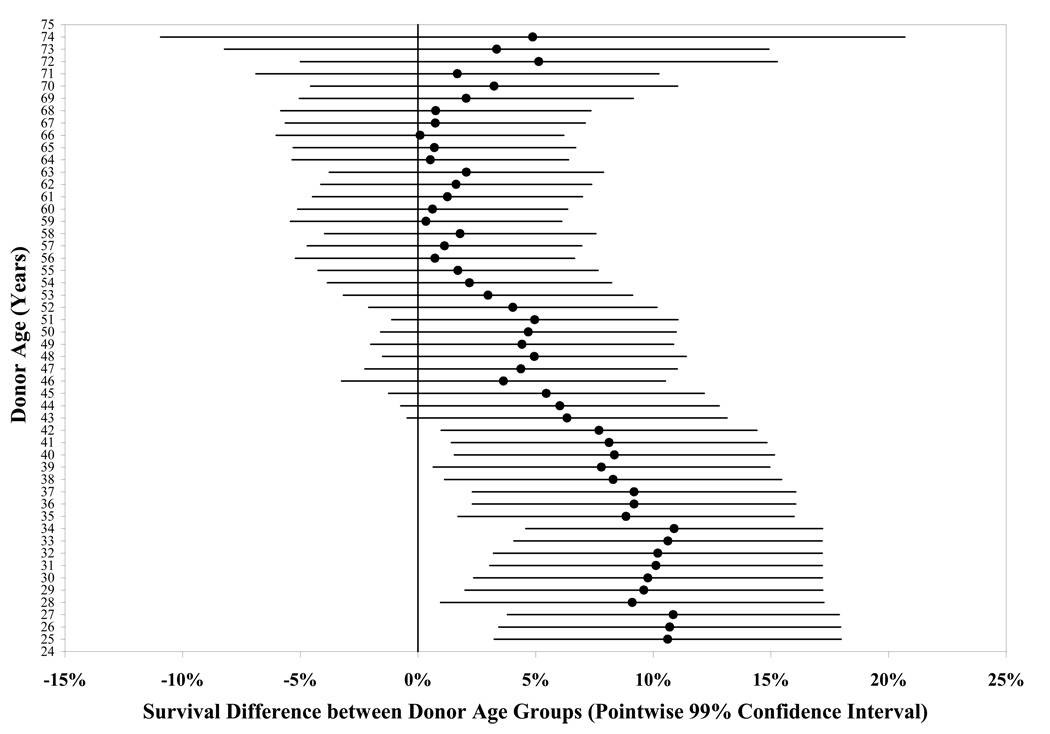
The 5-year cumulative probability of success was 86%: 86% in the <66.0 year donor age group and 86% in the ≥66.0 year donor age group (difference = 0%, upper limit of one-sided 95% confidence interval = 4%, Figure 2). Adjusting for baseline endothelial cell density had no appreciable effect on these results. In a statistical model with donor age as a continuous variable, there was not a significant relationship between donor age and outcome (P=0.11). However, in an exploratory analysis, there was a suggestion of a slightly higher success rate at the younger end of the range of donor ages (Table 2, Figure 3 and Figure 4 available at http://aaojournal.org). For instance, the graft survival rate was 93% when donor age was 12 to 40 years (N=127, 95% confidence interval 89% to 98%) compared with 85% when donor age was 41 to 75 years (N=963, 95% confidence interval 83% to 88%, P=0.001).
Figure 2.
Graft success by donor age group over time. For purposes of analysis, all failure or censoring events within the 5-year visit window (58 to 66 months) were mapped to month 60. The number of graft failures during the interval in parenthesis represents graft failures due to endothelial decompensation. CI is confidence interval.
Table 2.
Five-Year Graft Survival Rates According to Donor Age (N=1,090)
| Donor Age (years) |
N | Graft Failure (N) |
5 Year Graft Survival* (Pointwise 95% Confidence Interval) |
|---|---|---|---|
| 12 to <66 | 707 | 90 | 86% (83%, 89%) |
| 66 to <76 | 383 | 45 | 86% (82%, 90%) |
| 12 to <31 | 69 | 3 | 96% (91%, 100%) |
| 31 to <41 | 58 | 5 | 91% (83%, 98%) |
| 41 to <46 | 63 | 11 | 80% (70%, 91%) |
| 46 to <51 | 68 | 5 | 92% (86%, 99%) |
| 51 to <56 | 126 | 23 | 80% (73%, 87%) |
| 56 to <61 | 152 | 18 | 87% (82%, 93%) |
| 61 to <66 | 171 | 25 | 84% (79%, 90%) |
| 66 to <71 | 217 | 24 | 87% (82%, 92%) |
| 71 to <76 | 166 | 21 | 85% (79%, 91%) |
5-year Kaplan-Meier estimate
Figure 3 (on line).
