Abstract
Rationale
Central Ang II inhibits baroreflex and plays an important role in the pathogenesis of hypertension. However, the underlying molecular mechanisms are still not fully understood.
Objective
Our objective in the present study was to characterize the signal transduction mechanism of PI3-kinase involvement in Ang II-induced stimulation of central neuronal activity in cultured neurons and Ang II-induced inhibition of baroreflex in SHR versus WKY rats.
Methods and Results
Application of Ang II to neurons produced a 42% greater increase in neuronal firing in cells from the SHR than the WKY rat. Whilst in the WKY the Ang II-mediated increase in firing rate was abolished entirely by the PKC inhibitor GF109230, it was necessary to block both PKC and PI3K activity in the SHR. This was associated with an increased ability of Ang II to stimulate NADPH-oxidase-ROS mediated signaling involving phosphorylation of the p47phox subunit of the NADPH oxidase and was dependent on the activation of PI3 Kinase in the SHR. Inhibition of PI3 Kinase resulted in the reduction of levels of p47phox phosphorylation, NADPH oxidase activity, ROS levels and ultimately neuronal activity in cells from the SHR but not the WKY rat. In addition, in working heart-brainstem preparations, inhibition of PKC activity in the NTS in situ abolished the Ang II-mediated depression of cardiac and sympathetic baroreceptor reflex gain in the WKY. In contrast, PKC inhibition in the NTS of SHR only partially reduced the effect of Ang II on the baroreceptor reflex gain.
Conclusions
These observations demonstrate that PI3-Kinase in the cardiovascular brainstem regions of the SHR may be selectively involved in Ang II-mediated signaling that includes a reduction in baroreceptor reflex function, presumably via a NADPH-ROS mediated pathway.
Keywords: Hypertension, angiotensin II, PI3 kinase, reactive oxygen species, NADPH oxidase
Introduction
It is well established that the brain angiotensin system plays a critical role in the control of cardiovascular functions, including blood pressure regulation. Physiological actions of central angiotensin II (Ang II) include modulation of sympathetic pathways, increased secretion of vasoactive hormones such as vasopressin, and dampening of baroreceptor reflexes by stimulation of neurons in the cardioregulatory brain regions of the hypothalamus and the medulla oblongata1,2. The importance of brain Ang II and the Ang II type 1 receptor (AT1R) is further underscored by observations that hyperactivity of this hormone system is linked to numerous cardiovascular diseases such as hypertension and heart failure3.
The nucleus of the solitary tract (NTS), located in the dorsomedial medulla, is one central nervous region that is crucial for the effects of Ang II on arterial pressure and baroreceptor reflex function4. For example, Ang II injected into the NTS depresses the arterial baroreflex in normotensive animals and baroreceptor activity is crucial in chronic regulation of BP5,6. In addition, evidence indicates that the sensitivity of NTS neurons to Ang II is altered in animal models of hypertension7,8. These and other observations7,8 support the concept that a reduction in baroreceptor reflex sensitivity could produce long-lasting detrimental consequences for BP homeostasis.
In spite of the evidence that the sensitivity of the NTS neurons to Ang II is altered2,9,10, not much is known about the cellular/molecular basis of this alteration in the hypertensive condition, although this has been studied in normotensive rats9,11,12. We have previously reported that Ang II produces a greater degree of activation of neuronal cells from a brainstem/hypothalamus co-culture of spontaneously hypertensive rats (SHR) than the normotensive Wistar Kyoto (WKY) rats16,17. This enhanced response of SHR neurons to Ang II appears to be the result of the existence of an additional signal transduction pathway for the AT1R in the SHR that is linked to PI3 Kinase (PI3K)14. Given these observations, coupled with the demonstrated sympathoactivation in the SHR and importance of the NTS in Ang II-mediated BP regulation, we propose the following hypothesis: Ang II modulation of SHR dorsal medullary neurons involves activation of PI3K in a PI3K-NADPH oxidase-ROS signaling pathway and this has relevance to NTS control of baroreceptor reflex function. Thus, our objective in this study was to use a combination of neuronal cells in culture and a physiological in situ technique to provide evidence to support this hypothesis.
Materials and Methods
In vitro studies
Preparation of neuronal cultures, electrophysiological recordings and biochemical measurements
Neuronal cells in primary culture were established and electrophysiological recordings were made as described previously14. Intracellular ROS levels in WKY rat and SHR neurons, as well as the NADPH oxidase activity, and phosphorylated levels of p47phox were measured. Please refer to the online supplement at http://circres.ahajournals.org for details of these methods.
In situ studies
All animal procedures were carried out according to the United Kingdom Home Office Guidelines on Animals (Scientific Procedure) Act of 1986 and those approved by the University of Bristol’s and Florida’s Animal Care and Use Committee.
Measurement of cardiovascular autonomic functions in situ
The in situ, arterially-perfused working heart-brainstem preparation (WHBP), as described previously12, was used to assess the effect of Ang II in the NTS on cardiovascular autonomic functions before and after inhibition of PKC in the NTS of the SHR and WKY. To examine the effect of Ang II in the NTS on cardiovascular autonomic functions before and after inhibition of PI3K in the NTS of the SHR, a dominant negative construct of the p85α subunit of the PI3K (DNp85α) was delivered in vivo in a lentiviral vector driven by EF1 promoter (LV-EF1- DNp85α-IRES-eGFP)15 (Accompanying Supplement). The site of Ang II microinjection, the extent of the lentiviral transduction, and protein expression were all confirmed post-hoc. Confirmation of the in vivo lentiviral-mediated inhibition of PI3K and NADPH signaling was performed using Western blotting. Cardiovascular parameters were analyzed using Spike2 software. For detailed description of the methods please refer to the online supplement at http://circres.ahajournals.org.
