Abstract
Antigen-specific T cells play a major role in mediating the pathogenesis of a variety of autoimmune conditions as well as other diseases. In the context of experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis, we present here a general approach to the discovery of highly specific ligands for autoreactive cells. These ligands are obtained from a combinatorial library of hundreds of thousands of synthetic peptoids that is screened simultaneously against two populations of CD4+ T cells. Peptoids that recognize autoreactive T cells with extremely high specificity can be identified in the library. Since no specific knowledge is required regarding the nature of the native antigens recognized by the autoreactive T cells, this technology provides a powerful tool for the enrichment and inhibition of autoimmune cells in a variety of disease states.
Introduction
The molecular basis of many autoimmune diseases remains unknown. In general, the immune system recognizes a native molecule as a foreign antigen and mounts an attack on self-tissue harboring these molecules. But exactly why this occurs and the nature of the self-antigens that trigger and drive the process are often unclear. Due in part to this lack of a molecular-level understanding, the state of the art in the development of diagnostic agents and effective therapies for autoimmune diseases is far from optimal. Almost without exception, drugs employed to treat these conditions either inhibit an event downstream of the autoimmune response itself, such as inflammation, or attempt to modulate the immune system non-selectively [1], with significant undesirable side effects. Molecules that target autoreactive T cells directly, but ignore T cells that recognize foreign antigens, would valuable tools in medicine for the detection and enrichment of autoimmune T cells. In addition, these molecules could serve as the foundation for a novel drug development program aimed at eradicating these autoreactive cells without affecting the proper function of the immune system.
Multiple Sclerosis (MS) is an immune-mediated inflammatory disease of the central nervous system (CNS) that results in demyelination and neurologic disability [2]. The MS-like condition of EAE is induced in genetically susceptible strains of rodents by immunization with myelin proteins or peptides, or by passive transfer of myelin-specific CD4+ T cells [3]. Studies in EAE indicate that myelin-specific CD4+ T cells that have become activated in the periphery, and produce pro-inflammatory cytokines, play a major role in disease pathogenesis of MS [3]. Moreover, these T cells express T cell receptors that are believed to preferentially recognize myelin basic protein in the central nervous system of affected individuals leading to destruction of the myelin sheath and, ultimately, neurological deficit [3]. Therefore, a therapeutic strategy that specifically targets only autoreactive T cells would be interesting to investigate for MS.
Results and Discussion
A screen for specific autoreactive T cell ligands in EAE
As a first step towards exploring this possibility, we focused on the isolation of synthetic compounds capable of highly specific binding to autoreactive T cells in EAE. To accomplish this, we adapted a screening strategy developed previously in our laboratory for the isolation of peptoids (oligo-N-substituted glycines [4]) that bind to G protein-coupled receptors (GPCRs) with high specificity [5]. In this protocol, cells that do or do not express the target receptor, but are presumed to be otherwise identical, are labeled with red and green quantum dots, respectively, mixed together, and incubated with thousands of hydrophilic beads that display different peptoids (each bead displays a unique peptoid). Beads that bind only the red-labeled cells, but not the green cells are then collected, the presumption being that this reflects highly specific binding to the target receptor since the peptoid must ignore all other molecules on the cell surface in order to exclude the green cells and be scored as a “hit” (Figure 1A).
Figure 1. Identification of putative autoreactive T cell binding peptoids using a bicolor on-bead screening protocol.
