Abstract
Natural killer T (NKT) cells play a pivotal role in maintaining immune homostasis. They recognize lipid antigen in the context of CD1d molecules and subsequently produce cytokines that activate cells of both the innate and adaptive immune responses. Many studies examining patients with autoimmune disease or cancer have shown that there is a reduction in both NKT cell number and function. Due to the complexities of manipulating NKT cells in vivo, ex vivo expanded effector NKT cells would be an excellent therapeutic modality. To date, immunotherapy utilizing the NKT/CD1d system has been dependent on the use of autologous DC in the presence or absence of a synthetic glycolipid, α-galactocylceramide. Here we report a novel technique that facilitates the growth and analysis of NKT cells through the use of CD1d-expressing aAPC. CD1d-based aAPC can effectively propagate both canonical (iNKT cells) and noncanonical (Vα14−) NKT cells. Importantly, CD1d-Ig aAPC can expand NKT cells from cancer patients. Thus, CD1d-expressing aAPC will enhance our knowledge of NKT cell biology and could potentially be used as a novel tool in adoptive immunotherapeutic strategies.
Keywords: NKT cells, aAPC, CD1d
1. Introduction
Numerous studies have reported that circulating numbers of NKT cells are reduced in cancer patients (Kawano et al., 1999; Tahir et al., 2001; Fujii et al., 2003). In fact, a recent study examining a large cohort of cancer patients and healthy controls has shown that circulating NKT cell numbers were 47% lower in cancer patients compared to age and gender matched healthy controls (Molling et al., 2005). This reduction in NKT cell numbers was independent of tumor type or tumor load. It was shown that even after effective removal of the tumor by surgery or radiotherapy NKT cell numbers were not restored to a normal level. In addition, in two phase I clinical trials, patients injected with either α-GalCer (Giaccone et al., 2002) or α-GalCer loaded immature dendritic cells (Nieda et al., 2004), the immune system was activated, but only in patients with detectable NKT cell numbers. More recently, Chang and colleagues showed that multiple injections of α-GalCer loaded mature dendritic cells lead to sustained expansion of NKT cells and antigen specific T cells (Chang et al., 2005). However, these expanded NKT cells from cancer patients still exhibited reduced capacity for IFN-γ secretion compared to NKT cells from healthy controls. These studies show that cancer patients have a deficiency in both NKT cell number and function, which suggests that in vivo NKT cell modulation would be ineffective and suggest that adoptive immunotherapy by ex vivo expansion of effector NKT cells could be a more productive strategy.
The potential to utilize NKT cells for therapeutic purposes has significantly increased with the ability to stimulate and expand human NKT cells with α-GalCer and a variety of cytokines. One report has shown that α-GalCer stimulated NKT cells can be expanded in a cytokine supplemented media (Harada et al., 2005). Importantly, these cells retained their original phenotype, secreted cytokines, and displayed cytotoxic function against tumor cell lines. These data demonstrate that ex vivo expanded NKT cells remain functional and therefore can be used for adoptive immunotherapy.
Immunotherapy utilizing the NKT/CD1d system has been limited by the use of autologous antigen presenting cells in the presence or absence of α-GalCer. The quantity and quality of these stimulator cells can vary substantially. For example it has been shown that monocyte-derived DC from cancer patients, express reduced levels costimulatory molecules and produce less inflammatory cytokines (Bella et al., ; Onishi et al., 2002). Therefore, Shimizu, et al. recently reported using murine DC rather than autologous APC to test the function of NKT cells from CML patients (Shimizu et al., 2006). However, this system can only be used for in vitro testing since NKT cells cannot be expanded by murine DC and then given back to patients. A standardized system that relies on artificial Antigen Presenting Cells (aAPC) could produce the stimulating effects of DC without the pitfalls of allo- or xenogeneic cells.
Development of a non-cellular aAPC is important for its potential clinical value to expand ex vivo antigen specific T cells as part of an adoptive immunotherapy regimen, as well as its ability to characterize basic requirements for T cell activation. Our laboratory has developed MHC-Ig based aAPC, which have been shown to effectively expand CMV and MART-1 specific CTL (Oelke et al., 2003). In the present study we have utilized this concept and developed CD1d-Ig based aAPC, which can be used to replace autologous α-GalCer pulsed antigen presenting cells to generate effector NKT cells. Our data demonstrate that CD1d-Ig based aAPC can effectively propagate NKT cells from both healthy controls as well as cancer patients.
