Abstract
Toxoplasma gondii is an obligate intracellular protozoan parasite that can infect most warm-blooded animals and cause severe and life-threatening disease in developing fetuses and in immune-compromised patients. Although Toxoplasma was discovered over 100 years ago, we are only now beginning to appreciate the importance of the role that parasite modulation of its host has on parasite growth, bradyzoite development, immune evasion, and virulence. The goal of this review is to highlight these findings, to develop an integrated model for communication between Toxoplasma and its host, and to discuss new questions that arise out of these studies.
Keywords: Toxoplasma, host–parasite interactions, apicomplexan, immune response
Toxoplasma gondii is an obligate intracellular protozoan parasite that can infect most warm-blooded animals. It is ubiquitous throughout the world and estimated to infect approximately half of the world's population. Toxoplasma is a member of the phylum Apicomplexa, which encompasses intracellular parasites characterized by a polarized cell structure and two unique apical secretory organelles named micronemes and rhoptries (1).
Studies on Toxoplasma are spurred on for two important reasons. First, Toxoplasma can cause severe and life-threatening disease (e.g. encephalitis, retinitis, and myocarditis) in developing fetuses and in immune-compromised patients. Although current available drugs can treat Toxoplasma infections, they are poorly tolerated, have severe side effects, and cannot act against chronic Toxoplasma infections. In addition, resistance to some of these drugs has recently been noted (2–4). Second, Toxoplasma is used as a model system for other disease-causing Apicomplexan parasites including Plasmodium, the causative agent of malaria; Eimeria, which is the cause of poultry coccidiosis; and Cryptosporidium, which is another important opportunistic infection in AIDS patients (5, 6).
Toxoplasma has a complex life cycle consisting of a sexual cycle in its feline definitive hosts and an asexual cycle in its intermediate hosts (7). Intermediate hosts, including humans, can be infected by ingestion of oocysts shed in cat feces. Unlike most other Apicomplexan parasites, Toxoplasma can be transmitted between intermediate hosts by either vertical (mother–fetus) or horizontal (carnivorism) transmission.
Toxoplasma exists, in intermediate hosts, in two interconvertable stages: bradyzoites and tachyzoites. Bradyzoites are the slow-growing, transmissible, and encysted form that are dormant (8). Infections with bradyzoite-containing cysts occur upon ingestion of undercooked meat. The wall of these cysts is digested inside of the host stomach and the released bradyzoites, which are resistant to gastric peptidases, will subsequently invade the small intestine. Within the small intestine they convert into tachyzoites, which is the rapidly growing, disease-causing form. Tachyzoites, which can infect most nucleated cells, replicate inside a parasitophorous vacuole (PV), egress, and then infect neighboring cells. These tachyzoites activate a potent host immune response that eliminates most of the parasites. Some tachyzoites, however, escape destruction and convert back into bradyzoites. In the absence of an adequate immune response, tachyzoites will grow unabated and cause tissue destruction, which can be severe and even fatal. However, the inflammatory immune response induced by tachyzoites can cause immune-mediated tissue destruction. Therefore, a subtle balance between inducing and evading the immune response is crucial for Toxoplasma to establish a chronic infection.
The success of Toxoplasma as a widespread pathogen is due to the ease in which it can be transmitted between intermediate hosts. Once inside a host the parasite has developed powerful tools to modulate its host cell and to develop into a chronic infection that can evade the host's immune system as well as all known anti-toxoplasmotic drugs. The ability of the parasite to replicate within a host cell, evade immune responses, and undergo bradyzoite development requires that the parasite effectively modulates its host. Recent work from several laboratories indicates that there are two major types of communication between Toxoplasma and its host. The first type is critical for parasite growth or bradyzoite development and does not appear to differ among distinct Toxoplasma strains. The second type of communication between Toxoplasma and its host differs among distinct Toxoplasma strains and likely results in strain-specific differences in pathogenesis. The goal of this review will be to highlight these findings, to develop an integrated model for communication between Toxoplasma and its host, and to discuss new questions that arise out of these studies.
TOXOPLASMA HIJACKS HOST ORGANELLES AND CYTOSKELETON
Toxoplasma invasion is a complex process consisting of multiple, independently regulated steps. First, parasites attach loosely to the host cell's surface. This low affinity interaction is likely mediated by parasite surface proteins, most of which are GPI-linked proteins named SAGs (surface antigens), SRSs (SAG-related sequences), and SUSAs (SAG-unrelated surface antigens) (9, 10). Toxoplasma's unique ability to infect almost any nucleated cell and the large number of surface proteins expressed by tachyzoites suggests that loose attachment may be mediated by more than a single host molecule. Specific host receptors for any parasite surface protein have yet to be identified, although some SAGs appear to interact with sulfated glycosaminoglycans such as heparin (11–13).
Following attachment, an unknown signal triggers an increase in cytosolic calcium, which eventually stimulates microneme discharge. Although most of the players leading to microneme release are unknown, there is evidence that calcium-dependent protein kinases (CDPK) are involved; a Toxoplasma CDPK (TgCDPK1) that can regulate motility and invasion has been described (14) and purfalcamine, a compound that inhibits a Plasmodium CDPK, also inhibits Toxoplasma invasion (15). In addition to TgCDPK1, the Toxoplasma genome predicts the presence of several other CDPK that may also regulate microneme secretion and parasite invasion (16). At least 20 micronemal proteins have been identified and many of these are either transmembrane adhesins or accessory proteins for these adhesins (17).
Micronemal adhesin binding to host cells results in tight attachment between the parasite and the host cell (18). The parasite then uses a unique form of motility called gliding motility that is powered by the parasite's actomyosin machinery and is thought to be important for invasion (19, 20). At some point, a second unknown trigger stimulates parasites to exocytose proteins from a second apical secretory organelle called the rhoptry. Four proteins, (RON2, RON4, RON5, and RON8), localized to an elongated section of the rhoptries named the rhoptry neck, bind a micronemal protein named AMA1 after they are secreted (21, 22). Together, these proteins form the moving junction, which is a complex on the host cell plasma membrane that migrates down the length of the parasite as invasion proceeds. It has been hypothesized that at least one component of the RON2, 4, 5, 8 complex traverses across the host plasma membrane and is exposed to the host cytoplasm (21).
As a parasite penetrates into its host cell it enters into the PV that forms concomitantly with invasion. Morphological and electro-physiological studies demonstrated that the lipids used to develop the PV are largely derived from the host plasma membrane and not from intracellular host organelles such as lysosomes, endoplasmic reticulum, and Golgi (23). Toxoplasma actively defines the protein composition of the PV by preventing the accumulation of most host transmembrane, but not GPI-linked, proteins (24, 25). How this membrane partitioning is achieved is unknown as is its significance. It is, however, tempting to speculate that exclusion of host transmembrane proteins from the growing PV underlies the mechanism by which the parasite escapes the host endolysosomal system.
Although much progress has been made on defining the parasite machinery needed for invasion, little is understood about host cell structures involved in parasite invasion. Besides not knowing the host surface proteins that interact with micronemal adhesins or the moving junction complex, it is also not clear whether the parasite can invade any region of the plasma membrane or whether it searches for regions enriched in specific lipids and/or proteins.
Another outstanding question is whether the host plasma membrane, like the parasite's, needs to be anchored at the moving junction. The host actin cytoskeleton is one candidate to fill this role given its well-established role as a critical regulator of plasma membrane dynamics (26). In addition, two rhoptry proteins, Toxofilin and RON8, have been identified that interact with the host actin cytoskeleton and are exposed to the host cytoplasm during infection (21, 27, 28). But inhibitors of the actin cytoskeleton, which block parasite invasion, target parasite, but not host, actin (19). Thus, host actin may have a more subtle role during invasion and/or other host cytoskeletal elements like microtubules or intermediate filaments may also be important for invasion. If the host's cytoskeleton is important for invasion, how does the parasite act to regulate this structure? The most probable model involves a protein localized to the moving junction that interacts with the host cytoskeleton. Whether these are rhoptry proteins like Toxofilin and RON8 or a host protein is an unanswered but important question. A recent report showing that the host Arf6 GTPase localizes to the PV may provide some clues to these questions because this GTPase is an important regulator of membrane trafficking and the actin cytoskeleton (29, 30).
TOXOPLASMA NUTRIENT ACQUISITION AND METABOLISM DURING INTRACELLULAR GROWTH
All vacuolar pathogens face the challenge of scavenging nutrients from their host cells. Past biochemical studies coupled with bioinformatic analysis of the parasite's genome has identified a number of nutrients that Toxoplasma cannot synthesize de novo but must scavenge. These include small molecules such as glucose, arginine, iron, tryptophan, and purine nucleosides that can freely diffuse across the PV and then are presumably pumped into the parasite by membrane transporters (31–35). While most of these transporters have not yet been identified, a glucose transporter was cloned and characterized by heterologous expression in Xenopous oocytes (36).
