Abstract
By using a nonneuronal cell system, evidence has previously been provided that tetanus toxin (TT) intoxication occurs in macrophages, impairing their secretory activity as well as their antitumoral activity. In particular, both secreted and total lysozyme (LZM) activities are reduced by TT treatment, provided that GG2EE macrophages have been preexposed to gamma interferon (IFN-gamma). In an attempt to provide insight into the molecular mechanisms underlying this phenomenon, we focused our attention on the levels of LZM-specific transcripts. GG2EE macrophages preexposed to IFN-gamma exhibited augmented levels of LZM-specific mRNA. Such an effect was detected 1 h after removal of IFN-gamma, peaked at 3 h, and gradually decreased with time in culture. Exposure of IFN-gamma-pretreated GG2EE macrophages to TT resulted in the prevention of the IFN-gamma-mediated upregulation of LZM mRNA levels. The phenomenon was mediated by the holotoxin (> or = 1 micrograms/ml) and abrogated by preexposure of the macrophages to the C fragment of TT. Protein kinase C (PKC) and Ca(2+)-calmodulin-dependent PK were likely involved in the IFN-gamma-mediated upregulation of LZM mRNA levels and biological activity, as assessed by PK inhibitors. Furthermore, PK inhibitors mimicked TT in impairing LZM activity of GG2EE macrophages, thus suggesting that impairment of PKC and/or the Ca(2+)-calmodulin-dependent PK pathway(s) may be one of the events involved in TT intoxication of macrophages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasi E., Farinelli S., Varesio L., Bistoni F. Augmentation of GG2EE macrophage cell line-mediated anti-Candida activity by gamma interferon, tumor necrosis factor, and interleukin-1. Infect Immun. 1990 Apr;58(4):1073–1077. doi: 10.1128/iai.58.4.1073-1077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E., Mathieson B. J., Varesio L., Cleveland J. L., Borchert P. A., Rapp U. R. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985 Dec 19;318(6047):667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- Blasi E., Pitzurra L., Burhan Fuad A. M., Marconi P., Bistoni F. Gamma interferon-induced specific binding of tetanus toxin on the GG2EE macrophage cell line. Scand J Immunol. 1990 Sep;32(3):289–292. doi: 10.1111/j.1365-3083.1990.tb02922.x. [DOI] [PubMed] [Google Scholar]
- Blasi E., Radzioch D., Durum S. K., Varesio L. A murine macrophage cell line, immortalized by v-raf and v-myc oncogenes, exhibits normal macrophage functions. Eur J Immunol. 1987 Oct;17(10):1491–1498. doi: 10.1002/eji.1830171016. [DOI] [PubMed] [Google Scholar]
- Blasi E., Radzioch D., Varesio L. Inhibition of retroviral mRNA expression in the murine macrophage cell line GG2EE by biologic response modifiers. J Immunol. 1988 Sep 15;141(6):2153–2157. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chung L. P., Keshav S., Gordon S. Cloning the human lysozyme cDNA: inverted Alu repeat in the mRNA and in situ hybridization for macrophages and Paneth cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6227–6231. doi: 10.1073/pnas.85.17.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine R. V., Handler C. M., Simpson L. L., Sherwin J. R. Tetanus toxin inhibits neurotensin-induced mobilization of cytosolic protein kinase C activity in NG-108 cells. Toxicon. 1991;29(11):1351–1357. doi: 10.1016/0041-0101(91)90122-8. [DOI] [PubMed] [Google Scholar]
