Abstract
Background
We tested the hypothesis that nuclear factor κB (NFκB) activity contributes to vascular endothelial dysfunction with aging and obesity in humans.
Methods and Results
We conducted a randomized, double blind, placebo controlled, crossover study in 14 non-diabetic overweight or obese (body mass index ≥25 kg/m2) middle-aged and older (age 52-68 years) adults. Salsalate (non-acetylated salicylate, 4500 mg/d), a compound that inhibits NFκB activity, or placebo was administered for 4-day periods. Plasma salicylate concentrations reached the mid-therapeutic range (21.8 ± 1.1 mg/100 ml, P≤0.0001 vs. placebo) by day 4 of Salsalate treatment. Salsalate increased expression of the inhibitor of NFκB, and reduced total and nuclear expression of NFκB in endothelial cells obtained from the subjects (all P<0.05). Salsalate increased brachial artery flow-mediated dilation (FMD) by 74% (4.0 ± 0.4 to 6.6 ± 0.5%, P<0.001), but did not affect endothelium-independent dilation (P=0.83). The change in brachial FMD with Salsalate was inversely related to baseline FMD (r=−0.77, P<0.01). Infusion of vitamin C increased brachial FMD during placebo (P<0.001), but not after Salsalate (P=0.23). Salsalate reduced nitrotyrosine (P=0.06) and expression of NADPH oxidase p47phox (P<0.05) in endothelial cells obtained from the subjects, but did not influence plasma or endothelial cell inflammatory proteins.
Conclusions
Our findings provide the first direct evidence that NFκB, in part via stimulation of oxidative stress, plays an important role in mediating vascular endothelial dysfunction in overweight and obese middle-aged and older humans.
Keywords: endothelium-dependent dilation, brachial flow-mediated dilation, aging
Vascular endothelial dysfunction plays an important role in the increased risk of cardiovascular diseases (CVD) with aging 1, particularly in overweight and obese adults 2, 3. Inflammation and oxidative stress are thought to be important processes by which aging and increased adiposity lead to development of vascular endothelial dysfunction 4-7. However, the molecular mechanisms involved, particularly in humans, are poorly understood.
The nuclear transcription factor nuclear factor κB (NFκB) and its inhibitory protein, inhibitor of NFκB (IκB), comprise a tightly controlled system regulating the inflammatory and redox states of vascular endothelial cells 8, 9. Data from animal and cell culture models implicate NFκB in both age- and obesity-associated vascular endothelial dysfunction 10, 11. Recently, we found that endothelial cells obtained from overweight/obese 12 and older 13, 14 non-diabetic adults have greater protein expression of NFκB compared with normal weight and young adults, respectively. Moreover, NFκB was positively related to nitrotyrosine, a cellular marker of oxidative stress 13. Endothelial cell expression of the oxidant producing enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase also was greater in overweight/obese 12 and older 13 adults. Taken together, these observations suggest that vascular endothelial dysfunction in middle-aged and older adults with increased body fat may be mediated in part by NFκB activation, perhaps through the development of oxidative stress. However, there is no direct evidence for this concept in humans.
In the present study, we tested this hypothesis using a randomized, double blind, placebo controlled, crossover design in non-diabetic overweight/obese middle-aged and older adults. Salsalate (non-acetylated salicylate), a compound that inhibits NFκB activation by preventing phosphorylation of IκB and, thus, translocation of the p50/65 subunit of NFκB to the nucleus 9, 15-17, or placebo was administered for 4-day periods. Endothelium-dependent (brachial artery flow-mediated dilation, FMD) and –independent dilation were determined at the end of Salsalate and placebo administration, and endothelial cells were obtained for subsequent analysis of IκB, total and nuclear NFκB, and other inflammation-modulating proteins. Serum was assayed for metabolic risk factors, markers of inflammation and prostaglandin metabolism, and circulating vasoconstrictor factors. In a subset of the subjects, acute supraphysiological infusion of ascorbic acid (vitamin C) was used to assess oxidative stress-associated suppression of endothelium-dependent dilation under conditions of normal (placebo) and reduced (Salsalate) NFκB activation. Endothelial cells were analyzed for nitrotyrosine and NADPH oxidase.
METHODS
Subjects
Fourteen middle-aged and older (age 52-68 years) overweight and obese (body mass index, BMI, ≥25 kg/m2) men and women were studied. Subjects were non-smokers, non-diabetic and were free of other clinical diseases as assessed by medical history, physical examination, blood chemistry and resting and exercise ECG. Subjects were excluded if they were taking any prescription medications, herbal supplements, antioxidants or aspirin. Subjects had elevated abdominal girth (waist circumference ≥94 cm for men and ≥80 cm for women, 12/14) and/or plasma C-reactive protein (CRP) concentrations ≥1<10 mg/L (9/14), and one or more of the following: hypertriglyceridemia (≥150 mg/dL, 3/14), low high-density lipoprotein cholesterol (HDL-C, <40 mg/dl in men and <50 mg/dl in women, 5/14), pre-hypertension (systolic blood pressure 130-139 mmHg and/or diastolic blood pressure 85-89 mmHg, 4/14) or high fasting glucose (100-125 mg/dL, 2/14). Subjects performed no regular exercise (i.e., <30 minutes/day, <2 days/week) for at least the prior 2 years. All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Measurements
All measurements were performed at the University of Colorado at Boulder General Clinical Research Center (GCRC) after a 12-hour overnight fast and 24-hour abstention from alcohol.
Subject Characteristics
Body mass index (BMI) was calculated from height and weight to the nearest 0.1 kg, and waist and hip circumference were measured by anthropometry 18. Total body fat was determined using dual energy x-ray absorptiometry (DPX-IQ, GE/Lunar, Inc.) 19. Arterial blood pressure was measured during supine rest using a semi-automated device (Dynamap XL, Johnson and Johnson) and maximal oxygen consumption (VO2max), a measure of maximal aerobic exercise capacity, was determined as previously described 20.
Blood Analyses
Details can be found in the on-line Data Supplement.