But how Toxoplasma uses glucose as an energy source is, however, not clear. The parasite's genome indicates the presence of a full complement of glycolytic and Kreb's cycle enzymes. In addition, Toxoplasma can efficiently perform oxidative phosphorylation (37). In contrast, pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA is localized not to the mitochondrion but to another DNA-containing membrane-bound organelle, the apicoplast (38). Most significantly, parasite growth is only mildly affected in the absence of a functional Kreb's cycle. This is consistent with earlier findings showing that glucose is primarily broken down to lactic and acetic acids (39, 40). Thus, it appears that Toxoplasma generates most of its ATP via substrate-level phosphorylation. But some of the prescribed anti-toxoplasmotic drugs (e.g. atovaquone) act by inhibiting the parasite's mitochondrial electron transport (37). Therefore, it is possible that the slight affect on parasite growth seen in the citric acid cycle mutant may still have a dramatic affect on in vivo growth and virulence.
In contrast to glucose and other small nutrients that passively diffuse across the PV and then are pumped into the parasite, other nutrients are obtained by more active, parasite-driven mechanisms. For example, Toxoplasma redirects LDL-mediated cholesterol transport to the PV by redirecting host microtubules and microtubule-based transport towards the PV (41). Electron micrographs show that host microtubules push into the PV and form elongated membranous tubules that contain LDL-loaded cholesterol. These membrane tubules are wrapped with a parasite-derived protein complex that includes the dense granule protein GRA7. This wrapping appears similar to dynamin and dynamin-like proteins that facilitate pinching off of vesicles from larger membranes. Recombinant GRA7 in vitro can stimulate liposome tubulation, suggesting that GRA7 may be involved in driving PV tubulation in vivo (41). But how the parasite internalizes cholesterol once it reaches the PV is still unknown. In addition to microtubules, host intermediate filaments are also reorganized around the PV but whether this is important in nutrient acquisition is not known (42).
Dr. David Roos and his colleagues have spearheaded an in silico effort to use Toxoplasma genomic sequence data (available at http://toxodb.org/toxo/) to develop metabolic pathway reconstruction maps (see http://rooscompbio2.bio.upenn.edu/~fengchen/pathway_comparison_2/map01100.html). These studies have helped define many of the nutrients that the parasite can synthesize de novo; however, the parasite still scavenges some of these nutrients from its host. For example, host-derived lipoic acid is metabolized by the parasite's mitochondrion even though lipoic acid is synthesized and used in the apicoplast (43). Why the parasite needs to scavenge lipoic acid from host cells is not clear but could be because of a lack of a lipoic acid transporter to export the nutrient out of the apicoplast.
How does the parasite ensure that it has access to host nutrients synthesized in host mitochondria and other organelles? One possible way is to relocalize host mitochondria and endoplasmic reticulum, which synthesize some of the nutrients the parasite needs, to the PV (44). Recruitment of these organelles occurs quickly after a parasite invades a cell. Antisense RNA-based assays indicated that the ROP2 rhoptry protein acts to recruit mitochondria (45). ROP2 has an N-terminal domain that interacts with host mitochondria and a C-terminal portion that was originally proposed to span across the PV (45). This model has recently been challenged by the finding that proteins closely related to ROP2 are secreted into the host cytoplasm and interact with the PV as peripheral membrane proteins (46). Whether ROP2 and ROP2 family members (e.g. ROP3, ROP4, and ROP7) play similar functions in mediating organelle recruitment remains to be determined.
Another important, but less studied aspect of the interaction between Toxoplasma and its host is ensuring that the host has enough nutrients not only for the parasite but also for itself. Previous microarray studies surprisingly demonstrated an increase in host transcripts encoding glycolytic and mevalonate enzymes as well as the transferrin receptor (47, 48). As discussed above, these genes encode proteins that function in pathways needed to help the parasite satisfy its nutritional needs. It is, therefore, tempting to speculate that these changes in gene expression act to compensate for either differences in the metabolism or amounts of these nutrients within an infected cell.
The ability for the host to modulate nutrient pools is not only a property that the parasite may co-opt but also represents a critical mechanism for the host to restrict parasite growth. As an example, a key IFNγ effector gene in humans is indoleamine dioxygenase that functions to catabolize tryptophan, which the parasite cannot synthesize de novo (32). Thus, tryptophan starvation is a critical anti-toxoplasmotic pathway in some but not all hosts.
TOXOPLASMA MODULATION OF HOST CELL CYCLE
Regulating host cell cycle is a widely recognized mechanism for viruses to perfect their intracellular lifestyle (49). Evidence also indicates that this is not restricted to viruses and many microbial pathogens also manipulate their host's cell cycle. The most striking example among Apicomplexan parasites is Theileria, which transforms its host cells to facilitate its replication (50).
Two recent reports demonstrate that Toxoplasma is also able to dysregulate its host's cell cycle and causes host cells to arrest at the G2/M border (51, 52). This effect on the host cell cycle was independent of the type of host cell and occurred in dividing and senescent cells (52). Importantly, RNAi-mediated knockdown of a gene involved in regulating the host cell cycle (UHRF1), which causes cells to arrest in G1, resulted in a significant reduction in parasite growth (51). Initial characterization of a factor that modulates the host cell cycle during infection indicated that it is a heat-labile factor larger than 10 kDa (53). Surprisingly, this factor was secreted from infected cells and could act on neighboring uninfected cells. Why would Toxoplasma want to affect the cell cycle of both infected and uninfected cells? One reason could be that the parasite is acting extrinsically on neighboring cells to prepare them for infection (53). Early studies supporting this hypothesis showed that Toxoplasma preferentially infects cells in the S-phase (54–56). Alternatively, host cell structures with which the parasite interacts (e.g. the MTOC) may not be accessible at other stages of the cell cycle (41, 57).
TOXOPLASMA MODULATES HOST TRANSCRIPTION
Changes in host gene expression are among the most common and widespread that takes place after infection. To understand the importance of these changes in Toxoplasma-infected cells, several groups have used DNA microarrays to examine changes in transcript abundance after infection (47, 48, 58). These experiments indicated that the expression levels of more than 1000 host genes were modulated. These genes encode proteins involved in many different processes including inflammation, apoptosis, metabolism, and cell growth and differentiation. The challenge to these types of studies is defining how each host gene contributes to the host-pathogen interaction. As a first step, the genes were clustered into three functionally distinct classes: (i) `pro-host' – host genes required for host defense, (ii) `pro-parasite' – host genes required for parasite growth, and (iii) `bystander' – host genes incidentally regulated as a consequence of modulating the first two classes (47).
Potential pro-parasite genes upregulated by infection included those encoding glycolytic metabolic genes, transferrin receptor, and vascular endothelial growth factor. These candidate pro-parasite genes are regulated by a single host transcription factor named hypoxia-inducible factor 1 (HIF1) (59, 60). HIF1, which is the key transcription factor in a cell's response to decreased oxygen levels, is a heterodimer consisting of α and β subunits. Toxoplasma infection activates HIF1 and loss of the HIF1α subunit leads to a significant reduction in parasite growth at physiological oxygen levels (61). The parasite factor that activates HIF1 is currently unknown but does not appear to be a byproduct of parasite oxygen consumption because other hallmarks of hypoxic stress responses were not evident in Toxoplasma-infected cells (61). Rather HIF1 activation is mediated by a short-lived diffusible factor, which excludes a rhoptry-derived factor. This conclusion is also supported by the finding that upregulation of the transferrin receptor, which is another HIF1-target gene, is also mediated by a diffusible factor (48).
Besides HIF1, other host genes that encode transcription factors involved in cell growth and survival were also upregulated by infection. Among the earliest of these host genes were those encoding subunits of the EGR and AP-1 transcription factors (62, 63). In contrast to HIF1, upregulation of one of these genes, EGR2, required direct contact between the host cell and parasite and appears to involve a rhoptry-derived factor. EGR and AP-1 transcription factors regulate genes involved in cell growth, survival, and differentiation. Given the well-documented roles that the EGR and AP-1 transcription factors play in stress responses (64–66), the activation of these stress response transcription factors may be important to help host cells survive the stress of the infection.
Nuclear factor-κ B (NF-κB) is another transcription factor whose activity is modulated during infection (67–70). Although parasite modulation of NF-κB is debated in the Toxoplasma field, interest in this transcription factor was driven by two independent findings. First, loss of various NF-κB subunits leads to greater susceptibility during both acute and chronic infections (71–73). In addition, Toxoplasma prevents its host cell from undergoing apoptosis via NF-κB-dependent expression of pro-apoptotic genes (67, 74) (for a more detailed discussion of Toxoplasma regulation of host cell apoptosis, we direct readers to an excellent recent review (75)).
The NF-κB family is composed of five members: p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), RelB, and c-Rel (76). In unstimulated cells, homo- or hetero-dimers of NF-κB are sequestered in the cytoplasm by a family of inhibitors, called IκBs (inhibitor of κ B). Activation of NF-κB is initiated by the degradation of IκB proteins. This occurs primarily via activation of kinases called IκB kinases (IKKs). When activated, IKK phosphorylates two serine residues in IκB, which leads to its ubiquitination and degradation by the proteasome. The NF-κB complex is then free to enter the nucleus where it can induce expression of specific genes that have NF-κB-binding sites in their promoter (77).
