Summary
The dissemination of cancer cells from a primary tumor is conventionally viewed as a unidirectional process that culminates with the metastatic colonization of distant organs. Here we show that circulating tumor cells (CTCs) can also colonize their tumors of origin, in a process that we call “tumor self-seeding”. Self-seeding of breast cancer, colon cancer, and melanoma tumors in mice is preferentially mediated by aggressive CTCs, including those with bone, lung or brain metastatic tropism. The tumor-derived cytokines IL-6 and IL-8 acted as CTC attractants and the poor-prognosis markers MMP1/collagenase-1 and the actin cytoskeleton component fascin-1 as mediators of CTC infiltration into mammary tumors. Self-seeding can accelerate tumor growth, angiogenesis, and stromal recruitment through seed-derived factors including, in a breast cancer model, the chemokine CXCL1. Tumor self-seeding could explain the relationships between anaplasia, tumor size, vascularity and prognosis, and local recurrence seeded by disseminated cells following ostensibly complete tumor excision.
Introduction
Cancer progression is commonly segregated into processes of primary tumor growth and secondary metastasis. In this conventional model, the high cell density and rapid growth rate of primary tumors are attributed to an ability of cancer cells to sustain unlimited proliferation and to favorably influence their microenvironment. In contrast, metastasis is thought to depend on cancer cell dissemination and adaptation to distant organs (Chiang and Massagué, 2008; Langley and Fidler, 2007; Scheel et al., 2007). Shedding of tumor cells into the circulation may occur in large numbers and from early stages of tumor formation (Husemann et al., 2008; Pantel and Brakenhoff, 2004; Stoecklein et al., 2008). Yet overt metastasis is achieved only by a minority of these dispersed cells. Tight vascular wall barriers, unfavorable conditions for survival in distant organs, and a rate-limiting acquisition of organ colonization functions are just some of the impediments to the formation of distant metastasis (Nguyen et al., 2009). However, these impediments may be less stringent as regards the ability of CTCs to re-infiltrate their tumors of origin. The neovasculature of tumors is typically leaky (Carmeliet and Jain, 2000; Rafii et al., 2003), a feature that would facilitate not only the passage of tumor cells into the circulation but also their entry from the circulation back into the tumor. CTCs would likely need no further adaptation to thrive in the microenvironment of their source tumor. Based on these theoretical considerations, we have postulated that CTCs may re-infiltrate an establish tumor, enriching it with aggressive cells that have withstood a period of dissemination. This process, which we refer to as “tumor self-seeding”, could have consequences for tumor growth and the breeding of metastatic cell progenies (Norton and Massague, 2006).
In the present studies we sought experimental evidence for the existence, the features, the mediators, and the potential consequences of tumor self-seeding. Using human breast, colon and melanoma cancer cells, we investigated in mice the ability of malignant cells to seed a tumor from the circulation. The results led us to investigate whether metastatic cells have a superior ability to seed an established tumor, and whether tumor self-seeding depends on attraction signals from the tumor mass and infiltrative functions in the circulating seeds. Our results uncovered various mediators of such attraction and infiltration functions, including factors whose expression in primary tumors is associated with relapse in patients. These insights dissect tumor self-seeding into steps of CTC attraction and tumor infiltration, and highlight the implications of tumor self-seeding for cancer biology and clinical oncology.
Results
Seeding of established tumors by CTCs
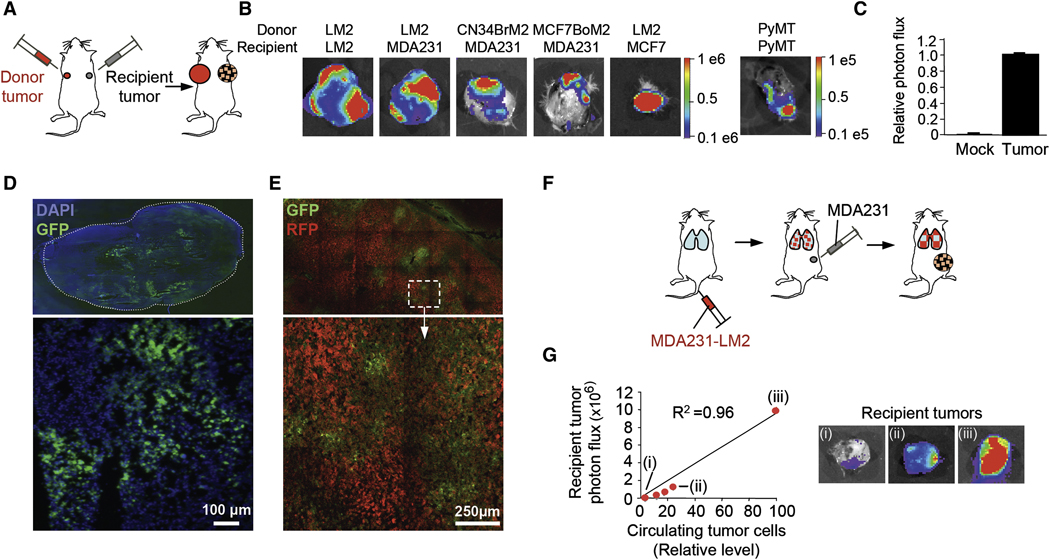
To investigate whether cancer cells that are shed into the circulation can re-infiltrate a primary tumor mass, we first used MDA-MB-231 (MDA231 for brevity), a breast cancer cell line from which metastatic subpopulations were previously isolated and characterized (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a). The lung metastatic derivative line MDA231-LM2 (Minn et al., 2005a), was transduced with a GFP-luciferase fusion vector and inoculated into one mammary gland in mice to form a “donor” tumor mass. Unlabeled MDA231-LM2 cells were inoculated into a contra-lateral mammary gland to form a “recipient” mass of the same tumor (Figure 1A). After 60 days, the recipient tumors were excised and examined for the presence of seeding cells by means of ex-vivo bioluminescence imaging (BLI). A majority (85%) of the recipient tumors showed extensive seeding by MDA231-LM2 cells (Figure 1B and Table 1). Tumors formed by the more indolent MDA231 parental population were as effective as MDA231-LM2 tumors at capturing seed cells (Figure 1B and Table 1). No seeding was observed in mock-inoculated mammary glands within the same time period (Figure 1C).
Figure 1. Seeding of established tumors by CTCs.
(A) A diagram of contralateral-seeding experiment. Unlabeled and GFP/luciferase-expressing breast cancer cells were injected into contralateral No. 2 mammary glands as a “recipient tumor” and a “donor tumor”, respectively.
(B) BLI of recipient tumors extracted from mice bearing the indicated GFP/luciferase-expressing donor tumors. Color-range bars: photon flux. LM2: a lung metastatic derivative of MDA231. MCF7-BoM2: a bone-metastatic derivative of MCF7, CN34-BrM2: a brain-metastatic derivative of pleural effusion CN34, PyMT: cells derived from mammary tumors developed in MMTV-PyMT transgenic mice.
(C) BLI of tumor-free and tumor-bearing mammary glands from mice bearing GFP/luciferase-expressing donor tumors. n=9–18.
(D) Frozen sections of seeded MDA231-LM2 tumors were visualized by fluorescence microscopy. An entire tumor section and a higher-magnification image (×10) of a selected field are shown.
(E) A contralateral-seeding experiment was performed with RFP- and GFP-expressing MDA231-LM2 cells. Frozen sections from RFP-labeled tumors were visualized under confocal microscopy at ×20.
