Abstract
T cell recruitment into inflamed skin is dependent on skin-homing receptor binding to endothelial (E)- and platelet (P)-selectin. These T cell receptors, or E- and P-selectin ligands, can be targeted by the metabolic fluorosugar inhibitor, 4-F-GlcNAc, to blunt cutaneous inflammation. Compelling new data indicate that, in addition to T cells, NK cells are also recruited to inflamed skin in allergic contact hypersensitivity (CHS) contingent on E- and P-selectin-binding. Using a model of allergic CHS, we evaluated the identity and impact of NK cell E-selectin ligand(s) on inflammatory responses and examined the oral efficacy of 4-F-GlcNAc. We demonstrated that the predominant E-selectin ligands on NK cells are P-selectin glycoprotein ligand-1 and protease-resistant glycolipids. We showed that, unlike the induced E-selectin ligand expression on activated T cells upon exposure to Ag, ligand expression on NK cells was constitutive. CHS responses were significantly lowered by orally administered 4-F-GlcNAc treatment. Although E-selectin ligand on activated T cells was suppressed, ligand expression on NK cells was insensitive to 4-F-GlcNAc treatment. These findings indicate that downregulating effector T cell E- and P-selectin ligand expression directly correlates with anti-inflammatory efficacy and provides new insight on metabolic discrepancies of E-selectin ligand biosynthesis in effector leukocytes in vivo.
Leukocyte accumulation in skin is associated with skin disorders, including atopic dermatitis, allergic contact hypersensitivity (CHS),3 psoriasis, and cutaneous T cell lymphoma. Thus, identifying the factors critical for the skin-tropic behavior of leukocytes can have a profound influence on the development of targeted anti-inflammatory approaches (1). Considerable efforts have been undertaken to understand how T cells traffic to skin by determining which molecular constituents elaborate T cell affinities for dermal postcapillary venules and/or elicit chemotactic movement into inflamed skin. Surprisingly, the importance of NK cells as effector cellular mediators in adaptive cutaneous immune responses has recently been described (2, 3) and, thus, identifying and characterizing the skin-homing features on NK cells has intensified.
Leukocytes traffic to cutaneous sites through coordinated adhesive and chemotactic signaling events, resulting in tethering, rolling, and firm adhesion on dermal post capillary venules and diapedesis through the endothelial surface into the papillary dermis. Among the group of adhesion molecules initiating leukocyte-endothelial cell attachment are a family of type I membrane proteins called selectins (4). P (platelet)-, E (endothelial)-, and L (leukocyte)-selectin mediate low-affinity binding interactions between leukocytes in flow and endothelial cells by engaging with counterreceptor ligands (4). The importance of E- and P-selectin, specifically, is clearly evident as they are constitutively expressed on dermal endothelium and are necessary for T cell recruitment into inflamed skin (5–7). Similarly, E- and P-selectin ligands are critical for T cell trafficking to inflamed skin (4, 8) and, together, are characteristically referred to as “skin-homing receptors.” In vivo models of T cell-mediated inflammation reveal that E- and P-selectin ligand function and skin-tropic behavior on T cells are dependent on α1,3 fucosyltransferases IV and VII (FT4/7) (7, 9, 10). These enzymes are responsible for generating sialyl Lewis X moieties on T cell membrane protein and lipid scaffolds, conferring ligand activity and the skin-homing designation of leukocytes (11–18). Prior studies using metabolic inhibitors of selectin ligand biosynthesis, namely 4-fluorinated N-acetylglucosamine (4-F-Glc-NAc), also show that preventing the formation of sialyl Lewis X moieties inhibits the recruitment of effector T cells to inflamed skin (19, 20). Collectively, the functional importance of T cell E- and P-selectin ligands and FT4/7 highlights the potential benefit of targeting these factors as anti-inflammatory strategies. In light of the proposition that NK cells can help elicit hapten-induced CHS responses, the significance of this functional activity heightens the importance of understanding the E- and P-selectin ligand repertoire on NK cells and how to control both T and NK cell recruitment into inflamed skin.
The ubiquitous P-selectin ligand on leukocytes, including NK cells (21), is P-selectin glycoprotein ligand-1 (PSGL-1), which has also been found to be a major E-selectin ligand on T cells. The predominant E-selectin ligands on T cells are PSGL-1 (15, 16, 22–24) and CD43 (16, 25–27), and are both involved in the dermal tropism of effector T cells in CHS. However, the expression and role of NK cell E-selectin ligands in allergic CHS is not fully understood (21, 28 –31) and is the impetus for studies performed in this research.
Using purified NK cells from mice and humans, we performed biochemical analyses to illuminate authentic candidate NK cell E-selectin ligand(s). Preparation of NK cell isolates from PSGL-1−/− or FT4/7−/− mice were included to evaluate the role of PSGL-1 and E-selectin ligands in NK and T cell recruitment associated with CHS. Our studies showed that the major NK cell E-selectin glycoprotein ligand was PSGL-1 and that NK cell glycolipids bearing both terminal sialic acid and fucosylated residues (or fucosylated neolactosphingolipids) were also major constituents bearing E-selectin ligand activity. In addition, we found that PSGL-1 was the principal P-selectin ligand on NK cells. Of particular significance, we found that, while 4-F-GlcNAc treatment interfered with CHS responses and E-selectin ligand synthesis on activated T cells, NK cell E-selectin ligand expression was resistant to 4-F-GlcNAc treatment. We also observed that 4-F-GlcNAc was orally active. These findings expand our view of the skin-homing receptor repertoire on NK cells and indicate that effector leukocytes are differentially sensitive to glyco-metabolic inhibitors, providing the opportunity to selectively manage adaptive cutaneous T cell-mediated inflammation.
Materials and Methods
Mice
Four- to six-week old, wild type (wt) or Rag1-deficient C57BL/6J mice, which were backcrossed 10-times into C57BL/6J inbred mice, were purchased from The Jackson Laboratories and housed in the animal facility in The Harvard Institutes of Medicine. C57BL/6 mice deficient in α1,3 fucosyltransferases IV and VII (FT4/7) were generated as previously described (9) and provided by Dr. John B. Lowe (Department of Pathology, Case Western Reserve University School of Medicine). C57BL/6 mice deficient in PSGL-1 were generated as previously described (22) and provided by Dr. Bruce Furie (Department of Medicine, Division of Hemostasis/Thrombosis, Beth-Israel Deaconess Medical Center).
Cells
Human hemopoietic KG1a cells (from American Type Culture Collection), which express E-selectin ligands CD44 and PSGL-1, were maintained in RPMI 1640 with glutamine/10% FBS/1% penicillin/streptomycin (Invitrogen Life Technologies) (32).
For isolation of fresh leukocytes from wt and mutant C57BL/6 mice in allergic CHS experiments, skin-draining lymph nodes (LN) (auricular and inguinal) or spleens were resected and minced with frosted cover slips. Leukocyte suspensions were released in ice-cold PBS, passed through a 40-μm cell strainer and washed with PBS. To lyse RBC in splenic preparations, the pellet was resuspended in 5 ml of RBC lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA (pH 7.2)) and incubated for 5 min at room temperature. Cells were washed in PBS and passed through a 40-μm cell strainer. Cells suspended in RPMI 1640/0.2% BSA were then plated onto tissue culture flasks (BD Biosciences) at 37°C for 30 min to deplete any nonleukocyte stromal cells, strained again, and washed with PBS. T cells were isolated by positive selection using anti-murine CD90 (Thy1.2) Ab-coated magnetic bead technology according to the manufacturer’s instructions (Miltenyi Biotec). T cell purity was confirmed by FACS analysis of CD3 expression. NK cells were then isolated from CD90-depleted cells using anti-CD49b (DX5) Ab-coated magnetic microbeads (Miltenyi Biotec). DX5+ CD90− NK cell purity was confirmed by FACS staining with anti-NK1.1 moAb PK136 (BD Biosciences).
Human NK cells were isolated from the whole blood of normal healthy volunteers in accordance with the Institutional Review Board(s) and principles expressed in the Helsinki Declaration. PBMC were first obtained from citrated-whole blood by density-gradient centrifugation as previously described (20). To prepare CD56+ CD3− NK cells, CD3+ T cells were first depleted from PBMC using CD3 immunomagnetic beads (Miltenyi Biotec) and then CD56+ cells were selected from CD3− cells using CD56 immunomagnetic beads (Miltenyi Biotec). NK cell purity was validated by flow cytometric analysis of CD56 (Miltenyi Biotec) and was consistently >95% positive.