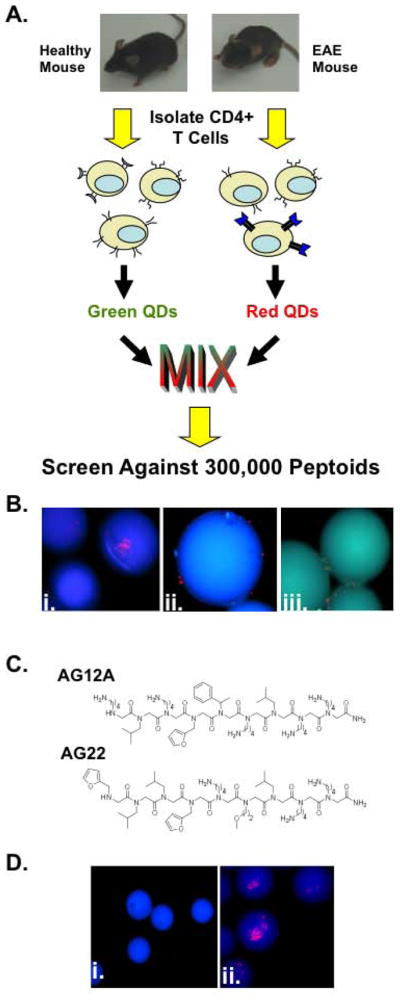
(A) Schematic representation of the peptoid screening protocol. CD4+ T cells isolated from EAE mice have an increased frequency of Vα2.3/Vβ8.2 MBP Ac1-11 specific T cell receptors compared to wild type healthy control littermates. Following isolation, T cells were differentially labeled with green and red quantum dots and screened against a bead-displayed peptoid library. Peptoid beads binding only cells from EAE mice were selected and sequenced. (B) Fluorescent microscopic images of peptoid beads after screening and washing (100X magnification; DAPI filter). i.and ii. Photographs depicting the 2 putative hit peptoid beads bound to CD4+ T cells from EAE mice (red stained cells). iii. Photograph depicting peptoid beads binding to CD4+ T cells from healthy mice and EAE mice. (C) Chemical structures of the two hits identified in the screen. (D) Fluorescent microscopic images of tentagel beads displaying AG12A bound to autoreactive T cells. i. CD4+ T cells from B10.PL wildtype control mice do not bind AG12A peptoid beads. ii. CD4+ T cells from Vα2.3/Vβ8.2 MBP Ac1-11 TCR transgenic mice bind to AG12A peptoid beads.
To apply this two-color screening technology to the present problem, EAE was induced in B10.PL mice by immunization with the myelin basic protein peptide Ac1-11 (MBP Ac1-11) and Complete Freund’s Adjuvant (CFA). Immunization with this myelin peptide results in activation and expansion of CD4+ T cells expressing the MBP Ac1-11 specific Vα2.3/Vβ8.2 TCR [6]. These animals, or mice immunized with CFA alone were sacrificed following the development of clinically definite EAE (supplementary figure 1A) and the CD4+ T cells were isolated. CD4+ T cells from EAE mice and controls were labeled with red and green-emitting quantum dots, respectively. The cells were then mixed together in a 1:1 ratio and incubated with a bead-displayed peptoid library containing approximately 300,000 peptoids (supplementary figure 1B). We hypothesized that the millions of different T cells in the overall population should all be present at low levels and that the two populations would be rather similar. The major exception, of course, would be an increased number of MBP Ac1-11 specific autoreactive T cells that expanded in response to immunization with the autoantigen in the EAE mice. This suggested that if a bead was found with only red cells, these were highly likely to be the autoreactive T cells (Figure 1A).
Following incubation with the peptoid beads, only beads binding red-labeled T cells were selected as possible hits. Using this methodology, we identified two putative hit peptoids that were observed to bind specifically to CD4+ T cells from EAE mice and not to T cells from the control animals (figure 1B panels i. and ii.). The peptoids on the two beads scored as hits were sequenced by Edman degradation [7] Their deduced structures are illustrated in Figure 1C. The two “hits” were found to have some sequence similarity, so we elected to focus on one of them (AG12A) for more detailed characterization. Neither had any obvious structural relationship to the antigenic peptide (Ac-ASQKRPSQRSK) other than the presence of some positively charged residues.
The AG12A peptoid is a ligand for EAE autoreactive T cells
To further evaluate the binding of AG12A to the autoreactive T cells, we took advantage of the existence of transgenic mice, in which the vast majority of CD4+ T cells express the MBP Ac1-11 specific TCR Vα2.3/Vβ8.2 [8]. AG12A was resynthesized on beads, as was a control peptoid (see Supplementary Figure 2). The beads were incubated with red quantum dot-labeled MBP Ac1-11 specific T cells. As shown in Figure 1D, CD4+ T cells from MBP Ac1-11 TCR transgenic mice bound to AG12A displayed on beads, where as WT CD4+ T cells did not (Figure 1D).