(F) A diagram to test mammary tumor seeding from lung metastases. GFP/luciferase-expressing MDA231-LM2 cells were injected intravenously. Once lung metastases were established, unlabeled MDA231 cells were injected into a mammary gland No. 2.
(G) Left: burden of CTCs derived from lung metastases in mice described in panel F. Relative levels of CTC were plotted against the luminescent signals of recipient tumors. Right: BLI of three representative recipient tumors (i, ii and iii) identified in the graph.
Table 1. Tumor seeding activity of various carcinoma and melanoma cell lines.
The table indicates the type of cancer, the relationship, species, and name of the cell lines used as donor and recipient pairs, the tumor site (MFP: mammary fat pad, SubQ: subcutaneous), and the fraction of tumors that showed seeding as determined by BLI. MDA231-LM2, a lung metastatic derivative of MDA231; MCF7-BoM2, a bone-metastatic derivative of MCF7; CN34-BrM2, a brain-metastatic derivative of pleural effusion CN34; SW620-LM1, a lung-metastatic derivative of SW620; A375-BoM2, a bone metastatic derivative of A375. PyMT: cells derived from a mammary tumor developed in MMTV-PyMT transgenic mice.
| Cancer | Relationship | Species | Donor tumor | Recipient tumor | Site | Seeding (%) |
|---|---|---|---|---|---|---|
| Breast | Homotypic | Human | MDA231-LM2 | MDA231-LM2 | MFP | 11/13 (85) |
| Breast | Homotypic | Human | MDA231-LM2 | MDA231 | MFP | 39/47 (83) |
| Breast | Homotypic | Mouse | 4T1 | 4T1 | MFP | 3/5 (60) |
| Breast | Homotypic | Mouse | 4T1 | 67NR | MFP | 11/11 (100) |
| Breast | Homotypic | Mouse | PyMT | PyMT endogenous | Breast | 8/11 (73) |
| Breast | Heterotypic | Human | CN34-BrM2 | MDA231 | MFP | 10/19 (53) |
| Breast | Heterotypic | Human | MCF7-BoM2 | MDA231 | MFP | 6/7 (86) |
| Breast | Heterotypic | Human | MDA231-LM2 | MCF7 | MFP | 7/7 (100) |
| Melanoma | Homotypic | Human | A375-BoM2 | A375 | SubQ | 8/11 (73) |
| Colon | Homotypic | Human | SW620-LM1 | SW620 | SubQ | 7/9 (77) |
Fluorescence microscopy analysis of MDA231 recipient tumors confirmed the presence of numerous GFP-positive MDA231-LM2 seeding cells as distinct patches typically encompassing less than a quarter of a tumor section (Figure 1D and data not shown). When recipient tumors were generated using red-fluorescent protein (RFP)-labeled cells, the infiltrating GFP+ cells were observed intermingling with resident RFP+ cancer cells and with unlabeled areas of presumptive tumor stroma (Figure 1E). qRT-PCR analysis of firefly-luciferase mRNA level in seeded recipient tumors revealed that seeder cells accounted for 5%–30% of the recipient tumor mass (data not shown).
To establish the generality of this seeding phenomenon, we performed similar experiments with different cancer cell lines. Recipient mammary tumors became seeded with high frequency (53% to 100% of mice) by donor tumors that were formed with bone metastatic (MCF7-BoM2), lung metastatic (MDA231-LM2), or brain metastatic (CN34-BrM2) cells from different subtypes of breast cancer (basal, estrogen receptor-negative MDA231 cells vs luminal, estrogen receptor-positive MCF7 cells) or patient-derived malignant cell cultures (CN34 cells) (Figure 1B and Table 1). Seeding of a recipient tumor by its own aggressive progeny was also observed between subcutaneous tumors formed by the human colon carcinoma line SW620 and its lung metastatic derivative SW620-LM1, and between the human melanoma line A375 and its bone metastatic derivative A375-BoM2 (Table 1). These experiments with human cell lines required the use of immunodeficient mice. However, seeding was also observed in immunocompetent mice using the syngeneic cell lines 4T1 and 67NR (Table 1). Derived from a spontaneous mouse mammary tumor, 4T1 is highly metastatic to lungs, liver, bones and brain whereas 67NR is poorly metastatic (Aslakson and Miller, 1992). Moreover, endogenous mammary tumors driven by the polyoma middle T oncogene (PyMT) in mice were efficiently seeded by PyMT tumor-derived cells placed in the circulation (Figure 1B; Table 1).
To determine if mammary tumors may be seeded with cells shed from metastatic lesions, we generated lung tumor colonies by tail-vein inoculation of labeled MDA231-LM2 cells and then implanted unlabeled MDA231 mammary tumors in the same mice (Figure 1F). Seeding of these mammary tumors by lung metastasis-derived cells was observed in 10/11 (91%) of mice (Figure 1G). The extent of seeding was proportional to the level of donor cells in the circulation (Figure 1G). Collectively, these results indicate that aggressive carcinoma and melanoma cells shed into the circulation can avidly seed an established tumor mass.
Preferential tumor seeding by metastatic cell progenies
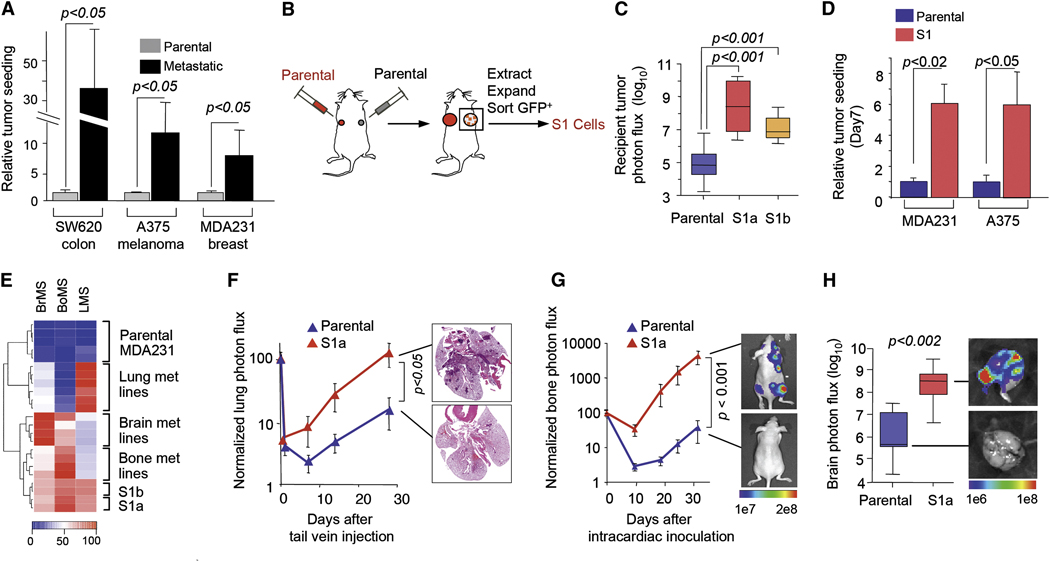
We compared the ability of metastatic derivatives from SW620, A375 and MDA231 with their corresponding parental lines to act as donor tumors. In contralateral homotypic tumor seeding assays, the metastatic derivatives showed an 8- to 35-fold higher seeding ability than their parental counterparts, as determined by BLI of homotypic recipient tumors (Figure 2A). Next we carried out in vivo selection experiments to isolate, from the parental MDA231 and A375 cell lines, the cells that most actively seed a tumor mass from the circulation. We inoculated contralateral mammary glands (MDA231) or contralateral flanks of mice (A375) with unlabeled (recipient) or GFP/luciferase-labeled (donor) cells, respectively (Figure 2B). Seeding of recipient tumors was detectable by BLI within 50 days of the inoculation. The seeded tumors were excised, dissociated, and placed in culture, and the GFP-positive seeder cell populations (MDA231-S1a, MDA231-S1b, and A375-S1) were obtained from these cultures by FACS. The tumor seeding ability of MDA231-S1a and MDA231-S1b as contralateral donor tumors was >100-fold higher than that of parental MDA231 (Figure 2C). The seeding ability of MDA231-S1a and A375-S1 from the circulation was 6-fold higher than that of the parental populations (Figure 2D). The S1 populations additionally showed an increased ability to pass across an endothelial cell layer in trans-well migration assays (Figure S1).