2. Materials and Methods
2.1. Peripheral Blood Mononuclear Cells (PBMC)
PBMC were isolated by Ficoll-Hypaque (Amersham Pharmacia Biotek, Uppsala, Sweden) density gradient centrifugation. In the initial studies CD3+ primary human T cells were isolated from the PBMC of healthy volunteers and ovarian cancer patients using the human Pan T cell isolation kit (Miltenyi). In later studies, CD3+CD161+ human T cells were isolated using the Pan T cell kit from Miltenyi, then the T cell enriched fraction was incubated with allophycocyanin-labeled CD161+ mAb (Pharmingen) (100µl/108 cells) for 20 min at 6–12°C, washed and then incubated with anti-mouse IgG1 microbeads (Miltenyi). All donors gave written informed consent before enrolling in the study. The Institutional Review Board of Johns Hopkins Medical Institutions approved this investigation.
2.2. Mice
Wild-type C57BL/6 mice were purchased from The Jackson Laboratory for analyses of liver mononuclear cells (MNC). All animal procedures were approved by the Johns Hopkins University School of Medicine’s Animal Care and Use Committee. Isolation of liver MNC was performed as described previously (Tupin and Kronenberg, 2006). In brief, hepatic portal veins were perfused with PBS, and then the livers were removed from mice and placed in PBS buffer containing 2% FBS and 0.02% sodium azide on ice. They were then minced and pressed through nylon cell strainers (70 micron, Falcon) and the resulting homogenate was resuspended in PBS buffer described above. Following centrifugation at 200 × g for 10 min at 4°C, cell pellets were resuspended in 25 ml of 37% Percoll solution in 50-ml conical tubes and centrifuged at 700 × g for 12 min at room temperature with the rotor brake on. The resulting cell pellet containing the MNC was treated with a RBC lysis solution for 2 min at room temperature, washed twice in PBS buffer, and resuspended in PBS buffer. Cell number and viability were determined by trypan blue dye exclusion.
2.3. Cell Lines
The Vα14+ NKT cell hybridoma cell lines DN32.D3, N38-2C12, N38-2H4, N38-3C3, and the CD1d-specific NKT cell hybridoma N37-1A12 (Vα5+), have all been described (Lantz and Bendelac, 1994; Brutkiewicz et al., 1995) (Burdin, 1998 #2) and were cultured in IMDM medium supplemented with 5% FBS and 2 mM l-glutamine.
2.4. Generation of artificial Antigen Presenting Cells
The preparation of CD1d-Ig based aAPC was performed according to the previously described method (Oelke et al., 2003) with minor modifications. Briefly, to conjugate CD1d-Ig dimer molecules to beads, 50 µg of CD1d-Ig (DimerXI; BD Biosciences) was added to 0.5 ml of washed epoxy beads (Dynabeads, M-450, Epoxy, 4×108 beads/ml) (Dynal) in sterile 0.1M Borate buffer, pH 7.0–7.4. The bead protein mixture was mixed with rotation and incubated for 24h at 4°C. Then the beads were washed twice with rotation in bead wash buffer and the protein expression was assessed by flow cytometry. All antibodies were purchased from BD Pharmingen, except PE-anti mouse IgG1 (CalTag). Then CD1d-Ig molecules were loaded with 5 µg/ml of either α-GalCer (purchased from Axxora, LLC) or OCH (kindly provided by Dr. Zhiping Li, Johns Hopkins School of Medicine) in 1 ml PBS containing 5 × 107 beads. CD1d-aAPC beads were stored at 4 °C for more than 3 months with no loss in activity. In the experiments examining costimulatory molecules 50µg of CD1d-Ig was bound either in the presence of 20µg mAb specific for CD28, CD44 or CD69 (BD Pharmingen) or in the absence of mAb. Beads without costimulatory mAb contained 70µg mCD1d-Ig to keep the total protein concentration constant.
2.5. Expansion of Primary human NKT cells
CD3+ T lymphocytes were enriched from PBMC using a Pan T cell isolation kit (Miltenyi). The resulting population, consisting of >90% CD3+ T cells, was used as responder cells and stimulated with α-GalCer-loaded hCDd1-Ig based aAPC. In brief, responder cells (104 cells/well) were co-cultured with 104 α–GalCer-loaded aAPC per well in a 96-well round-bottom plate in 200 µl/well complete RPMI medium (RPMI 1640 Medium, non-essential amino acids [Sigma-Aldrich], sodium pyruvate [Gibco, Invitrogen Corporation], vitamin solution [Gibco], 2-mercaptoethanol [Gibco], 10 µM ciprofloxacin [Serologicals Proteins Inc], 10% fetal calf serum [HyClone], and supplemented with 5% autologous plasma and 3% T-cell growth factor (TCGF) as previous described. No additional allogeneic feeder cells were used for induction or expansion of primary NKT cells, unless indicated. Medium and TCGF were replenished twice a week. On day 7 and weekly thereafter, NKT cells were collected, counted and re-plated at 104 NKT cells per well, together with 104 fresh α–GalCer-loaded aAPCs per well in complete medium supplemented with 3% TCGF.