Endothelial Cell Protein Expression
The procedures used for collection of venous endothelial cells and measurement of protein expression by quantitative immunofluorescence have been described in detail previously by our laboratory 12-14, 21. Two sterile J wires (Daig Corp., Minnetonka, MN) were advanced into an antecubital vein (~4 cm beyond the tip of the catheter) and retracted through an 18-gauge catheter. The wires were then transferred to a dissociation buffer solution and cells were recovered after a washing and centrifugation protocol. Collected cells were fixed with 3.7% formaldehyde and plated on poly-L-lysine coated slides (Sigma Chemical, St. Louis, MO) and then frozen at −80° C until analysis.
Cells were rehydrated and nonspecific binding sites were blocked with 5% donkey serum (Jackson Immunoresearch, West Grove, PA). Cells were incubated with monoclonal antibodies for NFκB p65, IκBα (both Novus, Littleton, CO), nitrotyrosine, NADPH oxidase p47phox, TNF-α, monocyte chemoattractant protein-1 (MCP-1) (all Abcam, Cambridge, MA), and cyclooxygenase (COX 1/2) (Cayman Chemical, Ann Arbor, MI). Cells were then incubated with an Alexaflour 555 fluorescent secondary antibody (Invitrogen Corp., Carlsbad, CA).
For analysis, slides were viewed using a fluorescence microscope (Eclipse 600, Nikon, Melville, NY) and cell images were digitally captured by a Photometrics CoolSNAPfx digital camera (Roper Scientific, Inc., Tucson, AZ). Endothelial cells were identified by staining for von Willebrand factor and nuclear integrity was confirmed using DAPI (4′,6′-diamidino-2-phenylindole hydrochloride). Once endothelial cells with intact nuclei were identified, they were analyzed using Metamorph Software (Universal Imaging Corp., Downingtown, PA). For an estimate of nuclear NFκB p65, the nuclear regions of endothelial cells were identified by DAPI staining, overlayed on the same cell’s Alexaflour 555 image and analyzed for fluorescent intensity of the Alexaflour within the DAPI region. Values for each protein are reported as a ratio of endothelial cell/human umbilical vein endothelial cell (HUVEC) average pixel intensity. The technician was blinded to the identity of the subject and experimental condition during the staining and analysis procedures. The reproducibility of our measurements of total and nuclear NFκB and IκBα recently was determined in 4 subjects in whom endothelial cells were collected at least a week apart 14. Reproducibility was defined as the absolute difference in endothelial cell mean fluorescent intensity in trial 1 vs. trial 2 divided by average mean fluorescent intensity for the trials. Mean values and range were: total NFκB 6.3% (4-8%), nuclear NFκB 7.1% (2-11%) and IκBα 6.8% (0.4-15%).
Brachial Artery FMD and Endothelium-Independent Dilation
Brachial artery FMD and endothelium-independent dilation in response to 0.4 mg sublingual nitroglycerin were determined using duplex ultrasonography (Powervision 5000, Toshiba, Inc.) with a linear array transducer as described previously by our laboratory13, 21-24. Details can be found in the on-line Data Supplement.
Study Design and Salsalate Administration
Subjects were randomly assigned oral doses of either Salsalate or placebo for 4 consecutive days using a randomized, crossover design. They received a standardized research diet prepared by the GCRC bionutritionist for 3 days before each of the 2 vascular assessment sessions. Both subjects and investigators were blind to treatment condition. Salsalate was administered in a total daily dose of 4500 mg: 2250 mg in the morning (taken in the GCRC) and 2250 mg in the evening (at home). This dosing regimen has been used clinically to inhibit NFκB by producing steady state plasma salicylate concentrations in the therapeutic range of 10-30 mg/100mL 17, 25, 26 while minimizing gastrointestinal and other side effects 27, 28. The treatment duration was chosen because ~72 hours is required to attain maximal plasma concentrations of salicylate 29, 30. Plasma salicylate concentrations were determined during the morning of day 0, 2 and 4 of Salsalate administration to ensure concentrations were below toxic levels (<30 mg/100 ml). Subjects were telephoned in the evenings to remind them to take the second dose and to monitor them for any signs or symptoms of toxicity (e.g., tinnitus, nausea, rash, etc.). If subjects reported any of these signs or symptoms, they were instructed to not take the evening dose and to have plasma levels measured in the morning.
Seventeen subjects were enrolled in the experimental protocol. Three subjects withdrew during the protocol for the following reasons: dizziness (n=1) or tinnitus (n=1) on day 3 of Salsalate treatment; development of gout during the placebo treatment (n=1). None of the 3 subjects had plasma salicylate concentrations in the toxic range.
Data Analysis
All data are presented as mean ± standard error. A paired t-test was performed to determine differences in outcomes during Salsalate vs. placebo. Bivariate pearson correlations were performed to assess relations of interest. Statistical significance was set a priori at P<0.05. The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
Baseline Status of Subjects
Baseline characteristics and serum factors are presented in Tables 1-3. Subjects were clinically overweight or obese as indicated by a BMI range of 26-42 kg/m2, and had elevated abdominal adiposity based on waist circumference (Table 1). VO2 max ranged from 19-36 ml/kg/min, reflecting maximal aerobic exercise capacity within the low-normal range. Resting blood pressure and metabolic risk factors varied among individuals, but mean levels were within the clinically normal ranges (Table 2). Mean plasma CRP concentration (2.0 ± 0.5 mg/L) was in the moderately elevated CVD risk category (Table 3), consistent with chronic low-grade inflammation 31.
Table 1.
Baseline subject characteristics
| Mean ± SE | Range | |
|---|---|---|
| n | 14 | --- |
| Sex (male/female) | 11 / 3 | --- |
| Age (years) | 61 ± 1 | 52 - 68 |
| Weight (kg) | 92 ± 4 | 82 - 131 |
| Body mass index (kg/m2) | 31 ± 2 | 26 - 42 |
| Waist circumference (cm) | 101 ± 4 | 80 - 127 |
| Hip circumference (cm) | 109 ± 3 | 101 - 135 |
| Waist:Hip ratio | 0.94 ± 0.03 | 0.74 - 1.09 |
| Body fat (%) | 35 ± 2 | 23 - 48 |
| VO2 max (ml/kg/min) | 28 ± 1 | 19 - 36 |
SE, standard error. VO2max, maximal exercise oxygen consumption
Table 3.