Survival Difference between Donor Age Groups by Sequential Donor Age Cutoff Groups. Each donor age value on the y-axis marks the cutoff for two age groups (e.g. 35 donor age cutoff value divides the cohort into two donor age groups: <35 years and ≥35 years), for which the survival difference is calculated. The survival difference estimate (represented by a dot in the figure, with the line representing the pointwise 99% confidence interval) is calculated by subtracting the Kaplan-Meier survival estimate in the older donor age group from the survival estimate in the younger donor age group. Positive survival difference indicates greater survival in the younger donor age group. The first cut point used for the analyses is at age 25 because of the small number of donors less than 25 years of age.
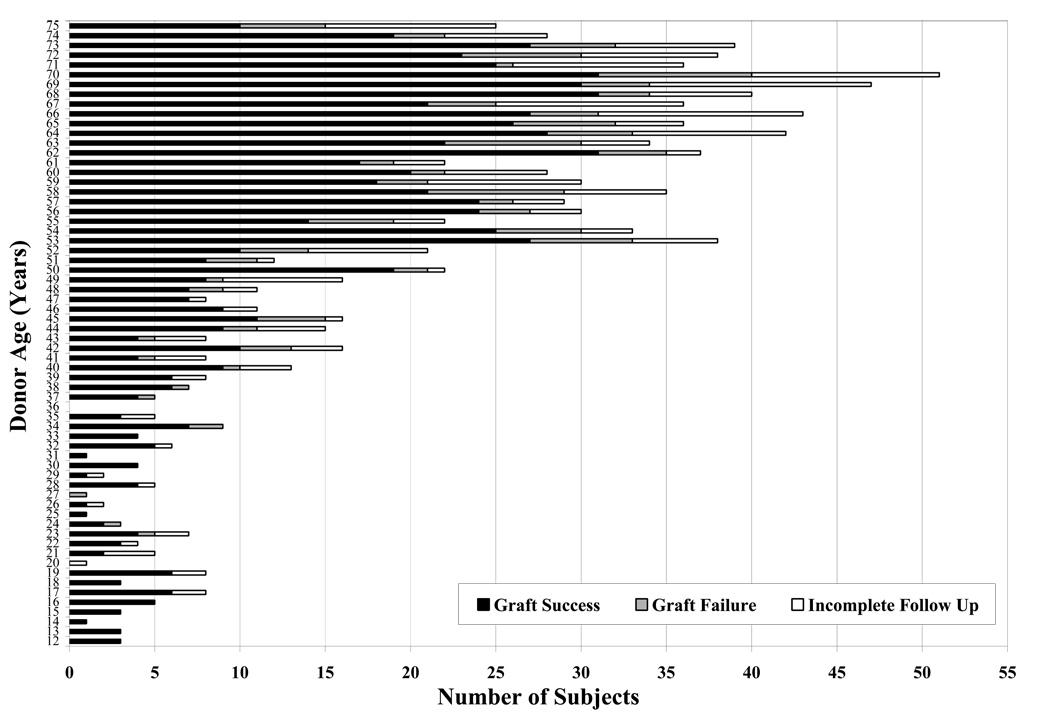
Figure 4 (on line).
Five-year Graft Status by Donor Age. The category “Graft Success” includes subjects who completed the 5-year examination; the category “Graft Failure” includes subjects who experienced graft failure within 5 years after surgery; the category “Incomplete Follow Up” includes subjects who died, withdrew, were lost to follow up prior to the end of the 5-year window, or did not complete a 5-year exam.
Among the 135 eyes with graft failures, a regraft was performed in 102 (76%) cases, while the other 33 (24%) cases met the cloudy cornea failure criteria defined for the study without a regraft as of the closure date of study data (30 had a cloudy cornea for at least three months and 3 had a cloudy cornea for less than three months without additional available follow up). Three graft failures were due to primary donor failure (donor ages 52, 35, and 27 years), 8 to uncorrectable refractive error, 48 to graft rejection, 46 to endothelial decompensation, and 30 to other causes (Table 3). At least one probable or definite graft rejection episode preceded graft failure in 23 of the 46 failures attributed to endothelial decompensation (4 definite and 19 probable) and in 18 of the 30 failures attributed to other causes (4 definite and 14 probable). The distribution of causes of failure between the donor age groups did not substantially differ (Table 3).
Table 3.
Causes of Graft Failure
| Donor Age Group* | |||
|---|---|---|---|
| Total N=1,090 |
<66.0 N=707 |
≥66.0 N=383 |
|
| Total Graft Failures | 135 (12%) | 90 (13%) | 45 (12%) |
| Primary Donor Failure | 3 (<1%) | 3 (<1%) | 0 |
| Refractive | 8 (<1%) | 5 (<1%) | 3 (<1%) |
| Graft Rejection | 48 (4%) | 32 (5%) | 16 (4%) |
| Non-rejection | 76 (7%) | 50 (7%) | 26 (7%) |
| Causes of Non-rejection Graft Failure | |||
| Endothelial decompensation | 46 | 30 | 16 |
| Infection | 15 | 11 | 4 |
| Epithelial defects | 6 | 3 | 3 |
| Glaucoma | 3 | 2 | 1 |
| Epithelial downgrowth | 2 | 2 | 0 |
| Corneal edema | 1 | 0 | 1 |
| Corneal thinning | 1 | 1 | 0 |
| Hypotony | 1 | 1 | 0 |
| Wound dehiscence | 1 | 0 | 1 |
DISCUSSION
In this study of 1,090 moderate risk cornea transplants, the overall five-year success rate was 86% for grafts performed with corneas from donors 12 to 65 years old as well as for grafts performed with corneas from donors 66 to 75 years old. The 95% confidence interval on the observed difference was well within the study’s pre-specified definition of non-inferiority.