Model of allergic contact dermatitis
As a model of allergic contact dermatitis (5), we induced CHS in mice by sensitizing abdominal skin on days 0 and 1 with 25 μl of 0.5% 2,4-dinitrofluorobenzene (DNFB) in a 4/1 solution of acetone to olive oil (vehicle) and then rechallenged with 0.25% DNFB on the ear (10 μl/side) on day 5. To demonstrate Ag-dependence, ear swelling was determined in mice sensitized with vehicle and then challenged with DNFB or in mice sensitized with DNFB and then challenged with vehicle. In addition, to evaluate the oral efficacy of the selectin ligand inhibitor, peracetylated 4-fluoro-N-acetylglucosamine (4-F-GlcNAc), mice were treated i.p. or orally (p.o.) from days 1–5 with drug vehicle (0.9% saline) or 100 mg/kg 4-F-GlcNAc. This dose of 4-F-GlcNAc administered i.p. has been shown to inhibit leukocyte E-selectin ligand expression and markedly blunt allergic CHS responses by i.p. administration (19). Ear swelling was determined by calculating the difference in ear thickness between days 5 and 6. Statistical significant differences between groups (10 mice/group) were ascertained using a Student’s paired t test. This experimental protocol was used routinely for generating fresh effector leukocytes and analysis of leukocyte E-selectin ligand expression as assayed by flow cytometry, parallel-plate flow chamber analysis, and Western blotting.
Flow cytometric analysis
All cells (1 × 106/test) were suspended in FACS buffer (PBS with Ca2+/Mg2+ containing 3% FBS and 0.05% NaN3). Where indicated, cells were pretreated with 0.2U/ml Vibrio Cholerae neuraminidase (Roche Diagnostics), a glycosidase that cleaves terminal sialic residues on the cell surface, or 0.1% bromelain (Sigma-Aldrich), a protease that cleaves cell surface protein, for either 1 h or 30 min, respectively, at 37°C. Cells were first blocked in 10% normal goat serum for 5 min and then incubated with 10 μg/ml recombinant mouse E-selectin-human IgG chimera (R & D Systems) in HBSS with Ca2+/Mg2+/10 mM HEPES with or without 250 mM EDTA for 30 min at 4°C. After washing in FACS buffer, cells were incubated with allophycocyanin-F(ab′)2 goat anti-human IgG Fcγ (Jackson ImmunoResearch Laboratories) for 20 min at 4°C, rinsed, and then blocked again with 10% normal rat serum (Sigma-Aldrich) for 5 min. For multiple marker analysis of NK cells, cells were then stained with PE-anti-mouse CD49b (DX5) and PE Cy7-anti-mouse NK1.1 (PK136) moAbs (both from BD Biosciences) for 20 min at 4°C. For multiple marker analysis of T cells, cells were then stained with PE-Cy5-anti-mouse TCR β-chain and FITC-anti-mouse L-selectin (MEL-14) (both from BD Biosciences) for 20 min at 4°C. Positive and negative controls were blocked with goat and rat serum and then stained with PE-anti-mouse CD8, allophycocyanin-anti-mouse CD3ε, PE Cy7-NK1.1, or FITC-anti-mouse CD31 Abs (all from BD Biosciences) or with FITC-anti-mouse CD90 (Miltenyi Biotec) Ab for 20 min at 4°C. For human NK/T cell staining, cells were suspended in FACS buffer, blocked in anti-human CD16 (clone 3G8) Ab (Biolegend), and then incubated with FITC-anti-human CD3 (BD Biosciences) and/or PE-anti-CD56 Abs (Miltenyi Biotec) for 30 min at 4°C. The absolute number of E-selectin ligand+ NK or T cells per inguinal LN (ILN) was determined by multiplying the percentage of E-selectin ligand+ DX5+ NK1.1+ cells or of CD90+ cells by the total number of leukocytes isolated per ILN. Samples were acquired with a BD FACSCanto flow cytometer, and data were analyzed with FlowJo software (Tree Star).
Immunoprecipitation/SDS-PAGE/Western blotting
KG1a cell lysate and lysates from murine and human leukocyte isolates were prepared as previously described (20). In some cases before lysate preparation, cells were treated with 0.1% bromelain (Sigma-Aldrich) for 30 min at 37°C. Protein concentrations were quantified using Bradford reagent (Sigma-Aldrich). For immunoprecipitation of PSGL-1, anti-human PSGL-1 moAb KPL-1 (BD Biosciences) was incubated with lysates and PSGL-1 immune complexes were eluted from protein-G agarose (Invitrogen Life Technologies) as previously described (33). Untreated or treated cell lysates or anti-PSGL-1 immunoprecipitates were resolved on reducing 4 –20% SDS-PAGE gradient gels (Bio-Rad) and transferred to Immunoblot polyvinylidene difluoride membrane (Bio-Rad) (34). After blocking in FBS for 1 h, blots were incubated with either rabbit polyclonal anti-sera raised against the N-terminal amino acid sequence (aa 42– 60) of mouse PSGL-1, anti-human PSGL-1 moAb KPL-1, E-selectin-Ig, or isotype Ab controls for 1 h at room temperature. To control for protein loading, blots were performed in parallel using anti-PSGL-1 moAb KPL-1 or anti-β-actin moAb C4 (BD Biosciences). Blots were washed with TBS/0.1% Tween 20 and incubated with corresponding alkaline phosphatase-conjugated anti-rabbit, -human or -mouse IgG (all at 1/2000) (Zymed) for 1 h at room temperature. Following several washes with TBS/0.1% Tween 20 and one final wash with TBS, blots were developed using alkaline phosphatase substrate Western Blue (Promega). These experiments were performed at least three times.
Parallel-plate flow chamber analysis
T cell rolling on mouse E-selectin-Ig chimera was analyzed using the parallel-plate flow chamber under physiologic shear stress (20, 35). To prepare E-selectin-Ig-coated plastic, E-selectin-Ig (50 ng/50 μl 0.1M NaHCO3) (pH 9.6) was pipetted on ten-twenty-nine petri dishes and allowed to adsorb overnight at 4°C. E-selectin-Ig solution was removed and replaced with FBS and incubated for ≥2 h at 37°C to block nonspecific binding sites. T cells were suspended at 1 × 107 cells/ml HBSS/10 mM HEPES/2 mM CaCl2 assay medium and infused into the chamber over selectin chimera. Cell tethering defined as a reduction in forward motion below hydrodynamic velocity for a minimum of two frames (0.07 s) was permitted at 0.3 dynes/cm2 for 1 min. Cell rolling defined as >5 cell diameters of forward movement below hydrodynamic velocity was then assessed. The number of rolling cells was assayed at 1 dyne/cm2 from the midpoint of the chamber viewing field (four fields/selectin spot, three different experiments) at 100× magnification. All experiments were observed in real time and videotaped for offline analysis. Rolling cells were enumerated on human IgG or using adhesion assay medium containing 5 mM EDTA to control for E-selectin-binding specificity.
Cell binding assay
To examine E- and P-selectin-binding activities of fresh human NK cells, we designed a cell adhesion assay using tissue culture-treated 96-well plates with black sides and clear bottoms (Corning). This system allowed for a qualitative analysis of limited numbers of NK cells. Wells were first coated with 10 μg/ml mouse E- or P-selectin-Ig (R & D Systems) or negative control human IgG for 24 h at 4°C. To block nonspecific binding, PBS/1% BSA was then added to the wells for 15 min at 4°C. KG1a or fresh CD56+CD3− NK cells were next labeled with a fluorescent dye, calcein AM (Molecular Probes), at 5 μg/ml in adhesion assay buffer (RPMI 1640/0.2% BSA) for 30 min at 22°C. Where indicated, cells were pretreated with 0.2 U/ml neuraminidase for 1 h or 0.1% bromelain for 30 min at 37°C or with 40 μg/ml anti-human PSGL-1 moAb KPL-1 or isotype control. Untreated or treated NK cells were then incubated with 10 μg/ml anti-CD16 moAb 3G8 (Biolegend) for 10 min to block nonspecific binding of NK cell FcγRIIIα receptor to Ig IgG as described previously (36). Cells suspended at 2 × 105 were added to wells in the presence or absence of 10 mM EDTA and incubated for 1 h at 4°C. Wells were washed three times with adhesion assay buffer. Fluorescence (485 nm excitation; 535 nm emission) was measured on a Wallac Victor (2) plate reader (PerkinElmer) at 0.1 s intervals at the Institute of Chemistry and Cell Biology Screening Facility of Harvard Medical School. Values represent percent cell binding of untreated cell binding subtracted from nonspecific cell binding to human IgG alone. Statistical significant differences with untreated control were ascertained using a repeated measures ANOVA with Dunnett post test.