Humoral factors
| Placebo | Salsalate | |
|---|---|---|
| C-reactive protein (mg/L) | 2.0 ± 0.5 | 2.2 ± 0.4 |
| Interleukin-6 (pg/ml) | 1.8 ± 0 2 | 1.3 ± 0.2 |
| Tumor necrosis factor-α (pg/ml) | 1.4 ± 0.2 | 1.5 ± 0.1 |
| Oxidized LDL (U/L) | 63 ± 3 | 61 ± 5 |
| Leptin (ng/ml) | 11.3 ± 2.3 | 13.1 ± 3.0 |
| Adiponectin (μg/ml) | 8.3 ± 1.4 | 8.2 ± 1.3 |
| Endothelin-1 (pg/ml) | 5.9 ± 0.2 | 5.3 ± 0.3 |
| Norepinephrine (pg/ml) | 251 ± 23 | 240 ± 18 |
| 6-keto prostanglandin F1α (pg/ml) | 238 ± 14 | 225 ± 13 |
| 11-dehydro thromboxane B2 (pg/ml) | 22.7 ± 4.2 | 36.0 ± 3.3* |
Values are mean ± standard error. LDL, low-density lipoprotein.
P<0.05 vs. Placebo.
Table 2.
Blood pressure and circulating metabolic factors
| Placebo | Salsalate | |
|---|---|---|
| Systolic blood pressure (mmHg) | 124 ± 3 | 118 ± 3* |
| Diastolic blood pressure (mmHg) | 72 ± 2 | 69 ± 2 |
| Total cholesterol (mg/dl) | 205 ± 12 | 180 ± 8* |
| LDL-cholesterol (mg/dl) | 130 ± 9 | 113 ± 10 |
| HDL-cholesterol (mg/dl) | 47 ± 3 | 47 ± 3 |
| Triglycerides (mg/dl) | 139 ± 18 | 81 ± 11* |
| Glucose (mg/dl) | 95 ± 2 | 84 ± 2* |
Values are mean ± standard error. LDL, low-density lipoprotein; HDL, high-density lipoprotein
P<0.05 vs. Placebo.
Oral Salsalate Increased Plasma Salicylate Concentrations to Therapeutic Levels
Plasma salicylate concentrations were undetectable at baseline, increased by Day 2 of Salsalate administration (13.5 ± 0.9 mg/100 ml, P<0.0001 vs. baseline) and attained mean concentrations in the mid-therapeutic range (21.8 ± 1.1 mg/100 ml) by Day 4 (P≤0.0001 vs. baseline and Day 2).
Salsalate Increased Expression of IκBα and Reduced Total and Nuclear NFκB in Vascular Endothelial Cells from the Subjects
Compared with placebo, Salsalate increased endothelial cell expression of IκBα (n=11) and reduced expression of total (n=13) and nuclear (n=13) NFκB (all P<0.05, Figures 1A-C). Thus, Salsalate treatment increased IκBα in endothelial cells from our subjects and this was associated with reduced NFκB activation.
Figure 1.
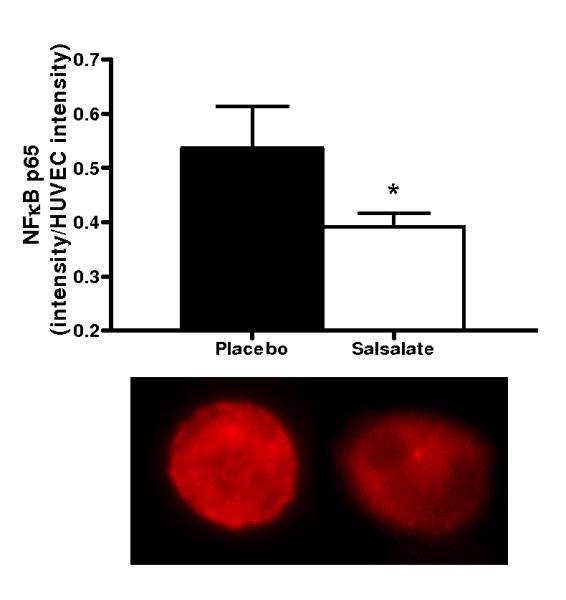
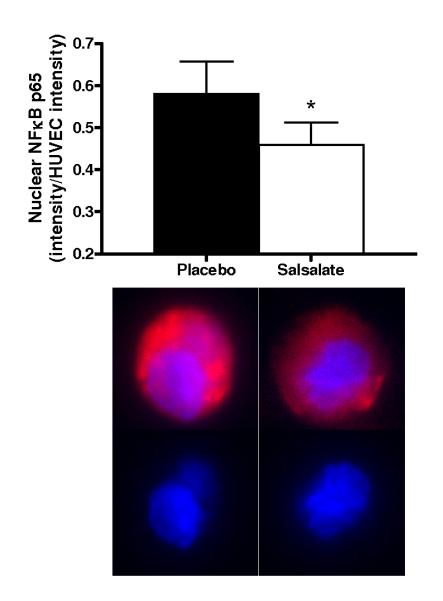
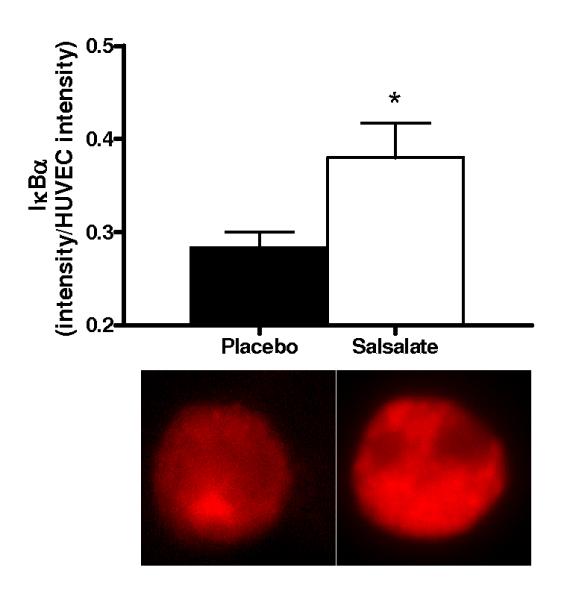
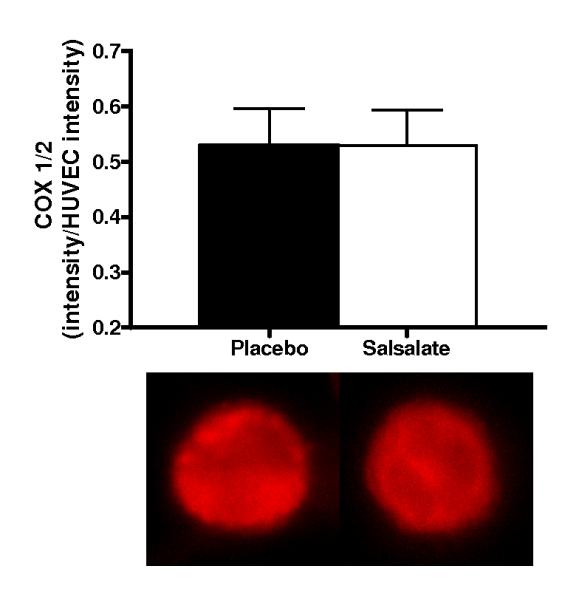
Protein expression of: A) whole cell nuclear factor kappa B (NFκB) p65 (n=13); B) nuclear NFκB p65 (n=13); C) inhibitor of NFκB (IκBα; n=11) and D) cyclooxygenase (COX 1/2; n=10) via quantitative immunofluorescent staining of endothelial cells obtained from an antecubital vein during placebo and Salsalate conditions. Mean ± SE values of fluorescent intensity adjusted for human umbilical vein endothelial cell (HUVEC) intensity are shown with examples of cells below each bar graph from individual subjects after placebo and Salsalate. For panel B, DAPI nuclear staining (blue) overlay on whole cell (red) NFκB p65 and DAPI stain alone are shown. *P<0.05 vs. Placebo.