3 Results
3.1. Generation of artificial Antigen Presenting Cells (aAPC)
Recent studies have shown that antigen-loaded CD1d-Ig fusion proteins can be used to characterize specific NKT cell populations (Sriram et al., 2005; Park et al., 2008). Here we first assessed the ability of α-GalCer loaded human CD1d-Ig to bind to human NKT cells. CD1d-Ig fusion proteins were loaded with α-GalCer, then purified Vα24+Vβ11+ human NKT cells were stained with either unloaded or loaded CD1d-Ig molecules. As shown in Supplemental Fig 1A, we found that α-GalCer loaded CD1d-Ig molecules bind specifically to canonical human NKT cells. We next tested the ability of α-GalCer loaded CD1d-Ig to stimulate a panel of well-characterized murine NKT cell hybridomas (Lantz and Bendelac, 1994; Bendelac et al., 1995; Burdin et al., 1998; Roberts et al., 2002). The recognition of human CD1d molecules by murine NKT cells has been reported (Burdin et al., 1998; Naidenko et al., 1999) and could be useful for optimizing our system. Therefore, plates were coated with α-GalCer loaded human CD1d-Ig molecules. We then examined the ability of these molecules to stimulate murine NKT cell lines. While all of the NKT cell lines responded non-specifically to the anti-CD3 coated plates, we found that α-GalCer loaded CD1d-Ig only stimulated the canonical Vα14+ NKT cell lines DN32.D3 and N38-3C3 (Supplemental Fig. 1B), but not the noncanonical, Vα5+ CD1d-specific cell line, N37-1A12 as previously reported (Burdin et al., 1998). Collectively, our data demonstrate that CD1d-Ig molecules bind to and stimulate NKT cells in an antigen-specific manner.
CD1d-Ig based aAPC were generated by coupling CD1d-Ig onto magnetic beads. For our initial prototype we used CD1d-Ig alone or in combination with anti-CD28 mAb (see Fig. 1A for aAPC schematic). To verify that CD1d1-Ig (Isotype IgG1) molecules and anti-CD28 (Isotype IgG2a) were indeed stably immobilized onto the surface of the magnetic beads we stained them with anti-mouse IgG1 or IgG2a antibodies respectively and analyzed by flow cytometry. As shown in Figure 1B, we found that CD1d-Ig and anti-CD28 antibodies were both detected on the surface of the magnetic beads. Moreover, when we examined the levels of CD1d in the presence and absence of anti-CD28 antibodies there were no detectable differences in amount of CD1d-Ig bound (data not shown). We further confirmed the presence of CD1d moiety using anti-CD1d antibodies (data not shown) which verified that the chimeric CD1d-Ig was intact.
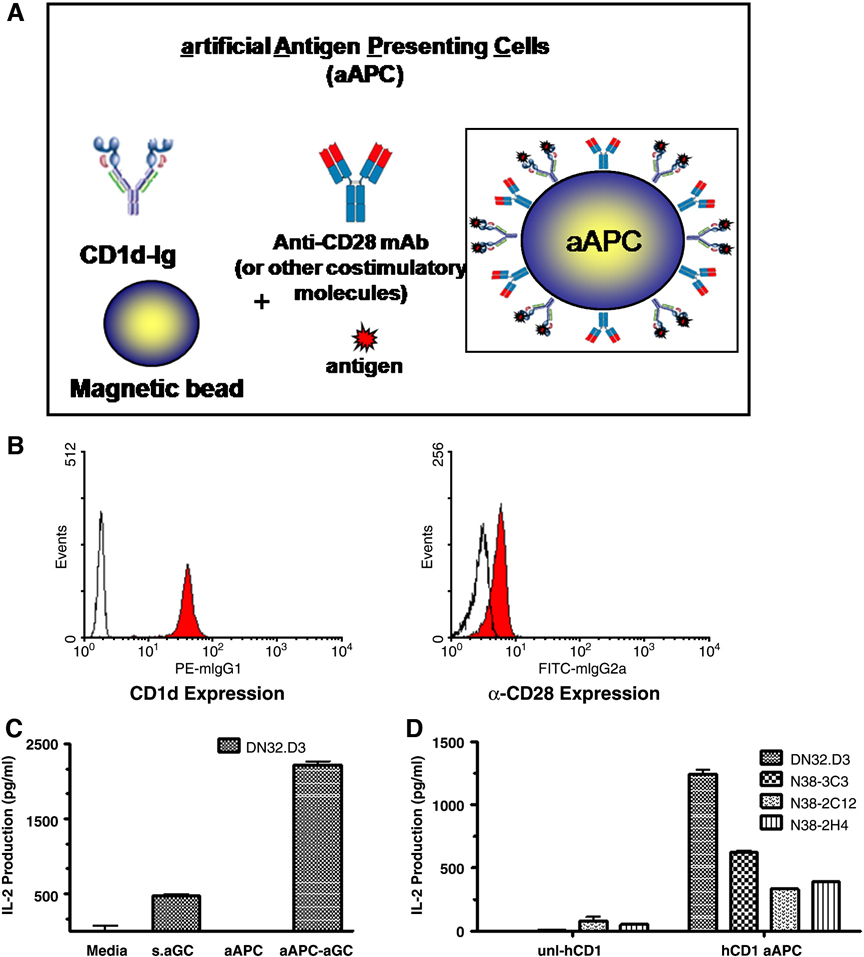
Fig. 1.
