Abstract
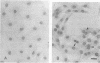
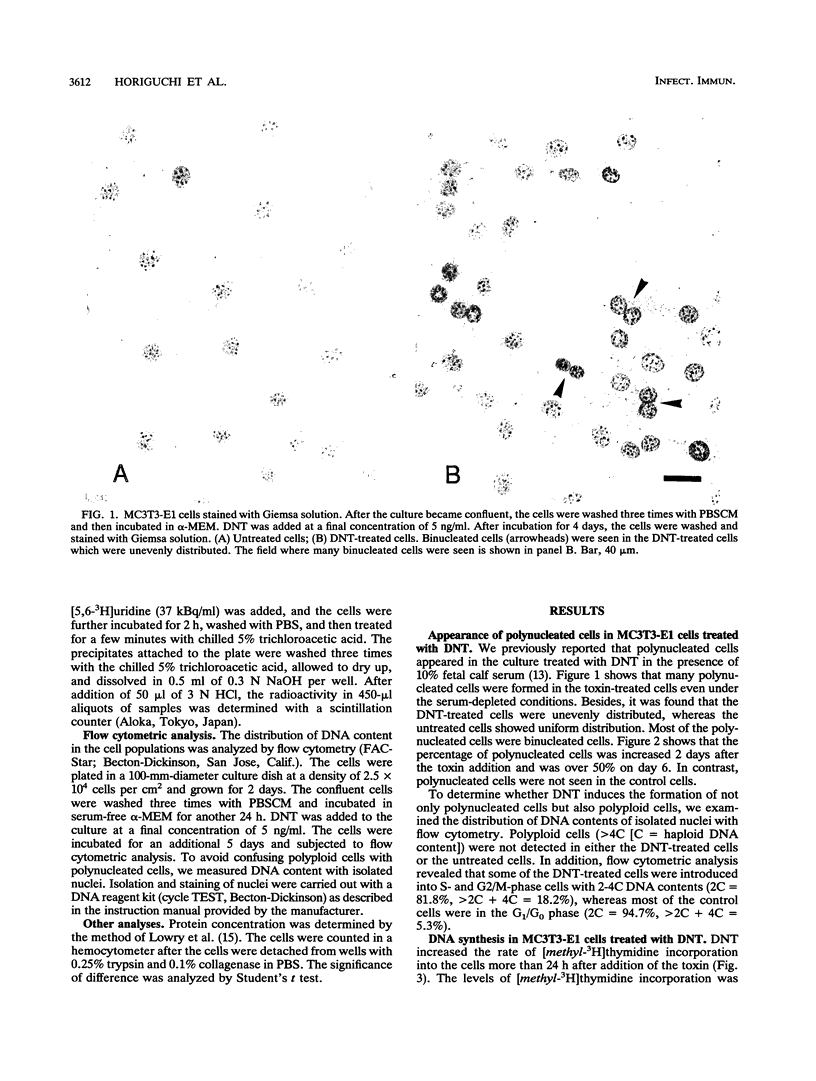
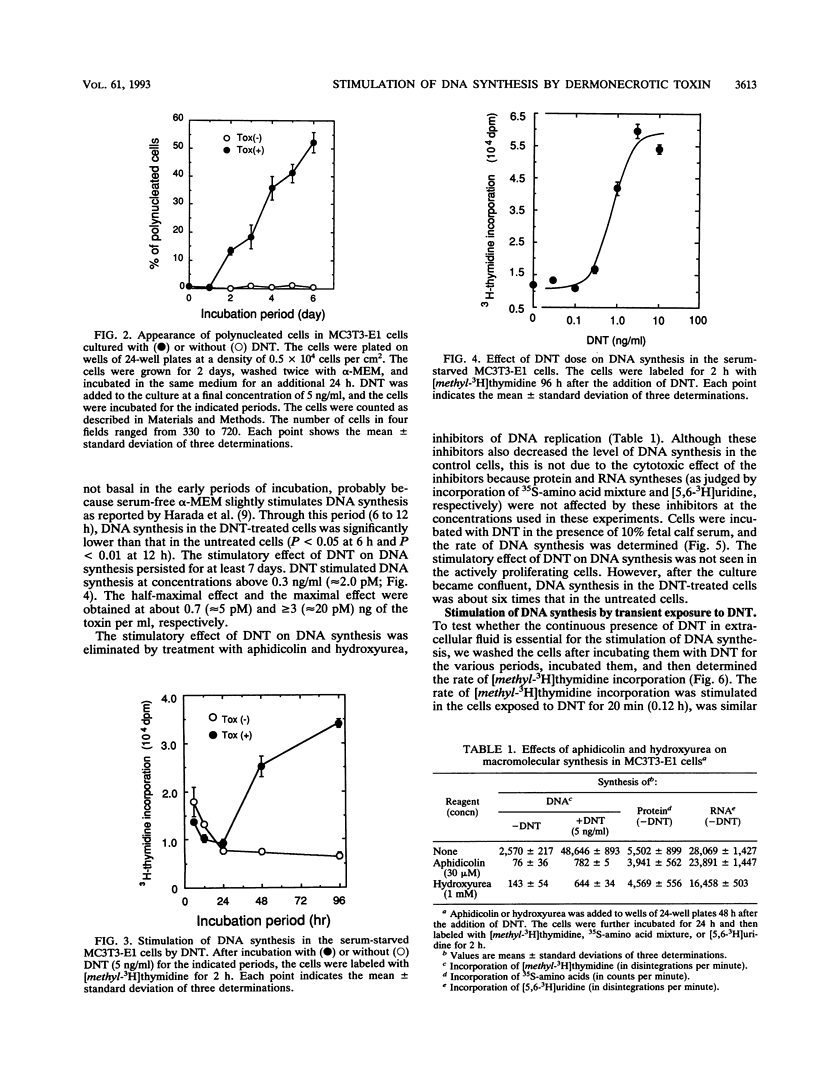
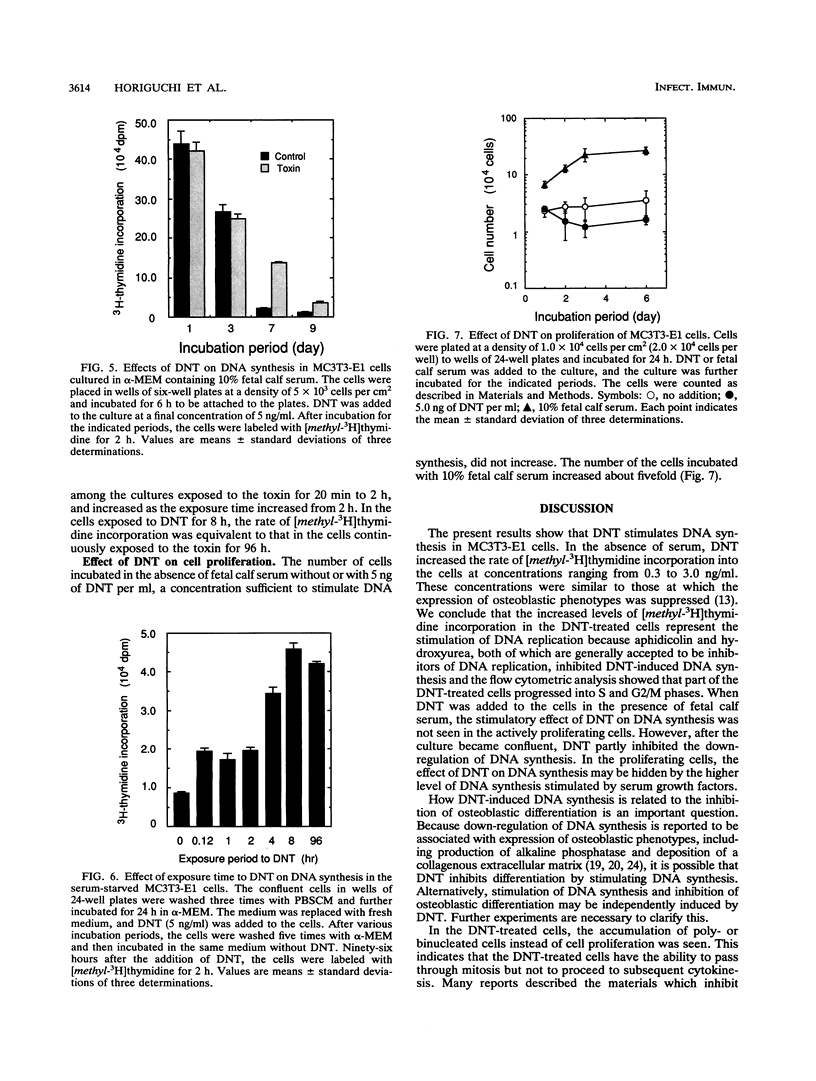
We investigated the effects of Bordetella bronchiseptica dermonecrotic toxin on DNA synthesis in MC3T3-E1 cells. The rate of [methyl-3H]thymidine incorporation increased in the toxin-treated cells more than 24 h after addition of the toxin under the serum-starved conditions. This effect was dependent on the toxin concentration ranging from 0.3 to 3 ng/ml and was eliminated by aphidicolin and hydroxyurea, inhibitors for DNA replication. In the toxin-treated culture, the number of cells did not increase but polynucleated cells appeared and their number increased to ca. 50% of the total number of cells 6 days after the toxin addition. From these results, we concluded that the toxin stimulates DNA replication in MC3T3-E1 cells without cell proliferation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M. R., Rimler R. B., Thurston J. R. Experimental model of atrophic rhinitis in gnotobiotic pigs. Infect Immun. 1991 Oct;59(10):3626–3629. doi: 10.1128/iai.59.10.3626-3629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominick M. A., Rimler R. B. Turbinate atrophy in gnotobiotic pigs intranasally inoculated with protein toxin isolated from type D Pasteurella multocida. Am J Vet Res. 1986 Jul;47(7):1532–1536. [PubMed] [Google Scholar]
- Duncan J. R., Ross R. F., Switzer W. P., Ramsey F. K. Pathology of experimental Bordetella bronchiseptica infection in swine: atrophic rhinitis. Am J Vet Res. 1966 Mar;27(117):457–466. [PubMed] [Google Scholar]
- Eliás B., Boros G., Albert M., Tuboly S., Gergely P., Papp L., Barna Vetró I., Rafai P., Molnár E. Clinical and pathological effects of the dermonecrotic toxin of Bordetella bronchiseptica and Pasteurella multocida in specific-pathogen-free piglets. Nihon Juigaku Zasshi. 1990 Aug;52(4):677–688. doi: 10.1292/jvms1939.52.677. [DOI] [PubMed] [Google Scholar]