Salsalate Did Not Affect Expression of COX 1/2 in Vascular Endothelial Cells from the Subjects
Salsalate did not influence endothelial cell expression of COX 1/2 (n=10; P=0.50, Figure 1D). Plasma concentration of 6-keto-prostanglandin F1α was not different (P=0.45), whereas plasma 11-dehydro thromboxane B2 was increased (P<0.01) after Salsalate (Table 3). These results suggest that Salsalate had no effect on COX 1/2 expression in endothelial cells or prostacyclin metabolism, and may have increased thromboxane A2 metabolism.
Salsalate Increased Endothelium-Dependent Dilation Without Affecting Endothelium–Independent Dilation
Compared with placebo, brachial artery FMD was 65% (Δ%) to 74% (Δmm) greater after Salsalate treatment in the overall group (n=14; P<0.001, Figures 2A and 2B). In contrast, Salsalate did not affect endothelium-independent dilation (n=11; P=0.83) (Figures 2C and 2D). Brachial artery FMD was greater after Salsalate in 13 of the 14 subjects (Figure 3A). The change in brachial artery FMD from placebo to Salsalate treatment was strongly inversely related to baseline FMD (n=14; Δ% and Δmm: both r=−0.77, P<0.01, Figure 3B). Salsalate did not affect baseline or peak shear rate (n=11; both P>0.05 vs. placebo). As such, FMD normalized for peak shear rate was 71% greater in the Salsalate condition (0.0024 ± 0.0002 vs. 0.0014 ± 0.0002 mm/sec−1, P<0.001). Based on a 2-tailed alpha level of 0.05, the power achieved for determining differences in brachial FMD with our 14 subjects was 99%. These results demonstrate that Salsalate selectively improved endothelium-dependent dilation, with the greatest improvements occurring in subjects with the most impaired baseline function.
Figure 2.
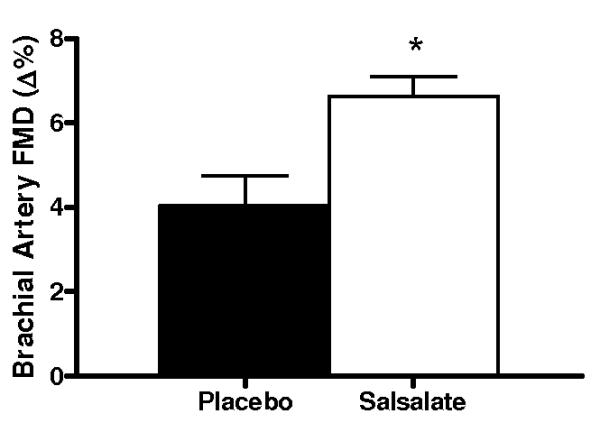
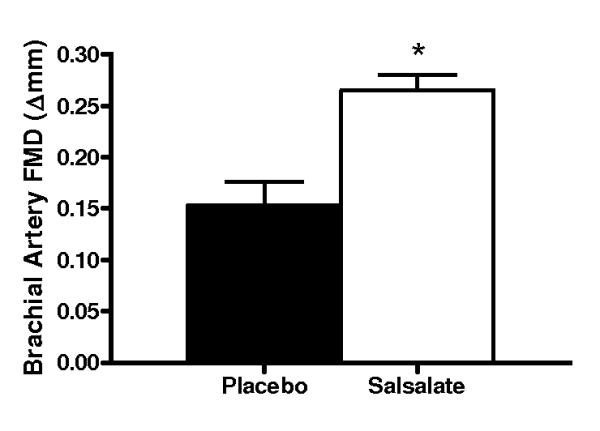
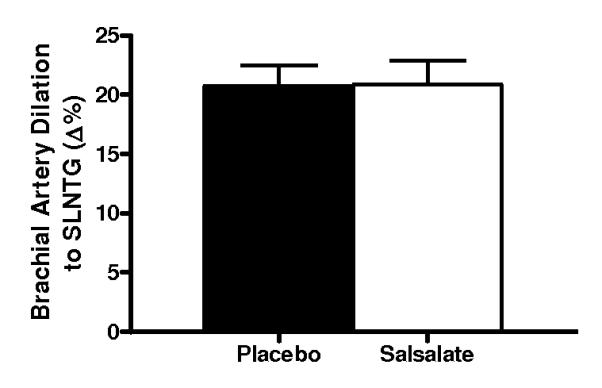
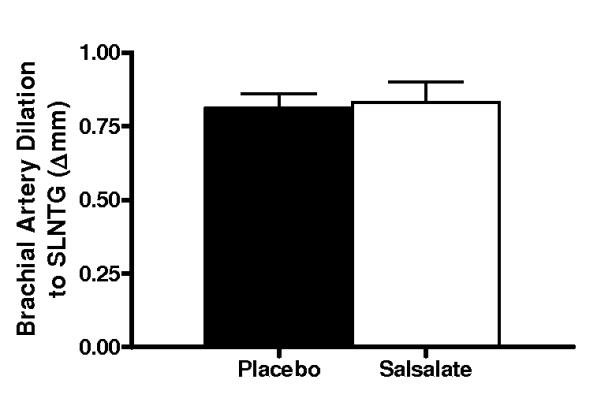
Endothelium-dependent dilation (n=14; brachial artery flow-mediated dilation, FMD; A, percentage change, Δ%; B, absolute change, Δmm) and endothelium independent dilation (n=11; brachial artery dilation with sublingual nitroglycerin, SLNTG; C, percentage change, Δ%; D, absolute change, Δmm) in subjects during placebo and Salsalate conditions. Values are mean ± SE. *P<0.05 vs. Placebo.