A solution phase binding experiment was also carried out using flow cytometry as the readout. Initial attempts to measure binding of AG12A to MBP Ac1-11 specific T cells resulted in a lower than expected amount of binding. This, we concluded, was probably due to the rapid dissociation of the receptor/ligand binding since there is no opportunity for avidity effects to stabilize the peptoid-T cell interaction as is the case when the peptoid is densely displayed on a bead surface. In an attempt to stabilize the binding of the peptoid to its target receptor on the T cells, we utilized a chemical cross-linking technique that involves the oxidation of dihydroxyphenylalanine (DOPA) attached to the peptoid to an ortho-quinone intermediate (see Supplementary Fig. 3 for the structures of the modified peptoids). This intermediate can then cross-link to nearby nucleophilic residues on the target receptor protein [9–11]. Biotin-DOPA-AG12A and a control biotin-DOPA peptoid were synthesized and incubated with CD4+ T cells from MBP Ac1-11 TCR transgenic mice or wildtype controls. Following addition of the oxidizing agent, sodium periodate, the reaction was quenched and the cross-linked cells were stained with fluorochrome-conjugated streptavidin and fluorochrome-conjugated anti-CD4+. Peptoid binding to the T cells was assessed by calculating the mean fluorescence intensity of CD4+/streptavidin+ cells. AG12A bound to MBP Ac1-11 specific T cells with a half maximal binding activity of approximately 40μM (Figure 2A and 2B; note that this cross-linking experiment does not monitor equilibrium binding, so this value may not correspond to the true KD). No significant interaction between biotinylated AG12A and wild-type T cells could be detected, nor did the biotinylated control peptoid bind to the Vα2.3/Vβ8.2 TCR transgenic T cells (Fig. 2B). However, a constant level of autofluorescence was seen in the peptoid treated wild-type T cells that was not present in the transgenic population. The reason for this autofluorescence is not completely understood, but is believed to be related to the DOPA mediated cross-linking reaction in this heterogeneous population, as it was not present in the initial flow cytometry experiments performed without cross-linking. In addition, with low doses of AG12A we found a small number of cells that displayed a high amount of binding to the transgenic T cells. This finding, we believe, was due to the difficulty associated with properly washing a cross-linked population of cells. Therefore, despite these minor technical issues, we believe that these data demonstrate clearly the specific binding of AG12A to the autoreactive T cell population.
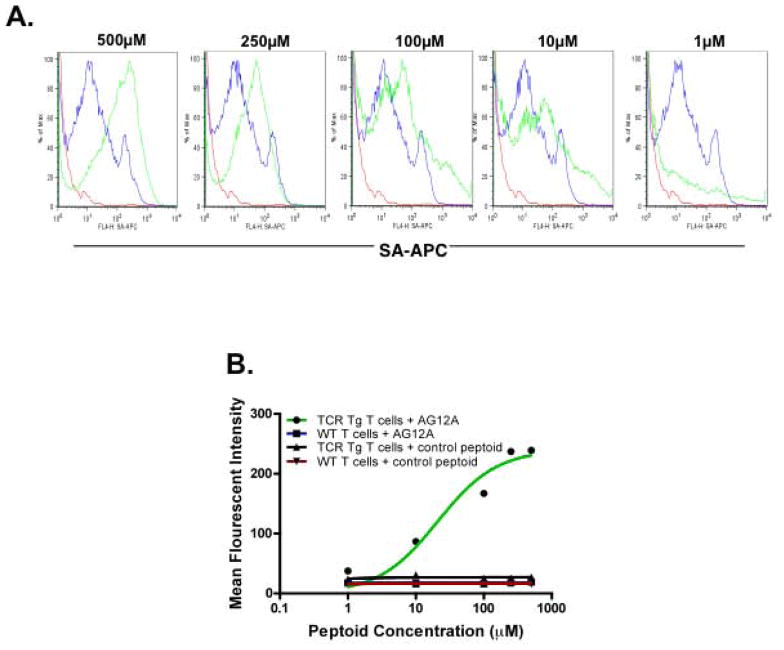
Figure 2. AG12A binds MBP Ac1-11 specific T cells with mid micromolar affinity and high specificity.