- Hakeda Y., Harada S., Matsumoto T., Tezuka K., Higashino K., Kodama H., Hashimoto-Goto T., Ogata E., Kumegawa M. Prostaglandin F2 alpha stimulates proliferation of clonal osteoblastic MC3T3-E1 cells by up-regulation of insulin-like growth factor I receptors. J Biol Chem. 1991 Nov 5;266(31):21044–21050. [PubMed] [Google Scholar]
- Hakeda Y., Hotta T., Kurihara N., Ikeda E., Maeda N., Yagyu Y., Kumegawa M. Prostaglandin E1 and F2 alpha stimulate differentiation and proliferation, respectively, of clonal osteoblastic MC3T3-E1 cells by different second messengers in vitro. Endocrinology. 1987 Dec;121(6):1966–1974. doi: 10.1210/endo-121-6-1966. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Mabuchi I. Effects of phalloidin microinjection and localization of fluorescein-labeled phalloidin in living sand dollar eggs. Cell Motil. 1982;2(2):103–113. doi: 10.1002/cm.970020203. [DOI] [PubMed] [Google Scholar]
- Hanada M., Shimoda K., Tomita S., Nakase Y., Nishiyama Y. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1979 Feb;41(1):1–8. doi: 10.1292/jvms1939.41.1. [DOI] [PubMed] [Google Scholar]
- Harada S., Matsumoto T., Ogata E. Role of ascorbic acid in the regulation of proliferation in osteoblast-like MC3T3-E1 cells. J Bone Miner Res. 1991 Sep;6(9):903–908. doi: 10.1002/jbmr.5650060902. [DOI] [PubMed] [Google Scholar]
- Hata R., Hori H., Nagai Y., Tanaka S., Kondo M., Hiramatsu M., Utsumi N., Kumegawa M. Selective inhibition of type I collagen synthesis in osteoblastic cells by epidermal growth factor. Endocrinology. 1984 Sep;115(3):867–876. doi: 10.1210/endo-115-3-867. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y., Nakai T., Kume K. Effects of Bordetella bronchiseptica dermonecrotic toxin on the structure and function of osteoblastic clone MC3T3-e1 cells. Infect Immun. 1991 Mar;59(3):1112–1116. doi: 10.1128/iai.59.3.1112-1116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi Y., Nakai T., Kume K. Purification and characterization of Bordetella bronchiseptica dermonecrotic toxin. Microb Pathog. 1989 May;6(5):361–368. doi: 10.1016/0882-4010(89)90078-8. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y., Nakai T., Kume K. Simplified procedure for purification of Bordetella bronchiseptica dermonecrotic toxin. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):39–43. doi: 10.1016/0378-1097(90)90255-o. [DOI] [PubMed] [Google Scholar]