- Eisel U., Jarausch W., Goretzki K., Henschen A., Engels J., Weller U., Hudel M., Habermann E., Niemann H. Tetanus toxin: primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986 Oct;5(10):2495–2502. doi: 10.1002/j.1460-2075.1986.tb04527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N. F., Lyness V. A. The complete nucleotide sequence of tetanus toxin. Nucleic Acids Res. 1986 Oct 10;14(19):7809–7812. doi: 10.1093/nar/14.19.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Dreyer F. Clostridial neurotoxins: handling and action at the cellular and molecular level. Curr Top Microbiol Immunol. 1986;129:93–179. doi: 10.1007/978-3-642-71399-6_2. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. L., Klempner M. S. Diminished activity of protein kinase C in tetanus toxin-treated macrophages and in the spinal cord of mice manifesting generalized tetanus intoxication. J Infect Dis. 1988 May;157(5):925–933. doi: 10.1093/infdis/157.5.925. [DOI] [PubMed] [Google Scholar]
- Ho J. L., Klempner M. S. Tetanus toxin inhibits secretion of lysosomal contents from human macrophages. J Infect Dis. 1985 Nov;152(5):922–929. doi: 10.1093/infdis/152.5.922. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Hidaka H. 1-(5-Isoquinolinesulfonyl)-2-methylpiperazine (H-7) is a selective inhibitor of protein kinase C in rabbit platelets. Biochem Biophys Res Commun. 1984 Nov 30;125(1):258–264. doi: 10.1016/s0006-291x(84)80362-9. [DOI] [PubMed] [Google Scholar]
- Keshav S., Chung P., Milon G., Gordon S. Lysozyme is an inducible marker of macrophage activation in murine tissues as demonstrated by in situ hybridization. J Exp Med. 1991 Nov 1;174(5):1049–1058. doi: 10.1084/jem.174.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J., Radzioch D., Young H. A., Varesio L. Differential inhibition of IL-1 and TNF-alpha mRNA expression by agents which block second messenger pathways in murine macrophages. J Immunol. 1988 Nov 1;141(9):3101–3105. [PubMed] [Google Scholar]
- Krieglstein K., Henschen A., Weller U., Habermann E. Arrangement of disulfide bridges and positions of sulfhydryl groups in tetanus toxin. Eur J Biochem. 1990 Feb 22;188(1):39–45. doi: 10.1111/j.1432-1033.1990.tb15368.x. [DOI] [PubMed] [Google Scholar]
- Marxen P., Bartels F., Ahnert-Hilger G., Bigalke H. Distinct targets for tetanus and botulinum A neurotoxins within the signal transducing pathway in chromaffin cells. Naunyn Schmiedebergs Arch Pharmacol. 1991 Oct;344(4):387–395. doi: 10.1007/BF00172577. [DOI] [PubMed] [Google Scholar]