Activation of NF-κB by Toxoplasma is an area of controversy. Type I strains can transiently block NF-κB nuclear translocation in murine macrophages and human fibroblasts even though IKK activation and IκB degradation is not inhibited (70). This block in NF-κB translocation results in a decrease in the expression of inflammatory response genes such as IL-12p40 and TNF-α (70, 78). In contrast, NF-κB is in the nuclei of Toxoplasma-infected murine fibroblasts and the transcription factor plays a role in the induction of anti-apoptotic genes (63, 79). In infected murine fibroblasts, IκBα is phosphorylated but remarkably does not appear to be degraded (67). Instead, phosphorylated IκBα is found at the PVM where it may be phosphorylated by a parasite-encoded kinase (67, 80). Differences between these studies might be due to different host cell types and/or host species being used but are most likely not due to differences in parasite strains because these studies all used a type I strain. In other work, however, differences in NF-κB activation have been associated with strain type: type II but not type I strains induce nuclear translocation of NF-κB in murine splenocytes (81) and bone marrow-derived macrophages (82). Furthermore, several microarray studies have demonstrated that cells infected with type II parasites have higher levels of NF-κB-regulated genes compared with uninfected cells (47, 83).
TOXOPLASMA–HOST COMMUNICATION DURING BRADYZOITE DEVELOPMENT
The ability of Toxoplasma to establish a chronic, asymptomatic infection by converting into transmissible tissue cysts that remain hidden from the host's immune system is an important feature of the parasite's life cycle that allows it to be such a successful and wide-spread pathogen (84). To establish a chronic infection, bradyzoites must form and the host must control tachyzoite proliferation. Bradyzoite development is a complex process that consists of changes in parasite gene and protein expression with the goal to slow its growth and escape detection by the host's immune system (85). Thus, there is a decrease in the expression of immunogenic surface proteins, metabolic enzymes, and an increase in the abundance of genes that act to facilitate entry into Go.
In vitro, bradyzoite development can be triggered by factors that mimic immune-derived stressors such as high pH, IFNγ, nitric oxide, high temperature, nutrient starvation, or by some drugs used to treat Toxoplasma infections (31, 86–88). Thus, the parasite senses stress signals and uses these to develop into a persistent state that is relatively resistant to these stresses.
A role for the host cell in signaling tachyzoite to bradyzoite conversion was demonstrated by taking advantage of a drug, a trisubstituted pyrrole, designated Compound 1, which stimulated in vitro bradyzoite development (89). This drug acts by upregulating the abundance of the host gene CDA1, whose over expression leads to cell cycle arrest.
In primary muscle cells and neurons Toxoplasma spontaneously differentiates with high frequency into encysted bradyzoites, without the need for external stressors (90–92). Interestingly, neurons and muscle cells are terminally differentiated and permanently withdrawn from the cell cycle, which is in contrast to the typical cells most researchers use for Toxoplasma experiments (e.g. primary fibroblasts, macrophages, Vero cells, etc.). Together, these data suggest a model in which tachyzoite growth is favored inside of growing cells; but, when tachyzoites cannot manipulate the host's cell cycle (e.g. CDA1 upregulation or infection of terminally differentiated cells) bradyzoite development initiates. How the parasite senses replicating from non-replicating cells is not clear. One possibility is that infection of a terminally differentiated cells results in slower parasite growth, which is an important step in bradyzoite development (93, 94).
Changes in parasite gene expression are an important facet of bradyzoite development and Toxoplasma surface proteins represent the largest group of stage-specific genes. For example, tachyzoites predominantly express SAG1, SAG2a, SAG3, SRS1, SRS2, and SRS3. In contrast, bradyzoites express a largely different repertoire of surface proteins including SAG2C, SAG2D, SAG2X, SAG2Y, SRS9, and SAG4. Many of these surface proteins are immunogenic and antibodies to them can be detected in sera from infected animals. SAG1 and SAG2 are considered the most immunogenic tachyzoite surface proteins and are believed to be critical in helping to limit tachyzoite proliferation (95–99). What the anti-Toxoplasma antibodies are doing is less clear because FcγRII- or complement-deficient mice that receive sera from infected mice are protected just as well as wild-type mice (100), suggesting that complement-mediated lysis of parasites is not important. On the other hand, IgG purified from sera of infected animals reduce parasite invasion, suggesting that these antibodies act by preventing invasion, which is a critical step in the parasite's life cycle (95, 100).
TOXOPLASMA ACTIVATION AND EVASION OF HOST IMMUNE RESPONSES
Anti-Toxoplasma immune responses are generated at two distinct times – acute infections after a host is initially infected and reactivated infections after parasites are released from cysts. Regardless of when or where the immune system encounters Toxoplasma, IL-12 release by dendritic cells, macrophages, and neutrophils is needed to stimulate IFNγ secretion from both T-cells and NK cells (101, 102).
IL-12 expression is triggered by Toxoplasma stimulating CCR5- and Toll-like receptor (TLR)-dependent signaling. CCR5 is a chemokine receptor expressed by multiple cell types including macrophages and dendritic cells (103). Mice lacking CCR5 express significantly lower amounts of IL-12 after Toxoplasma infection and CCR5+ dendritic cells treated with Toxoplasma extracts upregulate IL-12 (104, 105). Subsequent biochemical purification identified Toxoplasma cyclophillin-18 (C18) as a potential CCR5 ligand; but, the effect of C18 on CCR5-dependent IL-12 expression was relatively low, suggesting that other CCR5 ligands may be important for IL-12 expression.
TLRs are a family of at least 13 proteins that function to recognize pathogen-associated molecular patterns (106). TLR signaling is mediated by a series of adaptor proteins, the most prominent of which is MyD88. Several TLRs including TLR2, TLR4, and TLR11 bind Toxoplasma-derived factors (107–110). Unlike MyD88, which is essential for survival in parasite-infected animals, loss of individual TLRs has minimal impact on survival (107, 111–113). These data indicate that either TLR signaling is functionally redundant or that MyD88 acts downstream of other receptors. These potential receptors include the IL-1 and IL-18 receptors both of which interact with MyD88 (114) and whose ligands are upregulated by infection (115–118).
Because MyD88 is important for host resistance, significant effort has been placed on identifying parasite-derived TLR ligands. Thus far, ligands for TLR11 (the actin-binding protein profilin) as well as TLR2 and TLR4 (HSP70 and GPI lipid anchors) have been described (107, 108, 110). In mice, profilin binding to TLR11 is most critical for stimulating IL-12 production (107, 119). In addition, TLR11 is necessary for the development of CD4+ T-cell responses to profilin (120). But it is not clear whether profilin is important in human disease because TLR11 is not functionally expressed in humans (121).
Although MyD88 is critical for primary responses to infection, it is less clear what role it plays in immunity to either reactivated or secondary infections. Mice lacking MyD88 specifically in their T-cells are susceptible to intraperitoneal parasite infections (122). In addition, loss of MyD88 blocks the ability for mice to develop Th1 responses when vaccinated with Toxoplasma lysates (123). In contrast, MyD88 is not essential to develop protective immune responses to oral infections in mice vaccinated with an attenuated parasite strain (124). The basis for these differences is most likely due to the infection route (oral vs intraperitoneal) and type of vaccination (lysate vs attenuated strain). Elucidating the mechanistic basis for these differences is, however, critical for vaccine and drug development.
IFNγ, produced in response to parasite stimulation of IL-12 expression, is the critical cytokine for resistance to both acute and chronic Toxoplasma infections (125–127). Several IFNγ effectors important for resistance have been identified including the IFNγ-activated p47 family of GTPases (LRG47, IGTP, and IRG47), nitric oxide synthase, and indoleamine dioxygenase (32, 128, 129). These IFNγ target genes affect parasite growth by different mechanisms. For example, the GTPases stimulate autophagy (130) and indoleamine dioxygenase sequesters tryptophan (32). With the exception of indoleamine dioxygenase, loss of most of these effectors in mice affects resistance to either acute or chronic infections, suggesting that they are expressed at either temporally or spatially distinct phases.
Although these data have helped define how various IFNγ effectors protect against Toxoplasma in mice, some effectors are not present in humans; humans only have one p47 family member (131) and iNOS and nitric oxide, which are important for resistance in mice, appear to have no significant role in protection in human cells (132, 133). Thus, either the single human GTPase can function in place of the murine isoforms or other IFNγ effectors are important in humans. One possible candidate is indoleamine dioxygenase, which is critical for resistance to Toxoplasma in human cells but does not appear to play any significant role in murine-derived cells (134–136).
IFNγ regulates expression of its effector genes mainly through activation of the transcription factor STAT1 (137). When IFNγ binds to its receptor it triggers the activation of Janus kinases (JAK1 and JAK2). These kinases phosphorylate STAT1 on tyrosine701 residue, which subsequently leads to its dimerization, nuclear import, and binding to promoters of genes that contain consensus γ-activated sequence (GAS) sites. Some of the genes activated by STAT1 are other transcription factors such as interferon regulatory factor (IRF)-1 and the class II MHC transactivator (CTIIA). STAT1 alone or in combination with other transcription factors (such as IRF1) will induce the transcription of the effector genes important for resistance against Toxoplasma.