Schematic diagram of CD1d-based aAPC. (A) In this system, CD1d-Ig is used to provide the cognate antigen specific signal through the TCR (signal 1) and anti-CD28 Abs or other costimulatory molecules provide signal 2. These molecules can be easily loaded with lipid antigens, such as α-GalCer, simply by incubating them with an excess of the lipid of interest. (B) aAPC were analyzed for surface expression of CD1d-Ig and anti-CD28 by flow cytometry. Open histograms indicate isotype control; filled histograms represent the indicated antibodies. (C) DN32.D3 (5×104) NKT cell hybridomas were cocultured with media, soluble α-GalCer (100 ng/ml), or aAPC (2.5 × 105) in 96-well U- bottom plates for 20–24hr. Culture supernatants were harvested and standard sandwich ELISA was used to measure IL-2 production. (D) human CD1d-Ig Expressing aAPC can Stimulate IL-2 Production by NKT cells. Unloaded or α–GalCer loaded human CD1d-based aAPC were cocultured with the indicated Vαl4+ NKT cell hybridomas. Culture supernatants were harvested and standard sandwich ELISA was used to measure IL-2 production.
To determine whether murine CD1d-based aAPC were functional we stimulated the well characterized NKT cell hybridoma, DN32.D3 with the CD1d-Ig based aAPC and compared the level of activation to treatment with soluble antigen alone. aAPC stimulation resulted in a significant increase in NKT cell activation (Figure 1C), as determined by IL-2 released. We next examined whether aAPC consisting of human CD1d-Ig molecules loaded with α-GalCer could stimulate mouse NKT cells. Murine NKT cell lines have been reported to recognize human CD1d molecules (Burdin et al., 1998; Naidenko et al., 1999). The use of the easily maintained mouse NKT cell lines to optimize our system would be advantageous. As shown in Fig 1D, murine NKT cell lines were stimulated by the aAPC expressing human CD1d-Ig loaded with α-GalCer. These data demonstrate that mouse NKT cell lines can be stimulated by human CD1d molecules. Thus, we are able to use the well- characterized mouse NKT lines for quality control experiments on different batches of CD1d-Ig based aAPC. Taken together, these data suggest that aAPC utilizing CD1d1-Ig dimers provide high affinity, divalent interactions that can trigger TCR crosslinking.
3.2. The Function of Co-stimulatory Molecules can be Assessed using aAPC
CD1d-based aAPC can stimulate NKT cells, thus they can be used as a tool to determine the effect of costimulatory molecules on NKT cell activation. In these studies, the NKT cell population (CD1dtetra+ CD3+) was characterized for the expression of CD4, CD44 and CD69, thus freshly isolated mononuclear cells were harvested from the liver and spleen of C57BL/6 mice, stained and analyzed by flow cytometry (Supplemental Figs. 2A & B). We found that both CD4+ and CD4− NKT cells express CD44 and CD69.
Given the relatively high expression of CD44 and CD69, and the study by Larkin, et al. (Larkin et al., 2006) identifying CD44 as a co-stimulatory molecule, aAPC were generated to express either mouse CD1d-Ig alone or in combination with either anti-CD28, anti-CD44, or anti-CD69, using equal amounts of protein on all aAPC. CD1d-Ig aAPC were generated, loaded with α-GalCer and the aAPC were cocultured with NKT cell hybridomas. As shown in Figs. 2A & B, a modest increase was observed in the presence of anti-CD28, while little or no effect was observed with anti-CD69. However, the addition of anti-CD44 resulted in a 2.5–4 fold increase in cytokine production by the NKT cell hybridomas. In the next set of studies we used a homologue of α-GalCer, OCH, which has been shown to induce a Th2 cytokine bias compared to stimulation with α-GalCer (Miyamoto et al., 2001). When we examined the stimulatory capacity of OCH-loaded aAPC, the addition of anti-CD44 and anti-CD28 resulted in an increase in cytokine production; however, anti-CD69 abrogated NKT cell activation (Figs. 2C & D).