Figure 2. Preferential tumor seeding by metastatic cell progenies.
(A) Comparison of seeding activity of highly and poorly metastatic cells. Contralateral-seeding experiments were performed with the GFP/luciferase-expressing parental cancer cell lines, or with their lung metastatic (SW620-LM1, MDA231-LM2) or bone metastatic derivatives (A375-BoM2). Relative luminescent signals are plotted.
(B) A schematic diagram of isolation of seeder cell populations. For details see Experimental Procedures.
(C) Comparative tumor-seeding ability of in vivo-selected seeder cells (MDA231-S1a and S1b) and parental MDA231 cells in contralateral-seeding experiments as described in Figure1A. n= 6–8.
(D) Comparative tumor-seeding ability between parental and in vivo-selected seeder cells (MDA231-S1 and A375-S1) from the circulation as described in Figure 3A. n= 8–10.
(E) The gene expression profiles of parental MDA231, various metastatic derivatives and the seeder lines S1a and S1b were scored with gene expression classifiers for metastasis to lung (LMS), bone (BoMS) or brain (BrMS). Raw scores were scaled between 0 and 100.
(F) Left: Parental or MDA231 S1a cells were inoculated intravenously into mice. Lung colonization was measured by BLI and quantified. n=6–7. Right: representative histological staining (H&E) of lung sections from the experiment on the left.
(G, H) Cells were inoculated into the cardiac left ventricle. Bone colonization was measured by BLI and quantified; n=12. Brains were extracted and colonization was measured by BLI; n=5–7.
Error bars in all cases represent SEM and p values were based on two-tailed Mann-Whitney test; for details see Experimental Procedures.
We chose MDA231 for further analysis because the metastatic composition of this cell line has been extensively characterized. MDA231 was derived from the pleural fluid of a breast cancer patient with advanced metastatic disease, and it contains minority subpopulations that are highly metastatic to either the bones, the lungs, or the brain (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a). These subpopulations may represent the disseminated descendants of different metastatic lesions in the source patient, and are characterized by a differential expression of distinct gene sets. Several of these genes act as mediators of organ-specific metastasis in experimental systems and are associated with organ-specific relapse in independent cohorts of breast cancer patients (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a; Minn et al., 2005b).
A comparison of transcriptional profiles uncovered 72 genes (78 probe sets) whose mRNA level was at least 3-fold higher in the MDA231-S1a and -S1b cells than in parental MDA231 (Table S1). Interestingly, many of these are genes whose expression is characteristic of bone, lung and/or brain metastatic cell populations (Table S1) (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a). The anti-apoptotic gene BCL2A1 was also highly expressed in S1 cells (Table S1). Furthermore, the MDA231-S1a and -S1b transcriptomes were significantly enriched for the expression of bone metastasis (BoMS), lung metastasis (LMS) and brain metastasis (BrMS) gene expression classifiers (Figure 2E). These signatures were previously derived from human breast cancer cells that are selectively metastatic to one of these organs in mouse models and in patients (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a; Minn et al., 2005b). Indeed, when compared to parental MDA231 in metastasis assays in mice, the MDA231-S1a cells demonstrated a higher ability to colonize the lungs (Figure 2F), the bones (Figure 2G) and the brain (Figure 2H) from the circulation. Collectively, these results suggest that tumor self-seeding preferentially involves metastatic cancer cell progenies irrespective of their organ tropisms.
Tumor attraction and infiltration functions
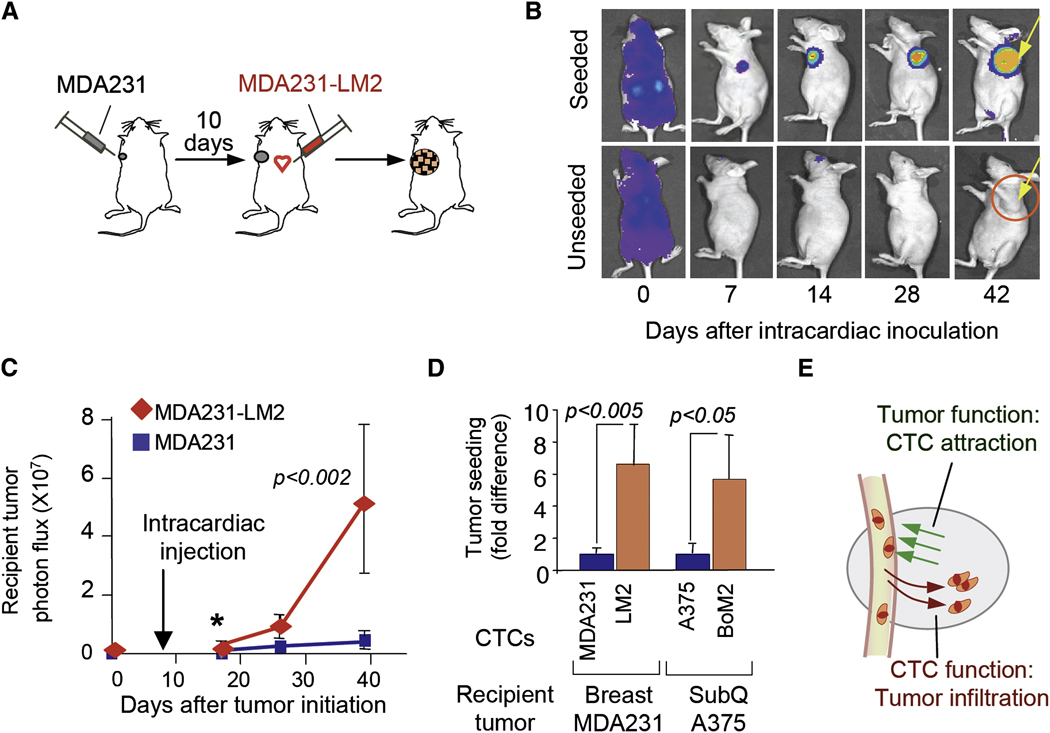
To define the basic functions required for tumor seeding by CTCs, we inoculated LacZ/GFP/Luciferase-expressing MDA231 cells (either parental or LM2) into the arterial circulation (via the left cardiac ventricle) of mice bearing unlabeled MDA231 mammary tumors (Figure 3A). This experimental protocol obviates possible differences in donor tumor growth rate or in donor tumor-derived systemic signals (McAllister et al., 2008) that might confound the interpretation of our results. The inoculated cells were rapidly distributed throughout the body (Figure 3B, day 0), and became extensively cleared within a few days except for cells that infiltrated the mammary tumors (Figure 3B, day 7 and beyond). These cells readily seeded the established mammary tumors but not the intact or mock-inoculated mammary glands (Figure 3B, day 42). Furthermore, the highly metastatic MDA231-LM2 cells were more effective at seeding the recipient tumors from the circulation than were the parental MDA231 cells (Figure 3C). This effect was already apparent within 10 days after inoculation (Figure 3D), before a marked outgrowth of the seeding cells took place within the recipient tumor mass. In similar experiments, the metastatic derivative A375-BoM2 seeded subcutaneous A375 melanoma tumors from the circulation more effectively than did the parental A375 cells (Figure 3D). These results suggested that tumor seeding by CTCs involves two distinct functions, namely, an ability of tumors to attract their own circulating progeny, and an ability of CTCs to infiltrate tumors in response to this attraction (Figure 3E).