Toxoplasma has developed ways to subvert IFNγ signaling thus allowing it to become a successful and widespread pathogen. Indeed, Toxoplasma-infected cells are significantly less responsive to IFNγ-induced upregulation of many genes, including MHC Class II, iNOS, and the p47 GTPases (83, 138). Because IFNγ activation of the STAT1 signaling pathway is essential for the control of Toxoplasma growth, much effort has focused on how Toxoplasma inhibits the STAT1-mediated transcription in IFNγ-stimulated cells. There is some evidence that Toxoplasma inhibits STAT1 by upregulating levels of suppressor of cytokine signaling (SOCS) proteins (139). SOCS are a family of eight proteins (SOCS1-7 and CIS) that are well-recognized attenuators of IFNγ-dependent signaling (140). These proteins affect IFNγ signaling by either inhibiting the catalytic activity of the JAKs (SOCS1, SOCS3) or by inhibiting recruitment of STATs (CIS). Toxoplasma infection of macrophages upregulates the abundance of SOCS-1, SOCS-3 and CIS mRNA levels. A role for these proteins in Toxoplasma immune evasion was established by demonstrating that when parasites infected macrophages stably overexpressing SOCS-1, SOCS-3, or CIS these macrophages could not produce nitric oxide or limit parasite growth in response to IFNγ (139). Furthermore, Toxoplasma has a reduced ability to downregulate IFNγ response genes (iNOS, MIG) in IFNγ-activated macrophages from SOCS-1−/− mice. All these effects are dependent on viable parasite invasion and correlated with the number of invasion events consistent with the hypothesis that they are caused by a factor that Toxoplasma secretes into the host cell. These downregulatory effects on IFNγ signaling were time-dependent and full inhibition was not achieved until 24 h post-infection, suggesting that Toxoplasma does not directly interfere with the initial IFNγ response but rather acts by up-regulating SOCS proteins (139). Supporting such a mechanism was the observation that the levels of total STAT1 were reduced in Toxoplasma-infected macrophages, which is consistent with the ability of SOCS to target STAT1 for degradation (139). However, others have not observed any differences in total STAT1 levels in Toxoplasma-infected cells stimulated with IFNγ (83, 141). These studies suggested that Toxoplasma interferes with the binding of STAT1 to GAS elements leading to decreased expression of STAT1 responsive genes. The basis for these differences in the mechanism underlying parasite inhibition of IFNγ is not clear, but could be because the work showing decreased STAT1 expression used high doses of type I parasites (139), while the other studies used lower doses of type II strains (83, 141).
TOXOPLASMA DISSEMINATION IN THE HOST
Toxoplasma disseminates rapidly from the initial site of infection to secondary lymphoid tissues and then on to other tissues (142, 143). As the key cells that traffic from infected tissues to the spleen and draining lymph nodes, dendritic cells are likely candidates as the `Trojan Horse' that Toxoplasma uses to disseminate. In support of this hypothesis, early in vitro studies demonstrated that parasites preferentially infect and replicate inside of monocytes and dendritic cells (144). In addition, infection of dendritic cells increased their migratory capacity (145–147). Dendritic cell trafficking was pertussis toxin sensitive, suggesting that Giα-dependent chemokine receptors are important for trafficking (147). Most importantly, parasitized dendritic cells adoptively transferred to uninfected mice disseminated more quickly than uninfected cells (147). A more detailed phenotyping of these dendritic cells surprisingly revealed that they were not conventional dendritic cells but rather were a novel population of CD11c+ cells that expressed both PDCA1 (a plasmacytoid dendritic cell marker) and CD11b (a myeloid cell marker) (148).
The above experiments used in vitro and intraperitoneal infection models to examine dendritic cell-based dissemination. But it is still not clear whether these dendritic cells are important for dissemination after an oral infection. A useful model to test this may be a transgenic mouse that expresses the diphtheria toxin receptor under control of the CD11c promoter. Injection of these mice with diphtheria toxin depletes almost all dendritic cells (149). Dissemination of GFP- or luciferase-expressing Toxoplasma could then be monitored in mock- or dipthera toxin-treated mice. Unlike conventional plasmacytoid dendritic cells that are resistant to diphtheria toxin because they express low levels of CD11c (150), the PDCA1+ cells in Toxoplasma-infected mice should be sensitive to the toxin because they expressed high levels of CD11c (151).
IMPACT OF PARASITE GENOTYPE ON TOXOPLASMA HOST SIGNALING
Toxoplasma isolates from humans and livestock in Europe and North America group primarily into one of three clonal lineages (types I, II, and III) that can be discriminated in mice by their virulence (152–154). Type I strains are very virulent (LD100 of one parasite). In contrast, types II and III strains are less virulent (LD50 ~103 and ~105, respectively (155)). In humans, all three lineages cause disease, but they appear to differ in the tissues they affect and when they infect people. For example, type I strains are more often associated with post-natally acquired ocular infections, whereas type II strains are more associated with congenital infections and toxoplasmic encephalitis (156).
Recently, it has become more appreciated that the various Toxoplasma strains differ profoundly in how they modulate host cell signaling pathways. For example, human foreskin fibroblasts (HFFs) infected with types I, II, or III strains have significantly different gene expression profiles (155). If the strain-specific regulation of a host gene has a genetic basis then it should segregate among F1 progeny derived from a cross between two strains that differ in regulation of that gene. Thus, human DNA microarrays were used to compare the transcriptional profile of HFFs infected with 19 unique F1 progeny derived from crosses between types II and III strains (157). These microarray experiments indicated that 3188 human cDNAs correlated with the allelic state of specific Toxoplasma genomic loci. Interestingly, the expression levels of 1176 of these cDNAs correlated with a single locus on Toxoplasma chromosome VIIb. This suggested that in the vicinity of that locus there was at least one polymorphic Toxoplasma gene whose product had a major effect on HFF gene expression. Pathway analysis identified that many of the host genes regulated by this locus were targets of the STAT6 and STAT3 transcription factors (158), suggesting that many of the differences in modulation of host gene expression by the different Toxoplasma strains were due to differences in STAT3/6 signaling.
Data supporting this hypothesis included STAT3/6 activation for prolonged times in cells infected with types I and III strains (155). In contrast, STAT3/6 were only transiently activated in cells infected with type II strains. Subsequently, ROP16 was identified as the Toxoplasma protein mediating the strain-specific differences in maintaining STAT3/6 activation. While the basis for the differences between the two ROP16 alleles is not yet known, it is not due to differences in the expression, host cell secretion, or nuclear localization of either allele.
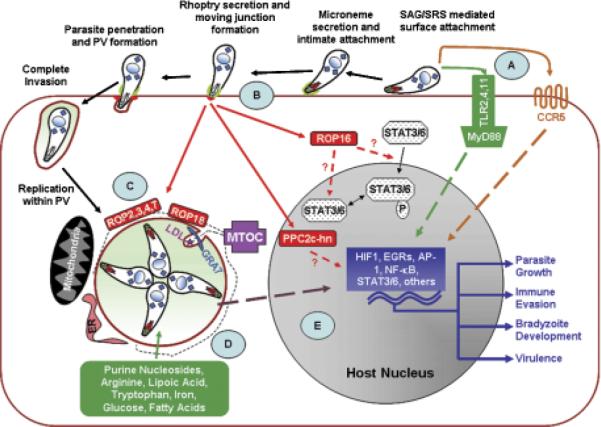
No other polymorphic parasite factors that regulate host gene expression are currently known. However, the data suggest that at least one gene exists on each Toxoplasma chromosome that is involved in the strain-specific regulation of host gene expression (155). Given the precedent for rhoptry-localized kinases and kinase-like proteins (e.g. ROP16, ROP18, and ROP2, 3, 4) as key mediators in regulating Toxoplasma host interactions, it is possible that other secreted kinases may fill a similar role in regulating other host genes. Indeed, a search of the Toxoplasma genome database (http://www.toxodb.org) predicts that more than 70 predicted genes encode proteins that have signal peptides and have significant homology to protein kinases (Fig. 1).
Fig. 1.
Overview of Toxoplasma host cell interactions. (A). Innate immune responses are initiated by Toll-like receptor (TLR) and CCR5 recognition of Toxoplasma-derived factors. (B). Parasite invasion is accomplished by the release of micronemal adhesins that interact with host surface factors. This is then followed by rhoptry secretion that results in the formation of the moving junction and in the release of parasite factors (e.g ROP2 family members, ROP16, ROP18, and PP2c-hn) that either interact with the parasitophorous vacuole (PV) (ROP2 family and ROP18) or are transported to the host cytoplasm (ROP16) or nucleus [PP2c-hn (177)]. Some of these factors (ROP16 and ROP18) are polymorphic virulence factors. (C). Intracellular parasites reorganize host mitochondria and endoplasmic reticulum as well as the host microtubule organizing center and cytoskeleton around the PV. Host microtubules associated with LDL-loaded cholesterol form membrane tubules that push into the PV and are wrapped with the dense granule protein GRA7. (D). Small soluble nutrients freely diffuse across the PV and then are taken up by the parasite presumably by membrane transporters. (E). Host transcription is regulated either by the parasite directly activating host transcription factors or by the parasite triggering host signaling cascades that culminate in activating the host transcription factors. Changes in host gene expression can act to either promote parasite growth, immune evasion, virulence, or bradyzoite development.