- Mochida S., Poulain B., Eisel U., Binz T., Kurazono H., Niemann H., Tauc L. Exogenous mRNA encoding tetanus or botulinum neurotoxins expressed in Aplysia neurons. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7844–7848. doi: 10.1073/pnas.87.20.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Okabe T., Sugimoto N., Matsuda M. Biochemical analyses of the inhibition of catecholamine release by tetanus toxin in digitonin-permeabilized chromaffin cells. Jpn J Med Sci Biol. 1990 Dec;43(6):266–267. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
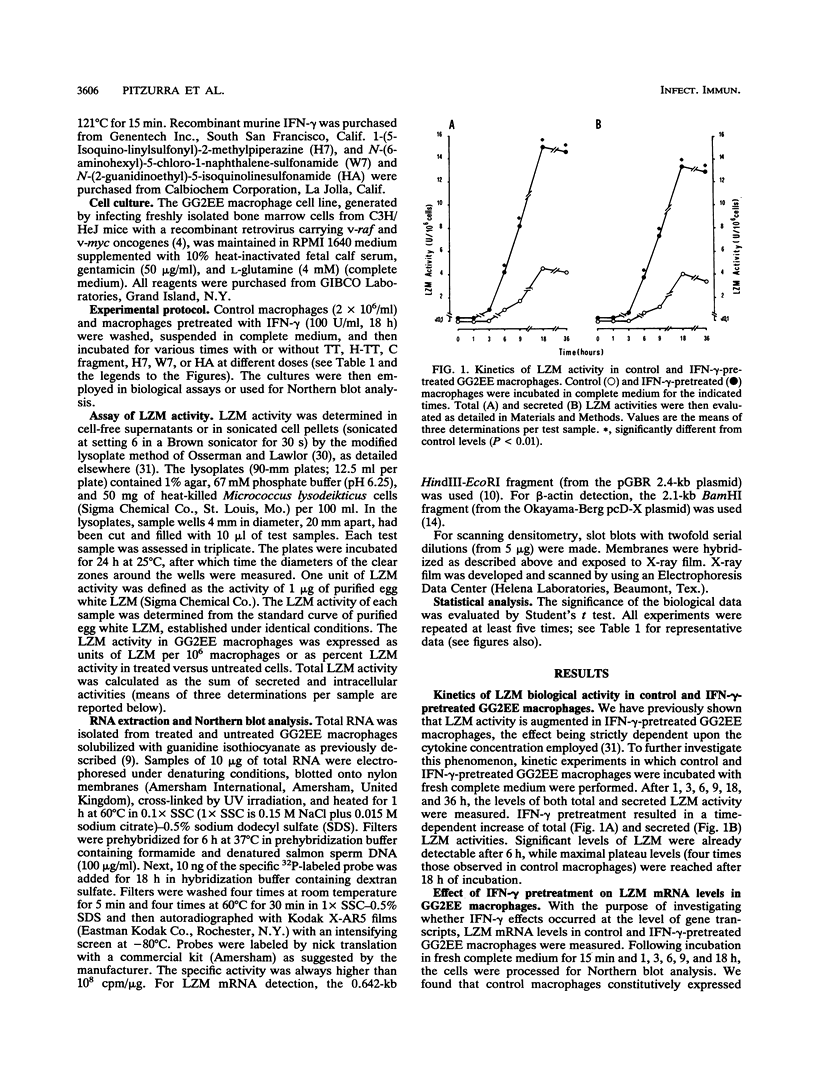
- Pitzurra L., Marconi P., Bistoni F., Blasi E. Selective inhibition of cytokine-induced lysozyme activity by tetanus toxin in the GG2EE macrophage cell line. Infect Immun. 1989 Aug;57(8):2452–2456. doi: 10.1128/iai.57.8.2452-2456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain B., Mochida S., Wadsworth J. D., Weller U., Habermann E., Dolly J. O., Tauc L. Inhibition of neurotransmitter release by botulinum neurotoxins and tetanus toxin at Aplysia synapses: role of the constituent chains. J Physiol (Paris) 1990;84(4):247–261. [PubMed] [Google Scholar]
- Poulain B., Mochida S., Weller U., Högy B., Habermann E., Wadsworth J. D., Shone C. C., Dolly J. O., Tauc L. Heterologous combinations of heavy and light chains from botulinum neurotoxin A and tetanus toxin inhibit neurotransmitter release in Aplysia. J Biol Chem. 1991 May 25;266(15):9580–9585. [PubMed] [Google Scholar]
- Poulain B., Tauc L., Maisey E. A., Wadsworth J. D., Mohan P. M., Dolly J. O. Neurotransmitter release is blocked intracellularly by botulinum neurotoxin, and this requires uptake of both toxin polypeptides by a process mediated by the larger chain. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4090–4094. doi: 10.1073/pnas.85.11.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Erulkar S. D., Lev-Tov A., Meiri H. Intracellular and extracellular calcium ions in transmitter release at the neuromuscular synapse. Ann N Y Acad Sci. 1978 Apr 28;307:583–598. doi: 10.1111/j.1749-6632.1978.tb41983.x. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Berry C. J., Rogers T. B. Studies on the intoxication pathway of tetanus toxin in the rat pheochromocytoma (PC12) cell line. Binding, internalization, and inhibition of acetylcholine release. J Biol Chem. 1989 Apr 5;264(10):5679–5686. [PubMed] [Google Scholar]
- Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B. R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992 Oct 29;359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G., Poulain B., Rossetto O., Benfenati F., Tauc L., Montecucco C. Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. EMBO J. 1992 Oct;11(10):3577–3583. doi: 10.1002/j.1460-2075.1992.tb05441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller U., Dauzenroth M. E., Meyer zu Heringdorf D., Habermann E. Chains and fragments of tetanus toxin. Separation, reassociation and pharmacological properties. Eur J Biochem. 1989 Jul 1;182(3):649–656. doi: 10.1111/j.1432-1033.1989.tb14874.x. [DOI] [PubMed] [Google Scholar]