Results
Induction of E-selectin ligand on T cells and NK cells in allergic CHS
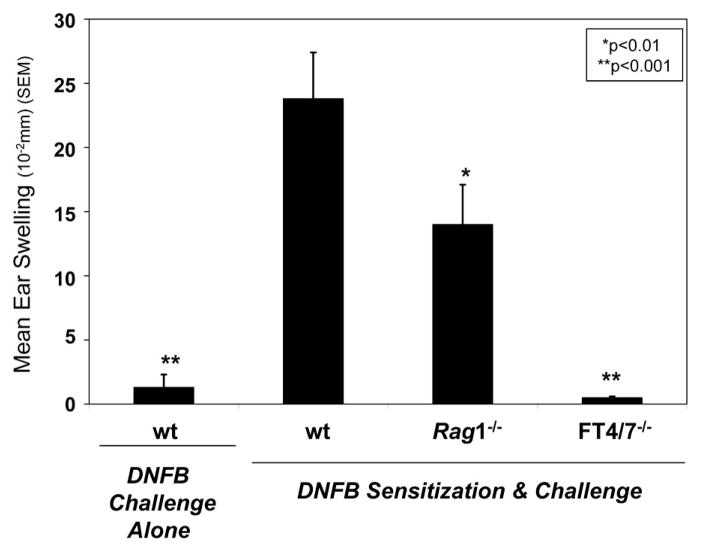
Effector leukocyte E- and P-selectin ligands are regulated by leukocyte α1,3 fucosyltransferases 4 and 7 (FT4/7) and are necessary for Ag-specific cutaneous inflammation and leukocyte recruitment into inflamed skin (4 –10). To validate the role of NK cells in allergic CHS, we performed NK cell-dependent CHS experiments with contact sensitizer DNFB in recombinase-activating gene 1-deficient (Rag1−/−) mice lacking T and B cells as previously described (2). The dependence of leukocyte FT4/7 in this model was also ascertained by performing CHS experiments in FT4/7−/− mice. Compared with DNFB-sensitized and challenged wt mice, CHS responses were evident, although significantly less in Rag1−/− mice (p < 0.01) and were attributable to E- and P-selectin ligand expression as mice lacking FT4/7 did not show any inflammation (p < 0.001) (Fig. 1).
FIGURE 1.
Allergic CHS in Rag1−/− or FT4/7−/− mice. CHS responses were induced in wt, Rag1−/−, or FT4/7−/− mice (10 mice/group) by sensitizing on days 0 and 1 with DNFB or vehicle control (negative control) and challenge applications on day 5 with DNFB. Ear thickness measurements were taken before and after challenge and expressed as mean (SEM). Wt mice receiving vehicle sensitization and DNFB challenge or FT4/7−/− mice sensitized and challenged with DNFB did not show any inflammation. Experiments were performed a minimum of three times. Statistically significant difference compared with ear swelling in wt mice sensitized and challenged with DNFB; Student’s paired t test, *, p < 0.01 and **, p < 0.001.
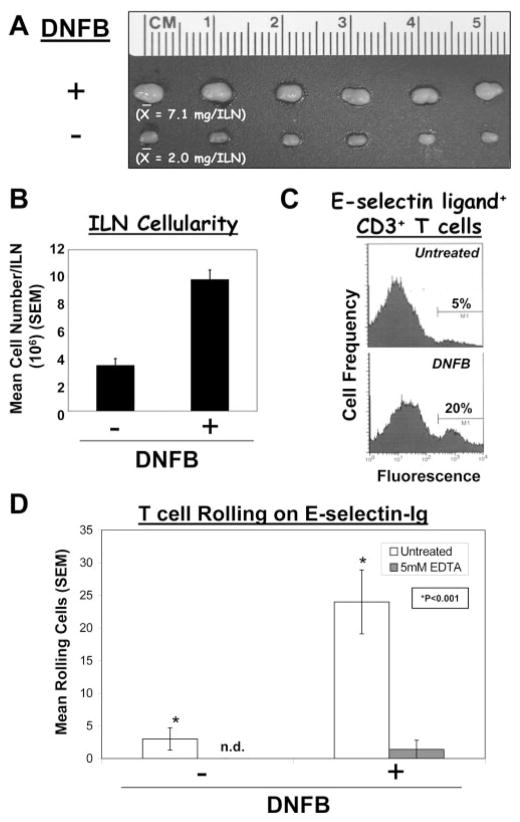
To investigate relevant E-selectin ligand+ effector leukocytes in this CHS inflammatory model, leukocytes were harvested from ILN-draining DNFB-sensitized abdominal skin. We first established that E-selectin-ligand expression was elevated on T cells after DNFB-sensitization. Skin-draining ILN from DNFB-sensitized mice were 3-fold larger and contained 3-fold greater number of cells than ILN from naive mice (Fig. 2, A and B), signifying the in situ T cell activation/expansion in and/or recruitment of naive lymphocytes to LN draining Ag-sensitized skin (10). Flow cytometry with E-selectin-Ig showed that a 4-fold higher percentage of E-selectin ligand+ CD3+ T cells in ILN draining DNFB-sensitized skin was observed in comparison with those cells in untreated mice (Fig. 2C). In addition, rolling of immunopurified CD90+ T cells on E-selectin-Ig was elevated 5-fold compared with that displayed by cells in ILN from untreated mice (p < 0.001) (Fig. 2D).
FIGURE 2.
Ag-dependent induction of T cell E-selectin ligand in CHS. CHS responses in mice were elicited by sensitization and challenge applications of DNFB. ILN draining DNFB or untreated skin were harvested, and T cells were assayed for E-selectin ligand activity by flow cytometry and parallel-plate flow analysis. ILN draining DNFB-treated skin were 3-fold larger and 3-fold more cellular than ILN draining untreated skin (A and B). C, Flow cytometry with E-selectin-Ig showed that gated CD3+ T cells from DNFB-treated mice expressed a 4-fold higher level of E-selectin ligand than on T cells from untreated mice. D, Parallel-plate flow analysis revealed that CD90+ T cells from ILN draining DNFB-sensitized skin rolled on E-selectin-Ig at a 5-fold greater level than T cells from untreated mice. Cell rolling activities expressed as mean (SEM) were inhibited with 5 mM EDTA and not evident on human IgG. Experiments were performed a minimum of three times. *, Statistically significant difference compared with untreated control; Student’s paired t test, p < 0.001.