Results
Ang II-induced increase in action potential firing rate in the SHR neuron is associated with increased NADPH oxidase-ROS signaling
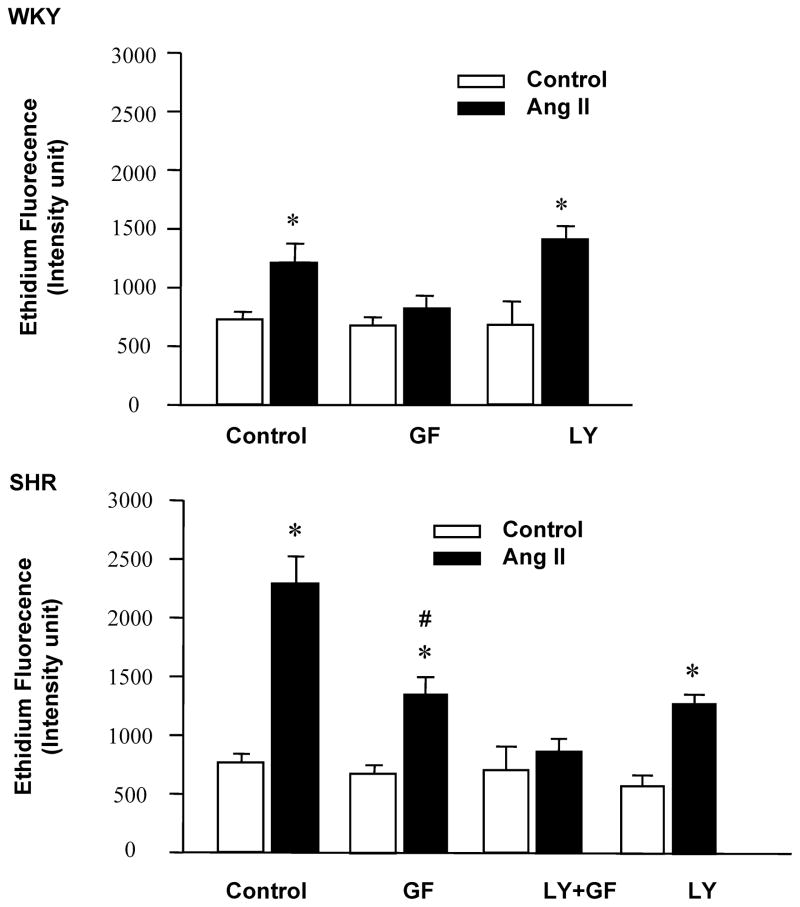
Our first objective was to determine if a greater increase in Ang II-stimulation of neuronal firing rates was associated with a greater response of Ang II on NADPH oxidase and ROS production in SHR versus WKY rat neurons by using the NADPH oxidase inhibitor, gp91ds-tat16. Although there was no significant difference in the basal neuronal firing between the two rat strains, Ang II caused a 42± 3% greater increase in this chronotropic effect in the SHR neuron than the WKY rat neuron (Figure 1). This increase was completely blocked by gp91ds-tat and not by scrambled gp91ds-tat. Comparison of ROS generation by DHE fluorogenic probe demonstrated insignificant difference in ethidium fluorescence at basal conditions between WKY rat and SHR neurons (see Figure S1 in the Supplement). However, treatment with 100nmol/L Ang II resulted in 61± 5% greater ethidium fluorescence in the SHR neurons compared to WKY rat neurons (Figure S1 in the Supplement).
Figure 1. gp91ds-tat attenuates chronotropic action of Ang II in SHR and WKY rat neurons.
A and B: Recordings of APs from SHR neurons under the following sequential treatments: Perfusion of PBS, followed by superfusion of Ang II (100 nmol/L); Washout of Ang II; Superfusion of gp91ds-tat (gp91ds, 5 μmol/L) or scrambled gp91ds-tat (Scra, 5 μmol/L); Superfusion of Ang II (100 nmol/L) plus gp91ds-tat (Ang II + gp91ds) or scrambled gp91ds-tat (Ang II + Scra).
C: Bar graph summarizing the effects of gp91ds-tat on the chronotropic action of Ang II in SHR and WKY rat neurons. Data are mean ± SE (n=11). *P<0.01 compared with respective control recordings. #P<0.05 compared recordings between SHR and WKY rat neurons under the same condition. D: Bar graph summarizing the effects of scrambled gp91ds-tat (5 μmol/L) on the chronotropic action of Ang II in SHR and WKY rat neurons. Data are mean ± SE (n=7). *P<0.01 compared with respective control recordings. #P<0.05 compared recordings between SHR and WKY rat neurons under the same condition.