When donor age was analyzed as a continuous variable, there was not a significant donor age effect over the range of 12 to 75 years included in the study. However, in an exploratory analysis, there was a slightly higher success rate in transplants performed with a cornea from donors at the younger end of the range of donor ages. This association remained even after adjusting for baseline endothelial cell density, which was slightly higher in corneas from younger donors. Since such a small proportion of the donor pool consists of corneas from young donors (less than 15% of donors are <40 years old1), this observation, even if real, has limited applicability.
The five-year success rate of 86% is higher than our originally projected rate of 80%, which was based on prior literature.15–23 Primary donor failures were rare (0.3% of cases). Approximately equal numbers of cases of graft failure were due to graft rejection and endothelial decompensation. Some cases classified as having endothelial decompensation had a prior, resolved episode of graft rejection.
Although our 5-year results indicate that there is no difference in the success rate of moderate-risk transplants according to donor age, results from our Specular Microscopy Ancillary Study indicate that among the successful cases, there is a slight association between donor age and endothelial cell loss, with the cell loss after 5 years being slightly lower in corneas from younger donors (r adjusted for baseline endothelial cell density = −0.19, 95% confidence interval −0.29 to −0.08).24 Whether this slight association between cell loss and donor age is of clinical importance is not known. Of greater importance, however, is the finding that irrespective of donor age, endothelial cell loss is substantial over the first five years after transplant even when the graft has been successful. Half of the successful cases experienced a cell loss of 70% or more and at five years more than half had an endothelial cell density <800 cells/mm2. Since the endothelial cell density is an indicator of the health of the cornea, we plan to follow this cohort for an additional 5 years in order to determine how the substantial decrease in endothelial cell density at 5 years impacts on the success rate over a longer time period.
Our results need to be interpreted in the context of the criteria applied in the selection of donor corneas for the study. In addition to the age range of 10 to 75, the eligibility criteria for the study donor corneas included an endothelial cell density of 2300 to 3300 cells/mm2 and an eye bank qualitative grading of good to excellent. The lower end of the endothelial cell density range was included to increase surgeon acceptance of the protocol, which required surgeons to be masked to specific information usually provided by the eye bank about a donor cornea, such as the endothelial cell density. Thus, we are unable to comment on whether the results would be the same with donor corneas that have lower endothelial cell densities.
Our cohort included only subjects with a corneal disease that was considered to be of moderate risk for graft failure, principally Fuchs’ dystrophy and pseudophakic corneal edema, to optimize our ability to evaluate the effect of donor age on graft outcome. With conditions such as keratoconus, the failure rate of transplantation is too low to be able to judge the effect of factors such as donor age without a huge, likely unfeasible sample size. With corneal conditions that have a low success rate, such as vascularized corneas or regrafts, graft failure is more likely to be due to recipient factors than to donor factors, which limits a study’s ability to evaluate the effect of donor factors on the success rate. Despite exclusion of these low-risk and high-risk indications for transplant, it is nevertheless reasonable to generalize the results of this study to corneal conditions that were not included, since there is not a biologically plausible reason to expect that the effect of donor age on outcome would be different with these conditions.
The CDS was designed to minimize the potential for bias by (1) masking the eye banks to the recipient age and diagnosis, thus avoiding a common pitfall in prior studies of matching donors and recipients particularly by age and (2) masking both subjects and surgeons to donor age, which eliminated the possibility that a subject’s medical care or the diagnosis of graft failure could be affected by knowledge of donor age. The definition of graft failure was based on the criteria used in the CCTS, and misclassification of graft failure was unlikely, as the cornea had to remain cloudy for at least 90 days or a regraft had to be performed in order for a subject to meet failure criteria. Subject retention over the 5-year period was excellent with only 5% of the subjects withdrawing from the study or being lost to follow up prior to five years.
The CDS results indicate that transplants using corneas from donors 66 to 75 years old that meet the study’s eligibility criteria have a 5-year graft survival rate similar to transplants using corneas from younger donors. These results support eye bank procurement of donor corneas through 75 years of donor age. We did not include corneas from donors 76 years or older, so we cannot comment on whether a similar success rate would occur when the donor age is greater than 75 years. The procurement of corneas from older donors will increase eye bank costs, because corneas from older donors are less likely to be suitable for transplantation than corneas from younger donors on the basis of criteria other than age. Using a minimum endothelial cell density of 2,000 cells/mm2 as a surrogate measure of corneal suitability for transplant, data from the Midwest Eye-Banks from 1994 to 1997 of over 5000 donor corneas showed that 95% of tissue from donors 1 to 60 years old, 88% of tissue from donors 61 to 70 years old, and 78% of tissue from donors over the age of 70 were considered suitable for transplant (Midwest Eye-Banks, unpublished data).