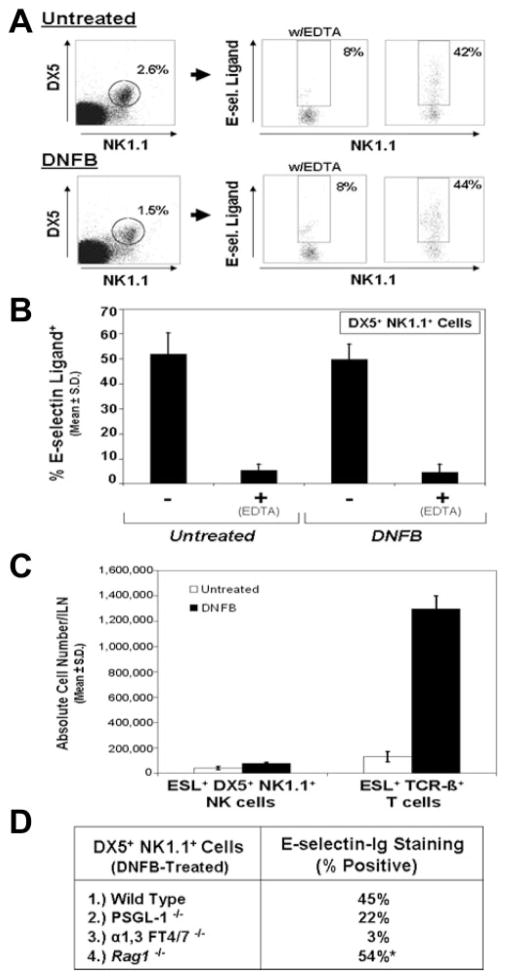
To analyze E-selectin ligand expression on fresh NK cells, we performed flow cytometry analysis and assayed E-selectin-Ig staining of cells positive for NK cell markers, DX5 (CD49b) and NK1.1 (CD161c), and negative for T cell marker, Thy1.2 (CD90). DX5+ NK1.1+ NK cells were negative for other markers, including CD3, CD31, and TCR-β-chain (data not shown). Compared with E-selectin-Ig staining in the presence of 5 mM EDTA, the percentage of E-selectin ligand+ NK cells (50%) was similar in cell isolates from ILN draining either untreated or DNFB-sensitized skin (Fig. 3, A and B). Even though ILN cellularity increased 3-fold after DNFB treatment, the absolute number of E-selectin ligand+ NK cells increased by only 1.8-fold, whereas the absolute number of E-selectin ligand+ T cells increased 10-fold (Fig. 3C). These results suggested that E-selectin ligand+ NK cells did not increase proportionately with ILN cellularity. Additional experiments analyzing E-selectin ligand expression on DX5+ NK1.1+ NK cells in PSGL-1−/−, FT4/7−/− or Rag1−/− mice were performed to determine whether expression of NK cell E-selectin ligand was altered in these mutant genotypes. Of note, due to the negligible level of leukocytes in ILN of FT4/7−/− and Rag1−/− mice, splenic leukocytes were used for analysis. Flow cytometric analysis revealed that the percentage of E-selectin ligand+ DX5+ NK1.1+ NK cells from DNFB-treated Rag1−/− mice was similar to wt controls; however, E-selectin-Ig staining on PSGL-1− DX5+ NK1.1+ NK cells was reduced by 50%, indicating that PSGL-1 may be a major ligand on NK cells (Fig. 3D). Moreover, E-selectin ligand expression on DX5+ NK1.1+ NK cells was dependent on FT4/7 (Fig. 3D). Interestingly, we found that a similar percentage of E-selectin ligand+ DX5+ NK1.1+ NK cells (45–55%) was observed in spleens from untreated or DNFB-treated wt and PSGL-1−/− mice (data not shown). This observation indicated that splenic tissue could be used as a source for isolating an adequate quantity of NK cells for lysate preparation and Western blotting experimentation.
FIGURE 3.
Invariable E-selectin ligand expression on NK cells in CHS. CHS responses using sensitization and challenge applications of DNFB were generated in wt, PSGL-1−/−, FT4/7−/−, or Rag1−/− mice. ILN draining DNFB- or vehicle-treated skin from wt and PSGL-1−/− mice or spleens from DNFB- or vehicle-treated FT4/7−/− and Rag1−/− mice were harvested, and ESL expression was assayed on DX5+ NK1.1+ NK cells by flow cytometry. A, In representative scatter plots, a comparable percentage of ESL+ DX5+ NK1.1+ NK cells was observed from both untreated and DNFB-treated mice. These percentages (mean ± SD) were consistently ~50% (B). Comparing the absolute number of ESL+ NK cells with ESL+ T cells from untreated or DNFB-treated mice showed that ESL+ T cells were 10-fold greater than ESL+ NK cells in DNFB-treated mice (C). In PSGL-1−/− mice, there was a 50% lower level of E-selectin-Ig staining on NK cells, while NK cells deficient in FT4/7 did not express any E-selectin ligand activity (D). Rag1−/− mice expressed a similar percentage of ESL+ DX5+ NK1.1+ NK cells as wt mice (D). *, Percent of DX5+ NK1.1+ gated cells in Rag1−/− mice was 10-fold higher than in wt controls. Experiments were performed a minimum of three times.
PSGL-1 is a major E-selectin glycoprotein ligand on NK cells
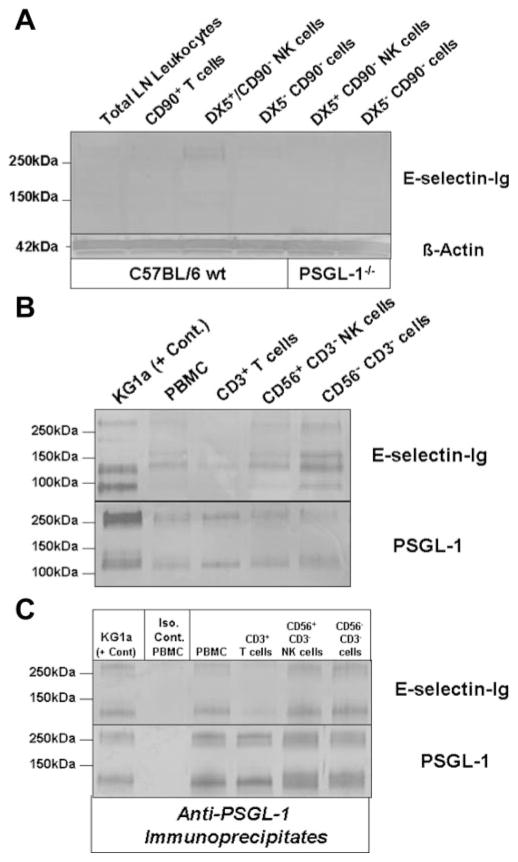
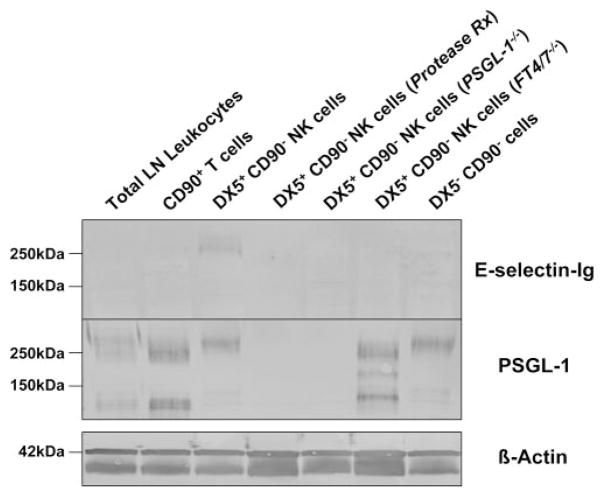
To identify the authentic E-selectin glycoprotein ligand on NK cells, we isolated fresh NK cells from LN draining DNFB-sensitized skin. After depleting T cells from total LN leukocytes with CD90 (Thy1.2) immunomagnetic beads, NK cells were enriched by positive selection with DX5 immunomagnetic beads. Lysates were prepared from unsorted total leukocytes, CD90+ T cells, DX5+ CD90− NK cells and DX5− CD90− leukocytes and analyzed by Western blotting with E-selectin-Ig. Flow cytometric analysis of NK1.1 confirmed that DX5+ CD90− cells were positive for NK1.1 Ag (data not shown). E-selectin-Ig blotting revealed that a prominent glycoprotein at 260 kDa was expressed only on DX5+ CD90− NK cells (Fig. 4A). In contrast, there was no detectable E-selectin glycoprotein ligand in CD90+ T cell lysate, due to the small percentage of E-selectin ligand+ T cells among total sorted CD90+ T cells (Fig. 3C). Nonetheless, the absence of E-selectin-Ig-stained DX5+ CD90− NK cell glycoprotein from PSGL-1−/− mice indicated that the 260 kDa band was likely PSGL-1 (Fig. 4A). Characteristically, PSGL-1 resolves on reducing SDS-PAGE gels as a monomer at 120 –130 kDa and/or as a dimer at 240 –260 kDa. Depending on the cellular source of PSGL-1, the ratio of fully reduced monomer PSGL-1 to unreduced dimer PSGL-1 is variable and implicit when investigating the expression of PSGL-1 by Western blotting.
FIGURE 4.