Involvement of PI3-K in Ang II-induced signaling and firing responses of SHR but not WKY rat neurons
Ang II treatment (100nmol/L) resulted in increases in NADPH oxidase activity in both WKY rat and SHR neurons. However, this stimulation was 61± 5% greater in the SHR neurons compared to WKY rat neurons (Figure 2). Our previous studies have shown that the firing response evoked by Ang II in neurons from WKY rats are mediated by NADPH-ROS signaling and completely blocked by inhibition of protein Kinase C14. This was confirmed in the present study i.e. Ang II stimulation of NADPH oxidase activity was completely abolished by the PKC inhibitor, GF109230 (1μmol/L) in neurons from WKY rats (Figure 2A). In contrast, this inhibitor only partially attenuated Ang II’s effect on NADPH oxidase in neurons from SHR (Figure 2A). GF109203 (1μmol/L) did not alter basal NADPH oxidase activity in neurons from either strain of rat.
Figure 2. Effects of blockage of PKC and PI3-kinase on NADPH oxidase activity in SHR and WKY rat neurons.
A: WKY rat neuronal cultures (filled bars) or SHR neuronal cultures (open bars) were treated under following conditions: Control; Ang II (100 nmol/L); GF109203 (GF, 1 μmol/L); GF 109203 plus Ang II; LY294002 (LY, 10 μmol/L); LY294002 plus Ang II; LY + GF + Ang II. Cells were collected and NADPH oxidase activity was measured and expressed as mean light emission count/mg protein/min. Data are mean ± SE (n=10). *P<0.05 significantly different from control treatment. #P<0.05 NADPH oxidase activity compared between SHR and WKY neurons treated under the same condition. B: Bar graphs summarizing the NADPH oxidase in WKY rat neuronal cultures (filled bars) or SHR neuronal cultures (open bars) were treated with PBS (ontrol, con) or Ang II (100 nmol/L) after 24 h incubation neurons with LV-EF1α-eGFP (LV-eGFP) or LV- EF1α-DNp85α-eGFP (LV-DNp85α). Data are mean ± SE (n=12). *P<0.05 significantly different from control treatment. #P<0.05 NADPH oxidase activity compared between Lenti-eGFP- or Lenti-DNp85α-treated neurons after Ang II treatment.
We next examined the role of PI3K. Treatment of WKY rat neuronal cells with 10μmol/L LY294002, an antagonist of PI3K14 did not influence Ang II-induced NADPH oxidase activity (Figure 2A). In contrast, LY294002 (10μmol/L) partially attenuated the stimulatory effect of Ang II on this enzyme in SHR neurons (Figure 2A). Finally, combined treatment of SHR neurons with 10μmol/L LY294002 and 1μmol/L GF109203 completely abolished ANG II-induced activation of NADPH oxidase activity (Figure 2A).
Neuronal cells were infected with LV-EFα-DNp85α-eGFP to determine the role of a selective PI3K inhibition on ROS and NADPH oxidase. This treatment causes a 77% decrease in Akt phosphorylation, a measure of PI3K activity (Figure S-2 in the Supplement). This was associated with a ~60% inhibition of Ang II-induced increase in ethidium fluorescence (Online Figure I) and NADPH oxidase activity (Figure 2B).
Neurons from WKY rat and SHR were treated with GF109203 (1μmol/L) and LY291002 (10μmol/L) to evaluate the role of PKC and PI3K on neuronal firing. As expected, Ang II treatment caused a greater increase in neuronal firing rates in neurons from SHR compared to WKY rats. In neurons from WKY rat treatment with GF109203 completely blocked this increase in firing, while LY294002 had no effect (Figure 3A). In contrast, individual treatment with GF109203 or LY294002 only partially attenuated Ang II-induced firing response in neurons from SHR. However, a combination of GF 109203 and LY294002 completely abolished Ang II action on neurons from the SHR (Figure 3B). Similar to the effects on NADPH oxidase, PKC inhibition completely abolished Ang II-induced increase on ROS in neurons from WKY rats and only partially inhibited ROS in neurons from SHR (Figure 4). Co-incubation with GF109203 and LY294002 was required to completely abolish Ang II’s effects on ROS generation in neurons from SHR (Figure 4).
Figure 3. Effect of blockade of PKC and PI3-kinase on the chronotropic action of Ang II in SHR and WKY rat neurons.
A: WKY neurons were treated under the following sequential conditions: Control (PBS), followed by superfusion of Ang II (100 nmol/L); Washout of Ang II; Superfusion of GF109203 (GF, 1 μmol/L) or LY29402 (LY, 10 μmol/L); Superfusion of GF109203 plus Ang II or LY29402 plus Ang II.
B: SHR neurons were treated under the following sequential conditions: Control (PBS), followed by superfusion of Ang II (100 nmol/L); Washout of Ang II; Superfusion of GF109203 (GF, 1 μmol/L) or LY29402 (LY, 10 μmol/L); Superfusion of GF109203 plus Ang II or LY29402 plus Ang II; Superfusion of GF109203 and LY29402 plus Ang II. Data are mean ± SE (n=11–13). *P<0.01 compared with respective control recordings. #P<0.05 compared with Ang II treatment. GF109203 and LY29402 alone had no significant effects on basal firing rate.
Figure 4. Blockade of PKC and PI3-kinase on Ang II-induced increases in intracellular ROS levels.
ROS level were detected using DHE. SHR (lower panel) and WKY rat (upper panel) neurons were treated under the following conditions: Ang II (100 nmol/L); Ang II plus GF109203 (GF, 1 μmol/L); Ang II plus LY294002 (LY, 10μmol/L) or Ang II plus combination of LY294002 with GF 10923 (LY+GF). GF109203 and LY294002 alone had no significant effect on the basal ROS level. Data are mean ± SE ethidium fluorescence (n=17–21 neurons used for quantification of fluorescence) and derived from three experiments and at least seven dishes in each experiment. *P<0.01 significantly difference from respective control. #P<0.05 compared with Ang II treatment.