Figure 3.
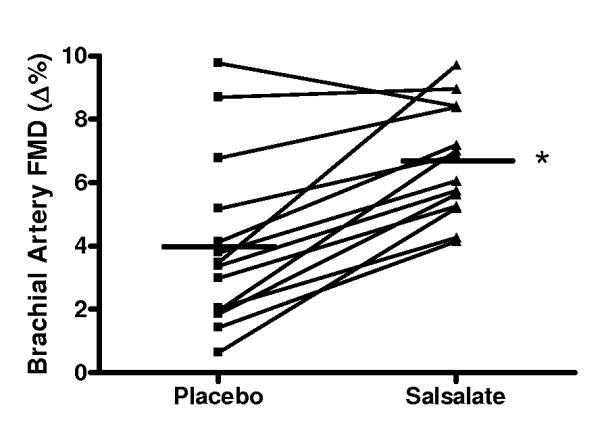
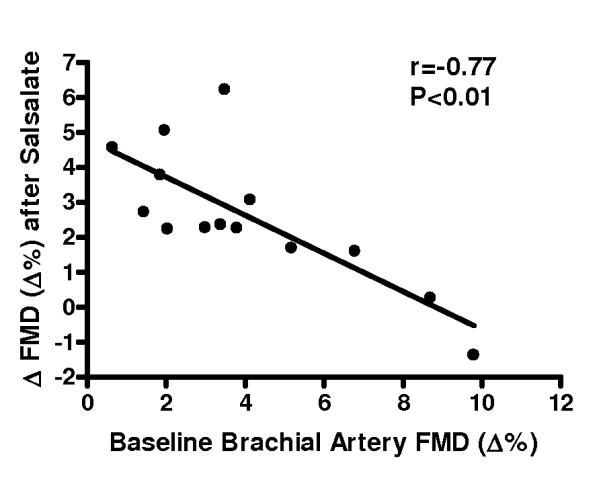
A, Individual values for endothelium-dependent dilation (n=14; brachial artery flow-mediated dilation, FMD; percentage change, Δ%) in subjects after placebo and Salsalate conditions; B, Relation between baseline (placebo condition) brachial artery FMD and the change (Δ) in FMD from placebo to Salsalate conditions. *P<0.05 vs. Placebo.
Salsalate Abolished Oxidative Stress Suppression of Endothelium-Dependent Dilation and Reduced NADPH Oxidase p47phox and Nitrotyrosine in Vascular Endothelial Cells from the Subjects
Compared with the saline control, acute infusion of vitamin C increased brachial artery FMD by ~40% during the placebo condition (P<0.001, Figure 4, left). In contrast, vitamin C infusion had no affect on FMD after Salsalate treatment (P=0.23) (Figure 4, right). Compared with the placebo condition, expression of NADPH oxidase p47phox (n=13; P<0.05) and nitrotyrosine (n=12; P=0.06) were reduced in endothelial cells obtained from the subjects after Salsalate treatment (Figures 5A and 5B). These observations indicate that Salsalate abolishes oxidative stress-associated inhibition of endothelium-dependent dilation and that this is associated with reduced expression of NADPH oxidase and decreased oxidative modifications in vascular endothelial cells.
Figure 4.
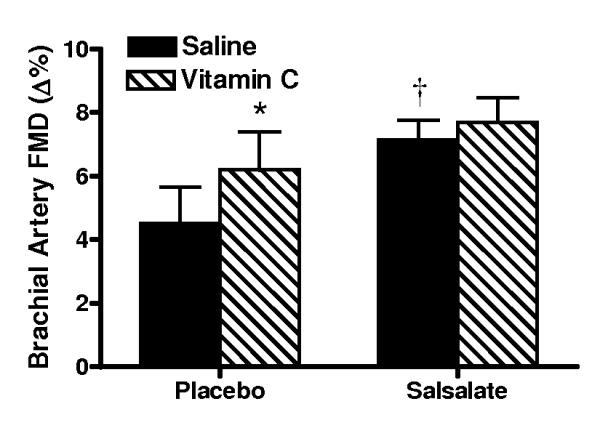
Endothelium-dependent dilation (n=8; brachial artery flow-mediated dilation, FMD) during acute intravenous infusion of saline and ascorbic acid (vitamin C) during placebo and Salsalate conditions. Values are mean ± SE. *P<0.05 vs. Saline. †P<0.05 vs. Placebo.
Figure 5.