Figure 3. Tumor attraction and infiltration functions.
(A) Unlabeled MDA231 cells were injected into a mammary gland No. 2. When tumors became palpable, LacZ/GFP/luciferase-expressing MDA231-LM2 cells were introduced into the circulation by intracardiac injection.
(B) BLI of mice with seeded and unseeded tumors. Arrow, recipient tumor.
(C) Comparative tumor-seeding ability of MDA231 and MDA231-LM2 cells from the circulation. Luminescent signals from recipient tumors at the indicated time points are shown.
(D) Luminescent signals of recipient tumors from mice injected with indicated cell lines were quantified 10 (MDA-231) and 5 (A375) days after injection. n=6–10.
(E) A diagram summarizing two functions involved in tumor self-seeding.
Error bars in all cases represent SEM and p values were based on two-tailed Mann-Whitney test.
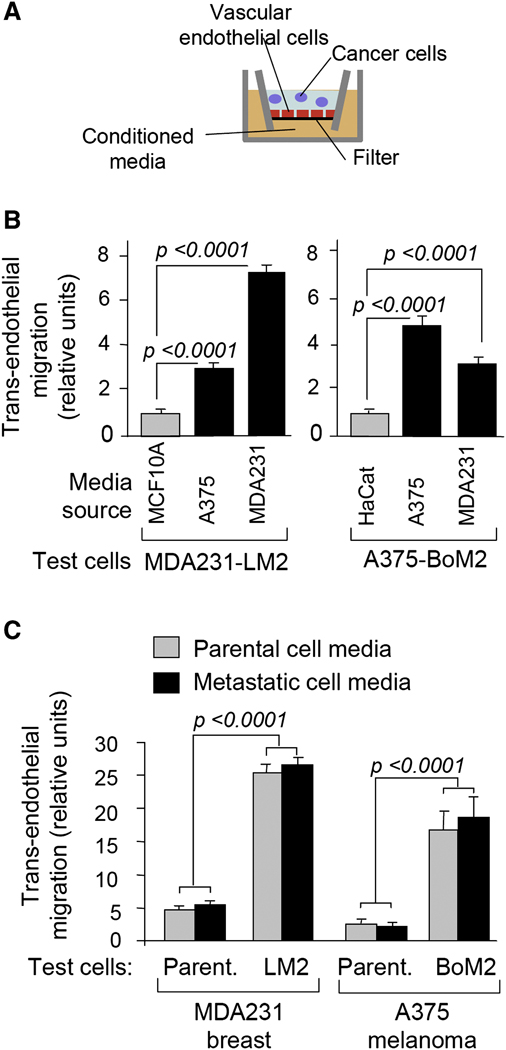
To gain further insight into these attraction and infiltration functions, we performed a trans-endothelial migration assay in which tumor cell conditioned media was placed in the bottom well of the chamber (Figure 4A). Media conditioned by MDA231 breast carcinoma or A375 melanoma cells were several-fold more active at stimulating the trans-endotheilal migration of MDA231-LM2 cells than were media conditioned by MCF10A cells, a human breast epithelial cell line derived from untransformed tissue (Figure 4B). Similarly, A375-BoM2 melanoma cells migrated through endothelial cell layers more actively in response to these cancer cell conditioned media than to media conditioned by HaCat cells (Figure 4B), a human keratinocyte cell line representing the most abundant cell type in skin epidermis. Media from MDA231 and MDA231-LM2 cultures were equivalent as a source of attraction in these experiments (Figure 4C), which is consistent with the equivalent ability of these two cell lines to act as recipient tumors in self-seeding assays (refer to Figure 1B and Table1).
Figure 4. Tumor-derived mediators of cancer cell attraction.
(A) Schematic diagram of the in vitro trans-endothelial migration assay. Conditioned media were placed in the bottom well. Test cells were plated in the top trans-well chamber. A confluent monolayer of human endothelial cells was present as indicated. Quantification was performed as described in the experimental procedures.
(B) Trans-endothelial migration of LM2 and A375-BoM2 cells in the presence of media conditioned by the indicated cell lines. n= 60–150.
(C) Trans-migration activity of tumor cells under indicated conditions was expressed as a fold difference relative to migration of parental MDA231 or A375 cells in the presence of media from non-tumorigenic cells (MCF10A conditioned media for MDA231; HaCat conditioned media for A375). n= 40–70.
Error bars in all cases represent SEM and p values were based on two-tailed Mann-Whitney test.
MDA231-LM2 cells are more active at migrating through endothelial cell layers compared to parental MDA231 cells (Gupta et al., 2007). Conditioned media from either MDA231-LM2 or MDA231 cells further stimulated the trans-endothelial migration of MDA231-LM2 cells (Figure 4C). Parental MDA231 cells showed low trans-endothelial migration activity even in the presence of media conditioned by tumor cells (Figure 4C). Similarly, the migration of A375-BoM2 cells through endothelial layers was several-fold more efficient than that of the parental A375 cells in the presence of conditioned media from A375 or A375-BoM2 (Figure 4C). These results demonstrated that cancer cells release signals that attract their progeny across endothelial layers. In addition, these results suggest that aggressive cancer cells are superior to their more indolent counterparts in their ability to migrate in response to these signals.