Phosphorylation of p47phox is critical in the activation of NADPH oxidase. Thus, our objective was to determine if PI3K-dependence of enhanced actions of Ang II in SHR neurons are reflected in the phosphorylation status of this NADPH oxidase subunit. Ang II treatment caused a 64% and 148% increase in phosphorylated p47phox in WKY rat and SHR neurons, respectively. PKC inhibitor GF109203 completely inhibited this phosphorylation in WKY rat neurons while only 50% inhibition was observed in SHR neurons (Figure 5). However, co-treatment with GF109203 and LY294002 completely blocked p47phox phosphorylation in SHR neurons (Figure 5).
Figure 5. Blockade of PKC and PI3-kinase on Ang II-induced P47phox phosphorylation in SHR and WKY rat neurons.
Phosphorylation of p47phox was measured by using immunoprecipitation with Anti-p47phox antibody followed by immunoblotting with anti-phosphoserine antibody in SHR and WKY rat neurons treated with the following conditions: control, Ang II (Ang, 100 nmol/L), Ang II plus GF109203 (Ang+GF, 1 μmol/L), and Ang II plus LY294002 (Ang+LY, 10 μmol/L) or Ang II plus combination of GF10923 with LY294002 (Ang+GF+LY). Bottom: representative blot showing the levels of phosphorylated p47phox and total p47phox; Top: bar graphs showing the mean level of phosphorylated p47phox in SHR and WKY neurons under each treatment condition. Data are mean±SE (n=5 experiments). * p <0.01 versus respective control. # p <0.05 versus Ang II treatment.
In situ confirmation of an additional Ang II signaling pathway regulating cardiovascular functions in the NTS of the SHR
At a comparable rat age, pump flow rate and perfusate vasopressin concentration, baseline PP was higher in SHR (81±2 mm Hg) than WKY (71±2 mm Hg; P<0.01), consistent with previous reports19. Additionally, we observed a significantly higher (integrated) SNA (17±2) in the SHR than WKY rat (6±2; P<0.001) after correcting for electrical noise. Heart rate was similar in both rat strains (WKY: 364±9 bpm; SHR: 334±11 bpm). Both cardiac and sympathetic baroreflex gains were not different in the SHR versus the WKY. In both strains of rat, microinjection of vehicle neither affected baseline parameters nor the baroreceptor reflex gain.
We tested the effect of PKC inhibition in the NTS of WKY and SHR on Ang II modulation of the baroreceptor reflex gain. In WKY, bilateral microinjection of Ang II into the NTS reduced cardiac baroreflex gain to 55±9% of its control value (P<0.05; Figure 6A and C). Following microinjection of GF109230, this Ang II-induced decrease in cardiac baroreflex gain was abolished, such that the gain was not different to control (Figure 6B and C). Similar results were observed for the sympathetic component of the baroreflex. Ang II in NTS of WKY rats reduced the baroreflex-mediated sympathoinhibition to 47±5% of its control value (P<0.01; Figure 6A and D), yet this effect was abolished following pre-treatment with GF109230 (Figure 6B and D). In contrast, in the SHR, pretreatment with GF109230 did not fully prevent the Ang II-mediated inhibition of baroreflex gain. In SHR, Ang II reduced cardiac baroreflex to 58±1% of control value in the vehicle-treated rats (P<0.05 versus control baroreflex gain, Figure 7A and C), and to 77±2% of control value after GF109230 treatment (P<0.05 versus control baroreflex gain; P<0.01 versus vehicle, Figure 7B and C). Sympathetic baroreflex gain in the vehicle pre-treated SHR was reduced to 73±7% of the control value following the Ang II injection (P<0.05 versus control baroreflex gain, Figure 7A and D). Following the GF109230 treatment, the sympathetic baroreflex gain in the SHR was similar to its control baroreflex value (n.s.; Figure 7B and D). These observations suggest that the depressant actions of Ang II in the NTS on both the cardiac and sympathetic limbs of the baroreflex are mediated entirely by a PKC-dependent pathway in the WKY rat, while in the SHR an additional cellular pathway exists.
Figure 6. Effect of Ang II in NTS on baroreflex of WKY rats pretreated with either vehicle or GF109203.
A and B: Examples of recordings of integrated SNA (μV), HR (bpm) and PP (mm Hg) for (A) vehicle- or (B) GF109203-treated WKY rats. Baroreceptors were stimulated by pressor ramps (~2–5 sec) before and after bilateral microinjections of Ang II (10 μM; 50–60nl/side). C and D: Group results for the cardiac (C) and sympathetic (D) baroreflex gain before and after Ang II injection in the NTS of WKY rats pre-treated with either vehicle (black bars) or GF109203 (white bars), presented as percentage of control baroreflex gain (i.e. the gain measured prior to any of the injections). *P<0.05 and **P<0.01 vs. control baroreflex gain; #P<0.05 Vs. Vehicle. bpm = beats/min
Figure 7. Influence of Ang II in NTS on baroreflex of SHR pretreated with either the vehicle or GF109203.