Fig. 2.
Expression of costimulatory molecules on aAPC. (A) DN32.D3 and (B) N38-3C3 NKT cells were cocultured with α-GalCer loaded aAPC. (C) DN32.D3 and (D) N38-3C3 NKT cells were cocultured with OCH loaded aAPC. (E) Primary NKT cells were cocultured with aAPC and IL-13 production or was measured. (F) Primary NKT cells were cocultured with aAPC and IL-4 production assessed. In these experiments, NKT cells were cocultured with media, soluble antigen (100 ng/ml), or aAPC (2.5 × 105) for 20–24hr. Culture supernatants were harvested and standard sandwich ELISA was used to measure IL-2, IL-13, or IL-4 production.
The expression of co-stimulatory molecules is not essential for the activation of hybridomas; therefore, it is possible that the presence of these molecules will have a greater effect on the activation status of primary NKT cells. Therefore, freshly isolated mononuclear cells were harvested from the livers of C57BL/6 mice. The NKT cell population (NK1.1+ CD3+) was purified and co-cultured with the panel of aAPC. As shown in Figs. 2E & F, compared to stimulation with CD1d-Ig alone, the costimulatory molecules enhanced cytokine production. Notably, anti-CD28 and CD44 significantly increased NKT cell activation.
3.3. CD1d-based aAPC can be used to expand NKT cells from healthy controls and cancer patients
To explore the potential of aAPC as a novel method for expanding primary human NKT cells, we obtained PBMC from several healthy volunteers and cancer patients. Bulk CD3+ T cells were isolated and stimulated with human CD1d-based aAPC in weekly cycles. As shown in Table 1, we found that α-GalCer loaded aAPC were able to expand the NKT cells population in both groups up to 120 fold. Notably, the percentage of NKT cells varied significantly in each individual, as has been reported in the literature. As expected a high initial population of Vα24+ cells, resulted in higher percentages following expansion. Importantly, we have demonstrated that CD1d-Ig based aAPC can be used to expand NKT cells from healthy controls and cancer patients.
Table 1.
aAPC mediated Expansion of NKT cells
| Initial Percent Positive (Vα14+) |
Following α–GalCer- aAPC stimulation |
Fold Expansion |
||
|---|---|---|---|---|
| Healthy Controls | 1 | 0.53 | 7.83 | 15.6 |
| 2 | 0.16 | 5.56 | 34.75 | |
| 3 | 0.01 | 0.09 | 9 | |
| Cancer Patients | 1 | 0.95 | 13.83 | 14.5 |
| 2 | <0.01 | 0.92 | 92 | |
| 3 | <0.01 | 12 | 120 |
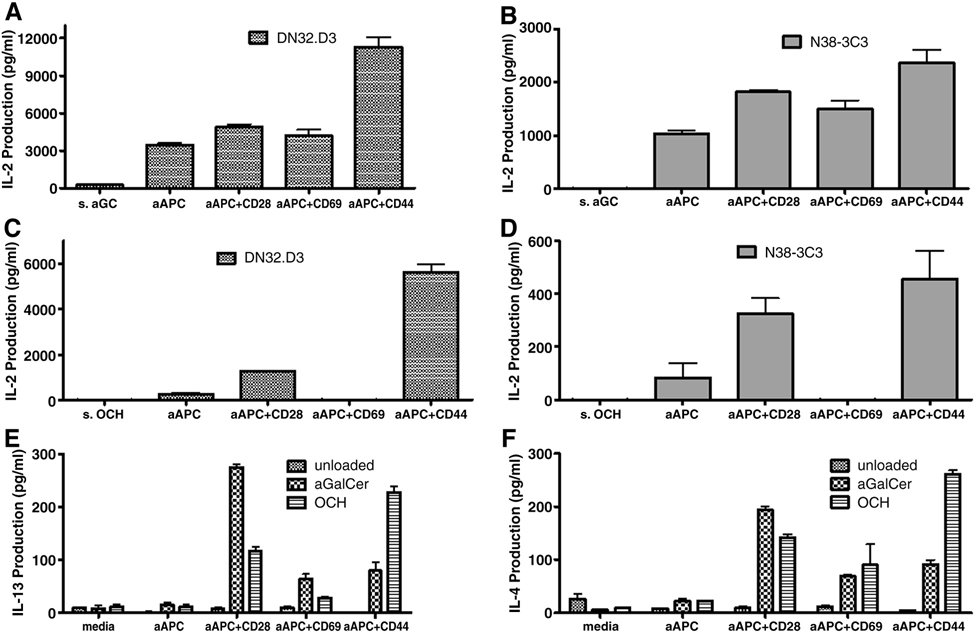
We also pulsed bulk irradiated autologous PBMC with α–GalCer to compare expansion rates between autologous stimulators and aAPC. In these studies, the CD3+ T cell population was enriched and the CD3− population was pulsed with a-GalCer (100ng/ml) and irradiated. Then the T cells were either stimulated with irradiated, α–GalCer- pulsed autologous PBMC (1:10 ratio) or with α–GalCer- loaded aAPC (1:1 ratio). The initial population of NKT cells (Vα24+Vβ11+) 0.16% increased to 7.21% following stimulation with α–GalCer- pulsed autologous PBMC, compared to stimulation with α–GalCer- loaded aAPC- 5.24%. Although the percent expansion was comparable between the groups, the total number of NKT cells was much higher in the group stimulated with aAPC due to a large expansion of the NKT cell culture (Figure 3).