(A) Flow cytometric analysis of Vα2.3/Vβ8.2 MBP Ac1-11 TCR transgenic vs. B10.PL wildtype CD4+ T cells in the presence of increasing concentrations of biotin-DOPA-AG12A. Cells were pre-incubated with 1μM, 10μM, 100μM, 250μM, or 500μM concentrations of biotin-DOPA-AG12A, cross-linked and stained with anti-CD4-PerCP-Cy5.5 and anti-streptavidin-allophycocyanin (APC). 2 color flow cytometry was used to determine the estimated binding affinity of biotinylated AG12A for autoreactive CD4+ T cells. The results are depicted as overlaying histograms with the green line representing Vα2.3/Vβ8.2 MBP Ac1-11 TCR transgenic T cells and the blue line representing B10.PL wildtype CD4+ T cells. The red line represents a no peptoid negative control. The mean fluorescent intensity (MFI) was determined for each concentration of peptoid tested using Flowjo software. Only Vα2.3/Vβ8.2 MBP Ac1-11 TCR transgenic T cells were found to bind AG12A. Results shown are representative of three independent experiments. (B) Binding isotherm of AG12A for Vα2.3/Vβ8.2 MBP Ac1-11 TCR transgenic T cells evaluated by flow cytometry. MFI for each concentration of peptoid tested was plotted for TCR transgenic T cells + AG12A, WT T cells + AG12A, TCR transgenic T cells + control peptoid, and WT T cells + control peptoid. The Kd was calculated using Graphpad Prism software and estimated to be approximately 40μM.
Ex vivo inactivation of autoreactive T cells using a ruthenium-peptoid conjugate
Using the screening conditions developed in our laboratory, peptoids are isolated routinely that bind their target with equilibrium dissociation constants in the mid to low micromolar range [12]. Therefore, the fact that AG12A binds to MBP Ac1-11 autoreactive CD4+ T cells with a half maximal binding activity of approximately 40 μm was unsurprising. Of course, for practical applications, particularly for the inhibition of autoreactive T cell proliferation, a higher affinity compound would be desirable and efforts are underway to optimize the structure of the peptoid. However, an alternative and far more rapid approach to the development of a potent inhibitor was developed recently in our laboratory ([13] and Lee, J., et al., submitted for publication). This approach involves conjugation of the peptoid to a ruthenium(II) tris-bipydridyl complex that is an efficient catalyst for the generation of singlet oxygen when irradiated with visible light. Singlet oxygen is a highly reactive species that will modify and inactivate most proteins, but which has a limited diffusion radius of only 40–80 Å. Thus, only proteins in the immediate vicinity of the ruthenium “warhead” are affected. When delivered to target proteins by the peptoid ligand, highly specific photo-triggered protein inactivation can be achieved.
MBP Ac-1-11 specific TCR transgenic T cells were incubated with increasing concentrations of the AG12A-ruthenium conjugate (Figure 3A) or a control peptoid-ruthenium conjugate (Supplementary Figure 2) and the cells were irradiated with visible light for ten minutes (<380 nm cut-off filter). Following irradiation, the T cells were activated with the autoantigen MBP Ac1-11 in the presence of antigen presenting cells. Cell proliferation was assessed using a tritiated thymidine assay. As shown in Figure 3B, the AG12A-ruthenium conjugate inhibited proliferation of MBP Ac1-11 specific autoreactive T cells potently at a concentration of 100nM (Figure 3B). This inhibition was not seen when CD4+ T cells from Myelin Oligodendrocyte Glycoprotein (MOG) 35–55 TCR transgenic mice were used (Figure 3C), demonstrating the specificity of AG12A for MBP Ac1-11 specific autoreactive T cells.
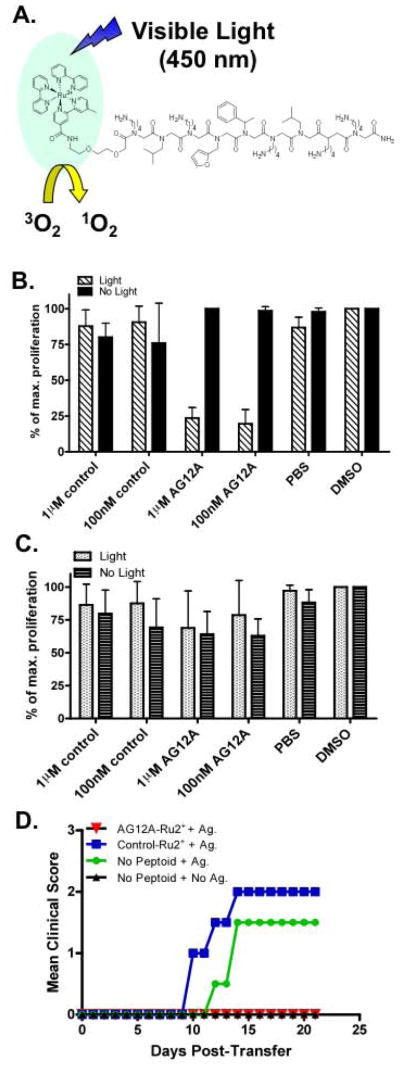
Figure 3. Specific inactivation of autoimmune T cells with a visible light-activated peptoid-“warhead” conjugate.