Tumor-derived mediators of cancer cell attraction
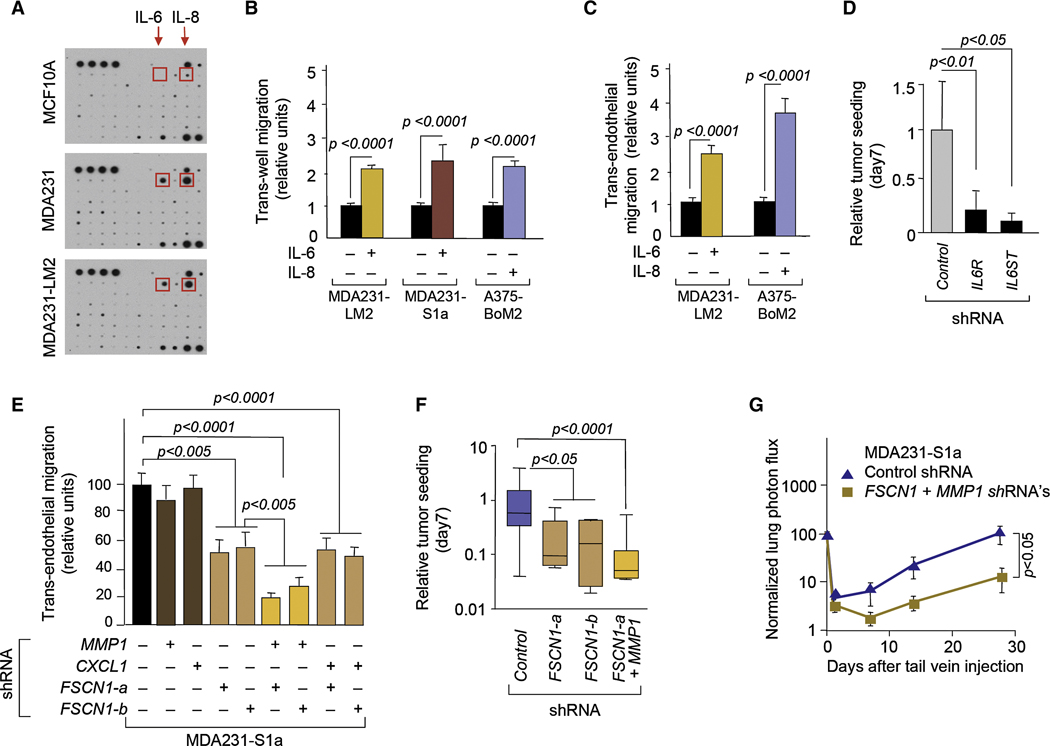
To identify candidate tumor-derived attractants for CTCs, we compared the secreted levels of 180 cytokines in conditioned media. This analysis uncovered several cytokines whose production was higher (IL-6, IL-8, Oncostatin M, and VEGF) or lower (CCL2) in MDA231 and its derivatives than in MCF10A cells (Figure 5A, Figures S2A and B). IL-6 and IL-8 showed the sharpest increase. IL-8 was also the most abundantly secreted cytokine in A375 melanoma cells compared to the HaCat cells. IL-6 and IL-8 are regulators of immune and inflammatory responses and have been implicated in tumor progression (Hodge et al., 2005; Kishimoto, 2005). Focusing on these two cytokines, the addition of recombinant IL-6 or IL-8 to media from the corresponding non-tumorigenic cells (MCF10A and HaCat, respectively) stimulated the trans-well migration of MDA231-LM2, MDA231-S1a and A375-BoM2 cells (Figure 5B). These effects were maintained or further increased in the presence of an endothelial cell layer (Figure 5C). IL-8 had little effect on MDA231-LM2 migration (data not shown), but these cells lack IL-8 receptors (CXCR-1 and 2) (Helbig et al., 2003).
Figure 5. Mediators of tumor infiltration by cancer cells.
(A) Cytokine antibody arrays (Raybiotech) probed with MCF10A, MDA231, and MDA231-LM2 conditioned media. The position of IL-6 and IL-8 are indicated. For additional annotation see Figure S2.
(B) Relative trans-well migration activity (without endothelial cell layer) of the indicated cell lines, with or without IL-6 or IL-8. n=89–110.
(C) Relative trans-endothelial migration activity of indicated cells with or without IL-6 or IL-8. n=84–124.
(D) Relative seeding actvity of MDA231-S1a cells expressing the indicated shRNA from the circulation. n=5–10.
(E) Trans-endothelial migration activity of indicated cell lines. n= 30–45.
(F) Relative seeding activity of MDA231-S1a cells expressing indicated shRNA from the circulation. n=5–8; error bars, maximum and minimum values.
(G) Lung colonization activity of MDA231-S1a cells expressing indicated shRNAs. n=6–7.
Error bars in all cases represent SEM and p values were based on two-tailed Mann-Whitney test unless indicated otherwise.
To determine the role of IL-6 on the attraction of MDA231 CTCs in vivo while averting the confounding effects of IL-6 on tumor vessels, we tested MDA231-S1a cells whose IL-6 receptor (IL6R) expression was inhibited with RNAi (Figure S3A). The knockdown of IL6R expression significantly decreased self-seeding ability of seeder cells (Figure 5C). The knockdown of IL6ST (gp130) (Figure S3A), a shared signal transducer for IL-6 cytokine family members including oncostatin M, strongly inhibited the seeding activity of MDA231-S1a (Figure 5D). Taken together, these results suggest that tumor-derived IL-6 and IL-8 can facilitate the self-seeding in these breast carcinoma and melanoma models by functioning as chemoattractants for circulating tumor cells.
Mediators of cancer cell infiltration of tumors
The enriched expression of various metastasis-associated genes in the MDA231-S1a and S1b populations indicated a preferential seeding of tumors by their metastatic cell progenies (refer to Figure 2E and Table S1). But this alone did not specifically link these genes to the ability of CTCs to infiltrate a tumor mass. To identify candidate mediators of this function, we searched this list for pro-invasive genes whose expression in primary breast tumors has been previously associated with relapse in breast cancer. These criteria were based on the rationale that expression of such mediators in primary tumor cells would endow these cells with a potential infiltrative advantage as they pass into the circulation.
These criteria were fulfilled by three genes (Table S1), namely, collagenase 1 (matrix metalloproteinase 1, MMP1), fascin 1 (FSCN1) and CXCL1. All three genes are implicated in invasion and infiltration and, most importantly, their expression in breast tumors is associated with priming of breast cancer cells for seeding of the lungs (MMP1, FSCN1, and CXCL1) and the brain (MMP1, FSCN1) (Bos et al., 2009; Minn et al., 2005a). These are among the top-ranked genes by association with lung relapse in the LMS signature in breast cancer patients (Minn et al., 2005a). MMP1 has been implicated in tumor cell invasion, vascular remodeling and pulmonary extravasation (Egeblad and Werb, 2002; Gupta et al., 2007). Fascin-1 is an actin cross-linking protein that functions in the organization of cytoplasmic microfilament bundles and dynamic, cortical cell protrusions including filopodia, lamellipodia and dendrites (Adams, 2004; Hashimoto et al., 2005). Fascin-1 has been implicated in the migration and invasiveness of malignant glioma and colorectal carcinoma cells (Hwang et al., 2008; Vignjevic et al., 2007). CXCL1 is a powerful mediator of leukocyte influx into sites of inflammation (Dhawan and Richmond, 2002; Kobayashi, 2008) and also participates in the recruitment of endothelial precursor cells for angiogenesis (Hristov et al., 2007).
Using qRT-PCR, we confirmed that the expression level of FSCN1, CXCL1 and MMP1 was 11-fold to >40-fold higher in the S1a and S1b seeder populations than in parental MDA231 (Figure S3B). To investigate the role of these genes in tumor seeding, we conducted trans-endothelial migration assays with MDA231-S1a cells in which the expression of these genes was inhibited with RNAi vectors (Figure S3C–E). Whereas the knockdown of MMP1 or CXCL1 singly had no effect on trans-endothelial migration, the knockdown of FSCN1 expression significantly decreased this activity (Figure 5E). The combined knockdown of MMP1 and FSCN1 further decreased this migration, while the combined knockdown of CXCL1 and FSCN1 did not (Figure 5E). Correlating with these effects, the ability of MDA231-S1a cells to infiltrate MDA231 mammary tumors from the circulation was significantly diminished by FSCN1 knockdown and was further decreased by a combined knockdown of MMP1 and FSCN1 (Figure 5F). The knockdown of MMP1 (Gupta et al., 2007) or FSCN1 (Figure S3F) had no effect on the growth of MDA231-LM2 cells as mammary tumors. The combined knockdown of MMP1 and FSCN1 in MDA231-S1a cells also inhibited the ability of these cells to colonize the lungs of mice from the venous circulation (Figure 5G). These results suggest that FSCN1 and MMP1, two genes whose expression in primary tumors is associated with distant relapse to lung and brain in breast cancer patients (Bos et al., 2009; Minn et al., 2005a), may also mediate the re-infiltration of mammary tumors by CTCs.