IMPACT OF PARASITE GENOTYPE ON TOXOPLASMA-INDUCED DISEASE
Virulence of type I strains is due in large part to over production of Th1 cytokines that cause tissue damage (116, 159, 160). In addition, type I parasites display enhanced migratory capacity across cellular barriers in vitro and in vivo, which may also contribute to virulence (161). Similarly, a virulent strain (named S23) derived from a cross between types II and III strains had a higher in vivo growth rate and disseminated more rapidly than an avirulent strain from the same cross (named S22) (162).
Recently, Taylor et al. (163) used QTL mapping of F1 progeny from a type I×III cross and identified a locus on chromosome VIIa that was tightly linked to virulence. The responsible gene was subsequently identified as ROP18. ROP18 is a rhoptry-localized, functional serine/threonine kinase that is secreted into host cells. Expression analysis indicated that ROP18 expression was significantly higher in type I than in type III strains. Importantly, transfer of the type I allele into the non-virulent type III strain increased virulence by more than four orders of magnitude. Virulence was dependent on the ROP18's kinase activity as transfer of a type I kinase dead mutant into a type III strain did not increase virulence. Although it is not clear how ROP18 functions, it is possible that its ability to increase parasite proliferation may enhance virulence (163, 164). Likewise, ROP18 substrates have yet to be identified although recombinant ROP18 can phosphorylate an unknown 70-kDa parasite protein, but not proteins in host cell extracts (164).
In a separate study, Grigg et al. (165) found that two out of 16 progeny from a cross between avirulent types II and III strains were surprisingly more virulent than either parental strain or other progeny. These differences in virulence were not due to faster growth because there were no apparent differences in invasion or growth rates between the virulent and avirulent progeny (165). To identify the virulence genes, mice were infected with the types II and III parental strains and with 40 unique F1 progeny of a type II×III cross. QTL mapping from these experiments identified five Toxoplasma genomic regions associated with virulence (VIR1–5) (166).
Using a candidate gene approach, we identified VIR3 as ROP18, which was the same rhoptry kinase identified by Taylor et al. (166). Subsequent sequence analysis of the ROP18 gene (including promoter regions) from types I–III strains revealed that type III strains have a unique 2.1-kb sequence inserted 85 bp upstream of the ATG start codon. It seems likely that this insertion (relative to types I and II) in the 5′-untranslated region – promoter of the type III ROP18 allele is involved in the major difference in expression of this locus in type III strains (163, 166).
Expressing the type II ROP18 allele in a type III strain increased virulence by four orders of magnitude (166), which is surprising because VIR3 was predicted to only account for ~10% of the variance in virulence between type II and III strains. One possible explanation for ROP18's surprisingly high impact on virulence lies in the fact that in the transgenic type III: ROP18II strain, ROP18 was expressed about eight times higher than in the wild-type type II strain (166).
Using a similar candidate gene approach, VIR4 was identified as ROP16 on chromosome VIIb. Interestingly, expression of a type I or III allele of ROP16 in a type II strain made that strain less virulent. At the moment, it is unknown why the type II strain became less virulent but is likely due to ROP16's role in sustained STAT3/6 activation (155).
The genes responsible for the VIR1, VIR2 and VIR5 QTLs have yet to be identified. VIR1 has been mapped to a region spanning ~0.98 Mb on the left end of chromosome XII. Two interesting candidate genes are found within this region: (i) a surface antigen (SAG3), and (ii) a secreted rhoptry-localized putative protein kinase (ROP5). VIR2 falls within a ~1.2 Mb interval on chromosome X, and contains 139 predicted genes. Two candidate genes in this locus (genes 42.m03493 and 42.m03409 in the ToxoDB database) are both predicted to have signal peptides and at least one transmembrane domain but lack homology to other known proteins or domains. Finally, the VIR5 QTL is found in a region on chromosome XII where there are very few differences between types II and III strains. It should also be noted that this QTL is linked to resistance to adenosine arabinoside, which is due to a loss of function mutation in the adenosine kinase gene (167). It has been reported that a lack of functional adenosine kinase can result in fitness defects (168). Because the type III parent carries the adenosine arabinoside marker and this drug was used to select 23 of the 41 recombinant progeny, it is possible that adenosine kinase is the gene responsible for VIR5.
CONCLUSIONS
Although Toxoplasma was discovered over 100 years ago (169, 170), we are only now beginning to appreciate the importance of the role that parasite modulation of its host has on parasite growth, bradyzoite development, immune evasion, and virulence. These discoveries are significant not only because they taught us about Toxoplasma-specific processes but also because they illuminate novel biological processes. Recent examples include hijacking of microtubule-based LDL-trafficking, invasion via the RON complex, rhoptry injection of virulence factors, and IFNγ-inducible GTPase-mediated autophagy. But many important questions remain about how Toxoplasma grows within its host. The development of new biochemical and genetic tools will facilitate studies to answer these questions. These tools include high-throughput RNAi and small molecule inhibitor screens that have already yielded important information about Toxoplasma invasion (171). In addition, the development of cosmid libraries to identify mutated genes in forward genetic screens will now allow many investigators to use chemical mutagenesis-based screens to identify parasite genes that modulate host cell functions (172).
Besides the topics we covered in this review, future Toxoplasma research will likely focus on understanding how epigenetic regulation of both parasite and host gene expression affect communication between Toxoplasma and its host. In addition, recent evidence has indicated that Toxoplasma infections can have significant effects on host behavior (173–176) and future work will likely aim to elucidate the molecular basis for these changes. Finally, the long-term goal of studying an important infectious disease like Toxoplasma is the development of protective vaccines as well the production of effective drug therapies that have few side effects. Understanding the basic biology underlying the interaction between Toxoplasma and its host will greatly aid in reaching these goals.
Acknowledgments
Our laboratories are funded by grants to I.J.B. from the National Institutes of Health (RO1AI069986) and the American Cancer Society (MBC-114461) and a Scientist Development Grant from the American Heart Association (0835099N) to J.P.S.
REFERENCES
- 1.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–99. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baatz H, Mirshahi A, Puchta J, Gumbel H, Hattenbach LO. Reactivation of toxoplasma retinochoroiditis under atovaquone therapy in an immunocompetent patient. Ocul Immunol Inflamm. 2006;14:185–7. doi: 10.1080/09273940600659740. [DOI] [PubMed] [Google Scholar]
- 3.Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, et al. The California Collaborative Treatment Group Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall TV, Joynson DH, Guy E, Hyde JE, Sims PF. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J Infect Dis. 2002;185:1637–43. doi: 10.1086/340577. [DOI] [PubMed] [Google Scholar]
- 5.Dubey JP. Toxoplasma, Neospora, Sarcocystis, and other tissue cyst-forming coccidia of humans and animals. In: Kreier JP, editor. Parasitic Protozoa. Academic Press Inc.; San Diego, CA: 1993. pp. 1–158. [Google Scholar]
- 6.Kim K, Weiss LM. Toxoplasma gondii: the model apicomplexan. Int J Parasitol. 2004;34:423–32. doi: 10.1016/j.ijpara.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubey JP. Toxoplasmosis – a waterborne zoonosis. Vet Parasitol. 2004;126:57–72. doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–58. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boothroyd JC, Hehl A, Knoll LJ, Manger ID. The surface of Toxoplasma: more and less. Int J Parasitol. 1998;28:3–9. doi: 10.1016/s0020-7519(97)00182-3. [DOI] [PubMed] [Google Scholar]
- 10.Pollard AM, Onatolu KN, Hiller L, Haldar K, Knoll LJ. Highly polymorphic family of glycosylphosphatidylinositol-anchored surface antigens with evidence of developmental regulation in Toxoplasma gondii. Infect Immun. 2008;76:103–10. doi: 10.1128/IAI.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Barria E, Boothroyd JC. A Toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J Biol Chem. 1999;274:1267–76. doi: 10.1074/jbc.274.3.1267. [DOI] [PubMed] [Google Scholar]
- 12.Carruthers VB, Hakansson S, Giddings OK, Sibley LD. Toxoplasma gondii uses sulfated proteoglycans for substrate and host cell attachment. Infect Immun. 2000;68:4005–11. doi: 10.1128/iai.68.7.4005-4011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He XL, Grigg ME, Boothroyd JC, Garcia KC. Structure of the immunodominant surface antigen from the Toxoplasma gondii SRS super-family. Nat Struct Biol. 2002;9:606–11. doi: 10.1038/nsb819. [DOI] [PubMed] [Google Scholar]
- 14.Kieschnick H, Wakefield T, Narducci CA, Beckers C. Toxoplasma gondii attachment to host cells is regulated by a Calmodulin-like domain protein kinase. J Biol Chem. 2001;276:12369–77. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 15.Kato N, Sakata T, Breton G, Le Roch KG, Nagle A, Andersen C, et al. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat Chem Biol. 2008;4:347–56. doi: 10.1038/nchembio.87. [DOI] [PubMed] [Google Scholar]