(A) Cartoon illustrating the photocatalytic destruction of the autoreactive TCR. AG12A was chemically coupled to Ru2+. Following incubation with the ruthenium peptoid complex, cells are irradiated with visible light (<380nm). Irradiation results in generation of singlet oxygen which will inactivate the target receptor. (B) CD4+ MBP Ac1-11 specific murine TCR transgenic T cells were isolated from B10.PL mice and incubated with 1μM or 100nM concentrations of AG12A-Ru2+, a control-Ru2+ peptoid, or solvent only (PBS or DMSO). Cells were either irradiated at <380nm for 10 minutes (hatched bars) or not exposed to light (black bars). Cultures were diluted with antigen presenting cells isolated from the spleens of wildtype B10.PL mice and stimulated with MBP Ac1-11 peptide at a final concentration of 10μg/ml. Proliferation was determined by adding [3H] thymidine to the cells for the final 16 hours of culture. Background levels of proliferation from cells that were not stimulated with antigen were subtracted from the results shown. (C) Same as panel (B) with the exception that CD4+ T cells used were isolated from MOG 35–55 specific 2D2 TCR transgenic mice. Proliferation of these cells was not affected by AG12A-Ru2+. (D) Treatment with AG12A-Ru2+ prevents adoptive transfer EAE. CD4+ T cells were isolated from MBP Ac1-11 specific TCR transgenic mice, incubated with 100nm AG12A-Ru2+ or control-Ru2+ peptoid and irradiated. Cells were then stimulated with antigen presenting cells and 10μg/ml MBP Ac1-11 peptide for 72 hours and transferred by i.p. injection to naïve B10.PL mice. Mice were monitored daily for clinical signs of EAE and mean clinical scores are depicted graphically for AG12A-Ru2+ (open circles), control-Ru2+ (open squares), antigen only (open triangles), and no antigen (stars) treated groups. All results shown are representative of 2 independent experiments.
In addition, we demonstrated that even in the absence of singlet oxygen production, peptoid AG12A is capable inhibiting proliferation of the MBP Ac1-11 specific autoreactive T cells in a dose-dependent fashion, but that it has no effect on the proliferation of mouse B cells or a different autoreactive T cell line that recognizes a different antigen (see Supplementary Fig. 4).
Photophoreresis therapies exist in which cells are removed from the patient, treated with a photoreactive drug, exposed to UV light, and re-infused back into the patient [14–16]. Thus, although the blue light required to trigger ruthenium tris-bipyridyl-catalyzed singlet oxygen production cannot penetrate significantly into a living organism, the ex vivo inactivation of autoimmune T cells by a peptoid-ruthenium conjugate seems feasible given this precedent. To test this theory and confirm that the autoreactive T cells have been rendered unresponsive following treatment with the peptoid-ruthenium conjugate and light, we used an adoptive transfer model of EAE. CD4+ T cells were isolated from MBP Ac1-11 TCR transgenic mice, treated with the AG12A-ruthenium conjugate or the control peptoid-ruthenium conjugate, irradiated with visible light, stimulated with MBP Ac1-11 peptide in the presence of antigen presenting cells, and injected back into naïve recipients. These animals were then observed for clinical signs of EAE. As anticipated, animals injected with antigen-stimulated autoreactive T cells that had been exposed to the control peptoid-ruthenium conjugate or no peptoid developed EAE (Figure 3D). When the T cells were neither stimulated with antigen nor exposed to a peptoid, adoptive transfer did not result in EAE, as expected. Strikingly, MBP Ac1-11 specific CD4+ T cells stimulated with antigen and treated with the AG12A-ruthenium conjugate did not induce EAE in the recipient animals (Figure 3D). This experiment demonstrates the feasibility of using autoreactive T cell-targeted ruthenium peptoid conjugates as potent photo-triggered inhibitors of autoimmune T cell activation ex vivo.