E-selectin-binding PSGL-1 is expressed on NK cells. A, Western blot analysis of E-selectin ligand expression was performed on DX5+ CD90− NK cell lysates (40 μg/lane) from both wt and PSGL-1−/− mice. Compared with total LN leukocyte, CD90+ T cell or DX5− CD90− cell lysates, a demonstrable E-selectin-Ig-stained band was evident in DX5+ CD90− NK cell lysate at 260 kDa, which was absent in DX5+ CD90− NK cell lysate from PSGL-1−/− mice. Protein loading was controlled for by staining lysates with anti-β-actin. B, Western blot analysis of E-selectin ligand expression was performed on control KG1a cell lysate and on total PBMC, CD3+ T cell, CD56+ CD3− NK cell, and CD56− CD3− cell lysates (30 μg/lane). Compared with E-selectin-Ig-stained bands principally represented by CD44 (95kDa) and PSGL-1 (130 and 260kDa) in KG1a lysate, there were also E-selectin-Ig-stained bands at 130 and 260 kDa in CD56+ CD3− NK cell lysates that comigrated with KG1a PSGL-1, in addition to another band at 155 kDa. Protein loading was controlled by staining lysates with anti-PSGL-1 moAb KPL-1. C, To confirm the E-selectin-binding function of PSGL-1, PSGL-1 was immunoprecipitated with anti-PSGL-1 moAb KPL-1 or isotype Ab from component cell lysates and Western blotted with E-selectin-Ig or KPL-1. As shown, PSGL-1 bound E-selectin-Ig in all immunoprecipitates (C). Experiments were performed a minimum of three times.
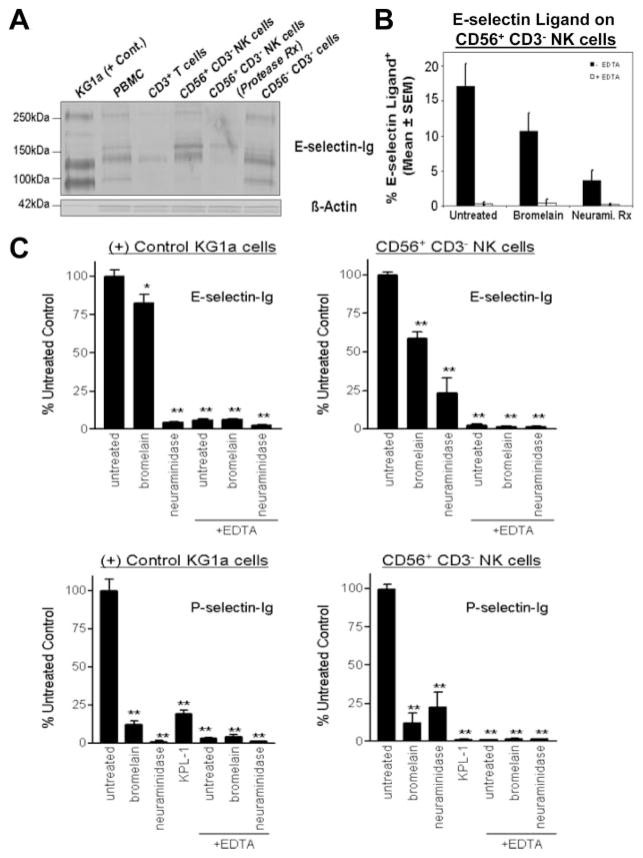
To better define the identity of PSGL-1 as a major E-selectin ligand on NK cells, we isolated human CD56+ CD3− NK cells from PBMC by immunomagnetic bead isolation and blotted lysates with E-selectin-Ig. After depleting CD3+ cells from PBMC, CD56+ cells were positively selected and used for flow cytometry and lysate preparation. Flow cytometry of CD56 and CD3 Ag confirmed that sorted CD3+ or sorted CD56+ CD3− cell isolates were >90% (data not shown). Western blotting CD56+ CD3− NK cell lysates with E-selectin-Ig showed that glycoproteins at 130, 155 and 260kDa were detected (Fig. 4B). Although CD56−CD3− cell lysates contained another band at 95 kDa, which is likely contributed by HCELL (CD44) on CD34+ HPC (32), CD3+ T cell and total PBMC lysates also expressed variable levels of E-selectin-binding proteins at 130, 155, and 260 kDa (Fig. 4B). Because E-selectin-Ig-stained protein at 130 and 260 kDa protein in KG1a cell lysate is largely represented by PSGL-1 (32, 34) (Fig. 4B), the E-selectin-Ig-stained protein at 130 and 260 kDa in CD56+CD3− NK cell lysate may also correspond to PSGL-1. The additional E-selectin glycoprotein ligand detected at 155 kDa may be compatible with a sialoglycoprotein localized to medial cisternae of the Golgi apparatus called E-selectin ligand-1 (ESL-1) (34).
To directly examine whether PSGL-1 expressed E-selectin-binding determinants on human NK cells, we immunoprecipitated PSGL-1 from CD56+CD3− NK cell lysate with moAb KPL-1 and blotted immunoprecipitates with E-selectin-Ig. Anti-PSGL-1 immunostaining was also performed to confirm the presence of PSGL-1 in immunoprecipitates. Compared with the isotype control immunoprecipitate and KG1a anti-PSGL-1 immunoprecipitate, all cell isolates from PBMC, including NK cells, expressed E-selectin-binding PSGL-1 (Fig. 4C).
Glycolipid E-selectin ligands are a major component on NK cells
In the Western blotting experiments described above, we observed that PSGL-1 was a prominent NK cell E-selectin ligand, though other glycoproteins, including CD43 and CD44, may have been undetectable by Western blotting with E-selectin-Ig. To analyze whether CD43 and CD44 also function as E-selectin ligands on NK cells, we pretreated DX5+ CD90− NK cells with a robust protease, bromelain, before lysate preparation. With the exception of ESL-1, an E-selectin ligand characteristically localized in the Golgi (34), bromelain digestion cleaves all cell surface E-selectin glycoprotein ligands on leukocytes (20, 33, 37). By flow cytometry, we first confirmed that bromelain treatment eliminated PSGL-1, CD43 and CD44 expression (data not shown). Subsequent Western blotting with E-selectin-Ig showed bromelain treatment of DX5+ CD90− NK cells before lysate preparation completely ablated E-selectin-Ig-staining of the PSGL-1 molecule at 260 kDa (Fig. 5). Interestingly, there was no detectable E-selectin-Ig-staining glycoprotein at 130 kDa and at 100 kDa, which correspond to CD43 and CD44, respectively. The absence of E-selectin-Ig-staining at 260 kDa in PSGL-1−/− DX5+ CD90− NK cell lysates confirmed that this glycoprotein was indeed PSGL-1 (Fig. 5). Furthermore, the absence of immunostained PSGL-1 in protease-treated NK cells validated the efficacy of bromelain to completely remove surface glycoprotein (Fig. 5). E-selectin-Ig-staining of PSGL-1 on NK cells from FT4/7−/− mice was also absent, indicating that α1,3 fucosylation was a key event for functional activity. The lack of E-selectin-binding determinants on resolved NK cell PSGL-1 from FT4/7−/− mice was highlighted by more rapid mobilization of PSGL-1 (Fig. 5).
FIGURE 5.
PSGL-1 is the principal E-selectin ligand on the NK cell surface. Lysates (30 μg/lane) were prepared of DX5+ CD90− NK cells from wt, PSGL-1−/−, or FT4/7−/− mice. Where indicated, DX5+ CD90− NK cells were treated with protease (0.1% bromelain) before lysate preparation. Western blot analysis of E-selectin ligand showed that protease treatment removed E-selectin-binding PSGL-1 (260kDa) and that PSGL-1 E-selectin-binding determinants were absent in FT4/7-deficient NK cells. Western blotting with anti-PSGL-1 moAb KPL-1 confirmed that PSGL-1 was eliminated on protease-treated cells and absent in PSGL-1−/− mice and that PSGL-1 was present in all other groups. Equivalent protein loading was validated by staining lysates with anti-β-actin moAb. Experiments were performed a minimum of three times.
Using human NK cells, we performed the analogous experiment, in which lysates from bromelain-treated CD56+ CD3− NK cells were blotted with E-selectin-Ig (Fig. 6A). Compared with E-selectin-Ig staining of untreated CD56+ CD3− NK cell lysate, there was a clear removal of 130 and 260 kDa stained protein, which likely correspond to the monomer and dimer forms of PSGL-1 as indicated in Fig. 4, B and C. Interestingly, the presence of the E-selectin-Ig-stained protein at 155 kDa was resistant to protease treatment, further indicating that this protein may be the intracellular E-selectin ligand, ESL-1 (34).
FIGURE 6.