In conclusion, our results indicate that the donor age pool should be expanded to 75 years. Surgeons and patients now have evidence that older donor corneas comparable in quality to those used in this study are suitable for transplantation.
Acknowledgments
Funding/Support: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services EY12728 and EY12358. Additional support provided by: Eye Bank Association of America, Bausch & Lomb, Inc., Tissue Banks International, Vision Share, Inc., San Diego Eye Bank, The Cornea Society, Katena Products, Inc., ViroMed Laboratories, Inc., Midwest Eye-Banks (Michigan Eye-Bank, Illinois Eye-Bank), Konan Medical Corp., Eye Bank for Sight Restoration, SightLife, Sight Society of Northeastern New York (Lions Eye Bank of Albany), Lions Eye Bank of Oregon
Appendix
CORNEA DONOR STUDY INVESTIGATOR GROUP
Listed in order of number of patients enrolled in the Cornea Donor Study are the clinical sites with city, state, site name, number of patients in parentheses and names of the investigators ordered alphabetically that participated in the study as part of the CDS Investigator Group.
CLINICAL SITES:
Southfield, MI; Michigan Cornea Consultants, PC (77): Christopher Y. Chow, MD, Steven P. Dunn, MD, David G. Heidemann, MD Albany, NY; Cornea Consultants of Albany (58): Michael W. Belin, MD, Robert L. Schultze, MD Seattle, WA (47): Matthew S. Oliva, MD, Walter M. Rotkis, MD Grand Rapids, MI; Verdier Eye Center, P.C. (41): David D. Verdier, MD Cleveland, OH; Case Western Reserve University and University Hospitals Case Medical Center (33): Jonathan H. Lass, MD, William J. Reinhart, MD, Joseph M. Thomas, MD Atlanta, GA; Eye Consultants of Atlanta, P.C. (30): Stephen M. Hamilton, MD, Gina C. Jayawant, MD, W. Barry Lee, MD Phoenix, AZ; Cornea Consultants of Arizona (28): Robert H. Gross, MD, Edward L. Shaw, MD Tampa, FL; Cornea and Eye Surface Center (28): Steven L. Maskin, MD Narberth, PA; Ophthalmic Subspecialty Consultants (26): Parveen K. Nagra, Irving M. Raber, MD Chicago, IL; University of Illinois at Chicago (25): Joel Sugar, MD, Elmer Tu, MD Fort Myers, FL; Eye Associates of Fort Myers (24): Mark S. Gorovoy, MD Lancaster, PA; Eye Physicians of Lancaster (24): Francis J. Manning, MD Scranton, PA; Northeastern Eye Institute (23): Thomas S. Boland, MD, Stephen E. Pascucci, MD Ann Arbor, MI; W.K. Kellogg Eye Center, The University of Michigan (21): Qais A. Farjo, MD, Roger F. Meyer, MD, H. Kaz Soong, MD, Alan Sugar, MD Charlotte, NC; Horizon Eye Care (21): Paul G. Galentine, MD, David N. Ugland, MD Langhorne, PA (21): Sadeer B. Hannush, MD San Diego, CA; Eye Care of San Diego (21): John E. Bokosky, MD Charleston, WV; Charleston Eye Care, PLLC (20): James W. Caudill, MD Chicago, IL; Northwestern University (20): Robert S. Feder, MD Colton, CA; Inland Eye Institute (20): John C. Affeldt, MD, Christopher L. Blanton, MD Cincinnati, OH; Cincinnati Eye Institute (20): Edward J. Holland, MD Dallas, TX; The University of Texas Southwestern Medical Center at Dallas (20): R. Wayne Bowman, MD, H. Dwight Cavanagh, MD, PhD, Mohamed-Sameh H. El-Agha, MD, James P. McCulley, MD Seattle, WA; Eye Associates N.W., Inc., P.S. (20): Thomas E. Gillette, MD Allentown, PA; Lehigh Valley Eye Center, P.C. (19): Alan B. Leahey, MD Madison, WI; Davis Duehr Dean Clinic (19): Christopher R. Croasdale, MD Louisville, KY (16): Richard A. Eiferman, MD