- 16.Nagamune K, Sibley LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Mol Biol Evol. 2006;23:1613–27. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- 17.Zhou XW, Kafsack BFC, Cole RN, Beckett P, Shen RF, Carruthers VB. The opportunistic pathogen Toxoplasma gondii deploys a diverse legion of invasion and survival proteins. J Biol Chem. 2005;280:34233–44. doi: 10.1074/jbc.M504160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carruthers V, Boothroyd JC. Pulling together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–9. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–9. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 20.Hakansson S, Morisaki H, Heuser J, Sibley LD. Time-lapse video microscopy of gliding motility in Toxoplasma gondii reveals a novel; biphasic mechanism of cell locomotion. Molecular Biology of the Cell. 1999:3539–47. doi: 10.1091/mbc.10.11.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straub K, Cheng S, Sohn C, Bradley P. Novel components of the Apicomplexan moving junction reveal conserved and coccidia-restricted elements. Cell Microbiol. 2009;11:590–603. doi: 10.1111/j.1462-5822.2008.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles. PLoS Pathog. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci USA. 1996;93:8413–8. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mordue DG, Desai N, Dustin M, Sibley LD. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring. J Exp Med. 1999;190:1783–92. doi: 10.1084/jem.190.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charron AJ, Sibley LD. Molecular partitioning during host cell penetration by Toxoplasma gondii. Traffic. 2004;5:855–67. doi: 10.1111/j.1600-0854.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 26.Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–34. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- 27.Poupel O, Boleti H, Axisa S, Couture-Tosi E, Tardieux I. Toxofilin, a novel actin-binding protein from Toxoplasma gondii, sequesters actin monomers and caps actin filaments. Mol Biol Cell. 2000;11:355–68. doi: 10.1091/mbc.11.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley PJ, Ward C, Cheng SJ, Alexander DL, Coller S, Coombs GH, et al. Proteomic analysis of rhoptry organelles reveals many novel constituents for host–parasite interactions in Toxoplasma gondii. J Biol Chem. 2005;280:34245–58. doi: 10.1074/jbc.M504158200. [DOI] [PubMed] [Google Scholar]
- 29.da Silva CV, da Silva EA, Cruz MC, Chavrier P, Isberg R, Mortara RA. ARF6, PI3-kinase and host cell actin cytoskeleton in Toxoplasma gondii cell invasion. Biochem Biophys Res Commun. 2008;378:656–67. doi: 10.1016/j.bbrc.2008.11.108. [DOI] [PubMed] [Google Scholar]
- 30.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 31.Fox BA, Gigley JP, Bzik DJ. Toxoplasma gondii lacks the enzymes required for de novo arginine biosynthesis and arginine starvation triggers cyst formation. Int J Parasitol. 2004;34:323–31. doi: 10.1016/j.ijpara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–12. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrotto J, Keister DB, Gelderman AH. Incorporation of precursors into toxoplasma DNA. J Protozool. 1971;18:470–3. doi: 10.1111/j.1550-7408.1971.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 34.Schwartzman JD, Pfefferkorn ER. Toxoplasma gondii: purine synthesis and salvage in mutant host cells and parasites. Exp Parasitol. 1982;53:77–86. doi: 10.1016/0014-4894(82)90094-7. [DOI] [PubMed] [Google Scholar]
- 35.Krug EC, Marr JJ, Berens RL. Purine metabolism in Toxoplasma gondii. J Biol Chem. 1989;264:10601–7. [PubMed] [Google Scholar]
- 36.Joet T, Holterman L, Stedman TT, Kocken CH, Van Der Wel A, Thomas AW, et al. Comparative characterization of hexose transporters of Plasmodium knowlesi, Plasmodium yoelii and Toxoplasma gondii highlights functional differences within the apicomplexan family. Biochem J. 2002;368:923–9. doi: 10.1042/BJ20021189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vercesi AE, Rodrigues CO, Uyemura SA, Zhong L, Moreno SNJ. Respiration and oxidative phosphorylation in the Apicomplexan parasite Toxoplasma gondii. J Biol Chem. 1998;273:31040–7. doi: 10.1074/jbc.273.47.31040. [DOI] [PubMed] [Google Scholar]
- 38.Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell. 2007;6:984–96. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohsaka A, Yoshikawa K, Hagiwara T. 1H-NMR spectroscopic study of aerobic glucose metabolism in Toxoplasma gondii harvested from the peritoneal exudate of experimentally infected mice. Physiol Chem Phys. 1982;14:381–4. [PubMed] [Google Scholar]
- 40.Fulton JD, Spooner DF. Metabolic studies on Toxoplasma gondii. Exp Parasitol. 1960;9:293–301. doi: 10.1016/0014-4894(60)90037-0. [DOI] [PubMed] [Google Scholar]
- 41.Coppens I, Dunn JD, Romano JD, Pypaert M, Zhang H, Boothroyd JC, et al. Toxoplasma gondii sequesters lysosomes from mammalian hosts in the vacuolar space. Cell. 2006;125:261–74. doi: 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 42.Halonen SK, Weidner E. Overcoating of Toxoplasma parasitophorous vacuoles with host cell vimentin type intermediate filaments. J Eukaryot Microbiol. 1994;41:65–71. doi: 10.1111/j.1550-7408.1994.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 43.Crawford MJ, Thomsen-Zieger N, Ray M, Schachtner J, Roos DS, Seeber F. Toxoplasma gondii scavenges host-derived lipoic acid despite its de novo synthesis in the apicoplast. EMBO J. 2006;25:3214–22. doi: 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinai A, Webster P, Joiner K. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110:2117–28. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 45.Sinai AP, Joiner KA. The Toxoplasma gondii protein ROP2 mediates host organelle association with the parasitophorous vacuole membrane. J Cell Biol. 2001;154:95–108. doi: 10.1083/jcb.200101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Hajj H, Lebrun M, Fourmaux MN, Vial H, Dubremetz JF. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cell Microbiol. 2007;9:54–64. doi: 10.1111/j.1462-5822.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 47.Blader IJ, Manger ID, Boothroyd JC. Micro-array analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem. 2001;276:24223–31. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 48.Gail M, Gross U, Bohne W. Transcriptional profile of Toxoplasma gondii-infected human fibroblasts as revealed by gene-array hybridization. Mol Genet Genom. 2001;265:905–12. doi: 10.1007/s004380100487. [DOI] [PubMed] [Google Scholar]
- 49.Zhao RY, Elder RT. Viral infections and cell cycle G2/M regulation. Cell Res. 2005;15:143–9. doi: 10.1038/sj.cr.7290279. [DOI] [PubMed] [Google Scholar]
- 50.Dobbelaere DA, Kuenzi P. The strategies of the Theileria parasite: a new twist in host-pathogen interactions. Curr Opin Immunol. 2004;16:524–30. doi: 10.1016/j.coi.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Brunet J, Pfaff AW, Abidi A, Unoki M, Nakamura Y, Guinard M, et al. Toxoplasma gondii exploits UHRF1 and induces host cell cycle arrest at G2 to enable its proliferation. Cell Microbiol. 2008;10:908–20. doi: 10.1111/j.1462-5822.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 52.Molestina RE, El-Guendy N, Sinai AP. Infection with Toxoplasma gondii results in dysregulation of the host cell cycle. Cell Microbiol. 2008;10:1153–65. doi: 10.1111/j.1462-5822.2008.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavine MD, Arrizabalaga G. Exit from host cells by the pathogenic parasite Toxoplasma gondii does not require motility. Eukaryotic Cell. 2008;7:131–40. doi: 10.1128/EC.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dvorak JA, Crane MS. Vertebrate cell cycle modulates infection by protozoan parasites. Science. 1981;214:1034–6. doi: 10.1126/science.7029713. [DOI] [PubMed] [Google Scholar]
- 55.Grimwood J, Mineo JR, Kasper LH. Attachment of Toxoplasma gondii to host cells is host cell cycle dependent. Infect Immun. 1996;64:4099–104. doi: 10.1128/iai.64.10.4099-4104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youn JH, Nam HW, Kim DJ, Park YM, Kim WK, Kim WS, et al. Cell cycle-dependent entry of Toxoplasma gondii into synchronized HL-60 cells. Kisaengchunghak Chapchi. 1991;29:121–8. doi: 10.3347/kjp.1991.29.2.121. [DOI] [PubMed] [Google Scholar]
- 57.Walker ME, Hjort EE, Smith SS, Tripathi A, Hornick JE, Hinchcliffe EH, et al. Toxoplasma gondii actively remodels the microtubule network in host cells. Microbes Infect. 2008;10:1440–9. doi: 10.1016/j.micinf.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaussabel D, Semnani RT, McDowell MA, Sacks D, Sher A, Nutman TB. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–81. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 59.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;407:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 60.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–46. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 61.Spear W, Chan D, Coppens I, Johnson RS, Giaccia A, Blader IJ. The host cell transcription factor hypoxia-inducible factor 1 is required for Toxoplasma gondii growth and survival at physiological oxygen levels. Cell Microbiol. 2006;8:339–52. doi: 10.1111/j.1462-5822.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 62.Phelps E, Sweeney K, Blader IJ. Toxoplasma gondii rhoptry discharge correlates with activation of the EGR2 host cell transcription factor. Infect Immun. 2008;76:4703–12. doi: 10.1128/IAI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molestina RE, Sinai AP. Host and parasite-derived IKK activities direct distinct temporal phases of NF-kappaB activation and target gene expression following Toxoplasma gondii infection. J Cell Sci. 2005;118:5785–96. doi: 10.1242/jcs.02709. [DOI] [PubMed] [Google Scholar]