Promotion of tumor growth and stroma recruitment by self-seeding
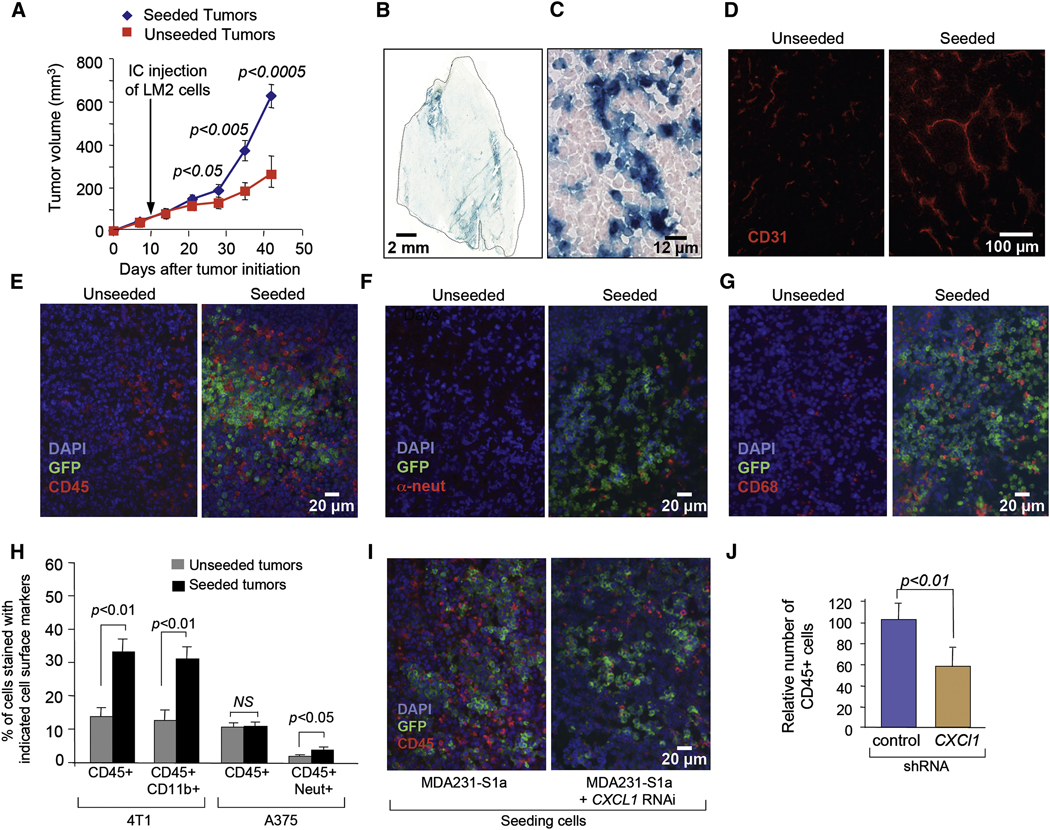
Aggressive cancer cells may promote tumor growth through the release of paracrine signals that enhance tumor angiogenesis and the recruitment of a supportive stroma (Carmeliet, 2005; Coussens and Werb, 2002; Joyce and Pollard, 2009; Tisty and Coussens, 2006). To determine if the seeding of a tumor mass by aggressive CTCs could affect the rate of tumor growth, we implanted MDA231 mammary tumors in mice and 10 days later we injected LacZ/GFP/Luciferase-expressing MDA231-LM2 cells into the circulation of these mice. As these tumors became seeded with MDA231-LM2 cells, the rate of tumor growth was accelerated compared to that of unseeded tumors (Figure 6A). The increase in the size of seeded tumors was reflected in an increase in the number of tumor cells and was not due to the less dense packing of cells (Figure S4A and S4B). Notably, β-galactosidase staining of histological sections revealed that the LacZ+ seeder cells represented 7% to 19% of the entire tumor mass which could not fully account for the larger size of these tumors (Figure 6B, C, and data not shown). Of note, the intrinsic proliferation rate of MDA231-LM2 is similar to that of the parental MDA231 population (Gupta et al., 2007) and the same applies to the MDA231-S1a and S1b lines (data not shown). These results argue that a small proportion of infiltrated MDA231-LM2 cells in a mammary tumor can augment the growth rate of the overall tumor cell population by paracrine effects on the stroma.
Figure 6. Promotion of tumor growth and stroma recruitment by self-seeding.
(A) Tumor volumes of MDA231-LM2-seeded and unseeded tumors at the indicated time points. n= 13–14.
(B, C) β-galactosidase staining of a typical tumor seeded with LacZ+ MDA231-LM2 cells. An entire tumor section (B) and a ×40 magnification (C) are shown.
(D) CD31 staining of sections from unseeded and seeded tumors.
(E) CD45 staining of sections from unseeded and seeded tumors.
(F) Anti-neutrophil staining of sections from unseeded and seeded tumors.
(G) CD68 staining of sections from unseeded and seeded tumors.
(H) Seeding experiments from the circulation were performed with 4T1 in syngeneic mice and with A375-BoM2 cells. Recipient tumors were analyzed by FACS. The percentage of stromal cells positively stained for the indicated markers are shown. P values were calculated based on two-tailed student’s t-test.
(I) Seeding experiments from the circulation were performed as described in Figure 3A with MDA231-S1a cells expressing indicated shRNAs. CD45 staining was performed on recipient tumors 19 days after the injection.
(J) Quantification of CD45 positive cells in tumors seeded by MDA231-S1a control and CXCL1 knockdown cells. n= 9.
Error bars in all cases represent SEM and p values were based on two-tailed Mann-Whitney test unless indicated otherwise.
The presence of seeder MDA231-LM2 cells in MDA231 tumors increased the average length and the extent of branching of tumor capillaries as determined subjectively (Figure 6D) and by quantitative digital imaging analysis of CD31-stained vasculature (Figure S4C). Moreover, the seeded tumors contained elevated numbers of infiltrating leukocytes, as determined by immunostaining for the pan-leukocyte marker CD45 (Figure 6E) (Charbonneau et al., 1988). Further analysis of recruited leukocytes revealed increased numbers of neutrophils as well as macrophages (CD68-positive cells) in the seeded tumors (Figure 6F and 6G).
We also investigated stroma recruitment in tumors seeded with 4T1 cells in a syngeneic mouse mammary tumor model, and A375-BoM cells in a melanoma model. FACS analysis of recipient tumors revealed that GFP+ A375-BoM2 seeder cells represent about 22–33% of the cells in seeded tumors whereas GFP+ 4T1 seeder cells accounted for 3–8 % (Figure S5A). Tumors seeded by 4T1 cells showed an increase in leukocyte recruitment (CD45+), the majority of which were CD11b+ myeloid cells (Figure 6H). Over 70% of the CD11b+ cells were also stained with F4/80, a pan macrophage marker (Figure S5B), which is consistent with previous analysis of stromal composition of 4T1 tumors (DuPre et al., 2007). Analysis of tumors seeded by A375-BoM2 revealed a limited but significant increase in neutrophil recruitment (CD45+ Neut+) (Figure 6H).