Burlington, MA; Lahey Clinic (15): Sarkis H. Soukiasian, MD Atlanta, GA; Emory University (14): R. Doyle Stulting, MD, PhD Baltimore, MD; The Johns Hopkins University School of Medicine (14): Walter J. Stark, MD N. Dartmouth, MA; Eye Health Vision Center (14): Kenneth R. Kenyon, MD, Richard C. Rodman, MD Dallas, TX; Cornea Associates of Texas (13): Walter E. Beebe, MD, Henry Gelender, MD Rochester, NY; University of Rochester (13): Steven S. Ching, MD, Ronald D. Plotnik, MD Tulsa, OK; The Eye Institute (13): Marc A. Goldberg, MD Atlanta, GA (12): Karen Sumers, MD Boston, MA; Center for Eye Research and Education (12): Nicoletta A. Fynn-Thompson, MD, Ann Z. McColgin, MD, Michael B. Raizman, MD Delray Beach, FL; Delray Eye Associates, P.A. (12): Steven I. Rosenfeld, MD Minneapolis, MN; Minnesota Eye Consultants, P.A. (12): Elizabeth A. Davis, MD, David R. Hardten, MD, Richard L. Lindstrom, MD Sacramento, CA; University of California, Davis (12): Mark J. Mannis, MD Tallahassee, FL; Eye Associates of Tallahassee (12): Jerry G. Ford, MD Cleveland, OH; The Cleveland Clinic Foundation (11): David M. Meisler, MD Indianapolis, IN; Price Vision Group (11): Kendall Dobbins, MD, Francis W. Price, Jr., MD, William G. Zeh, MD Pittsburgh, PA (11): Peter J. Berkowitz, MD Seattle, WA; Virginia Mason Medical Center (11): Thomas D. Lindquist, MD, PhD San Francisco, CA (10): Daniel F. Goodman, MD, Niraj P. Patel, MD Denver, CO; Colorado Eye Physicians and Surgeons (9): Abdulfatah M. Ali, MD, Richard F. Beatty, MD Iowa City, IA; University of Iowa (9): John E. Sutphin, MD, Ayad A. Farjo, MD, Kenneth M. Goins, MD Portland, OR; Northwest Corneal Services (9): Terry E. Burris, MD Pinellas Park, FL; Southeast Eye Institute, P.A. (9): Peter A. Shriver, DO Bangor, ME; Eastern Maine Eye Associates, P.A. (8): Cynthia A. Self, MD, Garth A. Wilbanks, MD Irvine, CA; University of California, Irvine (8): Roy S. Chuck, MD, PhD, Ronald N. Gaster, MD N. Dartmouth, MA; Southcoast Eye Care, Inc. (7): David W. Kielty, MD Galveston, TX; University of Texas Medical Branch at Galveston (6): Garvin H. Davis, MD, Stefan D. Trocme, MD (now at Case Western Reserve University and University Hospitals of Cleveland) Lexington, KY (6): Woodford S. Van Meter, MD Raleigh, NC (6): Patricia W. Smith, MD Memphis, TN; Associated Ophthalmic Specialists (6): Alan R. Schaeffer, MD Philadelphia, PA; Corneal Associates, P.C. (6): Elisabeth J. Cohen, MD, Peter R. Laibson, MD, Christopher J. Rapuano, MD Rochester, MN; Mayo Clinic College of Medicine (6): Keith H. Baratz, MD Lancaster, PA; Eye Doctors of Lancaster (5): Barton L. Halpern, MD, Mark A. Pavilack, MD (now at Tidewater Eye Center, Virginia Beach, VA) Lansdale, PA (5): Gerald B. Rosen, MD (now at Horizon Eye Care, Charlotte, NC) Minneapolis, MN; University of Minnesota (5): Donald J. Doughman, MD West Orange, NJ; Corneal Associates of New Jersey (5): Soo Mee Pak, MD, Theodore Perl, MD Columbia, MO; University of Missouri (4): John W. Cowden, MD Providence, RI; Rhode Island Eye Institute (4): Elliot M. Perlman, MD
Spokane, WA; Spokane Eye Clinic (4): Lance E. Olson, MD, Erik D. Skoog, MD Tacoma, WA; Pacific Cataract and Laser Institute (4): William D. Gruzensky, MD Nashville, TN; Cornea Consultants of Nashville, P.L.L.C. (3): Erich B. Groos, Jr., MD Salt Lake City, UT; University of Utah (3): Mark D. Mifflin, MD, Maureen K. Lundergan, MD Springfield, MA (3): Steven T. Berger, MD Boston, MA; Boston University School of Medicine (2): Kenneth C. Chern, MD Charleston, SC; Medical University of South Carolina (2): Kerry D. Solomon, MD Chicago, IL; Rush University Medical Center (2): Richard F. Dennis, MD, Jonathan B. Rubenstein, MD Palm Coast, FL; Atlantic Eye Center (2): Alexandra M. P. Kostick, MD Raleigh, NC (2): Samuel H. Santander, MD, MPH Beachwood, OH; The Cleveland Clinic Foundation (1): Allen S. Roth, MD Decatur, GA; Eye Physicians and Surgeons, P.C. (1): Laura A. Bealer, MD Los Angeles, CA (1): Jonathan I. Macy, MD Mount Pleasant, SC; Charleston Cornea & Refractive Surgery, P.A. (1): David G. O'Day, MD Portland, OR; Devers Eye Institute (1): Mark A. Terry, MD West Palm Beach, FL; Palm Beach Eye Clinic (1): Nunzio P. Sossi, MD, PhD Winston-Salem, NC; Wake Forest University School of Medicine (1): Keith A. Walter, MD
Listed in order of number of patients enrolled in the Cornea Donor Study are the eye banks with eye bank name, city, state, number of patients in parentheses and names of the eye bank directors and coordinators who participated in the study during the enrollment phase (D=Director, C=Coordinator).