- 64.Datta R, Taneja N, Sukhatme VP, Qureshi SA, Weichselbaum R, Kufe DW. Reactive oxygen intermediates target CC(A/T)6GG sequences to mediate activation of the early growth response 1 transcription factor gene by ionizing radiation. Proc Natl Acad Sci USA. 1993;90:2419–22. doi: 10.1073/pnas.90.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang RP, Wu JX, Fan Y, Adamson ED. UV activates growth factor receptors via reactive oxygen intermediates. J Cell Biol. 1996;133:211–20. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mechta-Grigoriou F, Gerald D, Yaniv M. The mammalian Jun proteins: redundancy and specificity. Oncogene. 2001;20:2378–89. doi: 10.1038/sj.onc.1204381. [DOI] [PubMed] [Google Scholar]
- 67.Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-{kappa}B by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated I{kappa}B to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–71. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- 68.Kim JM, Oh YK, Kim YJ, Cho SJ, Ahn MH, Cho YJ. Nuclear factor-kappa B plays a major role in the regulation of chemokine expression of HeLa cells in response to Toxoplasma gondii infection. Parasitol Res. 2001;87:758–63. doi: 10.1007/s004360100447. [DOI] [PubMed] [Google Scholar]
- 69.Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol. 2001;167:2193–201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- 70.Shapira S, Harb OS, Margarit J, Matrajt M, Han J, Hoffmann A, et al. Initiation and termination of NF-kappaB signaling by the intracellular protozoan parasite Toxoplasma gondii. J Cell Sci. 2005;118:3501–8. doi: 10.1242/jcs.02428. [DOI] [PubMed] [Google Scholar]
- 71.Caamano J, Alexander J, Craig L, Bravo R, Hunter CA. The NF-kappa B family member RelB is required for innate and adaptive immunity to Toxoplasma gondii. J Immunol. 1999;163:4453–61. [PubMed] [Google Scholar]
- 72.Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–59. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caamano J, Tato C, Cai G, Villegas EN, Speirs K, Craig L, et al. Identification of a role for NF-{kappa}B2 in the regulation of apoptosis and in maintenance of T cell-mediated immunity to Toxoplasma gondii. J Immunol. 2000;165:5720–8. doi: 10.4049/jimmunol.165.10.5720. [DOI] [PubMed] [Google Scholar]
- 74.Payne TM, Molestina RE, Sinai AP. Inhibition of caspase activation and a requirement for NF-{kappa}B function in the Toxoplasma gondii-mediated blockade of host apoptosis. J Cell Sci. 2003;116:4345–58. doi: 10.1242/jcs.00756. [DOI] [PubMed] [Google Scholar]
- 75.Laliberte J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci. 2008;65:1900–15. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 77.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 78.Kim L, Butcher BA, Denkers EY. Toxoplasma gondii Interferes with Lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from Endotoxin tolerance. J Immunol. 2004;172:3003–10. doi: 10.4049/jimmunol.172.5.3003. [DOI] [PubMed] [Google Scholar]
- 79.Sinai AP, Payne TM, Carmen JC, Hardi L, Watson SJ, Molestina RE. Mechanisms underlying the manipulation of host apoptotic pathways by Toxoplasma gondii. Int J Parasitol. 2004;34:381–91. doi: 10.1016/j.ijpara.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 80.Molestina RE, Sinai AP. Detection of a novel parasite kinase activity at the Toxoplasma gondii parasitophorous vacuole membrane capable of phosphorylating host IkappaBalpha. Cell Microbiol. 2005;7:351–62. doi: 10.1111/j.1462-5822.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 81.Dobbin CA, Smith NC, Johnson AM. Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-kappa B and nitric oxide. J Immunol. 2002;169:958–65. doi: 10.4049/jimmunol.169.2.958. [DOI] [PubMed] [Google Scholar]
- 82.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol. 2004;172:3686–94. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 83.Kim SK, Fouts AE, Boothroyd JC. Toxoplasma gondii dysregulates IFN-gamma-inducible gene expression in human fibroblasts: insights from a genome-wide transcriptional profiling. J Immunol. 2007;178:5154–65. doi: 10.4049/jimmunol.178.8.5154. [DOI] [PubMed] [Google Scholar]
- 84.Weiss LM, Kim K. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci. 2000;5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18:198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 86.Jones TC, Bienz KA, Erb P. In vitro cultivation of Toxoplasma gondii cysts in astrocytes in the presence of gamma interferon. Infect Immun. 1986;51:147–56. doi: 10.1128/iai.51.1.147-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bohne W, Heesemann J, Gross U. Induction of bradyzoite-specific Toxoplasma gondii antigens in gamma interferon-treated mouse macrophages. Infect Immun. 1993;61:1141–5. doi: 10.1128/iai.61.3.1141-1145.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soete M, Camus D, Dubremetz JF. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro. Exp Parasitol. 1994;78:361–70. doi: 10.1006/expr.1994.1039. [DOI] [PubMed] [Google Scholar]
- 89.Radke JR, Donald RG, Eibs A, Jerome ME, Behnke MS, Liberator P, et al. Changes in the expression of human cell division autoantigen-1 influence Toxoplasma gondii growth and development. PLoS Pathog. 2006;2:e105. doi: 10.1371/journal.ppat.0020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guimaraes EV, de Carvalho L, Barbosa HS. Primary culture of skeletal muscle cells as a model for studies of Toxoplasma gondii cystogenesis. J Parasitol. 2008;94:72–83. doi: 10.1645/GE-1273.1. [DOI] [PubMed] [Google Scholar]
- 91.Luder CG, Giraldo-Velasquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol. 1999;93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 92.Ferreira-da-Silva MD, Takacs AC, Barbosa HS, Gross U, Luder CG. Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite-to-bradyzoite conversion at higher rates than fibroblasts. Int J Med Microbiol. 2008 doi: 10.1016/j.ijmm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 93.Dzierszinski F, Nishi M, Ouko L, Roos DS. Dynamics of Toxoplasma gondii differentiation. Eukaryot Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Radke JR, Guerini MN, Jerome M, White MW. A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol. 2003;131:119–27. doi: 10.1016/s0166-6851(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 95.Mineo JR, McLeod R, Mack D, Smith J, Khan IA, Ely KH, et al. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;150:3951–64. [PubMed] [Google Scholar]
- 96.Khan IA, Smith KA, Kasper LH. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol. 1988;141:3600–5. [PubMed] [Google Scholar]
- 97.Handman E, Goding JW, Remington JS. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980;124:2578–83. [PubMed] [Google Scholar]
- 98.Sharma SD, Mullenax J, Araujo FG, Erlich HA, Remington JS. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983;131:977–83. [PubMed] [Google Scholar]
- 99.Parmley SF, Sgarlato GD, Mark J, Prince JB, Remington JS. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992;30:1127–33. doi: 10.1128/jcm.30.5.1127-1133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun. 2000;68:1026–33. doi: 10.1128/iai.68.3.1026-1033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–9. doi: 10.1073/pnas.90.13.6115. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Hieny S, et al. In the absence of endogenous IFN-gamma, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–54. [PubMed] [Google Scholar]
- 103.Mueller A, Strange PG. The chemokine receptor, CCR5. Int J Biochem Cell Biol. 2004;36:35–8. doi: 10.1016/s1357-2725(03)00172-9. [DOI] [PubMed] [Google Scholar]
- 104.Aliberti J, Sousa CRE, Schito M, Hieny S, Wells T, Huffnagel GB, et al. CCR5 provides a signal for microbial induced production fo IL-12 by CD8 alpha(1) dendritic cells. Nat Immunol. 2000;1:83–7. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 105.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–90. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 106.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 107.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–9. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 108.Mun HS, Aosai F, Norose K, Piao LX, Fang H, Akira S, et al. Toll-like receptor 4 mediates tolerance in macrophages stimulated with Toxoplasma gondii-derived heat shock protein 70. Infect Immun. 2005;73:4634–42. doi: 10.1128/IAI.73.8.4634-4642.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Debierre-Grockiego F, Azzouz N, Schmidt J, Dubremetz JF, Geyer H, Geyer R, et al. Roles of glycosylphosphatidylinositols of Toxoplasma gondii. Induction of tumor necrosis factor-alpha production in macrophages. J Biol Chem. 2003;278:32987–93. doi: 10.1074/jbc.M304791200. [DOI] [PubMed] [Google Scholar]
- 110.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J Immunol. 2007;179:1129–37. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 111.Mun HS, Aosai F, Norose K, Chen M, Piao LX, Takeuchi O, et al. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int Immunol. 2003;15:1081–7. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 112.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, et al. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 113.Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, et al. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–97. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 114.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1-and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 115.Cai G, Kastelein R, Hunter CA. Interleukin-18 (IL-18) enhances innate IL-12-mediated resistance to Toxoplasma gondii. Infect Immun. 2000;68:6932–8. doi: 10.1128/iai.68.12.6932-6938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol. 2001;167:4574–84. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 117.Chang HR, Grau GE, Pechere JC. Role of TNF and IL-1 in infections with Toxoplasma gondii. Immunology. 1990;69:33–7. [PMC free article] [PubMed] [Google Scholar]
- 118.Hunter CA, Chizzonite R, Remington JS. IL-1 beta is required for IL-12 to induce production of IFN-gamma by NK cells. A role for IL-1 beta in the T cell-independent mechanism of resistance against intracellular pathogens. J Immunol. 1995;155:4347–54. [PubMed] [Google Scholar]