A and B: Examples of recordings of integrated SNA (μV), HR (bpm) and PP (mm Hg) for (A) vehicle- or (B) GF109203-treated SHR. Baroreceptors were stimulated by pressor ramps (~2–5 sec) before and after bilateral microinjections of Ang II (10 μM; 50–60nl/side). C and D: Grouped values for the cardiac baroreflex gain (C) and sympathetic baroreflex gain (D) following Ang II injection in the NTS of the SHR pre-treated with either the vehicle (black bars) or GF109203 (white bars) presented as percentage of control baroreflex gain (i.e. the gain measured prior to any of the injections). *P<0.05 vs. control baroreflex gain; #P<0.05 and ##P<0.01 vs. Vehicle. bpm = beats/min
In vivo validation of the role of PI3K in the SHR
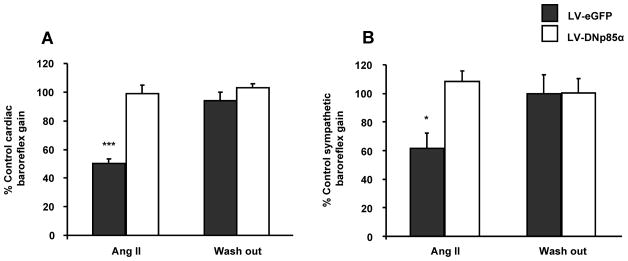
Considering our in situ results described above, and because our in vitro data implicating an additional role for PI3K in neurons from SHR but not in the WKY, we tested the functional implications of this on baroreflex gain in this rat strain only. We have used a combination of in vivo gene transfer and subsequently the in situ working heart-brainstem preparation to determine the cardiovascular implications of chronically blocking PI3K in the NTS on baroreceptor reflex gain of the SHR. At comparable perfusion rates and dose of vasopressin, there was no difference in cardio-respiratory parameters measured such as perfusion pressure, heart rate, and baroreceptor reflex gains (cardiac and sympathetic) in rats previously injected with LV-EF1α-DNp85α-eGFP compared to LV- EF1α-eGFP controls, which were also not different to untransfected rats described above. Bilateral microinjection of Ang II in the NTS resulted in a decrease in cardiac baroreflex gain in the LV- EF1α-eGFP group to 50±3% of the control value (P<0.001; Figure 8A). In contrast, in LV-EF1α-DNp85α-eGFP treated animals this Ang II-induced decrease in cardiac baroreflex gain was reduced such that the gain was comparable to the control values (Figure 8A). Similarly, Ang II in NTS of the LV- EF1α-eGFP treated group also reduced the baroreflex mediated sympathoinhibition to 62±10% of the control value (P<0.05; Figure 8B) - an effect not seen in rats in which the NTS was pre-transduced with LV-EF1α-DNp85α-eGFP, and where the gain was similar to the value established pre-Ang II injection (Figure 8B). Finally, LV-EF1α-DNp85α-eGFP treated NTS showed significant decreases in the PI3-kinase and NADPH oxidase activities (Online Figure II). These observations suggest that in the SHR the depressant actions of Ang II in NTS on both the cardiac and sympathetic limbs of the baroreflex are mediated by a PI3K-dependent pathway. Post-hoc histological analysis confirmed that expression of eGFP from both and LV-EF1α-DNp85α-eGFP and LV- EF1α–eGFP treated groups was comparable and restricted to the dorsal vagal complex (Online Figure III). Pontamine sky blue staining, used to indicate sites of microinjections of Ang II, were localized within areas expressing eGFP (Online Figure III).
Figure 8. Effect of Ang II in NTS on baroreflex of SHR pretreated with either the LV-DNp85α or LV-eGFP.
Grouped values for the cardiac baroreflex gain (A) and sympathetic baroreflex gain (B) following Ang II injection in the NTS of the SHR pre-treated with either the control virus (LV-eGFP, grey bars) or the virus blocking the PI3-K activity (LV-DNP85α, white bars). Data are presented as percentage of control baroreflex gain (i.e. the gain measured prior to the Ang II injection). *P<0.05 and ***P<0.001 vs. control baroreflex gain.
Discussion
The present study examined the role of PI3K on Ang II regulation of neuronal activity in the SHR NTS. Major findings are: (1) in dorsal medullary neurons from the WKY rat, Ang II-induced increase in firing is regulated by stimulation of PKC-NADPH oxidase-ROS mediated signaling pathway; (2) a second Ang II-signaling pathway involving PI3K has been identified in neurons from the SHR. This signaling kinase is also linked to NADPH oxidase-ROS pathway and results in a greater Ang II stimulation of neuronal firing in neurons from the SHR; (3) pharmacological inhibition of PKC in the NTS fully attenuates Ang II-induced decrease in cardiac and sympathetic baroreflex gain in the WKY, whereas this effect is only partial in the SHR. Collectively, these observations are consistent with our hypothesis that there is a greater sensitivity of NTS neurons to Ang II in the SHR, which may be a result of PI3K-mediated signaling transduction, and has direct relevance to control of baroreflex function.