Protease-resistant glycolipids represent a major component of NK cellular E-selectin ligand activity. Lysates (40 μg/lane) were prepared of sorted CD56+ CD3− NK cells and, where indicated, NK cells were treated with protease (0.1% bromelain) before lysate preparation. Western blotting showed that the major E-selectin-Ig-stained bands at 130 and 260 kDa in NK cell lysate were eliminated and a band at 155 kDa was reduced after protease treatment (A). Untreated and protease- or neuraminidase-treated NK cells were analyzed by flow cytometry with E-selectin-Ig. As shown, protease treatment of CD56+ CD3− NK cells reduced E-selectin-Ig staining by 40% and was inhibitable by co-incubating with 5 mM EDTA (B). Neuraminidase treatment confirmed that terminal sialic acid residues are necessary for NK cell E-selectin ligand activity (B). All E-selectin-Ig staining of NK cells was inhibitable by EDTA (B). In a cell binding assay system, P- and E-selectin-mediated binding of NK cells was quantified after pretreatment with bromelain, neuraminidase,or anti-PSGL-1 moAb KPL-1. Adhesion values expressed as the percentage of adhesion of untreated control were first normalized by subtracting cell binding values to human IgG alone. Control KG1a cell and CD56+ CD3− NK cell adhesion to P-selectin was markedly inhibited by bromelain and by anti-PSGL-1 moAb KPL-1 (C) (p < 0.001). In contrast, protease-resistant E-selectin ligand activity on KG1a and CD56+ CD3− NK cells was evident (C). Experiments were performed a minimum of three times. Statistically significant difference compared with untreated control; Repeated measures ANOVA with Dunnett post test, *, p < 0.05; **, p < 0.001.
To investigate whether eliminating surface glycoprotein affected the capacity of human NK cells to bind E-selectin, we assayed the binding activity of bromelain-treated CD56+ CD3− NK cells by performing flow cytometry with E-selectin-Ig. E-selectin-Ig staining of CD56+ CD3− NK cells treated with bromelain revealed that a major component of E-selectin-binding was protease resistant (60%) (Fig. 6B). In addition, we found that neuraminidase pre-treatment markedly reduced E-selectin-Ig staining, suggesting that terminal sialylation was a critical component of the E-selectin-binding determinant (Fig. 6B).
To validate that bromelain treatment showed a modest decrement in E-selectin ligand activity, we used a whole cell binding system to assay E and P-selectin ligand activities of limited numbers of sorted human NK cells. Adhesion to selectin-human IgG chimera was normalized to human IgG binding alone and expressed as percentage of untreated control. All cell binding was inhibitable by incubating with 5 mM EDTA and was significantly reduced by neuraminidase pretreatment (p < 0.001) (Fig. 6C). Consistent with flow cytometry data, CD56+CD3− NK cell E-selectin ligand activity was sensitive to neuraminidase and marginally reduced by bromelain treatment (reduced by 40%) (p < 0.001) (Fig. 6C). Of note, the adhesion of CD56+CD3− NK cells to P-selectin was significantly inhibited by bromelain, by neutralizing anti-PSGL-1 moAb KPL-1 and by neuraminidase (p < 0.001) (Fig. 6C). This indicated that PSGL-1 was the major P-selectin ligand on NK cells (21). Collectively, these results solidified prior observations (28) and implicated glycolipids as major effector membrane structures mediating NK cell adhesion to E-selectin.
Control of allergic CHS with orally administered 4-F-GlcNAc
Prior studies by our laboratory show that 4-F-GlcNAc is a metabolic inhibitor of N-acetyllactosamine and sialyl Lewis X biosynthesis, and, when administered i.p., can down-regulate E-selectin ligand expression on effector lymphocytes in association with suppressed CHS responses (19). Efficacious and toxic doses of 4-F-GlcNAc were established previously, although oral bioavailability of 4-F-GlcNAc has yet to be evaluated (19). In this study, we determined whether CHS responses and corresponding E-selectin ligand expression on effector T cells and NK cells in wt and Rag1−/− mice were modified by orally administered 4-F-GlcNAc. CHS responses were examined after implementing p.o. 4-F-Glc-NAc treatment from days 1–5. 4-F-GlcNAc efficacy was evaluated by assaying ear swelling and by assaying E-selectin ligand expression on NK cells by Western blotting and flow cytometry. In addition, E-selectin ligand expression on L-selectin+ or L-selectin− TCR-β-chain+ T cells from draining ILN was assayed to investigate the sensitivity of activated (effector) T cells to 4-F-GlcNAc. I.p. 4-F-GlcNAc treatment (100 mg/kg) markedly suppressed DNFB-dependent CHS responses in wt mice as determined by blunted ear swelling (p < 0.001) (Fig. 7A). Oral administration of 4-F-GlcNAc (100 mg/kg) was also efficacious (statistical significant difference compared with p.o. saline control; Student’s t test, p < 0.01). Interestingly, however, 4-F-GlcNAc treatments did not affect CHS responses in Rag1−/− mice, suggesting that effector NK cells in Rag1−/− mice were resistant to 4-F-GlcNAc treatment. In support of this notion, flow cytometry did not reveal a demonstrable reduction in E-selectin ligand expression on DX5+ NK1.1+ NK cells from 4-F-GlcNAc-treated wt and Rag1−/− mice (Fig. 7B). Similarly, Western blotting experiments showed that E-selectin-binding PSGL-1 on NK cells was evident in all treatment groups (Fig. 7C). To the contrary, E-selectin ligand expression on L-selectin− TCR-β+ T cells in DNFB-treated wt mice was significantly lowered in both i.p. and p.o. 4-F-GlcNAc-treated mice compared with corresponding saline treatment controls (p < 0.004 and p < 0.04, respectively) (Fig. 7D). Moreover, E-selectin ligand expression on L-selectin− TCR-β + T cells, notably in i.p. 4-F-GlcNAc-treated mice, was reduced to the basal level expressed on L-selectin− TCR-β+ T cells from untreated mice (Fig. 7D). Of note, the percentage and expression level of E-selectin ligand on L-selectin+ TCR-β+ T cells was unaffected by 4-F-GlcNAc treatment, which is likely due to the fact that L-selectin+ T cells recirculate among all lymphoid tissues.
FIGURE 7.
Oral efficacy of 4-F-GlcNAc on allergic CHS. CHS responses were generated by sensitizing wt or Rag1−/− mice on days 0 and 1 and challenged on day 5 with DNFB or vehicle control. Mice (10 mice/group) were administered i.p. or p.o. with 0.9% saline or 100 mg/kg 4-F-GlcNAc from days 1–5. Ear thickness measurements before and 24 h after DNFB-challenge were monitored to assess anti-inflammatory efficacy of 4-F-GlcNAc. DX5+ NK1.1+ NK cells from ILN of saline or treated wt and Rag1−/− mice and L-selectin− TCR-β + T cells from ILN of saline or treated wt mice were analyzed for E-selectin ligand expression by flow cytometry. Fresh DX5+ CD90− NK cells lysates were also prepared from saline or treated wt mice and analyzed for E-selectin ligand (PSGL-1) and β-actin expression by Western blotting. Both i.p. and p.o. 4-F-GlcNAc treatment caused significant reductions in mean ear swelling (statistically significant difference compared with saline treatment control; Student’s paired t test, **, p < 0.001 and *, p < 0.01, respectively), while CHS responses in Rag1-deficient mice were not affected (A). E-selectin ligand expression on DX5+ NK1.1+ NK cells from all groups was analyzed by flow cytometry and showed that ligand expression was relatively insensitive to 4-F-GlcNAc (B). Western blot analysis of E-selectin ligand and PSGL-1 expression on DX5+ CD90− NK cells (30 μg/lane) revealed that 4-F-GlcNAc did not ablate E-selectin-binding PSGL-1 (C). Anti-PSGL-1 and anti-B-actin immunoblots showed that loaded protein levels were similar (C). There was no staining evident in isotype control blots or in E-selectin-Ig blots performed in the presence of 5 mM EDTA. Flow cytometric analysis of E-selectin ligand expression on L-selectin− and TCR-β+ cells revealed that both i.p. and p.o. 4-F-GlcNAc treatments significantly reduced E-selectin ligand expression compared with saline treatment controls (statistically significant difference compared with corresponding saline treatment control; Student’s paired t test, **, p < 0.004; *, p < 0.04) (D). E-selectin ligand expression on L-selectin+ TCR-β+ T cells was unchanged following 4-F-GlcNAc treatment (D). Experiments were performed a minimum of three times.