EYE BANKS: Midwest Eye-Banks (192) [Ann Arbor, MI; Michigan Eye Bank, (145); Chicago, IL; Illinois Eye Bank, (47)]: Florence M. Johnston (D), Kyle L. Mavin (C), Kristen E. McCoy (C), Michael B. O'Keefe (C) Tissue Banks International (119) [Boston, MA; New England Eye & Tissue Transplant Bank (47); Indianapolis, IN; Indiana Lions Eye & Tissue Transplant Bank (22); Bismarck, ND; Lions Eye Bank of North Dakota, Inc. (19); Dayton, OH; Lions Eye Bank of West Central Ohio (11); Baltimore, MD; Medical Eye Bank of Maryland & Washington Eye Bank (4); Santa Ana, CA; Orange County Eye & Tissue Bank (4); Albuquerque, NM; New Mexico Lions Eye Bank (3); Los Angeles, CA; Doheny Eye and Tissue Transplant Bank (3); Orlando, FL; Medical Eye Bank of Florida (2); Oakland, CA; Northern California Transplant Bank (2); Springfield, NJ; Lions Eye Bank of New Jersey (2)]: Gerald J. Cole, MBA (D), Diane F. Johnston (C), Mark A. Jones (C), Sameera M. Farazdaghi, MPH (C), Elizabeth N. Walunas (C) Seattle, WA; SightLife (86): Monty M. Montoya, MBA (D), Bernie Iliakis (C), Rick D. McDonald (C), Misty L. Ostermiller (C), Cathy E. Saltwick (C) Tampa, FL; Central Florida Lions Eye & Tissue Bank, Inc. (73): Jason K. Woody (D, C) Allentown, PA; Northeast Pennsylvania Lions Eye Bank, Inc. (70): Mark H. Weaver (D), Michael J. Christ (C), Mark B. Gross (C) Minneapolis, MN; Minnesota Lions Eye Bank (61): Carol R. Engel (D), Raylene A. Dale(C), Stephanie K. Hackl(C), Elena J. Henriksen(C), Kathryn J. Kalmoe(C), Jennifer M. Larson(C), Jackie V. Malling(C), Brian J. Philippy (C) Albany, NY; Sight Society of Northeastern New York (58): Maryann Sharpe-Cassese, RN, MSN (D), Sue M. Hayes (C) Philadelphia, PA; Lions Eye Bank of Delaware Valley (58): Robert E. Lytle (D), David A. Rechtshaffen (C) Atlanta, GA; Georgia Eye Bank, Inc. (57): Bruce Varnum (D), Erin B. Angel (C), Matt D. Durell (C), Teresa R. Williams (C) Cleveland, OH; Cleveland Eye Bank (45): Susan V. Janssen (D), Brian E. Kraus (C), Marcy B. McLain (C), Jackie A. Rossi (C) Dallas, TX; Transplant Services Center UT Southwestern (33): Ellen L. Heck, MS, MA (D), Marilyn S. Hayes (C) Phoenix, AZ; Donor Network of Arizona (28): Gregory C. Davis (D), Tara L. Chavez (C), Lori D. Oswald (C), Noreen B. Ruiz (C) San Diego, CA; San Diego Eye Bank (26): Jeffrey G. Penta, MBA (D), Wayne E. Dietz (C), Jennifer L. Nary (C) Charleston, WV; Medical Eye Bank of West Virginia (21): Kenneth R. Sheriff (D), Nancy C. Driver (C) Charlotte, NC; Lifeshare of the Carolinas (21): William J. Faircloth (D), Paul E. Williams (C) Winston-Salem, NC; The North Carolina Eye Bank, Inc. (21): Kurt Weber, MA, MBA (D), Jerry W. Barker (C), Donna M. Bridges (C), Lee Chenier (C), Mark Soper (C) Redlands, CA; Inland Eye & Tissue Bank (20): Betsy Allen (D), Samantha J. Wright (C) Louisville, KY; University of Louisville Lions Eye Bank (16): James R. Martin (D), Anne J. Watson (C) Sacramento, CA; Sierra Eye & Tissue Donor Services-DCI (15): Greg McDonough, MS (D), Kristel D. Beilby (C) Rochester, NY; Rochester Eye & Human Parts Bank, Inc. (13): Linda K. Fraser (D), Tammi S. Sharpe (C) Pittsburgh, PA; Center for Organ Recovery and Education (11): Robert C. Arffa, MD, Michael A. Tramber (C) Portland, OR; Lions Eye Bank of Oregon (10): Barbara L. Crow (D), Matthew M. Fisher (C), Chris G. Stoeger (C) Aurora, CO; Rocky Mountain Lions Eye Bank (9): Edmund Jacobs (D), Michael P. Filbin (C), James I. Mather (C), Christopher M. McGriff (C), Eric E. Meinecke (C) Iowa City, IA; Iowa Lions Eye Bank (9): Patricia J. Mason (D), Garret D. Locke (C), Janice F. Reiter (C) Norfolk, VA; Lions Medical Eye Bank of Eastern Virginia, Inc. (7): David E. Korroch (D), Penelope M. Thomas (C) Galveston, TX; Southeast Texas Lions Eye Bank, Inc. (6): Wayne A. Lange (D, C), Rosemary F. Moore (C) Memphis, TN; Mid-South Eye Bank for Sight Restoration (6): Lee J. Williams (D), Yvette D. Friedhoff (C) Columbia, MO; Heartland Lions Eye Bank (4): Ronald J. Walkenbach, PhD (D), Jennifer E. Glover (C), Brenda A. Kafton (C), Kraig J. Lage (C) Charleston, SC; South Carolina Lions Eye Bank, Inc. (3): Brenda S. Horn (D), H. Tommy Bottoms (C), Ellen R. Kerns (C) Salt Lake City, UT; Utah Lions Eye Bank (3): Raymond Jessen, MPH (D, C), William H. Dennis (C)