The actions of Ang II in NTS in normotensive rats are likely to be different to those in hypertensive rats. In normotensive rats, we have shown previously that AngII mediated depression of the baroreflex involves AT1 receptor stimulation of endothelial nitric oxide synthase (eNOS) and NO generation20, 21. The latter pathway involved Gq protein-mediated activation of phospholipase C, which through 1,4,5-inositol triphosphate caused release of calcium from the IP3-sensitive intracellular stores and calcium-calmodulin formation11. Further, we found that NO via cyclic adenosine diphosphate ribose/ryanodine-sensitive stores increased intracellular calcium levels in GABAergic interneurones in NTS of normotensive rats in vitro, which we speculated could increase GABA release12. In addition, both Ang II and NO enhanced the magnitude of solitary tract evoked inhibitory post-synaptic potentials in NTS neurons in vitro22, 23. We have proposed that such a mechanism might cause a depression of the cardiac baroreflex in normotensive rats13, 28. Indeed, chronic depression of eNOS activity increased baroreceptor reflex gain in both WKY rats and SHR24, 25. However, for the hypertensive rat, we have no proof that this was a NO-soluble guanylate cyclase mediated effect unlike that in normotensive rats20. The present study compliments and advances our understanding of the mechanism by which Ang II acts in the NTS of the SHR and, importantly, identifies a possible functional significance of the PI3K cellular signaling pathway in this rat strain. We show for the first time that phosphorylation of the p47phox by a PKC-dependent mechanism which initiates this signaling cascade, leads to increased neuronal activity in WKY rat neurons. In addition, we have identified coupling of PI3K with this cascade, which is responsible for enhanced phosphorylation of p47phox, leading to a greater stimulation of neuronal firing and specific to neurons from the SHR. The mechanism by which PKC and PI3K mediate p47phox phosphorylation, and if both kinases phosphorylate the same serine residues in p47phox in these neurons, remains to be elucidated. However, our preliminary data with the use of decoy peptides based on the serine residues in the auto inhibitory region of the p47phox suggest that phosphorylation of serines 304 and 328 is important and both PKC and PI3K inhibitors completely attenuate phosphorylation of these two serine residues. Finally, regarding the Ang II- PKC-NADPH oxidase-ROS mediated signaling found in WKY rat neurons, we have not as yet identified a functional role for this but based on our previous data discussed above12,20–25 do not believe it important for NTS baroreflex control.
Our previous studies have established that the expression of the AT1R is 2–4 folds higher in the neurons from SHR compared with those from WKY rat1, 26. This increase was consistent with elevated levels reported in the cardiovascular-relevant brain regions of the adult SHR1. In addition, increased expression of the AT1R in the SHR neuron was associated with a greater firing rate response and neuromodulatory actions of Ang II in these strains of rat neurons14, 27, 28. The present study shows that PI3K inhibition preferentially diminishes the firing responses evoked by Ang II in the SHR by inhibiting phosphorylation of p47phox, NADPH oxidase activity, and levels of ROS. This inhibition reduces the firing response to the level observed in neurons from WKY rats exposed to Ang II. Complete attenuation of Ang II response in neurons from SHR is only accomplished by addition of a PKC inhibitor. These observations support the hypothesis that PI3K-linked phosphorylation is consistent with previous observations14, although its role in neurons of the SHR strain is novel.
Is this in vitro observation of any physiological relevance? Our in situ data suggest ‘yes’ to this question. Bilateral injection of a PKC inhibitor into the NTS of the WKY results in a complete attenuation of the Ang II-induced depressant effect on baroreflex gain (cardiac and sympathetic). However, this Ang II effect on both components of the baroreflex is only partially attenuated in the SHR. This suggests that an additional cellular pathway must exist in the NTS of SHR through which Ang II exerts its effects on the baroreflex. Based on our data present herein we suggest that this may be PI3K-dependent. This would complement our previous studies showing an Ang II-dependent elevation in PI3K in the brainstem and hypothalamic cardiovascular regions of the SHR compared to the WKY29, as well as our recent finding that chronic blockade of PI3K affects the spontaneous baroreflex gain and arterial pressure only in the adult SHR but not the WKY rat15. As PI3K blockade completely abolished the effect of exogenous Ang II in the NTS on the baroreceptor reflex in the SHR, we suggest that the AngII-PI3K pathway may operate upstream of the PKC signaling in the same neurons. Furthermore, the enhanced Ang II-PI3K signaling in the NTS of SHR may cause other downstream effects which may also add to the baroreflex depression, such as elevating the levels of NO in the NTS as mentioned above20, 21, 24, 25. Recent studies report a defective PI3K-Akt-NOS signaling pathway in the NTS and inflammatory effects in the vessel walls of SHR30, 31. Our recent work has also shown that specific inflammatory condition may exist in the NTS of the SHR that may contribute to the neurogenic hypertension and dampening of the baroreflex gain32, 33, 34. Furthermore, developmental differences in Ang II-PI3K signaling may exist between the P9 SHR used in vitro and the P21 SHR used in situ, as recently suggested19 which may also contribute to the perceived differences in the effects of the PI3K blockade. These apparent discrepancies may be related to the previously reported differences in AT1R signaling, as AT2R signaling is predominant in the neurons derived from neonatal brainstem, and shifts in favor of the predominantly AT1R-mediated signaling with age35. However, the absence of any effects of LV-DNp85 in the NTS on the cardiovascular variables in the adult WKY15 indicates that the suggested developmental change in Ang II signaling, if indeed it exists, may be present only in the SHR.