- Kimman T. G., Löwik C. W., van de Wee-Pals L. J., Thesingh C. W., Defize P., Kamp E. M., Bijvoet O. L. Stimulation of bone resorption by inflamed nasal mucosa, dermonecrotic toxin-containing conditioned medium from Pasteurella multocida, and purified dermonecrotic toxin from P. multocida. Infect Immun. 1987 Sep;55(9):2110–2116. doi: 10.1128/iai.55.9.2110-2116.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Kume K. Characterization of dermonecrotic toxin produced by serotype D strains of Pasteurella multocida. Am J Vet Res. 1984 Nov;45(11):2410–2413. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Samejima Y., Kume K. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect Immun. 1984 Nov;46(2):429–434. doi: 10.1128/iai.46.2.429-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Aronow M., Shalhoub V., Barone L. M., Wilming L., Tassinari M. S., Kennedy M. B., Pockwinse S., Lian J. B., Stein G. S. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990 Jun;143(3):420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- Quarles L. D., Yohay D. A., Lever L. W., Caton R., Wenstrup R. J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992 Jun;7(6):683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Higgins T., Chanter N., Lax A. J., Staddon J. M. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc Natl Acad Sci U S A. 1990 Jan;87(1):123–127. doi: 10.1073/pnas.87.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972 May;53(2):419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Lian J. B., Owen T. A. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990 Oct;4(13):3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- Sudo H., Kodama H. A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983 Jan;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]