Fig. 3.
CD1d-based aAPC can be used to expand human NKT cells. T cells (CD3+)were isolated from PBMC using magnetic bead separation. The cells were stimulated weekly with irradiated PBMC pulsed with 100ng/ ml α-GalCer (10:1 ratio) or a-GalCer loaded aAPC± anti CD28 (1:1 ratio). Data shown are after 2 weeks of stimulation.
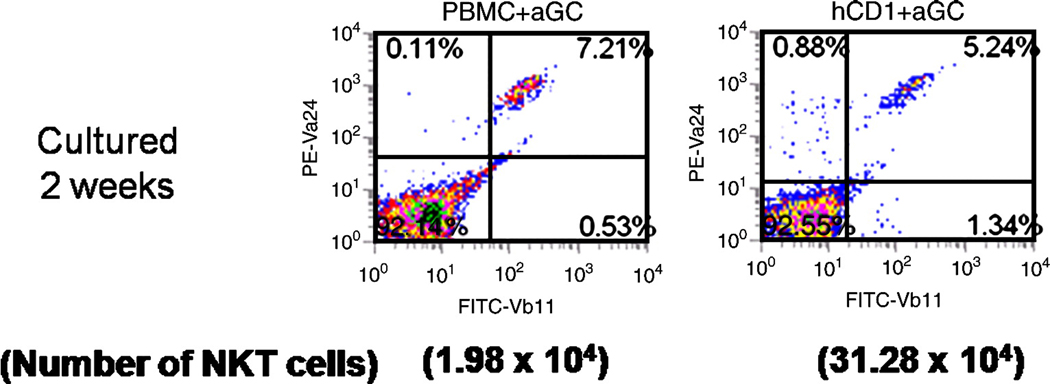
To further optimize our system, specifically to increase both the percentage and number of NKT cells we attempted to enrich for the initial percentage of NKT cells. After isolation of T cell population, the cells were stained with an anti-CD161 mAb. Anti-mouse IgG1 microbeads were used to separate the CD3+CD161+ cells. Although the NKT cell population (Vα24+Vβ11+) was still relatively low (<1%), after two weeks of stimulation with aAPC the percentage was approximately 60% , representing a 1200 fold expansion. Thus, the initial manipulation to enrich the iNKT population was helpful in generating an enhanced post-stimulation specific population. Overall, these studies demonstrate a novel technique to selectively activate and propagate NKT cells.
DISCUSSION
In this study, we have developed CD1d-Ig based aAPC, which can be used for the activation and expansion of NKT cells. It has been reported that soluble and plate bound forms of CD1d molecules loaded with lipid antigen are directly able to target NKT cells in vitro (Naidenko et al., 1999; Schumann et al., 2003; Sriram et al., 2005; Vasan et al., 2007). The physical interaction of NK cells with CD1d1 (Huang et al., 2004), but not NKT cell has been examined using a bead system. Since the engagement of the T cell receptor (TCR) by the CD1d-antigen complexes is a fundamental requirement of NKT cell activation, antigen:CD1d-Ig complexes, possibly along with appropriate costimulatory molecules, potentially offer a reliable method to isolate, activate, and expand effector NKT cell populations. Here, we have demonstrated that CD1d-Ig based aAPC, made by covalent coupling of CD1d-Ig and potential co-stimulatory molecules to magnetic beads, can be used as a standardized method for the propagation of NKT cells. Importantly, our system allows us to expand the NKT cell population from the PBMC of cancer patients. Moreover, our studies could have wide ranging applications because our system can be further modified to meet clinical standards so that the expanded cells can be given back to patients.