The finding that Ang II in NTS depresses the cardiac and sympathetic component of the baroreflex in SHR is confirmatory of previous work using normotensive rats studied using the same in situ preparation36, although baroreflex modulation of lumbar sympathetic activity by Ang II in NTS was less affected37. The present study in the WKY and SHR demonstrates that the depressant action of Ang II in NTS affects both autonomic limbs of the baroreflex. We suggest that Ang II driven PI3K activity in NTS neurons of SHR is a major modulator of one of the main homeostatic reflexes regulating arterial pressure. Interestingly, a prognostic indicator of morbidity and mortality for cardiovascular disease, including hypertension, is loss of HR variability and reduced baroreflex cardiac gain37, 38, 39. Our data provide a potential explanation for this especially as Ang II activity appears elevated in SHR brainstem21 and that both exogenous (but presumably endogenous) Ang II and circulating Ang II (independent of the area postrema) can depress cardiac baroreflex gain via actions at the level of the NTS21,41.
In conclusion, our studies demonstrate the presence of an exclusive Ang II-AT1R signaling system in dorsal medullary neurons of the SHR involving PI3-Kinase. This signaling is proposed to be involved in Ang II regulation of an impaired baroreflex in the SHR and thus may be a relevant target in the control of neurogenic hypertension.
Limitations
We acknowledge that usage of neuronal cultures in our in vitro experiments versus the P21 rats used in in situ may pose certain questions regarding comparability of the data. However, the viability of the in situ method is highly dependent on the age of the animal which limited these experiments to P21. Nevertheless the P21 rats are still juvenile and in our opinion may have limited comparability to in vitro situation. Therefore, even though here we may allude to slight developmental differences in Ang II-PI3K signaling in the NTS in vitro versus the P21 SHR rats, we point out that the main difference is in the role of PI3K signaling in the NTS of WKY and SHR.
Supplementary Material
Acknowledgments
All authors would like to dedicate this work to the late Dr Jeffrey T. Potts who supervised certain aspects of in vivo work to the end. We wish to thank Ms. Fan Lin for preparation of neuronal cultures.
Sources of Funding: This work was supported by grants from the NIH (HL33610) and the British Heart Foundation. JFRP is a receipt of a Royal Society Wolfson Research Merit Award. C. Sun is supported by American Heart Association (SDG 0635050N).
Abbreviations
- Ang II
angiotensin II
- AT1R
angiotensin II type 1 receptor
- AT2R
angiotensin II type 2 receptor
- BP
blood pressure
- DHE
dihydroethidium
- eNOS
endothelial nitric oxide synthase
- NADPH oxidase
nicotinamide adrenine dinucleotide phosphate-oxidase
- NT S
the nucleus of the solitary tract
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- SHR
spontaneously hypertensive rat
- WKY
Wistar Kyoto
- WHBP
working heart-brainstem preparation
Footnotes
Disclosures
None
References
- 1.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 3.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 4.Paton JF, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86:705–10. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 5.Heusser K, Tank J, Luft FC, Jordan J. Baroreflex failure. Hypertension. 2005;45:834–839. doi: 10.1161/01.HYP.0000160355.93303.72. [DOI] [PubMed] [Google Scholar]
- 6.Huang C, Yoshimoto M, Miki K, Johns EJ. The contribution of brain angiotensin II to the baroreflex regulation of renal sympathetic nerve activity in conscious normotensive and hypertensive rats. J Physiol. 2006;574:597–604. doi: 10.1113/jphysiol.2006.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thrasher TN. Unloading arterial baroreceptors causes neurogenic hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1044–R1053. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- 8.Thrasher TN. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr Hypertens Rep. 2006;8:249–254. doi: 10.1007/s11906-006-0058-z. [DOI] [PubMed] [Google Scholar]
- 9.Paton JF, Kasparov S. Differential effects of angiotensin II on cardiovascular reflexes mediated by nucleus tractus solitarii I. A microinjection study. J Physiol. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teschemacher AG, Wang S, Raizada MK, Paton JF, Kasparov S. Area-specific differences in transmitter release in central catecholaminergic neurons of spontaneously hypertensive rats. Hypertension. 2008;52:351–8. doi: 10.1161/HYPERTENSIONAHA.108.114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong LF, Polson JW, Murphy D, Paton JF, Kasparov S. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J. 2002;16:1595–1601. doi: 10.1096/fj.02-0099com. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Teschemacher AG, Paton JF, Kasparov S. The mechanism of nitric oxide action on inhibitory GABAergic signaling within the nucleus tractus solitarii. FASEB J. 2006;20:E821–E831. doi: 10.1096/fj.05-5547fje. [DOI] [PubMed] [Google Scholar]
- 13.Sun C, Sumners C, Raizada MK. Chronotropic action of angiotensin II in neurons via protein kinase C and CaMKII. Hypertension. 2002;39:562–566. doi: 10.1161/hy0202.103057. [DOI] [PubMed] [Google Scholar]
- 14.Sun C, Du J, Sumners C, Raizada MK. PI3-kinase inhibitors abolish the enhanced chronotropic effects of angiotensin II in spontaneously hypertensive rat brain neurons. J Neurophysiol. 2003;90:3155–3160. doi: 10.1152/jn.00222.2003. [DOI] [PubMed] [Google Scholar]