Significance
We have demonstrated here a combinatorial library screening protocol that is capable of yielding synthetic molecules that bind to antigen-specific autoimmune T cells. To the best of our knowledge, this is the first example of synthetic, unnatural molecules able to bind specifically to antigen-specific T cells without the requirement for MHC presentation. Moreover, an important feature of the screening technology by which these molecules were identified is that no knowledge of the native antigen recognized by the T cell is necessary. It is true that we took advantage of the well-characterized nature of the autoreactive T cells in EAE in order to validate the utility of AG12A, but the screen itself simply involved the identification of bead- displayed compounds that bind to cells that are much more abundant in one population than another. Therefore, this technology should constitute a powerful tool for the enrichment and inhibition of autoimmune cells in a variety of disease states.
Experimental Procedures
Bicolor on bead screening assay
To identify peptoids binding specifically to autoreactive TCRs, a bicolor on-bead screening assay was used as described previously [5] with minor modifications. Briefly, approximately 300,000 beads were swelled in DMF, washed with PBS, and equilibrated in complete RPMI 1640 media containing 3% BSA. CD4+ T cells isolated from either EAE or WT mice were resuspended in RPMI and labeled using quantum dots (Invitrogen) according to manufacturer’s instructions. CD4+ T cells from EAE mice were labeled with Qtracker 655 (red) and CD4+ T cells from WT mice were labeled with Qtracker 565 (green). Labeled cells were mixed in a 1:1 ratio with a total of approximately 10 × 106 of each cell type. The cells were then incubated with the peptoid bead library overnight in a 37° incubat or with 5% CO2 and gentle shaking. The beads were gently washed 2 times with RPMI media and were then visualized under a fluorescent microscope (Olympus BX-51) with excitation 340–380nm using a DAPI filter (100X total magnification). Beads binding only to red labeled cells were selected manually using a 20μl pipette. The “hit” beads were then washed, boiled with 1% SDS for 30 minutes and subjected to automated Edman sequencing.
Peptoid Library Synthesis
Details regarding design of the peptoid library have been published previously [5]. Briefly, the library was synthesized on TentaGel macrobeads (140–170μM diameter; substitution: 0.48 mmol/g resin; Rapp Polymere). Synthesis of the library was conducted using eight different amines resulting in a theoretical diversity of 262,144 compounds. A 9-mer library was synthesized using a microwave (1000 W) – assisted synthesis protocol and a split and pool method [17]. At the completion of library synthesis, beads were treated with a 95% TFA, 2.5% triisopropylsilane, and 2.5% water mixture for 2 hours to remove side chain protection groups and then neutralized with 10% diidoproplyethylamine in DMF. The beads were washed with dichloromethane, dried, and stored at 4°C until use.
Resynthesis of soluble peptoids
Resynthesis of peptoid ligands and scrambled control peptoids was conducted on Knorr amide MBHA resin (Novabiochem) using a standard microwave-assisted protocol [17] (1000 W microwave oven, 10% power delivered for 2 X 15 seconds with brief mixing in between). For biotinylated and biotin-DOPA peptoids, Fmoc-Glu(biotinyl-PEG)-OH (Novabiochem) and Fmoc-DOPA (Novabiochem) were subsequently coupled on Knorr amide MBHA resin by a standard peptide synthesis protocol using Fmoc chemistry [5]. A standard microwave-assisted protocol was used to create the peptoid portion of the molecules as described above. Peptoids were cleaved from the resin with 95% TFA, 2.5% triisopropylsilane, and 2.5% water for 2 hours, and purified using a Waters Breeze HPLC system. Mass of peptoids was detected using a MALDI-Voyager DE Pro mass spectrometer.
Mice
Female B10.PL mice and 2D2 MOG 35–55 TCR transgenic mice were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained in a federally approved animal facility at the University of Texas Southwestern Medical Center (Dallas, TX) in accordance with the Institutional Animal Care and Use Committee. B10.PL Vα2.3Vβ8.2 TCR transgenic mice were a kind gift from Dr. Olaf Stuve (UT Southwestern Medical Center, Dallas, TX) and were bred and maintained in our animal facility. All mice were between 7 and 10 weeks of age when experiments were performed.