To provide proof of principle that seeding cells can affect their host tumor through the release of specific leukocyte-recruiting signals, we turned our attention to the MDA231 model system and the seeder-derived chemokine CXCL1, which is a mediator of leukocyte influx into sites of inflammation (Galkina and Ley, 2007). Unlike the knockdown of MMP1 and FSCN1 expression, the knockdown of CXCL1 did not diminish the trans-endothelial migration of MDA231 cells (refer to Figure 5E) or the extent of seeding of MDA231 tumors with GFP+ MDA231-S1a cells (Figure 6I). However, CXCL1 knockdown in MDA231-S1a cells caused a significant decrease in the recruitment of CD45+ cells into the stroma of seeded MDA231 tumors (Figure 6I, J). These results are consistent with the expected consequences of an enrichment of a tumor mass with aggressive cancer cells that secrete stroma-recruiting signals.
Discussion
Based on previous considerations (Norton and Massague, 2006), here we present evidence that tumor self-seeding is a general phenomenon in experimental models of breast carcinoma, colon carcinoma and malignant melanoma. In these models, tumor masses become readily seeded by CTCs derived from a separate tumor mass, from metastatic lesions, or from direct inoculation. We observed self-seeding with homotypic models using the same cancer cell population as the recipient tumor mass, and with heterotypic models using cell lines from different patients and different breast cancer subtypes. While our focus is on seeding of a tumor by its own circulating progeny, we also observed cross-seeding between mammary and melanoma tumors (our unpublished results).
The features of tumor self-seeding indicate a process in which CTCs re-infiltrate a tumor mass based on distinct biological functions, a process that may foster tumor growth and the breeding of metastatic progenies. Unlike colonization of distant organs, self-seeding requires little, if any, additional adaptation of CTCs to the recipient microenvironment. However, self-seeding does select for cancer cell populations that are more aggressive than the bulk population of the primary tumor. As CTCs, the seeds have undergone selection for movement into, and survival in the circulation. Moreover, self-seeding is actively driven by the ability of CTCs to sense attraction signals from the tumor and to extravasate in response to such signals. These functions are represented in the most aggressive segment of a CTC population, including CTCs that may have already acquired a full complement of metastatic functions.
Tumor self-seeding selects for highly aggressive CTCs, as shown by our consistent observation that metastatic cell subpopulations are more efficient as seeders than their parental populations. Moreover, MDA231 seeder populations recovered from mammary tumors are a mixed population with a gene expression profile and a multi-organ metastatic phenotype that recapitulate those of various site-selective metastatic entities present in the MDA231 cell population. Bone, lung or brain metastatic subpopulations that can be segregated from each other in experiments of organ-specific metastases (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a), emerged as a mixed population when we selected for tumor-seeding cells. If seeding selects for highly aggressive segments of a CTC population, then seeding may foster the expansion of potentially metastatic populations in the compatible soil of the primary tumor.
Tumor self-seeding in mice carrying a large load of CTCs was not accompanied with de novo tumor formation in orthotopic sites (mammary glands or skin), suggesting that self-seeding requires tumor-derived attraction signals. Our evidence points at IL-6 and IL-8 as tumor-derived attractants of CTCs in breast carcinoma and melanoma models. IL-6 and IL-8 have been implicated in several tumorigenic processes including cancer cell chemoattraction (Arihiro et al., 2000; Wang et al., 1990; Waugh and Wilson, 2008). High serum levels of IL-6 indicate poor prognosis in breast, colon, and lung cancer (Esfandi et al., 2006; Knupfer and Preiss, 2007; Schafer and Brugge, 2007), and high expression of IL-8 in metastatic melanoma is associated with tumor load (Scheibenbogen et al., 1995; Ugurel et al., 2001). Inflammatory cells recruited to the tumor site can also be sources IL-6 (Balkwill et al., 2005; Grivennikov et al 2009). Thus, stroma-derived and cancer cell-derived factors may function in combination to attract CTCs back to a primary tumor.
The superior ability of aggressive cancer cells to infiltrate a tumor in response to this attraction argues that self-seeding also requires infiltration functions on the part of the CTCs. We show that MMP1 and fascin-1 expressed by breast cancer cells act as mediators of trans-endothelial migration and tumor seeding. Expression of MMP1 and FSCN1 in estrogen receptor-negative (ER−) breast tumors is associated with relapse to lungs and brain (Bos et al., 2009; Minn et al., 2005a). Our present and previous results (Gupta et al., 2007) are consistent with roles of MMP1 and fascin-1 in cancer cell extravasation– extravasation into distant organs for the development of metastases but also, as our present results suggest, extravasation into the tumor of origin.
The mediators of seed attraction and tumor infiltration involved in self-seeding may well be different depending on the tumor type. For example, although IL-6 secretion occurs in ER− breast cancer cells, no IL-6 expression is detected in various ER+ breast cancer cell lines (Sasser et al., 2007) or in A375 melanoma. Similarly, MMP1 and FSCN1 expression is associated with distant relapse in patients with ER− breast cancer but not with ER+ breast cancer (Minn et al., 2007; Minn et al., 2005a). In principle, seed-attracting signals could include chemoattractants secreted by tumor cells and/or by inflammatory cells, and tumor infiltration could involve any mediator of extravasation expressed in CTCs.
The interaction of aggressive cancer cells with the tumor stroma results in the release of signals that foster tumor growth, angiogenesis, invasion, and metastasis. These signals prominently include factors that recruit and activate inflammatory cells. Therefore, to the extent that tumor self-seeding recaptures highly aggressive segments of a CTC population it may result in a further enhancement of tumor growth through the action of seed-derived signals. Indeed, the seeding pattern of recipient tumors in our experiments was typically uneven and diffuse, with the seeding cells remaining a minority that mingled with resident cancer cells and tumor stroma. The seeding cells did not have an intrinsic proliferative advantage over the bulk population. Yet, seeded MDA231 mammary tumors grow faster, an increase that is not fully explained by the added mass of the seeder cells. Enhanced angiogenesis and increased recruitment of neutrophils and macrophages accompanied the seeded areas of these tumors, and seeder-derived CXCL1, which is another marker of poor prognosis in ER− breast cancer (Minn et al., 2007; Minn et al., 2005a), was partly responsible for this recruitment.
It would be premature to conclude at present that enhanced tumor growth is an obligate outcome of tumor self-seeding. The net effect of self-seeding would likely depend on variables such as the ratio of the tumor size to the size of the CTC population, the aggressiveness of the CTCs, the vascularity of the tumor, tumor micro-architecture and other factors that may change in the course of the disease. Self-seeding may provide harbor in a primary tumor for the expansion of cancer cell subpopulations that are primed for metastasis. As the shedding and attraction of CTCs by a tumor mass is a dynamic process, it is also conceivable that the presence of a substantial tumor mass could transiently decrease the load of aggressive cells in the circulation owing to their recapture by the tumor.
The present evidence provides clues that could elucidate certain enigmas in clinical oncology. The long-established association of large primary tumor size with poor prognosis in many types of cancer, thought to reflect the ability of larger cancers to release more cells of metastatic potential, may in addition reflect the ability of such aggressive cells to self-seed, promoting local-regional growth, acting in turn as a locus of expansion of these cells and priming for distant metastases. Similarly, the association of anaplasia with poor prognosis may be because micro-anatomical disorganization is a consequence of—and hence a marker of—assertive self-seeding. The hypervascularity of many cancers—and the association of such hypervascularity with poor prognosis—may similarly be explained. Our observation that a mammary tumor can be seeded by CTCs derived from lung metastatic nodules raises the possibility of reseeding after tumor excision as a potential cause of eventual local recurrence. Moreover, that the phenomenon of self-seeding is hereby linked to tumor-specific, and circulating cell-specific factors may create opportunities for the development of targeted therapies for the attrition of residual neoplastic cells from the breast and other organs.