COORDINATING CENTER: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD (Director), Mariya Dontchev, MPH, Robin L. Gal, MSPH, Craig Kollman, PhD, Lee Anne Lester, Shelly T. Mares, Yazandra A. Parrimon, Alandra Powe, Katrina J. Ruedy, MSPH, Heidi J. Strayer, PhD
SPECULAR MICROSCOPY READING CENTER: Case Western Reserve University and University Hospitals Case Medical Center, Cleveland, OH: Jonathan H. Lass, MD (Medical Director), Beth Ann Benetz, MA (Technical Director), Carmella Gentile (Head Technician), Stephanie Burke, Shannon Edwards, Lori Karpinecz
NATIONAL INSTITUTES OF HEALTH: National Eye Institute, Bethesda, MD: Maryann Redford, DDS, MPH, Mary Frances Cotch, PhD
DATA AND SAFETY MONITORING COMMITTEE: Marian Fisher, PhD (DSMC Chair), William Bourne, MD, Maryann Redford, DDS, MPH., Rabbi Samuel Fishman, Gary Foulks, MD, David C. Musch, PhD, MPH
STEERING COMMITTEE: Edward J. Holland, MD (Study Co-Chair, 1999–current), Mark J. Mannis, MD (Study Co-Chair, 1999–current), Mary Frances Cotch, PhD (1999–2001), Steven Dunn, MD (2001–2002), Ellen Heck, MS, MA (1999–2000), Florence Johnston (2000–2001, 2002–2004), Jonathan H. Lass, MD (1999–current), Thomas Lindquist, MD, PhD (2000–2001), Monty M. Montoya, MBA (2004–current), Maryann Redford, DDS, MPH (2001–current), Alan Sugar, MD (2004–current), Joel Sugar, MD (1999–2000), Jason Woody (2001–2002)
DATA ANALYSES ADVISORY COMMITTEE: Mark J. Mannis, MD, Edward J. Holland, MD, Michael W. Belin, MD, Steven Dunn, MD, Robert H. Gross, MD, Mark S. Gorovoy, MD, Stephen M. Hamilton, MD, Ellen Heck, MS, MA, Jonathan H. Lass, MD, Thomas Lindquist, MD, PhD, Francis J. Manning, MD, Steven L. Maskin, MD, Monty M. Montoya, MBA, Irving M. Raber, MD, Maryann Redford, DDS, MPH, Walter M. Rotkis, MD, Robert L. Schultze, MD, Walter J. Stark, MD, R. Doyle Stulting, MD, PhD, Alan Sugar, MD, Joel Sugar, MD, Bradley Tennant, David D. Verdier, MD, Jason Woody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A list of the members of the Cornea Donor Study Investigator Group is available at http://aaojournal.org
Conflict of Interest: None
Writing Committee: Lead Authors: Robin L. Gal, MSPH, Mariya Dontchev, MPH, Roy W. Beck, MD, PhD, Mark J. Mannis, MD, Edward J. Holland, MD, Craig Kollman, PhD Additional writing committee members (alphabetical): Steven P. Dunn, MD, Ellen L. Heck, MS, MA, Jonathan H. Lass, MD, Monty M. Montoya, MBA, Robert L. Schultze, MD, R. Doyle Stulting, MD, PhD, Alan Sugar, MD, Joel Sugar, MD, Bradley Tennant, David D. Verdier, MD
REFERENCES
- 1.Eye Bank Association of America. 2006 Eye Banking Statistical Report. Washington, DC: EBAA; 2006
- 2.Human Cells, Tissues, and Cellular and Tissue-Based Products; Donor Screening and Testing, and Related Labeling, Final Rule. Federal Register. 2007 Jun 19;72:33667–33669. [PubMed] [Google Scholar]
- 3.Cavanagh HD. Eye Banking 1995: danger and opportunity. Cornea. 1995;14:545–546. [PubMed] [Google Scholar]
- 4.Chu W, Dahl P, O'Neill MJ. Benefits of specular microscopy in evaluating eye donors aged 66 and older. Cornea. 1995;14:568–570. 634. [PubMed] [Google Scholar]
- 5.Mattern RM, Heck EL, Cavanagh HD. The impact on tissue utilization of screening donor corneas by specular microscopy at the University of Texas Southwestern Medical Center. Cornea. 1995;14:562–567. [PubMed] [Google Scholar]