Discussion
The molecular pathogenesis of cutaneous inflammation is critically dependent on the acquisition and use of skin-homing receptors (or E- and P-selectin ligands) on effector leukocytes (4, 8). Leukocyte E- and P-selectin ligands engage in low-affinity, Ca2+-dependent binding interactions with E- and P-selectin constitutively expressed on dermal postcapillary venules and initiate their recruitment into inflamed skin (1, 3–7). Functional activity of these ligands is dependent on the expression on sialo-fucosylated carbohydrate moieties synthesized by α2,3 sialyltransferase IV (32, 37, 38) and FT4/7 (9, 10). Identification of authentic leukocyte E- and P-selectin ligands has been vigorously investigated, as the implication of their neutralization may provide a means for management of chronic inflammatory conditions, such as atopic/allergic dermatitis and psoriasis, and cutaneous lymphomas.
Intriguing new findings show that NK cells can play an important role in allergic CHS responses (2). Even in the absence of T cells and B cells, these responses can be elaborated by skin-homing NK cells through a recruitment mechanism dependent on dermal E- and P-selectin and β2 integrins (2). Because the requirement for E- and P-selectin and β2 integrins are the key recruitment features of skin-homing leukocytes, we hypothesized that NK cell E- and P-selectin ligands are also critical for dermal tropic activity and could be targetable entities for anti-inflammatory exploitation. The identity of NK cell E-selectin ligand repertoire has yet to be formally ascertained and is the framework of our investigation in this study.
In this report, we analyzed the expression of native E-selectin ligands on fresh NK cells from both mice and humans using immunomagnetic bead technology and flow cytometric sorting. Multiple, yet complementary, assaying methods of cell isolates were used in this study to analyze NK cell E-selectin ligand expression due to the reagents available for probing human and murine selectin ligands and due to the limited numbers of NK cell isolates. NK cell E-selectin ligand expression was analyzed in the context of allergic CHS model. There is convincing evidence that induction of skin-tropic receptors (or E- and P-selectin ligands) on effector T cells in CHS models involves an Ag-driven priming event in LN draining Ag-treated or pathogen-infected skin (10, 12–14, 39). In fact, we previously demonstrate that E-selectin ligand on effector leukocytes isolated from LN draining DNFB-sensitized skin directly corresponds with allergic CHS reactions (19, 20, 33). To this end, we induced CHS in mice using DNFB and first investigated whether E-selectin ligand expression on NK cells from draining LN was up-regulated. We observed that the absolute number of E-selectin ligand+ NK cells in LN draining DNFB-treated skin did not expand proportionately with elevated LN cellularity, whereas a 10-fold induction of E-selectin ligand+ T cells was evidenced. However, the preponderance of NK cells was inherently positive for E-selectin ligand and, unlike the induced expression on T cells, NK cells expressed an invariable level of E-selectin ligand. A large percentage of NK cells (50%) was similarly E-selectin ligand positive in untreated and DNFB-treated mice. Using PSGL-1- or FT4/7-deficient mice in these studies, we found that NK cell E-selectin ligand activity was dependent on FT4/7 expression and largely contributed by PSGL-1 (50% of total) and by other E-selectin glycolipid ligand(s). Of note, a high frequency of E-selectin ligand+ NK cells (~50%) was also ascertained on cells isolated from splenic tissue independent of Ag sensitization. Although we argue that E-selectin ligands are necessary in the migration of NK cells to skin, the issue of tissue environment for priming skin-homing activity is complex and does not appear to be analogous to the hapten sensitization or T cell-dendritic cell priming occurring locally in draining LN. This point is highlighted by evidence that hapten-memory in CHS responses is transferable by the hepatic Ly49C-I+ subset of NK cells and that there is no difference in the frequency of Ly49C-I+ NK cells after hapten sensitization (2).
Analysis of NK cell E-selectin ligand identity was achieved by Western blotting NK cell isolates with E-selectin-Ig, a reagent commonly used for detecting functional E-selectin ligands (26, 33, 34). Western blotting with E-selectin-Ig showed that DX5+ CD90− NK cells expressed the E-selectin-binding glycoform of PSGL-1. This result was corroborated by evidence that PSGL-1 was the major E-selectin ligand on human CD56+ CD3− NK cells. There was a distinct E-selectin ligand noted at 155kDa in human CD56+ CD3− NK cell lysate that was resistant to protease pre-treatment, indicating that this protein may be expressed as an intracellular membrane protein and not a factor in cellular activity. This characteristic is reminiscent of the E-selectin ligand, ESL-1, a protease-resistant 150 kDa membrane protein characteristically localized to the medial cisternae of the Golgi apparatus (26, 34).
Although predominant E-selectin ligand activity on NK cells was ascribed to PSGL-1, the extent of residual activity after protease treatment was significant and suggested that other protease-sensitive ligands, such as CD43 and CD44, were not functional on NK cells. This residual activity, which consistently represented ~50% of total cellular E-selectin ligand activity in cell binding assays, may have a profound compensatory effect on maintaining dermal migratory activity in the absence of PSGL-1. The expression of protease-resistant E-selectin ligand activity on NK cells may elicit compensatory inflammatory activity in allergic CHS models performed in PSGL-1−/− CD43−/− mice (26, 27). Indeed, deficiencies of both FT4 and 7 are required for complete blunting of leukocyte E-selectin ligand formation and inflammatory activity (7, 9) and FT4 preferentially generates sialyl Lewis X moieties and E-selectin-binding determinants on glycolipids. These findings and others support our contention that protease-resistant E-selectin glycolipid ligand(s) is a key component of NK cellular activity (28). Further investigations are ongoing to provide direct evidence on which type of glycolipids bind E-selectin. We speculate that due to the requirement of sialyl Lewis X moieties, NK cell E-selectin glycolipid ligand(s) most likely belongs to a class called neolactosphingolipids.
Prior findings by our laboratory suggest that systemic administration of 4-F-GlcNAc can suppress allergic CHS by altering the metabolism of oligosaccharides necessary for functional expression of leukocyte E- and P-selectin ligands (19). There is strong evidence showing that 4-F-GlcNAc competes with natural-occurring N-acetylglucosamine and inhibits N-acetyllactosamine formation, preventing the synthesis of sialyl Lewis X and selectin-binding determinants on T cell membrane protein, including PSGL-1 and to a lesser degree CD43 (19, 20, 33). Hitherto, our analysis of leukocyte sensitivity to 4-F-GlcNAc treatment has focused on T cells. In view of the novel role of NK cells in allergic CHS (2, 3), the combinatorial influence of T and NK cell infiltration in the establishment of CHS suggests that diminution of E-selectin ligand expression on T cells as well as NK cells might account for anti-inflammatory efficacy of 4-F-GlcNAc. However, our findings reported in this study indicated that NK cell E-selectin ligand expression was not modified by 4-F-GlcNAc treatment. As expected, inhibition of T cell E-selectin ligand expression and CHS responses by 4-F-GlcNAc were noted. The differential efficacy implied that E- and P-selectin ligands on activated (L-selectin−) T cells may be more sensitive targets than those expressed on NK cells, suggesting that NK cells may exhibit a state of glyco-metabolic dormancy during the afferent phase of CHS. This point is strengthened by our observation the 4-F-GlcNAc treatment did not lower CHS responses in Rag1−/− mice, a mouse model analogous to Rag2−/− mice previously shown to develop NK cell-dependent CHS (2). Moreover, though 4-F-GlcNAc treatment significantly inhibited effector T cell E-selectin ligand expression, corresponding CHS responses in immunocompetent mice were not completely blunted by 100%. This indicated that NK cells in immunocompetent mice may have contributed to residual CHS responses. This residual activity was not as robust as that assayed in Rag1−/− mice, highlighting the possibility that T cells or other inflammatory mediators in a normal immunocompetent setting may regulate the effector function of NK cells in allergic CHS.
In summary, we provide novel and supportive evidence that PSGL-1 is the major E-selectin glycoprotein ligand on NK cells and assert that E-selectin glycolipid ligands play an important complementary role in NK cellular ligand activity. To our knowledge, this report shows for the first time that an orally efficacious selectin ligand inhibitor can effectively target inflammation-related synthesis of selectin-binding moieties on inflammatory cell membrane protein. We also demonstrate that effector leukocytes related to the elicitation of allergic CHS responses are differentially sensitive to this glycosylation inhibition approach in vivo. This distinction may offer the opportunity to selectively control adaptive immune responses without altering the tissue homing activity of leukocytes involved in other innate immunologic mechanisms. Our findings help rationalize the development of carbohydrate therapeutics for regulating effector T cell selectin ligand expression and for dampening cutaneous T cell-mediated inflammatory diseases. Further studies elucidating the sensitivity of other leukocytes, including neutrophils, dendritic cells and hemopoietic progenitor cells, to glycosylation inhibitors will help strategize the utility of glyco-metabolic antagonism as a targeted approach for treating leukocyte-associated pathologies.