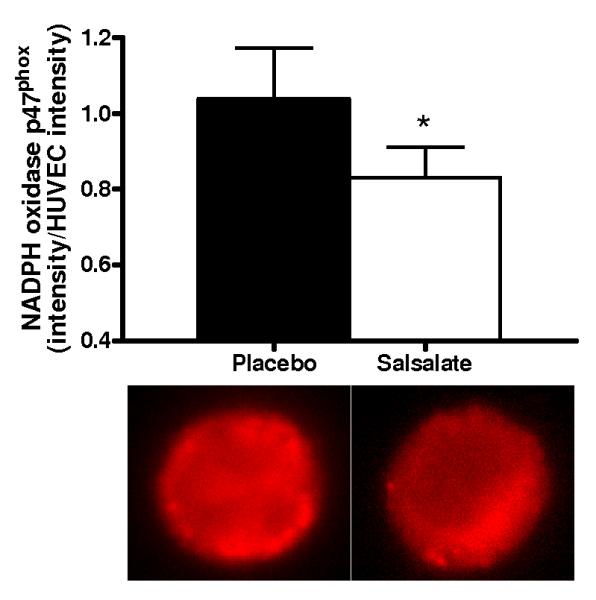
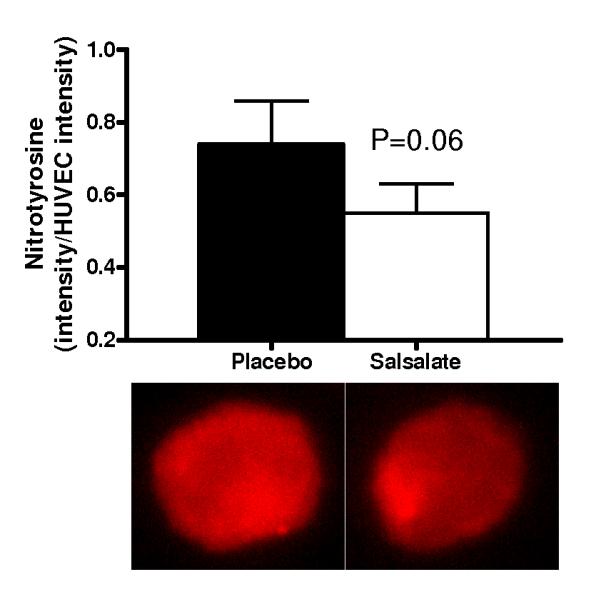
(A) Protein expression of NADPH oxidase p47phox (n=13) and (B) nitrotyrosine abundance (n=12) via quantitative immunofluorescent staining of endothelial cells obtained from an antecubital vein during placebo and Salsalate conditions. Mean ± SE values of fluorescent intensity adjusted for HUVEC intensity are shown with examples of cells below each bar graph from individual subjects after placebo and Salsalate. *P<0.05 vs. Placebo.
Salsalate Did Not Influence Inflammatory Cytokines in Vascular Endothelial Cells from the Subjects
Compared with placebo, endothelial cell protein expression of TNF-α (n=11; P: 0.67 ± 0.05 vs. S: 0.63 ± 0.04 TNF-α intensity/HUVEC intensity, P=0.25) and MCP-1 (n=10; P: 0.25 ± 0.03 vs. S: 0.24 ± 0.03 MCP-1 intensity/HUVEC intensity, P=0.31) was not affected by Salsalate.
Salsalate-Associated Improvements in Endothelium-Dependent Dilation Were Not Related to Other Factors at Baseline or in Response to Salsalate
Resting systolic blood pressure and fasting plasma total cholesterol and glucose were 5-12% lower, and plasma triglyceride concentrations were ~42% lower after Salsalate treatment compared with the placebo condition (all P<0.05, Table 2). Plasma LDL-C and HDL-C (Table 2), markers of inflammation and oxidative stress, and vasoactive proteins (Table 3) were not different after Salsalate (all P>0.05 vs. placebo), although plasma IL-6 tended to be lower (P=0.07). The changes in brachial FMD with Salsalate were not related to subject characteristics, blood pressure or serum factors at baseline, or to changes in plasma salicylate concentrations or any other factor in response to Salsalate administration (all P>0.05).
DISCUSSION
Based on data from cell culture, animal models and analysis of atherosclerotic plaques, NFκB is thought to play an important role in mediating endothelial dysfunction and increased risk of vascular diseases 10, 32-35. To our knowledge, however, there is no direct in vivo evidence in humans that NFκB contributes to chronically impaired vascular endothelial function with aging or obesity.
In the present study, we tested this hypothesis in a group of overweight and obese middle-aged and older adults who had elevated abdominal adiposity and at least one other risk factor for CVD. We found that short-term treatment with therapeutic doses of the NFκB inhibitor Salsalate increased expression of IκBα and reduced both total and nuclear NFκB in endothelial cells obtained from our subjects. The inhibition of NFκB by Salsalate was associated with mean improvements in brachial artery FMD of ~65-75%. The effect was selective for the vascular endothelium as endothelium-independent dilation was unchanged. Importantly, the improvement in brachial FMD with Salsalate was observed in all but one subject and was strongly inversely related to baseline function, indicating that NFκB-mediated suppression of endothelium-dependent dilation was greatest in the subjects with the most marked impairments in baseline vascular function. Infusion of supraphysiological concentrations of the antioxidant ascorbic acid improved brachial FMD during placebo control, but had no effect during Salsalate treatment, indicating that NFκB suppresses endothelium-dependent dilation at least in part via oxidative stress. Consistent with this notion, in endothelial cells obtained from our subjects Salsalate reduced expression of the p47phox subunit of the oxidant-producing enzyme NADPH oxidase as well as abundance of nitrotyrosine, a cellular marker of oxidative modification of proteins. Finally, improvements in FMD with Salsalate were not significantly related to any other baseline measure or to changes in any factor in response to treatment. Taken together, our findings provide the first direct evidence in humans that activation of NFκB contributes to vascular endothelial dysfunction in non-diabetic middle-aged and older adults with elevated total and abdominal body fatness, and chronic low-grade inflammation.
The present findings agree in part with the results of a recent non-randomized, non-controlled pilot intervention study in a small group of HIV-infected patients (mean age 38) in which brachial FMD was significantly increased after 8, but not 4 weeks of Salsalate treatment (1500 mg twice daily) 36. Salsalate administration did not affect plasma markers of inflammation including CRP, IL-6, soluble TNF-α receptor 1, MCP-1 or adhesion molecules. Consistent with these findings, in the present study Salsalate had no significant effects on plasma concentrations of CRP, IL-6, TNF-α, leptin or adiponectin. Together, the results suggest that inhibition of NFκB with Salsalate improves endothelium-dependent dilation in groups with impaired baseline function in the absence of clear reductions in circulating markers of inflammation.
Extensive in vitro evidence indicates that non-acetylated salicylic acids exert their cellular effects by inhibiting phosphorylation of IκB, thus preventing the translocation of NFκB to the nucleus 9, 15, 16. In agreement, here we present in vivo evidence that short-term systemic administration Salsalate increases IκBα expression in endothelial cells from human subjects with impaired baseline endothelial function. The increase in IκBα was associated with reduced nuclear expression of NFκB and a marked increase in endothelium-dependent dilation. The latter findings are consistent with previous observations from our laboratory that NFκB expression is increased in endothelial cells from overweight/obese 12 and older 13, 14 adults, and is inversely related to brachial artery FMD 13.