- 15.Zubcevic J, Waki H, Diez-Freire C, Gampel A, Raizada MK, Paton JF. Chronic blockade of phosphatidylinositol 3-kinase in the nucleus tractus solitarii is prohypertensive in the spontaneously hypertensive rat. Hypertension. 2009;53:97–103. doi: 10.1161/HYPERTENSIONAHA.108.122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–666. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- 17.Takeya R, Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol Cells. 2003;16:271–277. [PubMed] [Google Scholar]
- 18.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 19.Simms A, Paton JF, Pickering AE. Hierarchical recruitment of the sympathetic and parasympathetic limbs of the baroreflex in normotensive and spontaneously hypertensive rats. J Physiol. 2007;579:473–486. doi: 10.1113/jphysiol.2006.124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton JF, Lonergan T, Deuchars J, James PE, Kasparov S. Detection of angiotensin II mediated nitric oxide release within the nucleus of the solitary tract using electron-paramagnetic resonance (epr) spectroscopy. Auton Neurosci. 2006;126–127:193–201. doi: 10.1016/j.autneu.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Paton JF, Wang S, Polson JW, Kasparov S. Signaling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- 22.Kasparov S, Paton JF. Differential effects of angiotensin II in the nucleus tractus solitarii of the rat-- plausible neuronal mechanisms. J Physiol. 1999;521:227–238. doi: 10.1111/j.1469-7793.1999.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Paton JF, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii. Exp Physiol. 2007;92:371–382. doi: 10.1113/expphysiol.2006.036103. [DOI] [PubMed] [Google Scholar]
- 24.Waki H, Kasparov S, Wong L-F, Murphy D, Shimizu T, Paton JF. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J Physiol. 2003;546:233–242. doi: 10.1113/jphysiol.2002.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waki H, Murphy D, Yao ST, Kasparov S, Paton JF. Endothelial nitric oxide synthase in the nucleus tractus solitarii contributes to the hypertension in spontaneously hypertensive rats. Hypertension. 2006;48:644–650. doi: 10.1161/01.HYP.0000238200.46085.c6. [DOI] [PubMed] [Google Scholar]
- 26.Raizada MK, Lu D, Tang W, Kurian P, Sumners C. Increased angiotensin II type-1 receptor gene expression in neuronal cultures from spontaneously hypertensive rats. Endocrinology. 1993;132:1715–1722. doi: 10.1210/endo.132.4.8462471. [DOI] [PubMed] [Google Scholar]
- 27.Lu D, Yang H, Lenox RH, Raizada MK. Regulation of anigotensin II-induced neuromodulation by MARCKS in brain neurons. J Cell Biol. 1998;142:217–227. doi: 10.1083/jcb.142.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang H, Lu D, Yu K, Raizada MK. Regulation of neuromodulatory actions of angiotensin II in the brain neurons by the Ras-dependent mitogenactivated protein kinase pathway. J Neurosci. 1996;16:4047–4058. doi: 10.1523/JNEUROSCI.16-13-04047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veerasingham SJ, Yamazato M, Berecek KH, Wyss JM, Raizada MK. Increased PI3-kinase in presympathetic brain areas of the spontaneously hypertensive rat. Circ Res. 2005;96:277–279. doi: 10.1161/01.RES.0000156275.06641.b2. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao M, Lu PJ, Huang HN, Lo WC, Ho WY, Lai TC, Chiang HT, Tseng CJ. Defective phosphatidylinositol 3-kinase signaling in central control of cardiovascular effects in the nucleus tractus solitarii of spontaneously hypertensive rats. Hypertens Res. 2008;31:1209–18. doi: 10.1291/hypres.31.1209. [DOI] [PubMed] [Google Scholar]
- 31.Dai Q, Xu M, Yao M, Sun B. Angiotensin AT1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol. 2007;152:1042–8. doi: 10.1038/sj.bjp.0707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waki H, Gouraud SS, Maeda M, Paton JF. Specific inflammatory condition in nucleus tractus solitarii of the SHR: novel insight for neurogenic hypertension. Auton Neurosci. 2008;142:25–31. doi: 10.1016/j.autneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Paton JF, Waki H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci Biobehav Rev. 2009;33:89–94. doi: 10.1016/j.neubiorev.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Waki H, Liu B, Miyake M, Katahira K, Murphy D, Kasparov S, Paton JF. Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension. 2007;49:1321–7. doi: 10.1161/HYPERTENSIONAHA.106.085589. [DOI] [PubMed] [Google Scholar]
- 35.Sumners C, Tang W, Zelezna B, Raizada MK. Angiotensin II receptor subtypes are coupled with distinct signal-transduction mechanisms in neurons and astrocytes from rat brain. Proc Natl Acad Sci USA. 1991;88:7567–71. doi: 10.1073/pnas.88.17.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boscan P, Allen AM, Paton JF. Baroreflex inhibition of cardiac sympathetic outflow is attenuated by angiotensin II in the solitary tract nucleus. Neuroscience. 2001;103:153–160. doi: 10.1016/s0306-4522(00)00559-5. [DOI] [PubMed] [Google Scholar]
- 37.Polson JW, Dampney RA, Boscan P, Pickering AE, Paton JF. Differential baroreflex control of sympathetic drive by angiotensin II in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1954–R1960. doi: 10.1152/ajpregu.00041.2007. [DOI] [PubMed] [Google Scholar]
- 38.Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- 39.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 40.Villareal RP, Liu BC, Massumi A. Heart rate variability and cardiovascular mortality. Curr Atheroscler Rep. 2002;4:120–127. doi: 10.1007/s11883-002-0035-1. [DOI] [PubMed] [Google Scholar]
- 41.Tan PS, Killinger S, Horiuchi J, Dampney RA. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–R2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.