EXPERIMENTAL PROCEDURES
Additional methods can be found in the Supplemental DATA
Animal studies
All animal work was done in accordance with a protocol approved by the MSKCC Institutional Animal Care and Use Committee. Xenografts were performed on BALB/c nude, Athymic nu/nu (for reseeding experiments from the circulation) and NOD/SCID mice (for contralateral seeding experiments) age-matched between 5–8 weeks. Wild type BALB/c mice were used for studies in syngeneic model. For contralateral-seeding experiments, typically 5×105 unlabeled and GFP/Luciferse/TK expressing tumor cells, unless noted, were re-suspended in a 1:1 mixture of PBS and growth-factor-reduced Matrigel (BD Biosciences) and injected into contralateral No. 2 mammary glands (MDA231) or into flanks of NOD/SCID mice (A375 and SW 620) in a total volume of 50 µl. At necropsy, recipient tumors were extracted and imaged for luminescent signals with a Xenogen IVIS system. For experiments with CN34.BrM2, mice were intraperitoneally injected with etoposide (30mg/kg dissolved in DMSO, Sigma) 2 days prior to mammary gland injection. For reseeding experiments from the circulation, 2.5 × 105 and 5 × 105 unlabeled parental MDA231, 67NR and A375 cells were injected into a mammary gland No.2 or subcutaneously, respectively. Once the tumors became palpable (50–100mm3), 1×105 GFP/luiferase expressing MDA231-LM2, 4T1 or A375-BoM2 cells injected into the cardiac left ventricle in a total volume of 100 ul. Reseeding was monitored by bioluminescent imaging (BLI). Recipient tumors were extracted and imaged at the necropsy, typically 6–7 weeks after mammary gland injection. MMTV-PyMT transgenic mice in which mammary tumors are developed by the MMTV promoter-driven expression of the polyoma middle-T oncogene were used for reseeding experiment from the circulation. Cells derived from one such tumor were transduced with lentivirus encoding GFP/luciferase/TK and used for intracardiac injection. Tumors were harvested and imaged after 3 weeks. For reseeding from lung metastases, 2 × 105 MDA231-LM2 cells were injected into the lateral tail vein. After three weeks, 5 × 105 unlabeled parental MDA-MB-231 cells were injected into a mammary gland No. 2. After 3–4 weeks, tumors were extracted and imaged. Relative numbers of CTC from these mice were analyzed as previously described (Gupta et al., 2007) except that primers against luciferase were used to detect CTCs. Experimental metastasis assays and calculation of tumor size were done as previously described (Bos et al., 2009; Kang et al., 2003; Minn et al., 2005a).
In vivo selection of seeder cells
One million unlabeled and GFP/Luciferase/TK expressing parental MDA-MB-231 or A375 cells were injected as in contralateral seeding experiments. Fifty days after the injection, recipient tumors were extracted and imaged by BLI as previously described (Minn et al., 2005a). Two independent seeded tumors by MDA-MB-231 cells and one seeded tumor by A375 cells were minced and centrifuged in PBS containing antibiotics. Samples were then resuspended in DMEM containing 0.125% collagenase III and 0.1% of hyaluronidase and incubated at 37°C for 2.5 hours with occasional trituration. Subsequently, samples were trypsinized for 5 min, followed by centrifugation in DMEM with 10% FBS. Cells were filtered through a 40µm strainer and plated in a T75 flask with DMEM containing 10% FBS. Cells were expanded in culture for two passages and GFP-positive cells were isolated by fluorescence-activated cell sorting (FACS).
Trans-well migration assays
To generate conditioned media, 10^6 cells were plated on 6cm dish. The next day media was replaced with 0.2% FBS media without growth factors. After two days, media was collected, centrifugated, and used in trans-well migration assays as described previously (Gupta et al., 2007). Recombinant human IL-6 and IL-8 were purchased from R&D systems.
Cytokine antibody array
Cytokine antibody array was performed with conditioned media from MCF10A, MDA231, MDA231-S1a, MDA231-S1b, MDA231-LM2, MCF7, HaCat, A375, and A375-BoM2 according to manufacturer’s protocol. The complete array maps (Array 5, 9, and 10) can be found in http://www.raybiotech.com/map_all_m.asp#8.
Statistical analysis
For trans-well migration assays and animal studies in which a value is quantified relative to a normalized standard, standard deviation was calculated based on the following formula:
SEM was then calculated by using sd{f/g} /√ n.
Supplementary Material
Acknowledgments
We would like to thank F. Miller for cell lines, T. Shree for MMTV-PyMT mice, P. Bos, and S. Tavazoie for critical discussions, and D. Padua, G. Gupta and A. Insinga for contributions during the initiation of this work. This work was funded by grants from the National Institutes of Health (CA94060), the Hearst Foundation, and the Alan and Sandra Gerry Metastasis Research Initiative. M.Y.K. is supported by a Department of Defense Era of Hope postdoctoral fellowship. J.M. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
Gene expression data of S1 cells are deposited at GEO (GSE18833).
References
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590–596. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Arihiro K, Oda H, Kaneko M, Inai K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer. 2000;7:221–230. doi: 10.1007/BF02967464. [DOI] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- Balasubramanian S, Eckert RL. Keratinocyte proliferation, differentiation, and apoptosis--differential mechanisms of regulation by curcumin, EGCG and apigenin. Toxicol Appl Pharmacol. 2007;224:214–219. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69:4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H, Tonks NK, Walsh KA, Fischer EH. The leukocyte common antigen (CD45): a putative receptor-linked protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1988;85:7182–7186. doi: 10.1073/pnas.85.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9–18. [PMC free article] [PubMed] [Google Scholar]
- Eckert RL, Rorke EA. Molecular biology of keratinocyte differentiation. Environ Health Perspect. 1989;80:109–116. doi: 10.1289/ehp.8980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Esfandi F, Mohammadzadeh Ghobadloo S, Basati G. Interleukin-6 level in patients with colorectal cancer. Cancer Lett. 2006;244:76–78. doi: 10.1016/j.canlet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol. 2005;37:1787–1804. doi: 10.1016/j.biocel.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of CXC chemokine receptor 2 in t he homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circ Res. 2007;100:590–597. doi: 10.1161/01.RES.0000259043.42571.68. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Smith CA, Salhia B, Rutka JT. The role of fascin in the migration and invasiveness of malignant glioma cells. Neoplasia. 2008;10:149–159. doi: 10.1593/neo.07909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102:129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, Kreike B, Zhang Y, Wang Y, Ishwaran H, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–6745. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005a;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massague J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005b;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Norton L, Massague J. Is cancer a disease of self-seeding? Nat Med. 2006;12:875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- Rafii S, Avecilla ST, Jin DK. Tumor vasculature address book: identification of stage-specific tumor vessel zip codes by phage display. Cancer Cell. 2003;4:331–333. doi: 10.1016/s1535-6108(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21:3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Onder T, Karnoub A, Weinberg RA. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 2007;67:11476–11479. doi: 10.1158/0008-5472.CAN-07-1653. [DOI] [PubMed] [Google Scholar]
- Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5:179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel WT, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell. 2008;13:441–453. doi: 10.1016/j.ccr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, Ben-Ze'ev A, Robine S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- Wang JM, Taraboletti G, Matsushima K, Van Damme J, Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990;169:165–170. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.