The results of our study also provide insight into the mechanisms by which NFκB inhibition improves vascular endothelial function in this group. Excessive bioavailability of reactive oxygen species (oxidative stress) suppresses endothelium-dependent dilation in settings of increased adiposity and aging 7, 23, 37. In turn, NFκB stimulates the production of reactive oxygen species at least in part via activation of NADPH oxidase 38, 39. As such, we reasoned that inhibition of NFκB by Salsalate might improve endothelium-dependent dilation by reducing oxidative stress. In support of this, we found that infusion of ascorbic acid (vitamin C) acutely improved brachial artery FMD under placebo conditions, but not after Salsalate treatment. Moreover, that nitrotyrosine was reduced in endothelial cells obtained from our subjects after administration of Salsalate provides molecular evidence for reduced vascular endothelial oxidative stress following treatment. Salsalate also reduced NADPH oxidase, suggesting that reduced expression of this oxidant-producing enzyme could be one mechanism contributing to the decrease in oxidative stress. Together, our functional and molecular data are consistent with the idea that NFκB activation is associated with an upregulation of NADPH oxidase and oxidative stress in vivo in middle-aged and older adults with increased body fatness, and this, in turn, contributes to impaired endothelial function. However, reactive oxygen species also can activate NFκB 10, 40. The present data do not provide insight into the direction of the relation between these events.
In addition to modulating redox signaling in the cell, NFκB facilitates transcription of a large number of pro-inflammatory genes 41. As a result, NFκB also may suppress endothelium-dependent dilation via increased pro-inflammatory signaling. In the present study, Salsalate treatment did not influence plasma inflammatory markers. Insufficient numbers of endothelial cells were available from our technique to measure a wide array of inflammatory proteins in addition to those required to test our formal hypotheses. However, expression of the pro-inflammatory cytokines TNF-α and MCP-1 did not differ in the Salsalate and placebo conditions. Thus, although possible, we have no direct evidence that NFκB inhibited endothelium-dependent dilation through modulation of inflammatory signaling independent of oxidant effects.
Salsalate (i.e., sodium salicylate) is an aspirin-like compound, but lacks an acetyl group needed to directly inhibit COX-mediated production of prostaglandins 17, 42. In the present study, we established that Salsalate treatment did not affect COX 1/2 expression in vascular endothelial cells obtained from our subjects or plasma concentration of 6-keto-prostanglandin F1α , a marker of prostacylin metabolism. Plasma 11-dehydro thromboxane B2 was greater after Salsalate, suggesting that thromboxane A2 metabolism may have been increased. Together, these data indicate that Salsalate did not inhibit COX. Indeed, Salsalate produced an improvement in FMD despite a possible increase in circulating thromboxane, which has vasoconstrictor effects 43.
We measured blood pressure and a variety of serum factors that could have changed with Salsalate treatment and influenced vascular function. Salsalate did not affect the majority of these factors. However, systolic blood pressure, plasma total cholesterol and fasting glucose were modestly reduced, and a marked reduction in fasting plasma triglycerides was observed after only 4 days of Salsalate. These effects were not a result of changes in diet because subjects were placed on an identical research diet for 3 days prior to each experimental visit, thus ensuring similar total energy and macronutrient intake during the placebo and Salsalate conditions (see on-line Data Supplement Table I). The improvements in brachial FMD with Salsalate were not related to baseline levels or treatment-related changes in any factor. However, it is possible that such relations existed, but were not evident because of the small number of subjects. Therefore, we cannot rule out the possibility that the reductions in these selective risk factors contributed to improvements in FMD with Salsalate.
Because the acquisition of arterial endothelial cells would have required invasive arterial catheterization twice (placebo and Salsalate conditions) over a short period of time, venous sampling was used in the present study. However, we 13, 14 and others 44, 45 have shown that group- and treatment-associated differences in expression of NFκB, COX and other proteins can be assessed in endothelial cells obtained from peripheral veins. Moreover, Salsalate was administered systemically, and we were able to show the expected effects of this compound on expression of IκBα and total and nuclear NFκB in endothelial cells obtained from antecubital veins of our subjects.
We believe that our findings have important clinical relevance. Overweight and obese middle-aged and older adults constitute a significant portion of the population at elevated risk of CVD. Vascular endothelial dysfunction is considered a central feature of clinical vascular disorders. Despite this, the cellular and molecular mechanisms that contribute to impaired endothelial function in humans are incompletely understood. The present results provide unique and direct in vivo evidence supporting a key role for the transcription factor NFκB in mediating vascular endothelial dysfunction in humans and provide insight into the underlying mechanisms. Inhibition of NFκB with Salsalate improved brachial artery FMD in our subjects from 4.0 ± 0.4 to 6.6 ± 0.5%. By comparison, using the same method we find that brachial FMD averages 7.2% in young adult controls studied in our laboratory (sample n=62, 47M/15F, 22.7 ± 0.4 years of age). Thus, our results suggest that as much as 80% of the impairment in brachial artery FMD was restored by short-term treatment with Salsalate. Although we cannot definitively attribute this entire effect to inhibition of NFκB, our results strongly implicate NFκB activation and its associated mechanisms of action.
In summary, the present findings provide the first direct evidence in humans that NFκB, in part via stimulation of oxidative stress, plays an important role in mediating endothelial dysfunction in peripheral conduit arteries. Our results provide further support for the concept that inhibition of NFκB may be an effective therapeutic strategy in the prevention and treatment of age- and obesity-related vascular endothelial dysfunction in humans.