EAE induction
EAE was induced in WT B10.PL mice by subcutaneous injection over 4 sites in the flank with 50μg of myelin basic protein peptide MBP Ac1-11 emulsified in completed Freund’s adjuvant. Pertussis toxin was administered at the time of immunization and 48 hours later by i.p. injection. Mice were monitored daily for clinical signs of EAE and given a clinical score based on the following criteria: 0 = no disease, 1= limp tail, 2= hind limb weakness, 3= severe hind limb weakness/partial paralysis, 4= hind limb paralysis, 5= moribund, and 6= death due to EAE [18]
CD4+ T cell isolation
Spleens and lymph nodes were isolated from EAE, WT, or TCR transgenic mice and single cell suspensions were made by passing through a 70μm nylon cell strainer (BD Biosciences). CD4+ T cells were then isolated by negative selection using a CD4+ T cell enrichment kit (BD Biosciences) according to manufacturer’s instructions. Briefly, a biotinylated mouse CD4+ T lymphocyte enrichment cocktail was added to the cell suspension. Addition of this cocktail results in labeling of erythrocytes and leukocytes that are not CD4+ T cells. Following washing, magnetic streptavidin particles were added to the suspension and all labeled cells migrated toward a magnet, leaving the unlabeled CD4+ T cells in suspension. The CD4+ T cells were retained and all other cells discarded. Following isolation, cells were washed, counted and resuspended in complete RPMI 1640 media for downstream applications. The purity of the isolated T cells was analyzed by flow cytometry and determined to be greater than 95%.
Flow cytometry binding assay
Following isolation of CD4+ T cells from TCR transgenic mice and WT controls, cells were washed and resuspended in 0.1% BSA in PBS (FACS buffer). The cells were incubated with increasing concentrations (1μM, 10μM, 100μM, 250μM, or 500μM) of either the biotin-DOPA-AG12A peptoid or a biotin-DOPA-control peptoid and incubated for 30 minutes at 37°C. 5mM sodium periodate was added to the cells for 15–30 seconds to cross-link the peptoid to the target receptor. This reaction was quenched with DTT and the cells were washed twice with .1% BSA in PBS. Fc block (BD Biosciences) was added to the cells for 15 minutes on ice in order to reduce non-specific binding to Fc receptors. The cells were stained with 1μg anti CD4-PerCp Cy5.5 antibody and 0.02μg streptavidin-APC antibody (BD Biosciences) for 15 minutes on ice. The staining was followed by 2 washes with .1% BSA in PBS and the cells were run on a FACS Calibur flow cytometer to assess peptoid binding. The data were analyzed using Flowjo software (Treestar) to determine the mean fluorescent intensity and are shown as histograms. The mean fluorescent intensities (MFI) were plotted using Graphpad Prism software to determine an estimated Kd value and are depicted as a line graph.
Preparation of ruthenium-peptoid conjugates
Bis(2,2′-bipyridine)-4′-methyl-4-carboxybipyridine-ruthenium-bis(hexafluorophosphate), diisopropyl carbodiimide, and HOBt were dissolved in DMF and reacted with the previously generated deprotected peptoids for 2 hours at room temperature [13]. The compounds were washed and cleaved from the resin as described above and purified with HPLC. The mass of each peptoid was determined using a MALDI-Voyager DE Pro mass spectrometer.
Tritiated thymidine incorporation proliferation assay
Spleens from naïve Vα2.3/Vβ8.2 TCR transgenic mice or 2D2 MOG 35–55 TCR transgenic mice were harvested and single cell suspensions were made by pressing through a 70μm cell strainer (BD Biosciences). CD4+ T cells were isolated as described above and resuspended in phenol red-free complete RPMI media. 1X105 cells per well were plated in a 96 well plate and incubated with 1μM or 100nM concentrations of AG12A-Ru2+, control peptoid-Ru2+, DMSO, or PBS in quadruplicate. Cells were then irradiated for 10 minutes using a 150 W Xenon arc lamp (Oriel, Stamford, CT) as described previously [13]. Following irradiation, T cells were activated with 10μg/ml of MBP Ac1-11 and 3X105 antigen presenting cells per well. Cultures were maintained in 96-well flat-bottom plates for 96 h at 37°C in h umidified 5% CO2/air. The wells were pulsed with 0.5 μCi/well [methyl-3H]thymidine for the final 16 h of culture. Cells were harvested on glass filters and incorporated [methyl-3H]thymidine was measured with a Betaplate counter (PerkinElmer Wallac, Gaithersburg, MD). Background levels of proliferation from cells that were not stimulated with antigen were subtracted to determine the percent of maximum proliferation for each condition. The results were determined as means from quadruplicate cultures and are shown with SEM.