From this as well from our previous studies (Schutz et al., 2008; Durai et al., 2009), we know that our dimer based aAPC system can be used to evaluate different culture conditions, co-stimulatory- and death-signals. Furthermore, it allows for antigen-specific targeting of different immune cells. This is important because we have found, as have others, that some patients have barely detectable numbers of NKT cells (Table 1 and data not shown). In fact in a recent study (Croudace et al., 2008), examining the in vitro expansion of 25 healthy donors, it was found that growth rates were highly variable and did not correlate with age, gender, basal circulating levels or CD1d- ligand used.
To investigate significant decrease in the circulating NKT cell population in cancer patients, some preliminary studies were conducted. We examined whether PBMC from cancer patients were able to stimulate mouse NKT cell hybridomas at levels comparable to normal volunteers (data not shown). We found that when NKT cell hybridomas were cocultured with PBMC from three normal controls and one breast cancer patient, the PBMC from normal controls were able to stimulate the NKT cell hybridomas, however the PBMC from the breast cancer patient did not stimulate any of the NKT cells above background. Thus, it is possible that the low frequency of NKT cells in cancer patients may be caused by a lack of stimulation. Furthermore, it suggests that in contrast to CD1d-based aAPC, autologous DC from cancer patients may not offer the optimal level of stimulation. The lack of stimulation provided by cancer patient DC may explain why previous studies have found the absence of a significant in vivo response following either direct α-GalCer injections or adoptive transfer of autologous α-GalCer pulsed DC. In fact, Imataki and colleagues recently reported similar findings (Imataki et al., 2008).
We found that CD1d-Ig based aAPC can be used as a tool to examine the effect of specific costimulatory signals on NKT cell activation. As shown in Fig. 2C & D, we found that anti-CD44 had a strong costimulatory effect on the NKT cell hybridomas. A modest increase was observed in the presence of anti-CD28, while little or no effect was observed with anti-CD69. However, anti-CD28 had strong co-stimulatory effect on primary liver NKT cells, compared to stimulation with aAPC expressing anti-CD44 and anti-CD69. This could be a result of tissue specific differences in the NKT cell population, as well as a difference in primary cells and hybridomas. The NKT cell hybridomas used in this study were derived from thymi and the primary cells were isolated from the liver. It will be interesting to see if there are tissue specific differences that determine the co-stimulatory requirements for NKT cells (Lantz and Bendelac, 1994; Brutkiewicz et al., 1995) (Burdin, 1998 #2). In our human NKT cell expansion studies, we found similar percentages of Vα24+Vβ11+ cells (Figure 3) after two weeks of stimulation with autologous PBMC or aAPC; however, the total cell number was much lower in the group stimulated with pulsed autologous PBMC, as compared to aAPC based NKT cell expansion.
In conclusion, we have studied stimulation and expansion of NKT cells in healthy donors and cancer patients and modeled those studies on standard α-GalCer pulsed autologous APC mediated stimulation. We designed an artificial APC which is adaptable to any requirements we find necessary for optimal NKT cell proliferation. When aAPC are compared to standard autologous PBMC-based induction and expansion, the current standard for NKT cell expansion, aAPC compared favorably. Thus, aAPC represent a robust versatile technology useful for inducing and expanding NKT cells. Together these studies demonstrate that the CD1d-Ig based aAPC system has the potential to provide a better understanding of NKT cell biology which may lead to new strategies to enhance current approaches in cancer immunotherapy.
Supplementary Material
Fig. 4.
Expansion of CD3+CD161+ cells using aAPC. CD3+CD161+ cells were isolated from PBMC using magnetic bead separation. The sorted cells were stimulated weekly with α-GalCer loaded-aAPC. The initial population and the expanded population were stained for canonical NKT cells (Vα24+Vβ11+). Data shown are after 2 weeks of stimulation.