- 6.Moyes AL, Holland EJ, Palmon FE, et al. Tissue utilization at the Minnesota Lions' Eye Bank. Cornea. 1995;14:571–577. [PubMed] [Google Scholar]
- 7.Probst LE, Halfaker BA, Holland EJ. Quality of corneal donor tissue in the greater-than-75-year age group. Cornea. 1997;16:507–511. [PubMed] [Google Scholar]
- 8.Cornea Donor Study Group. Clinical profile and early surgical complications in the Cornea Donor Study. Cornea. 2006;25:164–170. doi: 10.1097/01.ico.0000164832.69668.4b. [DOI] [PubMed] [Google Scholar]
- 9.Eye Bank Association of America. Medical Standards. Washington, DC: EBAA; 2000. pp. 1–27. [Google Scholar]
- 10.Cornea Donor Study Group. Baseline donor characteristics in the Cornea Donor Study. Cornea. 2005;24:389–396. doi: 10.1097/01.ico.0000151503.26695.f0. [DOI] [PubMed] [Google Scholar]
- 11.Cornea Donor Study Group. An evaluation of image quality and accuracy of eye bank measurement of donor cornea endothelial cell density in the Specular Microscopy Ancillary Study. Ophthalmology. 2005;112:431–440. doi: 10.1016/j.ophtha.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Benetz BA, Gal RL, Ruedy KJ, et al. Specular Microscopy Ancillary Study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31:319–327. doi: 10.1080/02713680500536738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative Corneal Transplantation Studies Research Group. The Collaborative Corneal Transplantation Studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 14.Collaborative Corneal Transplantation Studies Research Group. Design and methods of the Collaborative Corneal Transplantation Studies. Cornea. 1993;12:93–103. doi: 10.1097/00003226-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Abbott RL, Forster RK. Determinants of graft clarity in penetrating keratoplasty. Arch Ophthalmol. 1979;97:1071–1075. doi: 10.1001/archopht.1979.01020010525001. [DOI] [PubMed] [Google Scholar]
- 16.Boisjoly HM, Tourigny R, Bazin R, et al. Risk factors of corneal graft failure. Ophthalmology. 1993;100:1728–1735. doi: 10.1016/s0161-6420(93)31409-0. [DOI] [PubMed] [Google Scholar]
- 17.Chang SD, Pecego JG, Zadnik K, et al. Factors influencing graft clarity. Cornea. 1996;15:577–581. [PubMed] [Google Scholar]
- 18.Forster RK, Fine M. Relation of donor age to success in penetrating keratoplasty. Arch Ophthalmol. 1971;85:42–47. doi: 10.1001/archopht.1971.00990050044007. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MS, Lempert SL, Brown SI. Significance of donor age in penetrating keratoplasty. Ann Ophthalmol. 1979;11:974–976. [PubMed] [Google Scholar]
- 20.Maguire MG, Stark WJ, Gottsch JD, et al. Collaborative Corneal Transplantation Studies Research Group. Risk factors for corneal graft failure and rejection in the Collaborative Corneal Transplantation Studies. Ophthalmology. 1994;101:1536–1547. doi: 10.1016/s0161-6420(94)31138-9. [DOI] [PubMed] [Google Scholar]
- 21.Price FW, Jr, Whitson WE, Johns S, Gonzales JS. Risk factors for corneal graft failure. J Refract Surg. 1996;12:134–143. doi: 10.3928/1081-597X-19960101-24. [DOI] [PubMed] [Google Scholar]
- 22.Vail A, Gore SM, Bradley BA, et al. Corneal Transplant Follow-up Study Collaborators. Corneal graft survival and visual outcome: a multicenter study. Ophthalmology. 1994;101:120–127. doi: 10.1016/s0161-6420(94)31376-5. [DOI] [PubMed] [Google Scholar]
- 23.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. Influence of advanced recipient and donor age on the outcome of corneal transplantation. Br J Ophthalmol. 1997;81:835–839. doi: 10.1136/bjo.81.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornea Donor Study Investigator Group. Donor age and corneal endothelial cell loss five years after successful cornea transplantation: specular microscopy ancillary study results. Ophthalmology. 2008 doi: 10.1016/j.ophtha.2008.01.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]