Adoptive Transfer
Spleens from naïve Vα2.3/Vβ8.2 TCR transgenic mice were harvested and single cell suspensions were prepared by pressing through a 70μm cell strainer (BD Biosciences). CD4+ T cells were isolated, treated with AG12A-Ru2+ or control peptoid-Ru2+, irradiated, and activated with MBP Ac1-11 as described above. After 72 h, the cells were washed with PBS and 10× 106 cells were injected i.p. into naive B10.PL mice. The mice were evaluated daily for clinical signs of EAE as previously described [18].
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Director’s Pioneer Award (DP1OD000663).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hemmer B, Hartung HP. Toward the development of rational therapies in multiple sclerosis: what is on the horizon? Ann Neurol. 2007;62:314–326. doi: 10.1002/ana.21289. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 3.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 4.Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Marlowe CK, et al. Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci U S A. 1992;89:9367–9371. doi: 10.1073/pnas.89.20.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. A peptoid “antibody surrogate” that antagonizes VEGF receptor 2 activity. J Amer Chem Soc. 2008;130:5744–5752. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 6.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10. PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 7.Alluri PG, Reddy MM, Bachhawat-Sikder K, Olivos HJ, Kodadek T. Isolation of protein ligands from large peptoid libraries. J Am Chem Soc. 2003;125:13995–14004. doi: 10.1021/ja036417x. [DOI] [PubMed] [Google Scholar]
- 8.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 9.Burdine L, Gillette TG, Lin HJ, Kodadek T. Periodate-triggered cross-linking of DOPA-containing peptide-protein complexes. J Amer Chem Soc. 2004;126:11442–11443. doi: 10.1021/ja045982c. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Burdine L, Kodadek T. Chemistry of periodate-mediated cross-linking of 3,4-dihydroxylphenylalanine (DOPA)-containing molecules to proteins. J Amer Chem Soc. 2006;128:15228–15235. doi: 10.1021/ja065794h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim HS, Cai D, Archer C, Kodadek T. Periodate-triggered cross-linking reveals Sug2/Rpt4 as the molecular target of a peptoid inhibitor of the 19S proteasome regulatory particle. J Amer Chem Soc. 2007;129:12936–12937. doi: 10.1021/ja075469+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodadek T, Reddy MM, Olivos HJ, Bachhawat-Sikder K, Alluri PG. Synthetic molecules as antibody replacements. Acc Chem Res. 2004;37:711–718. doi: 10.1021/ar030145l. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Yu P, Xiao X, Kodadek T. A general system for evaluating the efficiency of chromophore-assisted light inactivation (CALI) of proteins reveals Ru(II) tris-bipyridyl as an unusually efficient “warhead”. Mol Biosyst. 2008;4:59–65. doi: 10.1039/b712307h. [DOI] [PubMed] [Google Scholar]
- 14.Rostami AM, Sater RA, Bird SJ, Galetta S, Farber RE, Kamoun M, Silberberg DH, Grossman RI, Pfohl D. A double-blind, placebo-controlled trial of extracorporeal photopheresis in chronic progressive multiple sclerosis. Mult Scler. 1999;5:198–203. doi: 10.1177/135245859900500310. [DOI] [PubMed] [Google Scholar]
- 15.Besnier DP, Chabannes D, Mussini JM, Dupas B, Esnault VL. Extracorporeal photochemotherapy for secondary chronic progressive multiple sclerosis: a pilot study. Photodermatol Photoimmunol Photomed. 2002;18:36–41. doi: 10.1034/j.1600-0781.2002.180106.x. [DOI] [PubMed] [Google Scholar]
- 16.Cavaletti G, Perseghin P, Dassi M, Cavarretta R, Frigo M, Caputo D, Stanzani L, Tagliabue E, Zoia C, Grimaldi M, Isella V, Rota S, Ferrarese C, Frattola L. Extracorporeal photochemotherapy: a safety and tolerability pilot study with preliminary efficacy results in refractory relapsing-remitting multiple sclerosis. Neurol Sci. 2006;27:24–32. doi: 10.1007/s10072-006-0561-7. [DOI] [PubMed] [Google Scholar]
- 17.Olivos HJ, PGA, Reddy MM, Saloney D, Kodadek T. Microwave-assisted solid phase synthesis of peptoids. Org Lett. 2002;4:4057–4059. doi: 10.1021/ol0267578. [DOI] [PubMed] [Google Scholar]
- 18.Racke MK. Experimental autoimmune encephalomyelitis (EAE) Curr Protoc Neurosci . 2001;Chapter 9(Unit9):7. doi: 10.1002/0471142301.ns0907s14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.