Abbreviations used in this paper
- NKT
Natural killer T
- aAPC
artificial Antigen Presenting Cells
- α-GalCer
α-galactosylceramide
- MNC
mononuclear cells
- TCGF
T-cell growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bella SD, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J. Exp. Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croudace JE, Curbishley SM, Mura M, Willcox CR, Illarionov PA, Besra GS, Adams DH, Lammas DA. Identification of distinct human invariant natural killer T-cell response phenotypes to alpha-galactosylceramide. BMC Immunol. 2008;9:71. doi: 10.1186/1471-2172-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durai M, Krueger C, Ye Z, Cheng L, Mackensen A, Oelke M, Schneck JP. In vivo functional efficacy of tumor-specific T cells expanded using HLA-Ig based artificial antigen presenting cells (aAPC) Cancer Immunol Immunother. 2009;58:209–220. doi: 10.1007/s00262-008-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-gamma-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–622. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, Von Blomberg BM, Scheper RJ, Van Der Vliet HJ, Van Den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A Phase I study of the natural killer T-cell ligand α- galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- Harada Y, Imataki O, Heike Y, Kawai H, Shimosaka A, Mori S, Kami M, Tanosaki R, Ikarashi Y, Iizuka A, Yoshida M, Wakasugi H, Saito S, Takaue Y, Takei M, Kakizoe T. Expansion of alpha-galactosylceramide-stimulated Valpha24+ NKT cells cultured in the absence of animal materials. J Immunother. 2005;28:314–321. doi: 10.1097/01.cji.0000163593.66910.ad. [DOI] [PubMed] [Google Scholar]
- Huang MMS, Borszcz P, Sidobre S, Kronenberg M, Kane KP. CD1d1 Displayed on Cell Size Beads Identifies and Enriches an NK Cell Population Negatively Regulated by CD1d1. J Immunol. 2004;172:5304–5312. doi: 10.4049/jimmunol.172.9.5304. [DOI] [PubMed] [Google Scholar]
- Imataki O, Heike Y, Makiyama H, Iizuka A, Ikarashi Y, Ishida T, Wakasugi H, Takaue Y. Insufficient ex vivo expansion of Valpha24(+) natural killer T cells in malignant lymphoma patients related to the suppressed expression of CD1d molecules on CD14(+) cells. Cytotherapy. 2008:1–10. doi: 10.1080/14653240802072747. [DOI] [PubMed] [Google Scholar]
- Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, Akutsu Y, Motohashi S, Iizasa T, Endo H, Fujisawa T, Shinkai H, Taniguchi M. Antitumor cytotoxicity mediated by ligand-activated human Vα24 NKT cells. Cancer Res. 1999;59:5102–5105. [PubMed] [Google Scholar]
- Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. CD44 differentially activates mouse NK T cells and conventional T cells. J Immunol. 2006;177:268–279. doi: 10.4049/jimmunol.177.1.268. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, Scheper RJ, van den Eertwegh AJ. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- Naidenko OV, Maher JK, Ernst WA, Sakai T, Modlin RL, Kronenberg M. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J Exp Med. 1999;190:1069–1080. doi: 10.1084/jem.190.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–625. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- Onishi H, Morisaki T, Baba E, Kuga H, Kuroki H, Matsumoto K, Tanaka M, Katano M. Dysfunctional and Short-Lived Subsets in Monocyte-Derived Dendritic Cells from Patients with Advanced Cancer. Clinical Immunology. 2002;105:286–295. doi: 10.1006/clim.2002.5293. [DOI] [PubMed] [Google Scholar]
- Park J-E, Wu DY, Prendes M, Lu SX, Ragupathi G, Schrantz N, Chapman PB. Fine specificity of natural killer T cells against GD3 ganglioside and identification of GM3 as an inhibitory natural killer T-cell ligand. Immunology. 2008;123:145–155. doi: 10.1111/j.1365-2567.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ, Sriram V, Spence PM, Gui M, Hayakawa K, Bacik I, Bennink JR, Yewdell JW, Brutkiewicz RR. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–5414. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- Schumann J, Voyle RB, Wei B-Y, MacDonald HR. Cutting Edge: Influence of the TCR V{beta} Domain on the Avidity of CD1d:{alpha}-Galactosylceramide Binding by Invariant V{alpha}14 NKT Cells. J Immunol. 2003;170:5815–5819. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- Schutz C, Fleck M, Mackensen A, Zoso A, Halbritter D, Schneck JP, Oelke M. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111:3546–3552. doi: 10.1182/blood-2007-09-113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Hidaka M, Kadowaki N, Makita N, Konishi N, Fujimoto K, Uchiyama T, Kawano F, Taniguchi M, Fujii S. Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J Immunol. 2006;177:3484–3492. doi: 10.4049/jimmunol.177.5.3484. [DOI] [PubMed] [Google Scholar]
- Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- Tupin E, Kronenberg M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006;417:185–201. doi: 10.1016/S0076-6879(06)17014-7. [DOI] [PubMed] [Google Scholar]
- Vasan S, Poles MA, Horowitz A, Siladji EE, Markowitz M, Tsuji M. Function of NKT cells, potential anti-HIV effector cells, are improved by beginning HAART during acute HIV-1 infection. Int Immunol. 2007;19:943–951. doi: 10.1093/intimm/dxm055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.