Commentary
Middle-aged and older overweight and obese adults are at elevated risk of cardiovascular disease (CVD). This is attributable in part to vascular endothelial dysfunction, as indicated by impaired endothelium-dependent dilation (EDD). However, the cellular and molecular mechanisms involved are incompletely understood. The present study provides direct in vivo evidence supporting a key role for the proinflammatory, redox-sensitive transcription factor, nuclear factor κB (NFκB), in mediating vascular endothelial dysfunction in overweight/obese middle-aged and older adults. In 14 non-diabetic (body mass index ≥25 kg/m2) men and women aged 50-79 years (randomized, double blind, placebo controlled, crossover study), 4 days of treatment with oral Salsalate (non-acetylated salicylate, 4500 mg/d), a NFκB inhibitor, reduced total and nuclear NFκB in vascular endothelial cells and improved brachial artery flow-mediated dilation (FMD) by 74%. Improvements in brachial artery FMD with Salsalate were inversely related to baseline FMD (r=−0.77). Infusion of the antioxidant vitamin C improved brachial artery FMD during placebo, but not after Salsalate. Salsalate reduced nitrotyrosine, a marker of oxidative stress, and expression of the oxidant enzyme NADPH oxidase p47phox in vascular endothelial cells. Salsalate also reduced systolic blood pressure and improved plasma lipids and glucose. The present findings suggest that NFκB, partly via stimulation of oxidative stress, plays an important role in mediating endothelial dysfunction in peripheral conduit arteries in humans. Our results provide support for the concept that inhibition of NFκB may be an effective therapeutic strategy in the prevention and treatment of age- and obesity-related vascular endothelial dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Rhea Chiang for technical assistance.
FUNDING SOURCES This work was supported by National Institutes of Health awards AG006537, AG013038, AG015897, AG022241, AG000279 and RR00051.
Footnotes
DISCLOSURES None.
REFERENCES
- 1.Lakatta E, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Stevens J, Cai J, Pamuk E, Williamson D, Thun M, Wood J. Effect of age on the association between body mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung H, Sung B, Jung K, Zou Y, Yu B. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A, Ungvari Z, Edwards J, Kaminski P, Wolin M, Koller A, Kaley G. Aging- induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- 7.Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli P. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress. Protective effect of vitamin c. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 8.Read MA, Whitley MZ, Williams AJ, Collins T. NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J Exp Med. 1994;179:503–512. doi: 10.1084/jem.179.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce J, Read M, Ding H, Luscinskas F, Collins T. Salicylates inhibit IkB-a phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J of Immunol. 1996;156:3961–3969. [PubMed] [Google Scholar]
- 10.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 11.Kralisch S, Sommer G, Stangl V, Kohler U, Kratzsch J, Stepan H, Faber R, Schubert A, Lossner U, Vietzke A, Bluher M, Stumvoll M, Fasshauer M. Secretory products from human adipocytes impair endothelial function via nuclear factor kappaB. Atherosclerosis. 2008;196:523–531. doi: 10.1016/j.atherosclerosis.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation. 2007;115:627–637. doi: 10.1161/CIRCULATIONAHA.106.657486. [DOI] [PubMed] [Google Scholar]
- 13.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 14.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR. Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell. 2008 Sep 8; doi: 10.1111/j.1474-9726.2008.00438.x. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 16.Yin M, Yamamoto Y, Gaynor R. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IkB kinase-B. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 17.Wu K. Aspirin and salicylate. Circulation. 2000;102:2022–2023. doi: 10.1161/01.cir.102.17.2022. [DOI] [PubMed] [Google Scholar]
- 18.Christou DD, Gentile CL, DeSouza CA, Seals DR, Gates PE. Fatness is a better predictor of cardiovascular disease risk factor profile than aerobic fitness in healthy men. Circulation. 2005;111:1904–1914. doi: 10.1161/01.CIR.0000161818.28974.1A. [DOI] [PubMed] [Google Scholar]
- 19.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol. 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 20.DeSouza CA, Shapiro L, Clevenger C, Dinenno F, Monahan K, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 21.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol. 2007;102:63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 22.Eskurza I, Kahn Z, Seals DR. Xanthine oxidase does not contribute to impaired peripheral artery conduit artery endothelium-dependent dilatation with aging. J Physiol. 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskurza I, Monahan K, Robinson J, Seals DR. Effect of acute and chronic ascorbic acid augmentation on flow-mediated dilation with physically active and sedentary aging. J Physiol. 2004;556:215–224. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskurza I, Myerburgh L, Khan Z, Seals DR. Tetrahydrobiopterin augments endothelial-dependent dilation in sedentary but not habitually exercising older adults. J Physiol. 2005;568.3:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson MH, Menard HA, Kalish GH. Assessment of salsalate, a nonacetylated salicylate, in the treatment of patients with arthritis. Clin Ther. 1995;17:827–837. doi: 10.1016/0149-2918(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 28.Cryer B, Goldschmiedt M, Redfern JS, Feldman M. Comparison of salsalate and aspirin on mucosal injury and gastroduodenal mucosal prostaglandins. Gastroenterology. 1990;99:1616–1621. doi: 10.1016/0016-5085(90)90465-d. [DOI] [PubMed] [Google Scholar]
- 29.Dromgoole SH, Cassell S, Furst DE, Paulus HE. Availability of salicylate from salsalate and aspirin. Clin Pharmacol Ther. 1983;34:539–545. doi: 10.1038/clpt.1983.211. [DOI] [PubMed] [Google Scholar]
- 30.Dromgoole SH, Furst DE, Paulus HE. Metabolism of salsalate in normal subjects. J Pharm Sci. 1984;73:1657–1659. doi: 10.1002/jps.2600731147. [DOI] [PubMed] [Google Scholar]
- 31.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr., Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 32.Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- 33.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta SK, Johnson RM, Saha C, Mather KJ, Greenwald ML, Waltz JS, Rehman J, Dube MP. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. AIDS. 2008;22:653–655. doi: 10.1097/QAD.0b013e3282f470d2. [DOI] [PubMed] [Google Scholar]
- 37.Keaney JF, Jr., Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 38.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 39.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 40.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 41.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 42.Awtry E, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 43.Sellers MM, Stallone JN. Sympathy for the devil: the role of thromboxane in the regulation of vascular tone and blood pressure. Am J Physiol Heart Circ Physiol. 2008;294:H1978–1986. doi: 10.1152/ajpheart.01318.2007. [DOI] [PubMed] [Google Scholar]
- 44.Colombo P, Ashton A, Celaj S, Talreja A, Banchs J, Dubois N, Mariaccio M, Malla S, Lachmann J, Ware J, Le Jemtel T. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 45.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, DuBois NB, Ashton AW, Latif F, Jorde UP, Ware JA, LeJemtel TH. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111:58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