- 119.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 120.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+T cell response. Immunity. 2006;25:655–64. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 121.Zhang DK, Zhang GL, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 122.LaRosa DF, Stumhofer JS, Gelman AE, Rahman AH, Taylor DK, Hunter CA, et al. T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci USA. 2008;105:3855–60. doi: 10.1073/pnas.0706663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 2002;16:429–39. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 124.Sukhumavasi W, Egan CE, Warren AL, Taylor GA, Fox BA, Bzik DJ, et al. TLR adaptor MyD88 is essential for pathogen control during oral toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J Immunol. 2008;181:3464–73. doi: 10.4049/jimmunol.181.5.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yap GS, Sher A. Effector cells of both non-hemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumor necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J Exp Med. 1999;189:1083–92. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 127.Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;143:2045–50. [PubMed] [Google Scholar]
- 128.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Woude GF, et al. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med. 2001;194:181–8. doi: 10.1084/jem.194.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacMicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 2004;25:601–9. doi: 10.1016/j.it.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 132.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–4. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 133.Murray HW, Teitelbaum R, Hariprashad J. Response to treatment for an intracellular infection in a T cell-deficient host: toxoplasmosis in nude mice. J Infect Dis. 1993;167:1173–7. doi: 10.1093/infdis/167.5.1173. [DOI] [PubMed] [Google Scholar]
- 134.Schwartzman JD, Gonias SL, Pfefferkorn ER. Murine gamma interferon fails to inhibit Toxoplasma gondii growth in murine fibroblasts. Infect Immun. 1990;58:833–4. doi: 10.1128/iai.58.3.833-834.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halonen SK, Weiss LM. Investigation into the mechanism of gamma interferon-mediated inhibition of Toxoplasma gondii in murine astrocytes. Infect Immun. 2000;68:3426–30. doi: 10.1128/iai.68.6.3426-3430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Halonen SK, Chiu F, Weiss LM. Effect of cytokines on growth of Toxoplasma gondii in murine astrocytes. Infect Immun. 1998;66:4989–93. doi: 10.1128/iai.66.10.4989-4993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol. 2002;23:96–101. doi: 10.1016/s1471-4906(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 138.Luder CG, Walter W, Beuerle B, Maeurer MJ, Gross U. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1-alpha. Eur J Immunol. 2001;31:1475–84. doi: 10.1002/1521-4141(200105)31:5<1475::AID-IMMU1475>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 139.Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J Immunol. 2006;176:1840–7. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]
- 140.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 141.Lang C, Algner M, Beinert N, Gross U, Luder CG. Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-gamma-induced major histocompatibility complex class II gene expression. Microbes Infect. 2006;8:1994–2005. doi: 10.1016/j.micinf.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 142.Sumyuen MH, Garin YJ, Derouin F. Early kinetics of Toxoplasma gondii infection in mice infected orally with cysts of an avirulent strain. J Parasitol. 1995;81:327–9. [PubMed] [Google Scholar]
- 143.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29:487–96. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Channon JY, Seguin RM, Kasper LH. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect Immun. 2000;68:4822–6. doi: 10.1128/iai.68.8.4822-4826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Diana J, Vincent C, Peyron F, Picot S, Schmitt D, Persat F. Toxoplasma gondii regulates recruitment and migration of human dendritic cells via different soluble secreted factors. Clin Exp Immunol. 2005;141:475–84. doi: 10.1111/j.1365-2249.2005.02856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diana J, Persat F, Staquet MJ, Assossou O, Ferrandiz J, Gariazzo MJ, et al. Migration and maturation of human dendritic cells infected with Toxoplasma gondii depend on parasite strain type. FEMS Immunol Med Microbiol. 2004;42:321–31. doi: 10.1016/j.femsim.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 147.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–23. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 148.Bierly AL, Shufesky WJ, Sukhumavasi W, Morelli AE, Denkers EY. Dendritic cells expressing plasmacytoid marker PDCA-1 are Trojan horses during Toxoplasma gondii infection. J Immunol. 2008;181:8485–91. doi: 10.4049/jimmunol.181.12.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, et al. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–5. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 150.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med. 2007;204:1923–33. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, Yarovinsky F, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J Immunol. 2008;180:6229–36. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ajzenberg D, Banuls AL, Su C, Dumetre A, Demar M, Carme B, et al. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int J Parasitol. 2004;34:1185–96. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 153.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–6. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 154.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–5. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 155.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–7. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boothroyd JC, Grigg ME. Population biology of Toxoplasma gondii and its relevance to human infectio: do different strains cause different disease? Curr Opin Microbiol. 2002;5:438–42. doi: 10.1016/s1369-5274(02)00349-1. [DOI] [PubMed] [Google Scholar]
- 157.Sibley LD, LeBlanc AJ, Pfefferkorn ER, Boothroyd JC. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992;132:1003–15. doi: 10.1093/genetics/132.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ihle JN. The Stat family in cytokine signaling. Curr Opin Cell Biol. 2001;13:211–7. doi: 10.1016/s0955-0674(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 159.Gavrilescu LC, Denkers EY. IFN-gamma overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol. 2001;167:902–9. doi: 10.4049/jimmunol.167.2.902. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen TD, Bigaignon G, Markine-Goriaynoff D, Heremans H, Nguyen TN, Warnier G, et al. Virulent Toxoplasma gondii strain RH promotes T-cell-independent overproduction of proinflammatory cytokines IL12 and gamma-interferon. J Med Microbiol. 2003;52(Pt10):869–76. doi: 10.1099/jmm.0.04860-0. [DOI] [PubMed] [Google Scholar]
- 161.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195:1625–33. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Saeij JP, Boyle JP, Grigg ME, Arrizabalaga G, Boothroyd JC. Bioluminescence imaging of Toxoplasma gondii infection in living mice reveals dramatic differences between strains. Infect Immun. 2005;73:695–702. doi: 10.1128/IAI.73.2.695-702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–80. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 164.El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF. ROP18 is a Rhoptry Kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grigg ME, Bonnefoy S, Hehl AB, Suzuki Y, Boothroyd JC. Success and virulence in Toxoplasma as the result of sexual recombination between two distinct ancestries. Science. 2001;294:161–5. doi: 10.1126/science.1061888. [DOI] [PubMed] [Google Scholar]
- 166.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–3. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sullivan WJ, Jr., Chiang CW, Wilson CM, Naguib FN, el Kouni MH, Donald RG, et al. Insertional tagging of at least two loci associated with resistance to adenine arabinoside in Toxoplasma gondii, and cloning of the adenosine kinase locus. Mol Biochem Parasitol. 1999;103:1–14. doi: 10.1016/s0166-6851(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 168.Chaudhary K, Darling JA, Fohl LM, Sullivan WJ, Jr., Donald RG, Pfefferkorn ER, et al. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J Biol Chem. 2004;279:31221–7. doi: 10.1074/jbc.M404232200. [DOI] [PubMed] [Google Scholar]
- 169.Nicolle C, Manceaux L. Sur une infection a corps de Leishman (ou organismes voisins) du gondi. C R Seances Acad Sci. 1908;147:763–6. [Google Scholar]
- 170.Nicolle C, Manceaux L. Sur une protozoaire neouveau du gondi. C R Seances Acad Sci. 1909;148:369–72. [Google Scholar]
- 171.Carey KL, Westwood NJ, Mitchison TJ, Ward GE. A small-molecule approach to studying invasive mechanisms of Toxoplasma gondii. Proc Natl Acad Sci USA. 2004;101:7433–8. doi: 10.1073/pnas.0307769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, et al. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 2008;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci USA. 2007;104:6442–7. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mortensen PB, Yolken RH, Lindum B, Sorensen T, Hougaard D, Pedersen BN. Toxoplasmosis and other early infections in relation to schizophrenia risk. Schizophrenia Bull. 2005;31:232–3. [Google Scholar]
- 175.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267:1591–4. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Flegr J, Zitkova S, Kodym P, Frynta D. Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology. 1996;113:49–54. doi: 10.1017/s0031182000066269. [DOI] [PubMed] [Google Scholar]
- 177.Gilbert LA, Ravindran S, Turetzky JM, Boothroyd JC, Bradley PJ. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryot Cell. 2007;6:73–83. doi: 10.1128/EC.00309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]