Footnotes
Abbreviations used in this paper: CHS, contact hypersensitivity; P, platelet; E, endothelial; L, leukocyte; PSGL-1, P-selectin glycoprotein ligand-1; wt, wild type; p.o., orally; LN, lymph nodes; DNFB, 2,4-dinitrofluorobenzene; ILN, inguinal LN; ESL-1, E-selectin ligand-1.
Disclosures
The authors have no financial conflict of interest.
This work was supported by an American Cancer Society Grant RSG-06 – 024-01-CSM (to C.J.D.), National Institutes of Health/National Cancer Institute Grants CA102913 (to C.J.D.) and CA118124 (to C.J.D.), and National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 5P30 AR042689 –14 (to T.S.K.).
References
- 1.Zollner TM, Asadullah K, Schon MP. Targeting leukocyte trafficking to inflamed skin: still an attractive therapeutic approach? Exp Dermatol. 2007;16:1–12. doi: 10.1111/j.1600-0625.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell-and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 3.Boehncke WH, Schon MP, Giromolomi G, Griffiths C, Bos JD, Thestrup-Pedersen K, Cavani A, Nestle F, Bonish BK, Campbell JJ, Brakebusch C, Nickoloff B. Leukocyte extravasation as a target for anti-inflammatory therapy: which molecule to choose? Exp Dermatol. 2005;14:70–80. doi: 10.1111/j.0906-6705.2005.290a.x. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 5.Staite ND, Justen JM, Sly LM, Beaudet AL, Bullard DC. Inhibition of delayed-type contact hypersensitivity in mice deficient in both E-selectin and P-selectin. Blood. 1996;88:2973–2979. [PubMed] [Google Scholar]
- 6.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- 7.Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, von Andrian UH. Specialized contributions by α (1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665– 676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- 8.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Homeister JW, Thall AD, Petryniak B, Maly P, Rogers CE, Smith PL, Kelly RJ, Gersten KM, Askari SW, Cheng G, et al. The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity. 2001;15:115–126. doi: 10.1016/s1074-7613(01)00166-2. [DOI] [PubMed] [Google Scholar]
- 10.Smithson G, Rogers CE, Smith PL, Scheidegger EP, Petryniak B, Myers JT, Kim DS, Homeister JW, Lowe JB. Fuc-TVII is required for T helper 1 and T cytotoxic 1 lymphocyte selectin ligand expression and recruitment in inflammation, and together with Fuc-TIV regulates naive T cell trafficking to lymph nodes. J Exp Med. 2001;194:601– 614. doi: 10.1084/jem.194.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knibbs RN, Craig RA, Natsuka S, Chang A, Cameron M, Lowe JB, Stoolman LM. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8+ T cells specific for a skin-tropic virus. J Clin Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez JC, Kwok WW, Wald A, McClurkan CL, Huang J, Koelle DM. Expression of cutaneous lymphocyte-associated antigen and E-selectin ligand by circulating human memory CD4+ T lymphocytes specific for herpes simplex virus type 2. J Infect Dis. 2005;191:243–254. doi: 10.1086/426944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 16.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 18.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Dimitroff CJ, Kupper TS, Sackstein R. Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J Clin Invest. 2003;112:1008–1018. doi: 10.1172/JCI19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitroff CJ, Bernacki RJ, Sackstein R. Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen expression: implications in modulating lymphocyte migration to skin. Blood. 2003;101:602– 610. doi: 10.1182/blood-2002-06-1736. [DOI] [PubMed] [Google Scholar]
- 21.Yago T, Tsukuda M, Fukushima H, Yamaoka H, Kurata-Miura K, Nishi T, Minami M. IL-12 promotes the adhesion of NK cells to endothelial selectins under flow conditions. J Immunol. 1998;161:1140–1145. [PubMed] [Google Scholar]
- 22.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J Exp Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata T, Furie BC, Furie B. P-, E-, and L-selectin mediate migration of activated CD8+ T lymphocytes into inflamed skin. J Immunol. 2002;169:4307– 4313. doi: 10.4049/jimmunol.169.8.4307. [DOI] [PubMed] [Google Scholar]
- 24.Mangan PR, O’Quinn D, Harrington L, Bonder CS, Kubes P, Kucik DF, Bullard DC, Weaver CT. Both Th1 and Th2 cells require P-selectin glycoprotein ligand-1 for optimal rolling on inflamed endothelium. Am J Pathol. 2005;167:1661–1675. doi: 10.1016/S0002-9440(10)61249-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042– 8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J Immunol. 2007;178:2499–2506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- 27.Alcaide P, King SL, Dimitroff CJ, Lim Y-C, Fuhlbrigge RC, Luscinskas FW. The 130-kDa glycoform of CD43 is an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Derm. 127:1964–1972. doi: 10.1038/sj.jid.5700805. In press. [DOI] [PubMed] [Google Scholar]
- 28.Pinola M, Renkonen R, Majuri ML, Tiisala S, Saksela E. Characterization of the E-selectin ligand on NK cells. J Immunol. 1994;152:3586–3594. [PubMed] [Google Scholar]
- 29.Ohmori K, Fukui F, Kiso M, Imai T, Yoshie O, Hasegawa H, Matsushima K, Kannagi R. Identification of cutaneous lymphocyte-associated antigen as sialyl 6-sulfo Lewis X, a selectin ligand expressed on a subset of skin-homing helper memory T cells. Blood. 2006;107:3197–3204. doi: 10.1182/blood-2005-05-2185. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiyama J, Yoshino T, Toba K, Harada N, Nishiuchi R, Akagi T, Furukawa T, Takahashi M, Fuse I, Aizawa Y, Harada M. Induction and characterization of cutaneous lymphocyte antigen on natural killer cells. Br J Haematol. 2002;118:654– 662. doi: 10.1046/j.1365-2141.2002.03608.x. [DOI] [PubMed] [Google Scholar]
- 31.Hunger RE, Yawalkar N, Braathen LR, Brand CU. The HECA-452 epitope is highly expressed on lymph cells derived from human skin. Br J Dermatol. 1999;141:565–569. doi: 10.1046/j.1365-2133.1999.03031.x. [DOI] [PubMed] [Google Scholar]
- 32.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Descheny L, Gainers ME, Walcheck B, Dimitroff CJ. Ameliorating skin-homing receptors on malignant T cells with a fluorosugar analog of N-acetylglucosamine: P-selectin ligand is a more sensitive target than E-selectin ligand. J Invest Dermatol. 2006;126:2065–2073. doi: 10.1038/sj.jid.5700364. [DOI] [PubMed] [Google Scholar]
- 34.Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, Pienta KJ, Kutok JL, Rubin MA. Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res. 2005;65:5750–5760. doi: 10.1158/0008-5472.CAN-04-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL. Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res. 2004;64:5261–5269. doi: 10.1158/0008-5472.CAN-04-0691. [DOI] [PubMed] [Google Scholar]
- 36.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc γ RIIIa (CD16): differential ligand binding. J Immunol. 1997;159:3849–3857. [PubMed] [Google Scholar]
- 37.Underhill GH, Zisoulis DG, Kolli KP, Ellies LG, Marth JD, Kansas GS. A crucial role for T-bet in selectin ligand expression in T helper 1 (Th1) cells. Blood. 2005;106:3867–3873. doi: 10.1182/blood-2005-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dagia NM, Gadhoum SZ, Knoblauch CA, Spencer JA, Zamiri P, Lin CP, Sackstein R. G-CSF induces E-selectin ligand expression on human myeloid cells. Nat Med. 2006;12:1185–1190. doi: 10.1038/nm1470. [DOI] [PubMed] [Google Scholar]
- 39.Kantele A, Savilahti E, Tiimonen H, Iikkanen K, Autio S, Kantele JM. Cutaneous lymphocyte antigen expression on human effector B cells depends on the site and on the nature of antigen encounter. Eur J Immunol. 2003;33:3275–3283. doi: 10.1002/eji.200324311. [DOI] [PubMed] [Google Scholar]